Abstract
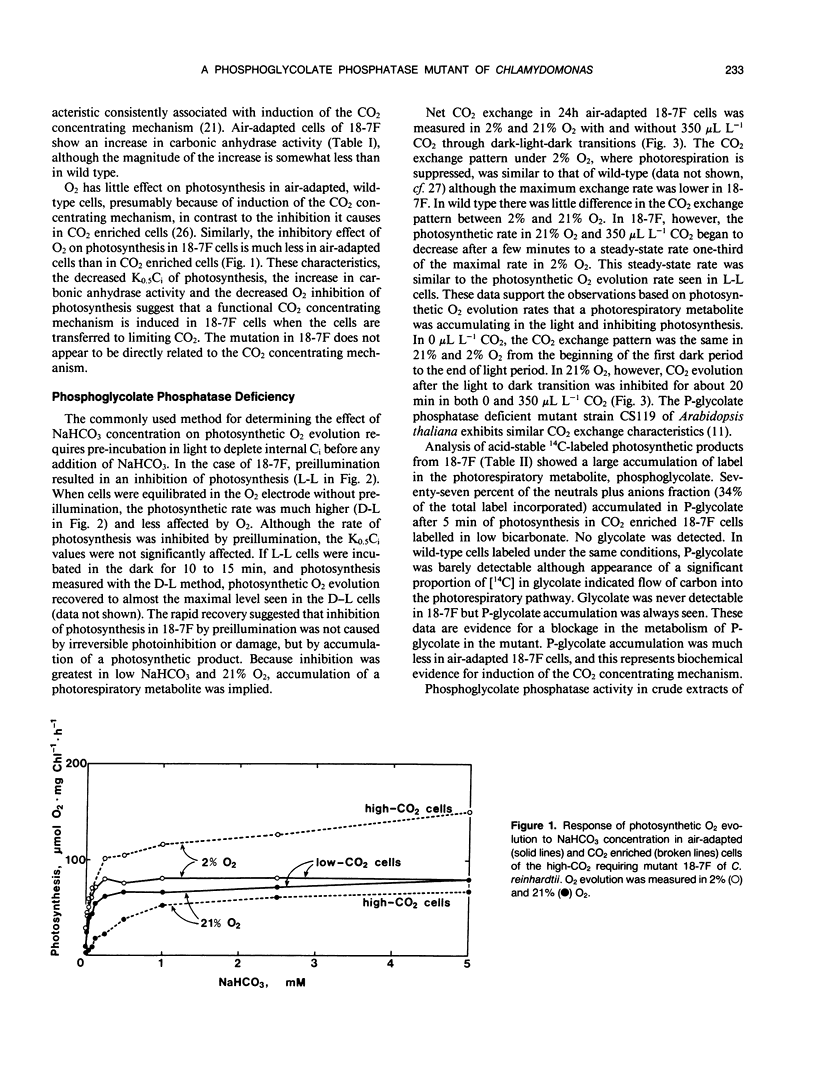
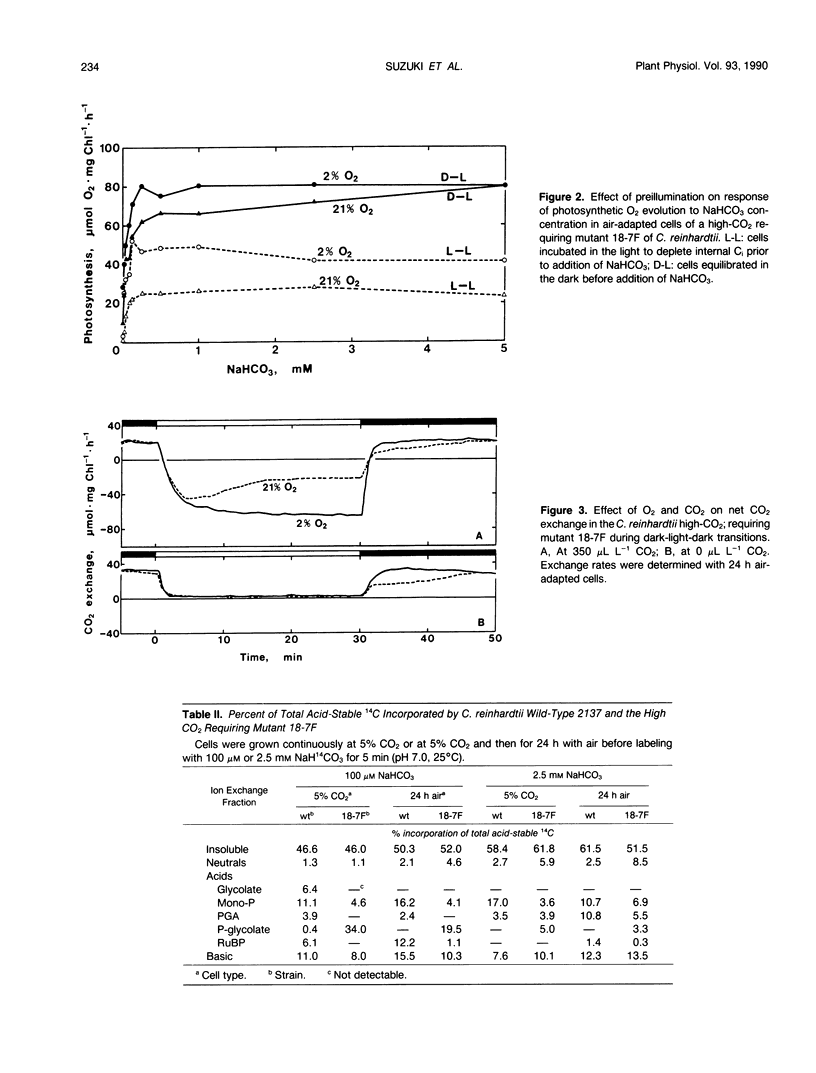
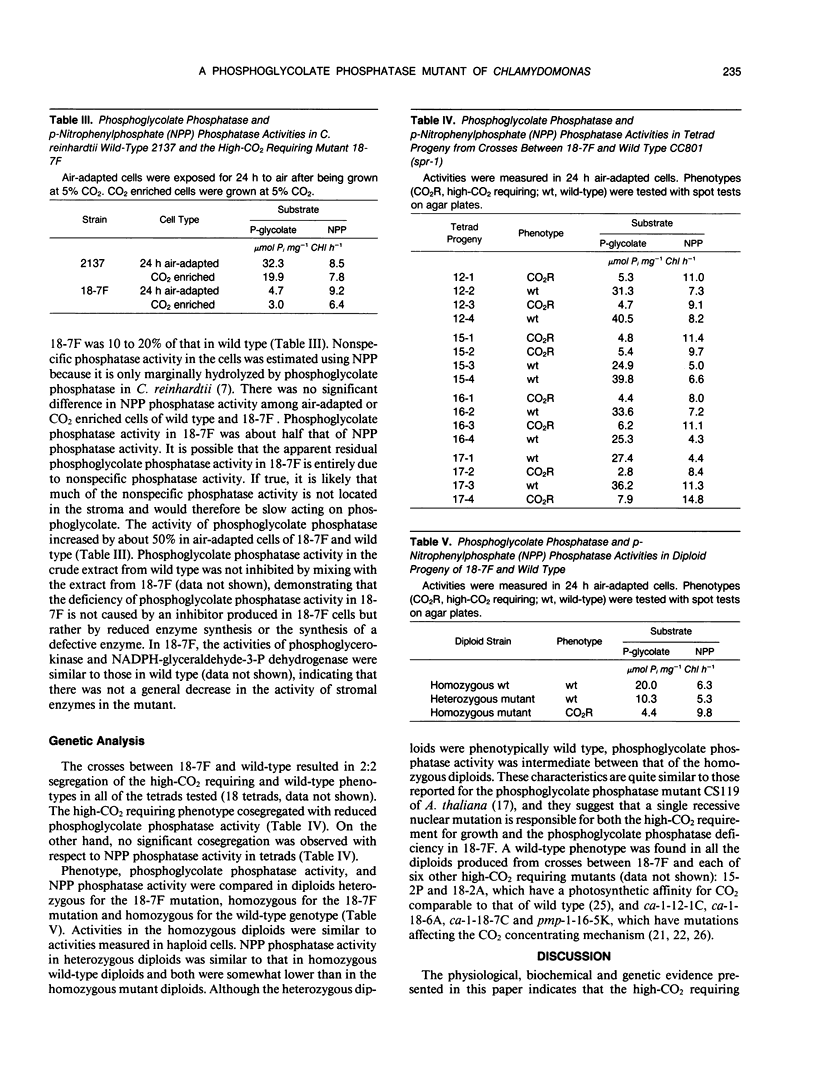
A mutant strain of Chlamydomonas reinhardtii, designated 18-7F, has been isolated and characterized. 18-7F requires a high CO2 concentration for photoautrophic growth in spite of the apparent induction of a functional CO2 concentrating mechanism in air-adapted cells. In 2% O2 the photosynthetic characteristics of 18-7F and wild type are similar. In 21% O2, photosynthetic O2 evolution is severely inhibited in the mutant by preillumination in limiting CO2, although the apparent photosynthetic affinity for inorganic carbon is similar in preilluminated cells and in cells incubated in the dark prior to O2 evolution measurements. Net CO2 uptake is also inhibited when the cells are exposed to air (21% O2, 0.035% CO2, balance N2) for longer than a few minutes. [14C]Phosphoglycolate accumulates within 5 minutes of photosynthetic 14CO2 fixation in cells of 18-7F. Phosphoglycolate does not accumulate in wild type. Phosphoglycolate phosphatase activity in extracts from air-adapted cells of 18-7F is 10 to 20% of that in wild-type Chlamydomonas. The activity of phosphoglycolate phosphatase in heterozygous diploids is intermediate between that of homozygous mutant and wild-type diploids. It was concluded that the high-CO2 requiring phenotype in 18-7F results from a phosphoglycolate phosphatase deficiency. Genetic analyses indicated that this deficiency results from a single-gene, nuclear mutation. We have named the locus pgp-1.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E. Chloroplast and cytoplasmic enzymes. II. Pea leaf triose phosphate isomerases. Biochim Biophys Acta. 1971 Apr 14;235(1):237–244. doi: 10.1016/0005-2744(71)90051-9. [DOI] [PubMed] [Google Scholar]
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husic H. D., Tolbert N. E. Properties of Phosphoglycolate Phosphatase from Chlamydomonas reinhardtii and Anacystis nidulans. Plant Physiol. 1985 Oct;79(2):394–399. doi: 10.1104/pp.79.2.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan A., Berry J. A. Glycolate Excretion and the Oxygen to Carbon Dioxide Net Exchange Ratio during Photosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 1981 Feb;67(2):229–232. doi: 10.1104/pp.67.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Y., Harel E., Kaplan A. Adaptation of the Cyanobacterium Anabaena variabilis to Low CO(2) Concentration in Their Environment. Plant Physiol. 1983 Jan;71(1):208–210. doi: 10.1104/pp.71.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Husic H. D., Tolbert N. E., Kitayama M., Manuel L. J., Togasaki R. K. Isolation and Characterization of a Mutant of Chlamydomonas reinhardtii Deficient in the CO(2) Concentrating Mechanism. Plant Physiol. 1989 Mar;89(3):897–903. doi: 10.1104/pp.89.3.897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroney J. V., Wilson B. J., Tolbert N. E. Glycolate Metabolism and Excretion by Chlamydomonas reinhardtii. Plant Physiol. 1986 Nov;82(3):821–826. doi: 10.1104/pp.82.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Paech C., Dybing C. D. Purification and degradation of ribulose bisphosphate carboxylase from soybean leaves. Plant Physiol. 1986 May;81(1):97–102. doi: 10.1104/pp.81.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Jeffrey M. Membrane-Associated Polypeptides Induced in Chlamydomonas by Limiting CO(2) Concentrations. Plant Physiol. 1989 Jan;89(1):133–137. doi: 10.1104/pp.89.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Reduced Inorganic Carbon Transport in a CO(2)-Requiring Mutant of Chlamydomonas reinhardii. Plant Physiol. 1983 Oct;73(2):273–276. doi: 10.1104/pp.73.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreitzer R. J., Mets L. Photosynthesis-deficient Mutants of Chlamydomonas reinhardii with Associated Light-sensitive Phenotypes. Plant Physiol. 1981 Mar;67(3):565–569. doi: 10.1104/pp.67.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K., Spalding M. H. Adaptation of Chlamydomonas reinhardtii High-CO(2)-Requiring Mutants to Limiting CO(2). Plant Physiol. 1989 Jul;90(3):1195–1200. doi: 10.1104/pp.90.3.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder T., Spalding M. H. Imazaquin and chlorsulfuron resistance and cross resistance in mutants of Chlamydomonas reinhardtii. Mol Gen Genet. 1988 Aug;213(2-3):394–399. doi: 10.1007/BF00339608. [DOI] [PubMed] [Google Scholar]
- Wintermans J. F., de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965 Nov 29;109(2):448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]


