Abstract
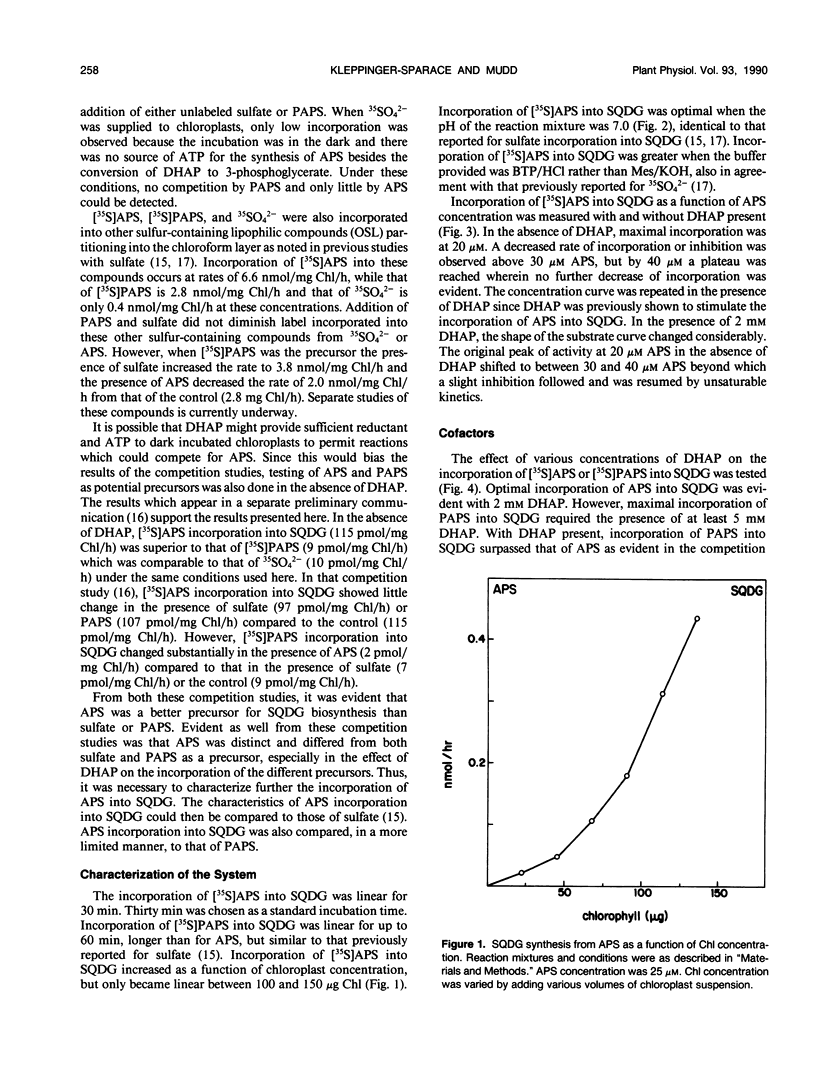
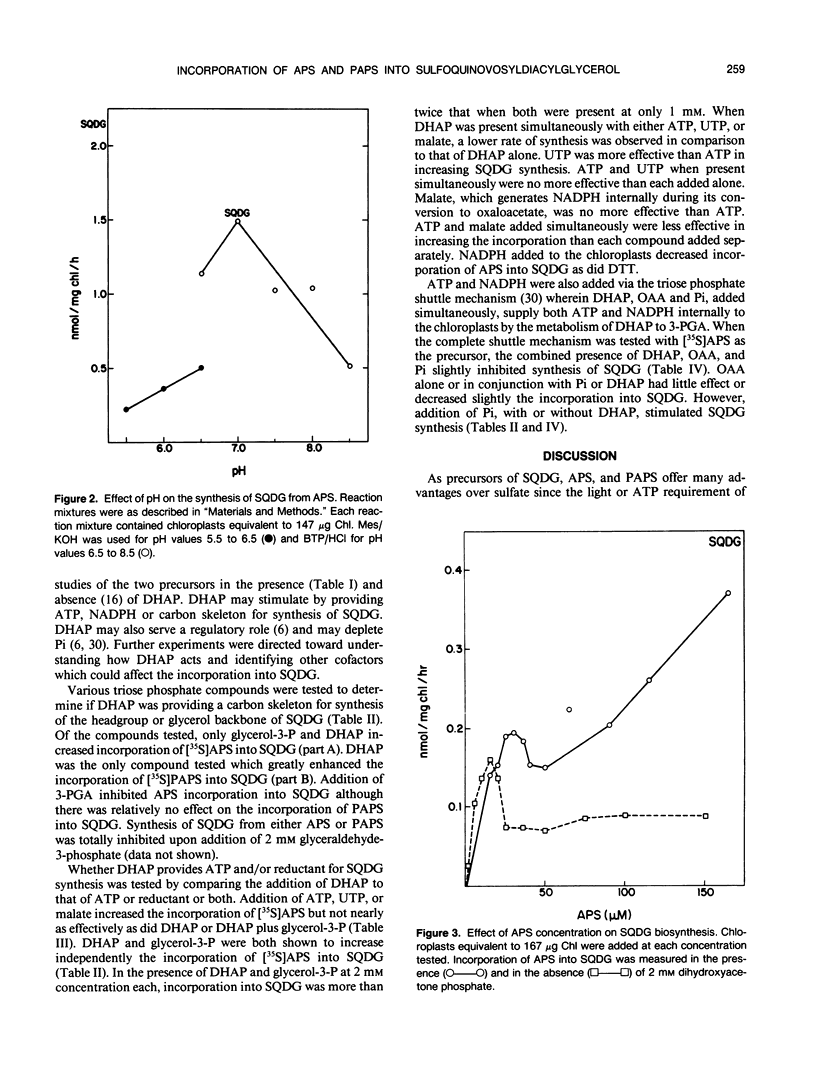
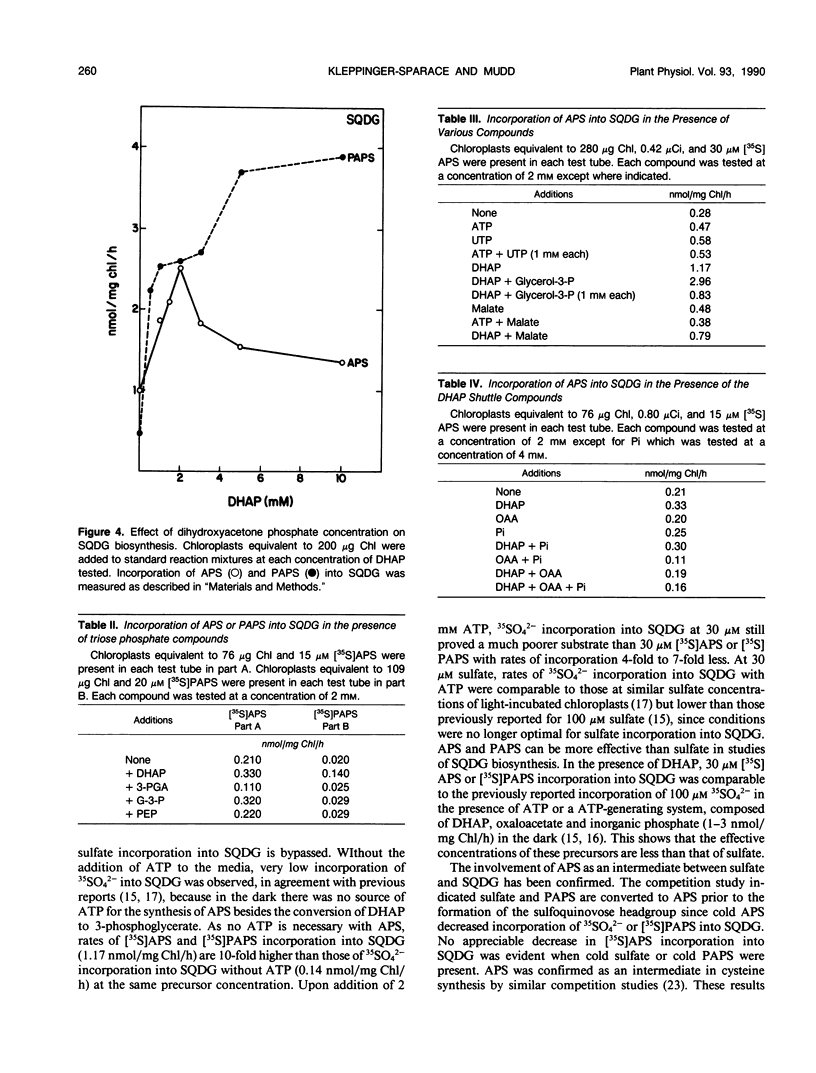
Adenosine-5′-phosphosulfate (APS) and adenosine-3′-phosphate 5′-phosphosulfate (PAPS) have been used as precursors of sulfoquinovosyldiacylglycerol (SQDG) in intact chloroplasts incubated in the dark. Competition studies demonstrated APS was preferred over PAPS and SO42−. Rates of SQDG synthesis up to 3 nanomoles per milligram of chlorophyll per hour were observed when [35S]APS and appropriate cofactors were supplied to chloroplasts incubated in the dark. The pH optimum for utilization of APS was 7.0. The incorporation was linear for at least 30 minutes. ATP and UTP stimulated the incorporation of sulfur from APS into SQDG, but the most stimulatory additions were DHAP and glycerol-3-P. The concentration curve for APS showed a maximum at 20 micromolar in the absence of DHAP and 30 micromolar in the presence of DHAP. The optimum concentration of DHAP for conversion of APS into SQDG was 2 millimolar. Rates of synthesis up to 4 nanomoles per milligram of chlorophyll per hour were observed when [35S]PAPS was the sulfur donor and appropriate cofactors were supplied to chloroplasts. Optimal rates for conversion of sulfur from PAPS into SQDG occurred with concentrations of DHAP between 5 and 10 millimolar. DHAP was by far the most effective cofactor, although ATP and UTP also stimulated the utilization of PAPS for SQDG biosynthesis. In general, triose phosphates, including glycerol-3-P were not effective cofactors for SQDG biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAHAM A., BACHHAWATBK Effect of light and streptomycin on the incorporation of sulfate into sulfolipids in Euglena gracilis. Biochim Biophys Acta. 1963 Feb 19;70:104–106. doi: 10.1016/0006-3002(63)90729-7. [DOI] [PubMed] [Google Scholar]
- BENSON A. A. THE PLANT SULFOLIPID. Adv Lipid Res. 1963;1:387–394. doi: 10.1016/b978-1-4831-9937-5.50016-8. [DOI] [PubMed] [Google Scholar]
- Burnell J. N., Anderson J. W. Adenosine 5'-sulphatophosphate kinase activity in spinach leaf tissue. Biochem J. 1973 Jun;134(2):565–579. doi: 10.1042/bj1340565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver K. A., Hope A. B., Walker D. A. Adenine nucleotide status, phosphoglycerate reduction and photosynthetic phosphorylation in a reconstituted chloroplast system. Biochem J. 1983 Jan 15;210(1):273–276. doi: 10.1042/bj2100273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee R. W., Byerrum R. U., Gerber D. W., Tolbert N. E. Differential inhibition and activation of two leaf dihydroxyacetone phosphate reductases : role of fructose 2,6-bisphosphate. Plant Physiol. 1988 Jun;87(2):379–383. doi: 10.1104/pp.87.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J. L. Synthesis of sulphoquinovosyl diacylglycerol by higher plants. Biochim Biophys Acta. 1975 Aug 25;398(2):224–230. doi: 10.1016/0005-2760(75)90138-1. [DOI] [PubMed] [Google Scholar]
- Herzog B., Stitt M., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : III. Properties of the Cytosolic Fructose 1,6-Bisphosphatase. Plant Physiol. 1984 Jul;75(3):561–565. doi: 10.1104/pp.75.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyard J., Forest E., Blée E., Douce R. Characterization of elemental sulfur in isolated intact spinach chloroplasts. Plant Physiol. 1988 Dec;88(4):961–964. doi: 10.1104/pp.88.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppinger-Sparace K. F., Mudd J. B. Biosynthesis of Sulfoquinovosyldiacylglycerol in Higher Plants: The Incorporation of SO(4) by Intact Chloroplasts in Darkness. Plant Physiol. 1987 Jul;84(3):682–687. doi: 10.1104/pp.84.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppinger-Sparace K. F., Mudd J. B., Bishop D. G. Biosynthesis of sulfoquinovosyldiacylglycerol in higher plants: the incorporation of 35SO4 by intact chloroplasts. Arch Biochem Biophys. 1985 Aug 1;240(2):859–865. doi: 10.1016/0003-9861(85)90096-7. [DOI] [PubMed] [Google Scholar]
- Sawhney S. K., Nicholas D. J. Effects of adenine nucleotides and phosphate on adenosine triphosphate sulphurylase from Anabaena cylindrica. Biochem J. 1977 Apr 15;164(1):161–167. doi: 10.1042/bj1640161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. H., Anderson J. W. Purification, properties and substrate specificity of adenosine triphosphate sulphurylase from spinach leaf tissue. Biochem J. 1972 Mar;127(1):237–247. doi: 10.1042/bj1270237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Lilley R. M., Heldt H. W. Adenine nucleotide levels in the cytosol, chloroplasts, and mitochondria of wheat leaf protoplasts. Plant Physiol. 1982 Oct;70(4):971–977. doi: 10.1104/pp.70.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werdan K., Heldt H. W., Milovancev M. The role of pH in the regulation of carbon fixation in the chloroplast stroma. Studies on CO2 fixation in the light and dark. Biochim Biophys Acta. 1975 Aug 11;396(2):276–292. doi: 10.1016/0005-2728(75)90041-9. [DOI] [PubMed] [Google Scholar]
- Zenker H., Brandt H. P. Netzhautarterienverschluss und Wetter. Klin Monbl Augenheilkd. 1966;148(2):238–244. [PubMed] [Google Scholar]


