Abstract
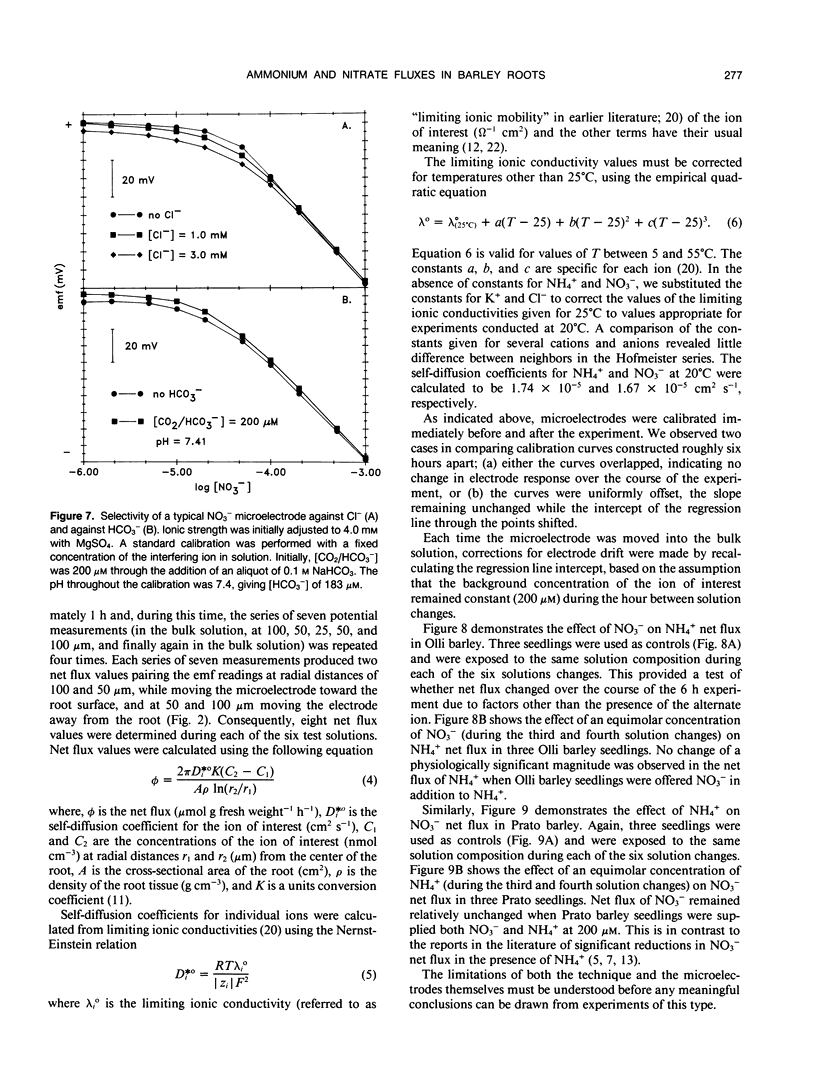
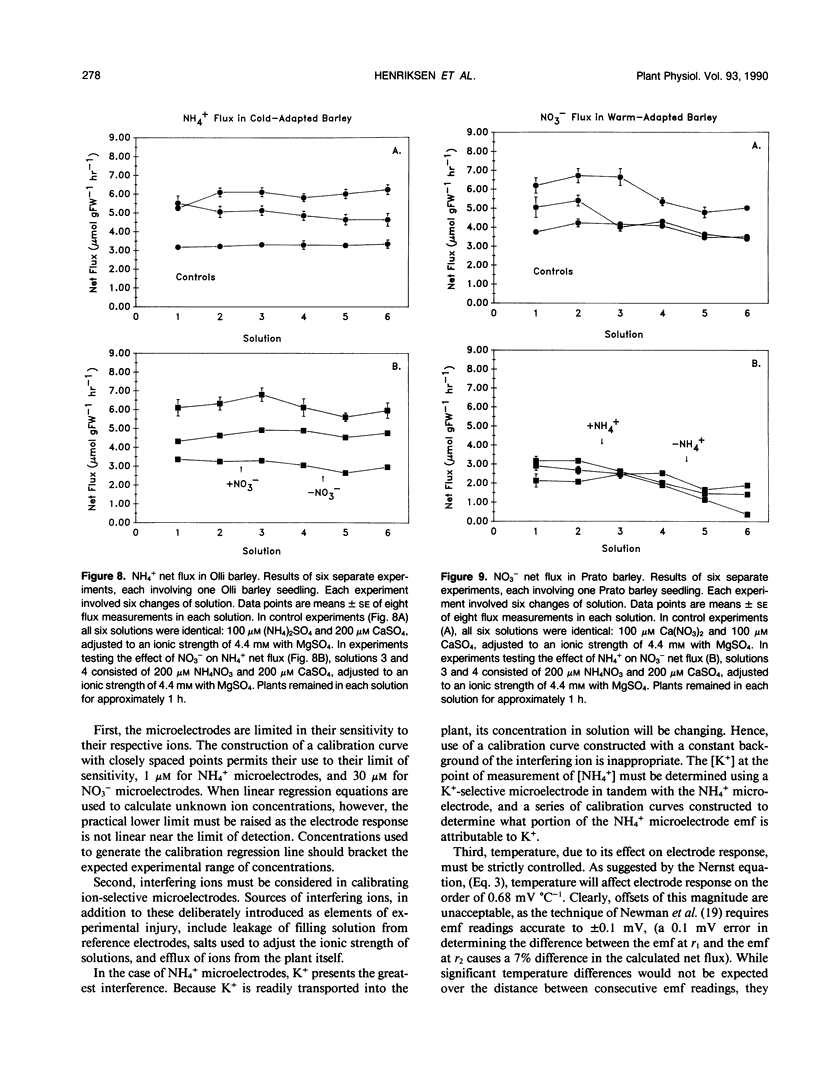
Neutral carrier-based liquid membrane ion-selective microelectrodes for NH4+ and NO3− were developed and used to investigate inorganic nitrogen acquisition in two varieties of barley, Hordeum vulgare L. cv Olli and H. vulgare L. cv Prato, originating in cold and warm climates, respectively. In the present paper, the methods used in the fabrication of ammonium- and nitrate-selective microelectrodes are described, and their application in the study of inorganic nitrogen uptake is demonstrated. Net ionic fluxes of NH4+ and NO3− were measured in the unstirred layer of solution immediately external to the root surface. The preference for the uptake of a particular ionic form was examined by measuring the net flux of the predominant form of inorganic nitrogen, with and without the alternative ion in solution. Net flux of NH4+ into the cold-adapted variety remained unchanged when equimolar concentrations (200 micromolar) of NH4+ and NO3− were present. Similarly, net flux of NO3− into the warm-adapted variety was not affected when NH4+ was also present in solution. The high temporal and spatial resolution afforded by ammonium- and nitrate-selective microelectrodes permits a detailed examination of inorganic nitrogen acquisition and its component ionic interactions.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom A. J., Finazzo J. The influence of ammonium and chloride on potassium and nitrate absorption by barley roots depends on time of exposure and cultivar. Plant Physiol. 1986 May;81(1):67–69. doi: 10.1104/pp.81.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrelli M. J., Carlini W. G., Dewey W. C., Ransom B. R. A simple method for making ion-selective microelectrodes suitable for intracellular recording in vertebrate cells. J Neurosci Methods. 1985 Oct-Nov;15(2):141–154. doi: 10.1016/0165-0270(85)90051-2. [DOI] [PubMed] [Google Scholar]
- Deane-Drummond C. E., Glass A. D. Short Term Studies of Nitrate Uptake into Barley Plants Using Ion-Specific Electrodes and ClO(3): II. Regulation of NO(3) Efflux by NH(4). Plant Physiol. 1983 Sep;73(1):105–110. doi: 10.1104/pp.73.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass A. D., Thompson R. G., Bordeleau L. Regulation of NO(3) Influx in Barley : Studies Using NO(3). Plant Physiol. 1985 Feb;77(2):379–381. doi: 10.1104/pp.77.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackown C. T., Jackson W. A., Volk R. J. Restricted nitrate influx and reduction in corn seedlings exposed to ammonium. Plant Physiol. 1982 Feb;69(2):353–359. doi: 10.1104/pp.69.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackown C. T., McClure P. R. Development of accelerated net nitrate uptake : effects of nitrate concentration and exposure time. Plant Physiol. 1988 May;87(1):162–166. doi: 10.1104/pp.87.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman I. A., Kochian L. V., Grusak M. A., Lucas W. J. Fluxes of h and k in corn roots : characterization and stoichiometries using ion-selective microelectrodes. Plant Physiol. 1987 Aug;84(4):1177–1184. doi: 10.1104/pp.84.4.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi S., Morgunov N., Boulpaep E. L. Submicron tip breakage and silanization control improve ion-selective microelectrodes. Am J Physiol. 1985 Nov;249(5 Pt 1):C514–C521. doi: 10.1152/ajpcell.1985.249.5.C514. [DOI] [PubMed] [Google Scholar]


