Summary
Background
Tobacco cessation is proven to be the most effective and cost-effective strategy for smokers to reduce their risk of smoking-related disease and premature death. Providing effective, efficient, safe, and patient-centred tobacco cessation treatment to reach those who need them is a significant challenge. To date, only a few nationwide studies in China have assessed the overall clinical care practice and treatment outcome of tobacco cessation.
Methods
This a prospective, nationwide, multicenter, cohort study covering all Eastern China, Northwest China, Central China, North China, Southwest China, Northeast China, and South China. Participants who were current smokers aged 18–85 years attending clinic for smoking cessation were included. All the participants were treated with 3-month cessation treatment and followed up for 3 months. Data were collected prospectively using online system. The primary outcome was 7-day point abstinence rate at 24 weeks, validated biochemically by an expired carbon monoxide level of less than 10 ppm. The participants lost to follow-up or not providing validation were included as non-abstainers.
Findings
A representative sample of 3557 participants were recruited and 2943 participants were included into this analysis. These participants had mean age of 53.05 years, and 94.8% were males, with 75.8% showing symptoms of tobacco dependence. A total of 965 (32.8%) participants were treated with Bupropion + behavioural counselling, followed by 935 (31.8%) with behavioural counselling, 778 (26.4%) with Varenicline + behavioural counselling, 135 (4.6%) with alternative treatments + behavioural counselling, and 130 (4.4%) with nicotine replacement therapy (NRT) + behavioural counselling. After 3-month treatment and 3-month follow-up, 21.74% of the participants quit smoking at 24 weeks. In the multivariable-adjusted analyses, quitting smoking was significantly associated with female, higher socioeconomic status, poor health condition, different treatment received, and less smoking intensity. The tobacco cessation treatment varied widely across different areas of China. In particular, the areas with higher usage of cessation medication were associated with better cessation treatment outcome.
Interpretation
The CNTCCS is the first large-scale nationwide cohort study of smoking cessation in China. Rich data collected from this prospective cohort study provided the opportunity to evaluate the clinical practice of tobacco cessation treatment in China.
Funding
Chinese Academy of Medical Sciences (CAMS) Initiative for Innovative Medicine (CAMS 2021-I2M-1-010), Heilongjiang Provincial Science and Technology Key Program (2022ZXJ03C02), and National Key R&D Program of China (grant no. 2017YFC1309400).
Keywords: Smoking, Smoking cessation, Cohort study, China National Tobacco Cessation Cohort Study (CNTCCS)
Research in context.
Evidence before this study
We searched PubMed and China National Knowledge Infrastructure database for articles published up to March 8, 2023 using the terms “tobacco cessation”, “cohort study”, “real world study”, and “China”. We screened papers by reviewing abstracts to identify full-text reports that were relevant to the study aims. As a result, we found there was no nationwide cohort study of tobacco cessation in China and world, and the current real-world study of tobacco cessation were mostly based on Caucasians populations.
Added value of this study
To the best of our knowledge, China National Tobacco Cessation Cohort Study (CNTCCS) is the first and largest cohort study for tobacco cessation in China. Our data indicated that approximately 1 out of 5 smokers quit smoking after 6 months; successful quitting smoking was significantly associated with female, higher socioeconomic status, poor health condition, different treatment received, and less smoking intensity. The tobacco cessation treatment varied widely across different areas of China. In particular, the areas with higher usage of cessation medication were associated with higher cessation treatment outcome.
Implications of all the available evidence
The data obtained could be used to better understand the “real world” situation of tobacco cessation practice in China. It will also serve as an evidence-based platform for conducting future research, which will ultimately improve the treatment and management provided to smokers.
Introduction
Tobacco use is a significant threat to human health and social development, causing an estimated 8 million annual deaths worldwide from a wide range of chronic non-communicable diseases.1 China, which accounts for more than 20% of the world population, consumes more than 40% of the world's total cigarettes, with more than one million annual deaths from tobacco.2 Moreover, approximately half of current smokers in China are nicotine dependent, translating to 183.5 million adults.3
Tobacco cessation is proven to be the most effective and cost-effective strategy for smokers to reduce their risk of smoking-related disease and premature death.4 Smokers who give up smoking before the age of 40 can reduce their risk of dying from smoking-related diseases by 90% compared to that of persistent smokers.5 In addition, tobacco cessation could also improve quality of life and increase lifespan.6
Currently, evidence-based tobacco cessation strategies include population-level interventions (brief advice, quit lines, mCessation), individual specialist approaches (behavioral support, cessation clinics) and pharmacologic interventions (nicotine replacement therapies and non-nicotine pharmacotherapies).7 Implementing these measures has been shown to result in a 2–15% increase in the proportion of tobacco users who quit tobacco use for 6 months or more, over no intervention.8
However, providing effective, efficient, safe, and patient-centred tobacco cessation treatment to reach those who need them is a significant challenge. To date, only a few nationwide studies9, 10, 11 have discussed the clinical care practice of tobacco cessation in China.
To help fill the evidence gap, a nationwide multicenter cohort study, China National Tobacco Cessation Cohort Study (CNTCCS), was initiated in December 2017. As the first national cohort study of tobacco cessation in China, CNTCCS aimed to (1) evaluate the treatment outcome and longitudinal trends of tobacco cessation during 6-month follow-up period, (2) identify the predictors of successful tobacco cessation; (3) measure the regional-level variation in cessation treatment after adjusting for provider, practice, and patient characteristics; (4) promote the standardization of diagnosis and treatment of tobacco cessation; and (5) provide real-world evidence for policy-making of tobacco control in China.
This study described the baseline characteristics of the participants recruited before June of 2021, and analyzed the treatment outcomes of the participants whose 6-month follow-up data were available. In addition, this study aimed to propose several key measures for improving tobacco cessation practice in China. By providing a detailed analysis of baseline characteristics and treatment outcomes, this study could help inform China public health policy in the future.
Methods
Study design
The China National Tobacco Cessation Cohort Study (CNTCCS) is an ongoing nationwide multicenter prospective cohort study of tobacco cessation with a 6-month follow-up period in a real-world setting. Currently a total of 27 centers (hospitals) dispersing all 7 areas of China (Eastern China. Northwest China, Central China, North China, Southwest China, Northeast China, and South China) participated in this study. A complete list of CNTCCS members and sites were presented in Appendix Table S1. The study protocol was presented in Appendix Material.
The CNTCCS has complied with all relevant ethical regulations, and was approved by Institutional Review Boards at all study centers. Informed consent was obtained from all participants of the study. The CNTCCS was registered in the Chinese Clinical Trial Registry (No. ChiCTR1800016919, URL: http://www.chictr.org.cn).
Study participants
The CNTCCS has recruited consecutive smokers who seek cessation treatment since December 2017. The smokers were included into CNTCCS if they met all the following criteria: (1) voluntarily participated in CNTCCS; (2) aged 18–85 years; (3) current smoking; (4) willing to quit smoking; (5) expired air carbon monoxide no less than 10 ppm; (6) signed the informed consent form.
The smokers were excluded if they had severe cardiovascular diseases (eg, acute myocardial infarction), cerebrovascular diseases (eg, stroke), neuropsychiatric disorders severe impairment of liver and kidney function (eg, renal failure), being pregnant or breastfeeding, and had severe psychiatric illness (eg, seizure and anorexia), or were allergic to cessation medications.
All patients were informed of the nature and aims of the study and signed an informed consent form.
Baseline data collection
A standard data collection protocol was developed by the steering committee. All study physicians had participated the China Training Courses for Stop-Smoking Specialists,12 and given a specific training by China–Japan Friendship Hospital during the kick-off meeting of CNTCS.
For each participant, a baseline survey was conducted to collect information. The demographics information included gender, age, ethnicity, education, marital status, income, and residential location; health-related characteristics included self-reported health status, blood pressure, body weight, medical history (such as CVD, cancer, respiratory diseases, depression, Anxiety), and alcohol use; tobacco-related characteristics included diagnosis of tobacco dependence, number of cigarettes smoked per day, and duration of smoking; Fagerstrom Test for Nicotine Dependence (FTND),13 and expired carbon monoxide (CO) reading.
The diagnostic criteria of tobacco dependence3 were based on international criteria (ICD-10, DSM-4) and tailored to Chinese population according to China Clinical Guideline for Tobacco Cessation (2015 version): tobacco dependence was diagnosed if they had 3 or more of the following 6 symptoms or signs: (1) craving, or a strong desire or urge to use tobacco; (2) a persistent desire or unsuccessful efforts to cut down or control tobacco use; (3) experiencing tobacco withdrawal symptoms (such as irritability, frustration, anger, anxiety, difficulty concentrating, increased appetite, restlessness, insomnia) after abrupt cessation of tobacco use, or reduction in the amount of tobacco used; (4) tolerance, defined as the need for markedly increased amounts of tobacco to achieve the desired effect; (5) given up or reduced important social, occupational, or recreational activities because of tobacco use; and (6) continued tobacco use despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by tobacco.
All the CO level was measured by using Bedfont Micro Smokerlyzers (Bedfont Scientific, UK), which was calibrated per year. All the study centers used the same machine.
Tobacco cessation treatment
The tobacco cessation treatment was categorized as Varenicline + behavioural counselling, Bupropion + behavioural counselling, nicotine replacement therapy (NRT) + behavioural counselling, behavioural counselling and alternative treatments (treatments not recommended by China Clinical Guideline for Tobacco Cessation, including acupuncture and traditional Chinese herb decoction) + behavioural counselling.
For the benefits of the participants, although CNTCCS was an observational study, we required all the study centers to provide tobacco cessation treatment based on China Clinical Guideline for Tobacco Cessation (2015 version).14 The keys of tobacco cessation treatment included:
-
•
All the participants should be screened for tobacco dependence.
-
•
All the participants are suggested to set a target quit date (TQD) within 2 weeks after medication.
-
•
Simply encouraging participants to stop smoking is insufficient. All participants should be provided with evidence-based treatment to help them stop.
-
•
All the participants are advised to receive 12 weeks of treatment.
-
•
The clinician should follow both currently-available scientific evidence and patient's preference for a proper choice of one therapy over another, paying particular attention to those patients having contraindications to these drugs due to the presence of specific comorbidities.
-
•
For the participants who are prescribed with medications, the medication use is explained at baseline assessment. Participants are encouraged to use their medications from the next day, and to stop smoking completely from their TQD onward.
-
•
The standard practice was to prescribe a one-month supply. However, if participants specifically requested a three-month supply, the physicians had the discretion to accommodate this request.
Follow-up data collection
All the participants were required to make face-to-face visits at study site at 1, 2, 4, 6, 9, 12, and 24 weeks after initiation of treatment. Participants were also included in a WeChat discussion group where they receive reminders for follow-up visits. Each follow-up visit takes approximately 30–45 min.
The follow-up questionnaire included self-reported information on smoking status, medication use, and CO readings. The follow-up questionnaires were identical for all follow-up periods. The interviewers entered the data collected into the case report form (CRF).
At each site, all data from each CRF were manually checked for completeness and correctness by a research staff. Beijing Natureself Technology Development Co., Ltd, an independent organization, served as the on-line centre of aggregated de-identified data. An online system of Case Report Form (CRF) developed by the expert advisory panel were used for data collection (https://jieyan.einmatrix.com).
Outcome assessments
The primary outcome (successful smoking quitting) is 7-day point abstinence rate at 24 weeks, validated biochemically by an expired carbon monoxide level of less than 10 ppm.15
Secondary outcomes included validated 7-day point abstinence rate at 1 week, 2 weeks, 4 weeks, 6 weeks, 9 weeks and 12 weeks; the CO-validated abstinence rate in different subgroups; risk factors of successful quit rate at 24 weeks; treatment usage rate across different regions of China. For all abstinence outcomes, participants lost to follow-up or not providing validation will be included as non-abstainers.
Statistical analysis
Based on our earlier study16 and previous large trials,17, 18, 19 we estimated the quit rate in this study was 30% in the Varenicline group and 20% in the behavioral counselling group. Then sample size of 300 per group was needed to provide 80% power, assuming a 20% loss to follow-up. As such, the total sample size was a minimum of 1500.
The intent-to-treat (ITT) approach was applied in this analysis, and the participants who lost contact were recognized to be smokers. The measurement data are presented as means (SD). The t-test was used for comparisons which met Gaussian distribution and homogeneity of variance, whereas the nonparametric test was used for comparisons which did not meet homogeneity of variance. The categorical variables were presented with numbers (percentages), and chi-square test was used for numeral data comparisons. We utilized logistic regression analysis to measure the relationship between potential influencing factors and successful smoking quitting (primary outcome), represented with OR value at the 95% confidence interval.
SPSS 19.0 statistical software (SPSS, Inc.) was used for statistical analysis. P < 0.05 was regarded as statistical significance. The authors had no access to information that could identify individual participants during or after data collection.
Patient and public involvement
Participants of the CNTCCS were not involved in setting the research question or the outcome measures, nor were they involved in developing plans for recruitment, design, or implementation of the study. No participants were asked for advice on interpreting or writing up of results. We intended to engage participants and the public to disseminate the results of our study.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, and data interpretation, or writing of the report. All authors had full access to all the data in the study and accepted the responsibility to submit it for publication.
Results
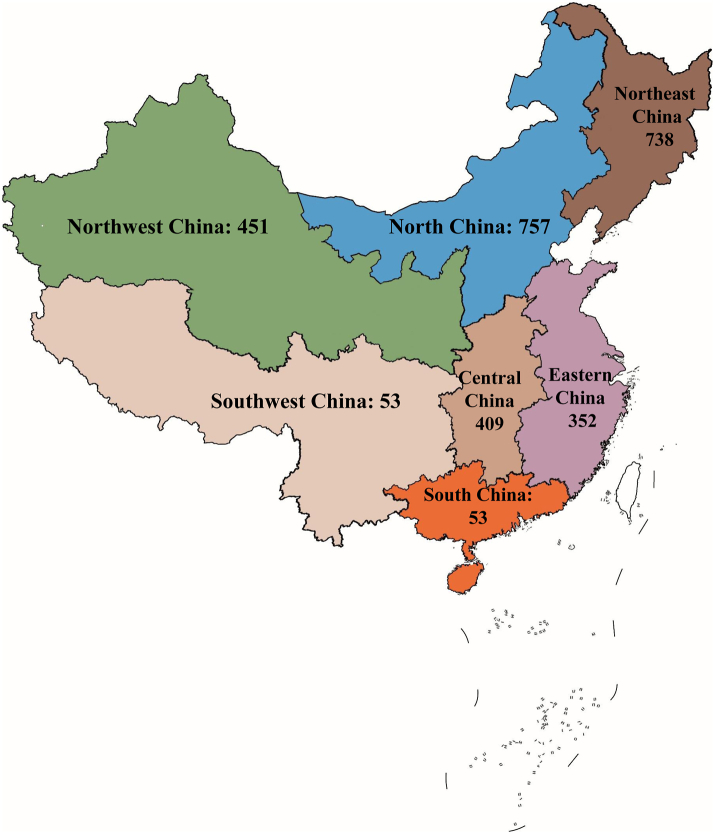
Between December 2017 and January 2022, a total of 3918 adults were screened for eligibility, and 3557 participants who met the study criteria were recruited. Of these, 614 participants who provided unreliable data were excluded, and 2943 participants were included in this analysis. The geographical distribution of the participants is shown in Fig. 1. The flow chart of participants is shown in Appendix Fig. S1.
Fig. 1.
Geographical distribution of the participants (N = 2943).
The baseline characteristics of participants are summarized in Table 1. Overall, 94.8% of the participants were male; the average age (SD) was 53.05 (13.72) years; 90.5% were married, 40.1% had education level of college and higher, and 68.5% had monthly income less than 5999 RMB. Regarding the residual location, 25.7% lived in North China while only 1.8% lived in Southwest China. Totally, 80.6% of participants self-reported average or good health status; the average systolic pressure was 126.20 (22.91) mmHg, while the average diastolic pressure was 77.97 (10.51) mmHg; the average body weight was 72.06 (20.75) Kg; 58.9% of the participants reported alcohol use; 30.6% had respiratory diseases, 10.0% had CVD, and 4.4% cancers. As for the smoking behavior, 75.8% of the participants were diagnosed with nicotine dependence; the average number of cigarettes smoked per day (SD) was 19.10 (9.04), the average smoking duration (SD) was 28.66 (13.42) years; the average FTND (SD) was 4.80 (2.45) points.
Table 1.
Baseline characteristics of study participants.
| Characteristics | With nicotine dependence N = 2231 | Without nicotine dependence N = 712 | Total N = 2943 |
|---|---|---|---|
| Gender, n% | |||
| Men | 2127 (95.3) | 663 (93.1) | 2790 (94.8) |
| Women | 104 (4.7) | 49 (6.9) | 153 (5.2) |
| Age (year) | |||
| Less than 40 | 528 (23.7) | 130 (18.3) | 658 (22.4) |
| 41–50 | 469 (21.0) | 111 (15.6) | 580 (19.7) |
| 51–60 | 597 (26.8) | 165 (23.2) | 762 (25.9) |
| 61 and above | 637 (28.6) | 306 (43.0) | 943 (32.0) |
| Mean (SD) | 52.07 (13.38) | 56.13 (14.33) | 53.05 (13.72) |
| Ethnicity, n% | |||
| Han | 2095 (93.9) | 665 (93.4) | 2760 (93.8) |
| Others | 136 (6.1) | 47 (6.6) | 183 (6.2) |
| Marriage, n% | |||
| Single | 164 (7.4) | 47 (6.6) | 211 (7.2) |
| Married | 2025 (90.8) | 638 (89.6) | 2663 (90.5) |
| Separated/divorced/widowed | 42 (1.9) | 27 (3.8) | 69 (2.3) |
| Education, n% | |||
| Primary school or less | 211 (9.5) | 93 (13.1) | 304 (10.3) |
| Middle and high school | 1079 (48.4) | 381 (53.5) | 1460 (49.6) |
| College and higher | 941 (42.2) | 238 (33.4) | 1179 (40.1) |
| Monthly income (RMB) | |||
| <1000 | 101 (4.5) | 39 (5.5) | 140 (4.8) |
| 1000–2999 | 431 (19.3) | 158 (22.2) | 589 (20.0) |
| 3000–5999 | 959 (43.0) | 327 (45.9) | 1286 (43.7) |
| 6000–9999 | 475 (21.3) | 119 (16.7) | 594 (20.2) |
| >10,000 | 265 (11.9) | 69 (9.7) | 334 (11.3) |
| Residual location, n% | |||
| Eastern China | 322 (14.4) | 30 (4.2) | 352 (12.0%) |
| Northwest China | 388 (17.4) | 63 (8.8) | 451 (15.3%) |
| Northeast China | 515 (23.1) | 223 (31.3) | 738 (25.1%) |
| South China | 149 (6.7) | 34 (4.8) | 183 (6.2%) |
| North China | 563 (25.2) | 194 (27.2) | 757 (25.7%) |
| Central China | 260 (11.7) | 149 (20.9) | 409 (13.9%) |
| Southwest China | 34 (1.5) | 19 (2.7) | 53 (1.8%) |
| Self-reported health status, n% | |||
| Poor | 455 (20.4) | 119 (16.7) | 574 (19.5) |
| Average | 731 (32.8) | 232 (32.6) | 963 (32.7) |
| Good | 1045 (46.8) | 361 (50.7) | 1406 (47.8) |
| Blood pressure, mean (SD) | |||
| Systolic pressure | 125.63 (13.40) | 128.00 (40.05) | 126.20 (22.91) |
| Diastolic pressure | 77.70 (10.51) | 78.81 (10.47) | 77.97 (10.51) |
| Alcohol use, n% | |||
| Yes | 1333 (59.7) | 400 (56.2) | 1733 (58.9) |
| No | 898 (40.3) | 312 (43.8) | 1210 (41.1) |
| Body weight, mean (SD) | 72.11 (22.90) | 71.87 (11.74) | 72.06 (20.75) |
| Diseases at baseline, n% | |||
| Respiratory diseases | 698 (31.3) | 203 (28.5) | 901 (30.6) |
| CVD | 224 (10.9) | 50 (7.0) | 274 (9.3) |
| Cancer | 106 (4.8) | 23 (3.2) | 129 (4.4) |
| Other chronic diseases | 467 (20.9) | 146 (20.5) | 613 (20.8) |
| Depression | 65 (2.9) | 23 (3.2) | 88 (3.0) |
| Anxiety | 68 (3.0) | 25 (3.5) | 93 (3.2) |
| Cigarettes smoked per day | |||
| 1–9 | 122 (5.5) | 95 (13.3) | 217 (7.7) |
| 10–19 | 666 (29.9) | 368 (51.7) | 1034 (35.1) |
| 20–29 | 1185 (53.1) | 226 (31.7) | 1411 (47.9) |
| 30 and above | 258 (11.6) | 23 (3.2) | 281 (9.5) |
| Mean (SD) | 20.06 (9.16) | 16.09 (7.92) | 19.10 (9.04) |
| Smoking duration (year) | |||
| 1–9 | 159 (7.1) | 53 (7.4) | 211 (7.2) |
| 10–19 | 462 (20.7) | 116 (16.3) | 578 (19.6) |
| 20–29 | 541 (24.2) | 155 (21.8) | 696 (23.6) |
| 30 and above | 1069 (47.8) | 388 (54.5) | 1457 (49.5) |
| Mean (SD) | 27.98 (12.98) | 30.77 (14.49) | 28.66 (13.42) |
| FTND | |||
| 0–3 | 513 (23.0) | 404 (56.7) | 917 (31.2) |
| 4–6 | 991 (44.4) | 263 (36.9) | 1254 (42.6) |
| 7 and above | 727 (32.6) | 45 (6.3) | 772 (26.2) |
| Mean (SD) | 5.34 (2.31) | 3.13 (2.13) | 4.80 (2.45) |
| Treatment provided | |||
| Bupropion + behavioural counselling | 736 (33.0) | 229 (32.2) | 965 (32.8) |
| Behavioural counselling | 721 (32.3) | 214 (30.1) | 935 (31.8) |
| Varenicline + behavioural counselling | 576 (25.8) | 202 (28.4) | 778 (26.4) |
| Alternative treatments + behavioural counselling | 98 (4.4) | 37 (5.2) | 135 (4.6) |
| NRT + behavioural counselling | 100 (4.5) | 30 (4.2) | 130 (4.4) |
Note: Data are shown as number (%) or mean (SD). FTND: Fagerstr€om Test for Tobacco dependence.
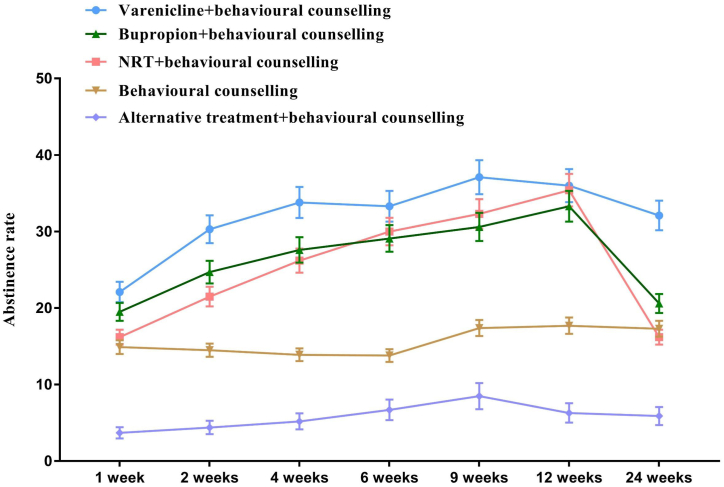
Of all the participants analyzed, 965 (32.8%) participants were treated with Bupropion + behavioural counselling, followed by 935 (31.8%) with behavioural counselling, 778 (26.4%) with Varenicline + behavioural counselling, 135 (4.6%) with alternative treatments + behavioural counselling, and 130 (4.4%) with nicotine replacement therapy (NRT) + behavioural counselling. After 3-month treatment and 3-month follow-up, 866 (29.4%) participants lost contact, and 21.74% of the participants quit smoking at 24 weeks. Specifically, the CO-validated 24-week abstinence rate was 32.1% in the Varenicline group + behavioural counselling, 20.6% in the bupropion group + behavioural counselling, 17.3% in the behavioural counselling group, 16.2% in the NRT group + behavioural counselling, and 5.9% in the alternative treatments group + behavioural counselling (Fig. 2, Appendix Fig. S3 and Appendix Table S2).
Fig. 2.
Comparison of abstinence rate between different treatments at different time points. Note: Bars represent proportion or mean and error bars 95% CI. NRT = Nicotine replacement therapy.
Moreover, the CO-validated abstinence rate at 24 weeks was 35.3% in women and 21.0% in men (P < 0.05), highest (32.1%) in those aged less than 40 years and lowest (16.14%) in those aged 51–60 years (P < 0.05), highest (24.8%) in those with education level of college and higher and lowest (17.8%) in those with education level of primary school or less (P < 0.05), and 33.99% in those without nicotine dependence and 17.84% in those with nicotine dependence (P < 0.05) (Appendix Table S2 and Appendix Fig. S2).
The treatment adherence was shown in Appendix Table S3. Overall, 2601 participants (88.38%) set target quit date (TQD), and 2915 (99.05%) received at least 1 dose of treatment. However, only 1336 (45.40%) received more than 80% of allocated medication and 1004 (34.11%) still using allocated treatment at 3-month follow-up. None used non-allocated treatment at any time point.
In the multivariable-adjusted analyses (Table 2), according to the model 2, the CO-validated abstinence rate at 24 weeks was significantly associated with women, younger age, being Han ethnicity, higher education level and monthly income, alcohol use, greater systolic and diastolic pressure, having cancer and respiratory diseases at baseline, different place of residence, different treatment received (for alternative treatments + behavioural counselling, OR: 0.228, 95% CI: 0.108–0.483; for NRT + behavioural counselling, OR: 1.082, 95% CI: 0.786–1.357; for Bupropion + behavioural counselling, OR: 1.213, 95% CI: 0.955–1.541; for Varenicline + behavioural counselling, OR: 2.179, 95% CI: 1.721–2.761), without nicotine dependence, lower cigarettes smoked per day, shorter smoking duration, and lower FTND.
Table 2.
Adjusted ORs for successful quitting at week 24.
| Characteristics | Number of cases | Model 1 |
Model 2 |
||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| Gender | |||||
| Men | 2790 | Ref | Ref | ||
| Women | 153 | 2.112 (1.491–2.993) | 0.000 | 1.985 (1.358–2.900) | 0.045 |
| Age | |||||
| 61 and above | 658 | Ref | Ref | ||
| 51–60 | 580 | 0.792 (0.616–1.019) | 0.070 | 0.916 (0.700–1.197) | 0.520 |
| 41–50 | 762 | 1.085 (0.838–1.405) | 0.535 | 1.466 (1.096–1.961) | 0.010 |
| Less than 40 | 943 | 1.955 (1.553–2.462) | 0.000 | 2.675 (1.990–3.594) | 0.000 |
| Ethnicity | |||||
| Others | 183 | Ref | Ref | ||
| Han | 2760 | 2.173 (1.362–3.467) | 0.001 | 2.117 (1.305–3.434) | 0.002 |
| Marriage | |||||
| Married | 2663 | Ref | Ref | ||
| Single | 211 | 0.782 (0.564–1.084) | 0.139 | 0.881 (0.626–1.240) | 0.467 |
| Separated/divorced/widowed | 69 | 0.717 (0.359–1.431) | 0.345 | 0.663 (0.324–1.358) | 0.261 |
| Education | |||||
| Primary school or less | 304 | Ref | Ref | ||
| Middle and high school | 1460 | 1.139 (0.822–1.580) | 0.434 | 1.453 (1.017–2.076) | 0.040 |
| College and higher | 1179 | 1.214 (0.856–1.721) | 0.276 | 1.878 (1.262–2.793) | 0.002 |
| Monthly income (RMB) | |||||
| <1000 | 140 | Ref | Ref | ||
| 1000–2999 | 589 | 0.923 (0.655–1.300) | 0.647 | 0.905 (0.633–1.293) | 0.583 |
| 3000–5999 | 1286 | 1.239 (0.908–1.691) | 0.176 | 1.284 (0.923–1.785) | 0.138 |
| 6000–9999 | 594 | 1.787 (1.098–2.908) | 0.020 | 2.504 (1.720–3.646) | 0.000 |
| >10,000 | 334 | 2.004 (1.422–2.822) | 0.000 | 2.195 (1.286–3.746) | 0.004 |
| Self-reported health status | |||||
| Good | 1406 | Ref | Ref | ||
| Average | 963 | 1.308 (1.017–1.681) | 0.036 | 1.018 (0.614–1.687) | 0.946 |
| Poor | 574 | 1.345 (1.033–1.752) | 0.028 | 1.179 (0.731–1.901) | 0.500 |
| Systolic pressure at baseline | 2943 | 1.013 (1.006–1.020) | 0.000 | 1.008 (1.001–1.015) | 0.035 |
| Diastolic pressure at baseline | 2943 | 1.018 (1.010–1.027) | 0.000 | 1.018 (1.009–1.027) | 0.000 |
| Alcohol use at baseline | |||||
| No | 1210 | Ref | Ref | ||
| Yes | 1733 | 1.632 (1.365–1.952) | 0.000 | 1.663 (1.390–1.989) | 0.000 |
| Body weight | 2943 | 1.002 (0.998–1.005) | 0.440 | 1.001 (0.997–1.005) | 0.568 |
| CVD at baseline | |||||
| No | 274 | Ref | Ref | ||
| Yes | 2669 | 1.133 (0.840–1.529) | 0.413 | 1.008 (0.573–1.772) | 0.979 |
| Cancer at baseline | |||||
| No | 129 | Ref | Ref | ||
| Yes | 2814 | 2.646 (1.475–4.748) | 0.001 | 3.109 (1.273–7.591) | 0.013 |
| Respiratory diseases at baseline | |||||
| No | 901 | Ref | Ref | ||
| Yes | 2669 | 1.133 (0.933–1.377) | 0.209 | 1.783 (1.222–2.601) | 0.003 |
| Depression at baseline | |||||
| No | 88 | Ref | Ref | ||
| Yes | 2855 | 0.795 (0.356–1.775) | 0.576 | 0.737 (0.193–2.818) | 0.653 |
| Anxiety at baseline | |||||
| No | 93 | Ref | Ref | ||
| Yes | 2850 | 0.929 (0.550–1.570) | 0.785 | 1.157 (0.318–4.216) | 0.825 |
| Residual location | |||||
| South China | 183 | Ref | Ref | ||
| Northeast China | 738 | 1.021 (0.632–1.650) | 0.933 | 0.821 (0.497–1.357) | 0.442 |
| Southwest China | 53 | 1.048 (0.449–2.447) | 0.914 | 0.730 (0.299–1.779) | 0.489 |
| North China | 757 | 1.836 (1.153–2.923) | 0.011 | 1.855 (1.129–3.046) | 0.015 |
| Central China | 409 | 1.973 (1.210–3.217) | 0.006 | 1.523 (0.906–2.561) | 0.112 |
| Northwest China | 451 | 2.393 (1.481–3.868) | 0.000 | 2.136 (1.278–3.568) | 0.004 |
| Eastern China | 352 | 2.711 (1.662–4.422) | 0.000 | 3.078 (1.828–5.182) | 0.000 |
| Treatment received | |||||
| Behavioural counselling | 935 | Ref | |||
| Alternative treatments + behavioural counselling | 135 | 0.251 (0.120–0.526) | 0.000 | 0.228 (0.108–0.483) | 0.000 |
| NRT + behavioural counselling | 130 | 1.159 (0.820–1.420) | 0.124 | 1.082 (0.786–1.357) | 0.426 |
| Bupropion + behavioural counselling | 965 | 1.237 (0.980–1.560) | 0.073 | 1.213 (0.955–1.541) | 0.114 |
| Varenicline + behavioural counselling | 778 | 2.255 (1.793–2.836) | 0.000 | 2.179 (1.721–2.761) | 0.000 |
| Nicotine dependence | |||||
| With nicotine dependence | 2231 | Ref | Ref | ||
| Without nicotine dependence | 712 | 2.542 (2.090–3.091) | 0.000 | 2.683 (2.183–3.297) | 0.000 |
| Cigarettes smoked per day | |||||
| 30 and above | 281 | Ref | Ref | ||
| 20–29 | 1411 | 1.024 (0.967–1.084) | 0.412 | 1.135 (1.082–1.191) | 0.000 |
| 10–19 | 1034 | 1.116 (1.047–1.191) | 0.001 | 1.214 (1.122–1.315) | 0.000 |
| 1–9 | 217 | 1.313 (1.184–1.457) | 0.000 | 1.349 (1.269–1.433) | 0.000 |
| Smoking duration | |||||
| 30 and above | 1457 | Ref | Ref | ||
| 20–29 | 696 | 2.105 (1.535–2.887) | 0.000 | 1.557 (1.124–2.156) | 0.008 |
| 10–19 | 578 | 3.162 (2.114–4.731) | 0.000 | 1.868 (1.347–2.591) | 0.000 |
| 1–9 | 212 | 9.116 (5.669–14.660) | 0.000 | 2.970 (1.906–4.629) | 0.000 |
| FTND | |||||
| 7 and above | 772 | Ref | Ref | ||
| 4–6 | 1254 | 6.191 (4.102–9.343) | 0.000 | 1.870 (1.016–3.442) | 0.044 |
| 0–3 | 917 | 9.640 (6.043–12.571) | 0.000 | 3.556 (1.915–6.604) | 0.000 |
Note: Model 1 was adjusted for gender and age; model 2 was adjusted for gender, age, ethnicity, marriage, education, monthly income, self-reported health status, location, treatment received, and nicotine dependence. FTND: Fagerstr€om Test for Tobacco dependence.
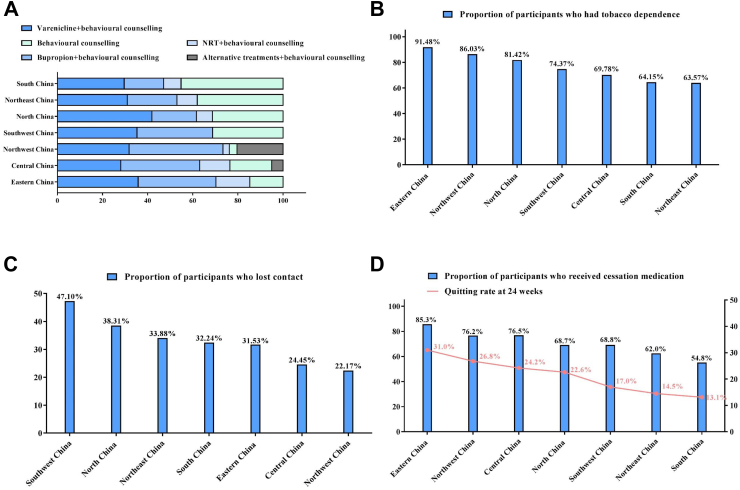
Lastly, a regional disparity in tobacco cessation practice was observed. As shown in Fig. 3A, for Varenicline, the highest usage rate was observed in North China (41.9%); for Bupropion, the highest usage rate was observed in Northwest China (41.6%); for NRT, the highest usage rate was observed in Eastern China (15.0%), and for behavioural counselling, the highest usage rate was observed in South China (45.2%). As shown in Fig. 3B, the proportion of participants who had tobacco dependence ranged from highest in Eastern China (91.48%) and lowest in Northeast China (63.57%). As shown in Fig. 3C, the rate of lost contact was highest in Southwest China (47.10%) and lowest in Northwest China (22.17%). As shown in Fig. 3D, the abstinence rate at 24 weeks was highest in Eastern China (31.0%), followed by 26.8% in Northwest China, 24.2% in Central China, 22.6% in North China, 17.0% in Southwest China, 14.5% in Northeast China, and 13.1% in South China. Combined with the rate of medication use, it was suggested that the areas with higher usage of cessation medication were associated with higher cessation treatment outcome (Appendix Table S2).
Fig. 3.
Comparison of different areas of China. Note: (A) Comparison of different cessation medication uses at different areas of China. (B) Comparison of participants who had tobacco dependence at different areas of China. (C) Comparison of participants who lost contact at different areas of China. (D) Comparison of cessation medication usage and treatment outcome at different areas of China.
Discussion
To the best of our knowledge, CNTCCS is the first and largest cohort study for tobacco cessation in China. In the present study, we introduced the design and protocol of CNTCCS, and described the characteristics, clinical care practice and outcomes of the participants for whom 6-month follow-up data were available. The data obtained could be used to better understand the “real world” situation of tobacco cessation practice in China. It will also serve as an evidence-based platform for conducting future research, which will ultimately improve the treatment and management provided to smokers.
First, one of the key purposes of CNTCCS was to evaluate the treatment outcome of tobacco cessation during 6-month follow-up period. In CNTCCS, we found that approximately 1 out of 5 smokers quit smoking after 6 months, which was similar to the 23.0% in a real-world study of 1560 participants in US,20 23.7% in a real-world study of 2802 participants in US,21 and 28.8% in a real-world study of 235 participants in Thailand.22 Specifically, Varenicline showed the best efficacy, which was consistent with many previous studies and recommendations of several clinical guidelines7,14; Bupropion is also welcomed as it is relatively cheap and may prevent weight gain after smoking cessation.23 The relatively low preference for NRT among Chinese patients may be attributed to the limited availability of NRT in most Chinese hospitals24; in addition, while e-commerce is popular in China and offers the potential for patients to buy NRT at a lower cost online, there is still a prevailing skepticism among Chinese patients when it comes to the quality and authenticity of medications available through online platforms. This mistrust in online medication purchases contributes to their hesitation in choosing NRT as a smoking cessation aid. It should be noted that acupuncture and herbal remedies were provided in CNTCCS. Although current evidence suggests that acupuncture and herbal remedies may increase the chance of stopping smoking,25,26 there is no consistent, bias-free evidence, and as some study centers are located in the hospitals of traditional Chinese medicine, they are more interested to use these methods to quit smoking. Nevertheless, we call for strong action to improve the adherence to the guideline of tobacco cessation.
Second, we identified several predictors of successful tobacco cessation. Among them, tobacco dependence should be highly valued. Tobacco dependence is the key barrier to successful smoking cessation.27 In CNTCCS, nearly 75% of the participants showed symptoms of tobacco dependence, which was higher than 49.7% in a nationwide surrey in China which used the same criteria for screening tobacco dependence.3 This difference is not surprising and could be explained by “hardcore smokers” theory: smokers who are willing to seek treatment often had greater smoking intensity, particularly tobacco dependence.28 However, nearly 30% of the dependent smokers were treated only by behavioural counselling. This is worrying because, as recommended by several clinical guideline, dependent smokers should be treated by intensive cessation treatment, particularly the first-line medications, rather than simply being encouraged to stop smoking.7,14
Third, a key finding of our study is that the tobacco cessation treatment varied widely across different areas of China. Possible reasons included variations of economic level across different regions, the lack of familiarity with tobacco cessation guidelines, physician's own smoking status, etc. Importantly, our study showed that the areas with higher usage of cessation medication were associated with higher cessation treatment outcome, which highlighted the importance of the availability and affordability of the cessation medications. A previous study found that tobacco cessation medications are unavailable and have limited availability in India; even when available, medications are unaffordable for most patients.29 This is very similar to China. Moreover, previous study has estimated that medical coverage of any nicotine replacement therapy products increases the usage by 20%.30 As such, our finding suggested that geographical targeting is required for more successful tobacco prevention and control in China, and we strongly recommend inclusion of tobacco cessation medication into national and provincial health insurance in China.
Our study has important implications. The CNTCCS required all the study centers to provide the participants with evidence-based treatment, which might overrate the quality of tobacco cessation practice, and even violate the observational nature of cohort study, but this action indeed greatly improved the overall treatment quality of tobacco cessation. In the meantime, it generally would be optimal to analyze a clinical and genetic biobank to learn about disease natural history and characteristics, and compare the efficacy among different treatments. However, in China there is no biobank that has longitudinal data on tobacco cessation. With the clinical longitudinal data (particularly the treatment method and efficacy) and blood sample of approximately 3000 participants, CNTCCS provides a good start to establishing a “China Tobacco Cessation Biobank”, thus giving a robust support for future study of tobacco cessation in China and even the world.
Our study has several strengths, including representative large sample of approximately 3000 participants, longitudinal clinical data collected from nationwide practices enrolled in CNTCCS, well-validated questionnaires, and stringent quality control process.
However, we acknowledge several limitations. First, the selection of participating sites, although covering all areas of China, was by convenience in nature. Second, the included study sites may represent the hospitals with more resources and expertise than county-level or even more grassroots-level hospitals. As the CNTCCS continuously proceeds, more study sites will be included to overcome these limitations. Third, the site participation in CNTCCS is voluntary; this possible selection bias means that the actual practice situation is very likely to be even lower in the non-CNTCC Shospitals. Fourth, although included in the ITT analysis, nearly 30% of the participants lost contact. This might be due to the pandemic of COVID-19. Future studies may consider using mobile health methods to collect data during follow-up windows. Fifth, as this was a real-world study, it was difficult to explore the sole efficacy of medication therapy. Sixth, although we have considered many factors, other potentially important factors such as BMI, whether smokers were referred to the cessation services by healthcare professionals, how much the participants paid for treatments, the types of NRT used and whether single or combined NRT was used, were not assessed. Seventh, several important demographic and clinical information might be subject to recall bias from patients.
Conclusion
To the best of our knowledge, the CNTCCS is the first and largest nationwide cohort study of tobacco cessation in China and elsewhere. Rich data collected from this prospective cohort study provided the opportunity to evaluate the quality of care for tobacco cessation practice in China.
Contributors
All authors were involved in the planning of the study, literature review, data collection, interpretation of the findings, and manuscript preparation. Chen Wang and Dan Xiao conceived and designed the study. Dan Xiao supervised the study. Zhao Liu, Rui Qin, Xue-Jun Hu, Li-Jun Liu, Su-Qin Xu, Guo-Chao Shi, Hong Zhou, Jing Bai, Chun-Mei Zhang, Yong Qi, Wei Zhou, Shu-Hua Lan, Jin Tong, Tong-Sheng Su, Qiang Wang, Xin-Yan Yang, De-Jun Sun, Li-Ming Zhu, Xiao-Yang Chen, Hong Chen, Yu-Peng Xie, Zhi-Hua Xiao, Yan-Bin Chen, Bo Zhao, Qiu-Ge Wu, Wen-Li Chen, Dong-Yan LI, Hongbo Liu, An-Qi Cheng, Zi-Yang Cui, Liang Zhao, Jin-Xuan Li, Xiao-Wen Wei, Xin-Mei Zhou, and Zheng Su Contributed to the acquisition of data. Dan Xiao, Zhao Liu, Kian Fan Chung, and Zheng-Ming Chen drafted the report. Zhao L did the statistical analysis. All authors revised the report and approved the final version before submission. Dan Xiao is the guarantor and attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data sharing statement
Data will be available upon reasonable request to the corresponding author immediately following publication to anyone wishing to access the data.
Declaration of interests
All authors have completed the ICMJE uniform disclosure form, and all the authors declared no conflicts of interest; no financial relationships with any organisations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgments
The authors would like to thank the participants of China National Tobacco Cessation Cohort Study (CNTCCS). The authors appreciate every supporter who contributed to the CNTCCS.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100826.
Appendix A. Supplementary data
References
- 1.World Health Organization . World Health Organization; Geneva: 2019. WHO report on the global tobacco epidemic, 2019. [Google Scholar]
- 2.Chen Z., Peto R., Zhou M., et al. Contrasting male and female trends in tobacco-attributed mortality in China: evidence from successive nationwide prospective cohort studies. Lancet. 2015;386:1447–1456. doi: 10.1016/S0140-6736(15)00340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Z., Li Y.H., Cui Z.Y., et al. Prevalence of tobacco dependence and associated factors in China: findings from nationwide China Health Literacy Survey during 2018-19. Lancet Reg Health West Pac. 2022;24 doi: 10.1016/j.lanwpc.2022.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NIH State-of-the-Science Panel National Institutes of Health State-of-the-Science conference statement: tobacco use: prevention, cessation, and control. Ann Intern Med. 2006;145:839–844. doi: 10.7326/0003-4819-145-11-200612050-00141. [DOI] [PubMed] [Google Scholar]
- 5.Pirie K., Peto R., Reeves G.K., et al. The 21st century hazards of smoking and benefits of stopping: a prospective study of one million women in the UK. Lancet. 2013;381(9861):133–141. doi: 10.1016/S0140-6736(12)61720-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doll R., Peto R., Boreham J., et al. Mortality in relation to smoking: 50 years' observations on male British doctors. BMJ. 2004;328(7455):1519. doi: 10.1136/bmj.38142.554479.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patnode C.D., Henderson J.T., Coppola E.L., et al. Interventions for tobacco cessation in adults, including pregnant persons: updated evidence report and systematic review for the US preventive services task force. JAMA. 2021;325(3):280–298. doi: 10.1001/jama.2020.23541. [DOI] [PubMed] [Google Scholar]
- 8.West R., Raw M., McNeill A., et al. Health-care interventions to promote and assist tobacco cessation: a review of efficacy, effectiveness and affordability for use in national guideline development. Addiction. 2015;110(9):1388–1403. doi: 10.1111/add.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin H., Xiao D., Liu Z., et al. National survey of smoking cessation provision in China. Tob Induc Dis. 2019;17:25. doi: 10.18332/tid/104726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fong G.T., Yuan J., Craig L.V., et al. Achieving the goals of healthy China 2030 depends on increasing smoking cessation in China: comparative findings from the ITC project in China, Japan, and the Republic of Korea. China CDC Wkly. 2021;3(22):463–467. doi: 10.46234/ccdcw2021.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang Y., Elton-Marshall T., Fong G.T., et al. Quitting smoking in China: findings from the ITC China Survey. Tob Control. 2010;19(Suppl 2 (Suppl_2)):i12–i17. doi: 10.1136/tc.2009.031179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang C.M., Xiao D., West R., et al. Evaluation of 3-day smoking cessation training course for doctors from 38 cities in China. Chin Med J (Engl) 2012;125(7):1338–1340. PMID: 22613611. [PubMed] [Google Scholar]
- 13.Fagerstrom K.O., Heatherton T.F., Kozlowski L.T. Nicotine addiction and its assessment. Ear Nose Throat J. 1990;69(11):763–765. [PubMed] [Google Scholar]
- 14.China National Health and Family Planning Commission . People's Medical Publishing House; Beijing: 2015. China clinical guidelines for tobacco cessation (2015 version) [Google Scholar]
- 15.Cahill K., Stevens S., Perera R., et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;2013(5):CD009329. doi: 10.1002/14651858.CD009329.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin R., Liu Z., Zhou X., et al. Adherence and efficacy of smoking cessation treatment among patients with COPD in China. Int J Chron Obstruct Pulmon Dis. 2021;16:1203–1214. doi: 10.2147/COPD.S301579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koegelenberg C.F., Noor F., Bateman E.D., et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312:155–161. doi: 10.1001/jama.2014.7195. [DOI] [PubMed] [Google Scholar]
- 18.Anthenelli Robert M., Benowitz Neal L., West R., et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387:2507–2520. doi: 10.1016/S0140-6736(16)30272-0. [DOI] [PubMed] [Google Scholar]
- 19.Baker Timothy B., Piper Megan E., Smith Stevens S., et al. Effects of combined varenicline with nicotine patch and of extended treatment duration on smoking cessation: a randomized clinical trial. JAMA. 2021;326:1485–1493. doi: 10.1001/jama.2021.15333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotz D., Brown J., West R. Prospective cohort study of the effectiveness of smoking cessation treatments used in the "real world". Mayo Clin Proc. 2014;89(10):1360–1367. doi: 10.1016/j.mayocp.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watkins S.L., Thrul J., Max W., et al. Real-world effectiveness of smoking cessation strategies for young and older adults: findings from a nationally representative cohort. Nicotine Tob Res. 2020;22(9):1560–1568. doi: 10.1093/ntr/ntz223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lertsinudom S., Kaewketthong P., Chankaew T., et al. Smoking cessation services by community pharmacists: real-world practice in Thailand. Int J Environ Res Public Health. 2021;18(22) doi: 10.3390/ijerph182211890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howes S., Hartmann-Boyce J., Livingstone-Banks J., et al. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2020;4(4) doi: 10.1002/14651858.CD000031.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann-Boyce J., Chepkin S.C., Ye W., et al. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database Syst Rev. 2018;5(5) doi: 10.1002/14651858.CD000146.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y.Y., Liu Z., Wu Y., et al. Efficacy of acupuncture is noninferior to nicotine replacement therapy for tobacco cessation: results of a prospective, randomized, active-controlled open-label trial. Chest. 2018;153(3):680–688. doi: 10.1016/j.chest.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Wang J.H., van Haselen R., Wang M., et al. Acupuncture for smoking cessation: a systematic review and meta-analysis of 24 randomized controlled trials. Tob Induc Dis. 2019;17:48. doi: 10.18332/tid/109195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benowitz N.L. Nicotine addiction. N Engl J Med. 2010;362:2295–2303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa M.L., Cohen J.E., Chaiton M.O., et al. "Hardcore" definitions and their application to a population-based sample of smokers. Nicotine Tob Res. 2010;12(8):860–864. doi: 10.1093/ntr/ntq103. [DOI] [PubMed] [Google Scholar]
- 29.Sarma S., Harikrishnan S., Baldridge A.S., et al. Availability, sales, and affordability of tobacco cessation medicines in Kerala, India. Circ Cardiovasc Qual Outcomes. 2017;10(11) doi: 10.1161/CIRCOUTCOMES.117.004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lei L., Liu F. Medicaid coverage and use of nicotine replacement treatment. Econ Hum Biol. 2021;40 doi: 10.1016/j.ehb.2020.100938. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.