Summary
In this scoping review, we offer a comprehensive understanding of the current and recent epidemiology, challenges, and emerging issues related to bacterial sexually transmitted infections (STIs) in the WHO European Region. We endeavour in collating data from both EU/EEA and non- EU/EEA countries, thereby giving a complete picture of the region which highlights the higher notification rates in Northern and Western countries than other regions, likely due to differences in testing, access to testing, and surveillance capacity. We provide an up-to-date review on the current knowledge of determinants and persistent inequities in key populations as well as the use of molecular epidemiology for identifying transmission networks in gonorrhoea and syphilis, and detecting chlamydia mutations that evade molecular diagnosis. Finally, we explore the emerging STIs in the region and the evolving transmission routes of food and waterborne diseases into sexual transmission. Our findings call for harmonized STI surveillance systems, proactive strategies, and policies to address social factors, and staying vigilant for emerging STIs.
Keywords: Sexually transmitted infections, Chlamydia, Gonorrhoea, Syphilis, Epidemiology, Key populations, Men who have sex with men, Emerging, Europe
Key messages.
Bacterial STIs notifications across the WHO European Region
-
•
Our review includes epidemiological data from 49 countries in the WHO European Region, encompassing reports from both EU/EEA countries and non- EU/EEA countries.
-
•
Considerable regional variations exist in bacterial STI notifications among countries in the WHO European Region. Countries in Northern and Western Europe have higher notification rates per 100000 population compared to other regions. These variations can be attributed to differences in surveillance systems performance, STI testing policies, access to testing, and the availability of sensitive diagnostic techniques and algorithms.
-
•
Temporal trends over the past decade show a slow increase in the case notification rate for Chlamydia trachomatis (CT), alongside a considerable increase for Neisseria gonorrhoeae (NG) and syphilis in EU/EEA countries, as opposed to decreasing trends in non- EU/EEA countries.
-
•
In 2021 in the EU/EEA, CT cases were mainly among 15-24-year-olds (60%), NG predominantly affected those aged 15–34 (66%), and syphilis cases were more common in individuals over 35 (55%). CT notifications were high among heterosexuals of both genders, NG and syphilis were more common in men, mostly in men who have sex with men.
STIs in key populations and people in vulnerable situations
-
•
A combination of factors/determinants, including biological factors, sexual behaviours, social connections, and structural barriers, contribute to the spread of HIV/STIs particularly among key populations.
-
•
Men who have sex with men in Europe have demonstrated a substantial increase in bacterial STIs over the past decade. While data on transgender and gender diverse individuals remain limited, transgender women exhibit a high epidemiological risk for STIs.
-
•
Displaced populations such as migrants and refugees experience sub-optimal access to health and prevention services, and sex workers have both limited access and are highly susceptible to STIs due to unsafe working conditions and criminalisation of sex work.
-
•
The challenges for STI management observed from epidemics like COVID-19 should serve as lessons informing the development of effective interventions to mitigating their impact and enhance future public health preparedness.
Insights from molecular epidemiology
-
•
NG cases, as revealed by molecular epidemiology, are predominantly acquired locally, and distinct NG transmission networks exist among men who have sex with men and among heterosexual populations.
-
•
T. pallidum's slow single nucleotide polymorphism acquisition limits molecular studies on transmission; however, combination of genomic data with meta-data revealed distinct syphilis transmission networks for men who have sex with men and heterosexual men.
-
•
In CT, molecular epidemiology has detected emerging variants like the nvCT (2006) and F-nvCT (2019) that initially evaded NAAT detection platforms in use at that time, resulting in undiagnosed infections and the subsequent national or regional spread of the variant.
Emerging STIs and emerging routes of sexual transmission for infectious pathogens
-
•
Zoonotic origins of sexually transmissible pathogens, such as HIV and Monkeypox virus, demonstrate the potential for pathogens to cross species barriers and adapt to human hosts, leading to widespread transmission and epidemics.
-
•
Shigella and Hepatitis A virus, traditionally transmitted through food and waterborne routes, have recently established sexual transmission routes, primarily among men who have sex with men.
-
•
Other emerging pathogens like Zika virus and Ebola virus can be transmitted via genital fluids, while Hepatitis C virus can be transmitted through contact with blood during sex.
-
•
Biological agents with the potential of being sexually transmitted can vary across regions and over time. Monitoring these potential emerging STIs is also crucial.
Search strategy and selection criteria.
This is a non-systematic narrative review. References for this review were identified through searches of PubMed, Embase and Science Direct with the search terms (“gonorrhoea” OR “Neisseria gonorrhoeae”), OR (“chlamydia” OR “Chlamydia trachomatis”), OR (“syphilis” OR “Treponema pallidum), OR (“sexually transmitted infection” OR “STI”), AND (“Europe” OR “epidemiology” OR “incidence” OR “prevalence” OR “notifications” OR “surveillance” OR “key populations” OR “men who have sex with men” OR “transgender” OR “migrants” OR “sex workers” OR “molecular epidemiology” OR “emerging”). The publication year was limited from 1995 until July 2023. We did not use language restrictions. Articles were also identified through searches of the authors’ own files. The final reference list was generated based on originality and relevance to the broad scope of this review.
Introduction
Sexually transmitted infections (STIs) pose a significant public health burden in the World Health Organization (WHO) European Region.1 In this narrative review, our aim is to examine the current epidemiological trends and multilevel determinants of (re-)emerging STIs in both the European Union/European Economic Area (EU/EEA) and non- EU/EEA countries, focussing on bacterial STIs such as Chlamydia trachomatis (CT), Neisseria gonorrhoeae (NG), and Treponema pallidum (syphilis) infections. Our aim is to shed light on the current epidemiological situation to identify opportunities for reducing the burden of these infections and improving sexual health outcomes across the region.
The paper is part of a series of articles that cover, besides STIs epidemiology in Europe, subjects such as STI prophylaxis and vaccines, effective HIV combination prevention,2 the role of online STI testing services and self-sampling,3 and antimicrobial resistance in STIs.4
Regional variations in notification rates
The global prevalence of bacterial STIs among individuals aged 15–49 years is annually estimated by the WHO using data from regions with robust case-based surveillance systems and population-based studies. In 2020, the overall world prevalence was 3.2% [2.7–3.9%] for CT, 0.7% [0.5–1.1%] for NG, and 0.6% [0.5–0.7%] for syphilis.5 The WHO European Region exhibited the lowest STI prevalence among all WHO regions for both genders, with values of 2.7% [2.2–3.5] for CT, 0.3% [0.1–0.5] for NG, and 0.11% [0.09–0.13] for syphilis. Additionally, lymphogranuloma venereum (LGV, CT serovar L1-L3) has been associated with several outbreaks in Europe; the infection, although notifiable, is considerably underreported.6,7
We conducted a search for STI surveillance records in 53 countries within the WHO European Region (Table 1). We retrieved data from The European Surveillance System8 for 26 of 27 EU Member States (except for Austria), the United Kingdom and two EEA countries; from a WHO Regional Office for Europe report9 for 11 non-EU/EEA countries; and from national reports for Andorra10, Austria,11 Switzerland,12 the Republic of Moldova,13 the Russian Federation,14 Kyrgyzstan,15 Bosnia-Herzegovina,16 Montenegro,17 Israel.27 Data sources could not be identified for four countries (Monaco, San Marino, Turkmenistan and Türkiye). Liechtenstein which is a EEA country started to report to the European Surveillance system in 2020.
Table 1.
Notification ratesa per 100,000 population for chlamydia, gonorrhoea, and syphilis in countries of the WHO European region, 2019.
| Countries in the WHO European Regionb | Chlamydia | Gonorrhoea | Syphilis | Data source |
|---|---|---|---|---|
| EU/EEA Countries grouped by un geoscheme regionsd | ||||
| Northern Europe | ||||
| Denmark | 614.53 | 38.06 | 6.22 | SAID8 |
| Estonia | 80.31 | 5.89 | 2.79 | SAID |
| Finland | 293.24 | 10.96 | 4.55 | SAID |
| Iceland | 502.81 | 34.17 | 10.64 | SAID |
| Ireland | 182.72 | 57.32 | 15.19 | SAID |
| Latvia | 65.05 | 6.88 | 3.91 | SAID |
| Lithuania | 8.9 | 2 | 4.2 | SAID/LMH18 (syphilis) |
| Norway | 533.88 | 31.98 | 3.85 | SAID |
| Sweden | 340.01 | 31.72 | 4.21 | SAID |
| United Kingdom | 388.47 | 116.05 | 13.11 | SAID |
| Western Europe | ||||
| Austria | n/a | 18.03 | 6.53 | SA11 |
| Belgium | 72.01 | 22.89 | 14.51 | SAID |
| France | 35.40 | 5.61 | 1.68 | BSP19 (CT)/SAID |
| Germany | n/a | n/a | 9.55 | SAID |
| Luxemburg | 7.17 | 3.91 | 8.14 | SAID |
| Monacoc | n/a | n/a | n/a | n/a |
| Netherlands | 104.52 | 39.84 | 8.49 | SAID |
| Switzerlandc | 143.2 | 45.3 | 8.4 | OFSP12 |
| Southern Europe | ||||
| Andorrac | 12.3 | 8.6 | 2.5 | GA10 |
| Cyprus | 0.11 | 0.23 | 3.54 | SAID |
| Croatia | 3.68 | 0.98 | 0.69 | SAID |
| Greece | 0.58 | 1.87 | 4.13 | SAID |
| Italy | 1.85 | 1.36 | 3.05 | SAID |
| Malta | 64.84 | 32.62 | 19.25 | SAID |
| Portugal | 7.66 | 10.98 | 4.66 | SAID |
| San Marinoc | n/a | n/a | n/a | n/a |
| Slovenia | 19.08 | 10.72 | 2.6 | SAID |
| Spain | 38.54 | 21.79 | 10.4 | SAID |
| Eastern Europe | ||||
| Bulgaria | 1.73 | 0.31 | 6.86 | SAID |
| Czech Republic | n/a | 15.47 | 5.92 | SAID |
| Hungary | 9.34 | 13.97 | 8.06 | SAID |
| Poland | 1.1 | 0.74 | 4.28 | SAID |
| Romania | 0.07 | 0.17 | 2.78 | SAID |
| Slovakia | 14.31 | 6.77 | 5.1 | SAID |
| Non- EU/EEA Countries grouped by un geoscheme regionse | ||||
| Eastern Europe | ||||
| Belarus | 43.3 | 8.1 | 4.3 | Barbaric et al.9/NSCRB20 |
| Republic of Moldova | 71.7 | 25 | 54.2 | BSM13 |
| Russian Federation | n/a | 7.7 | 15 | FSSS14 |
| Ukraine | 31.6 | 7.4 | 5.7 | Barbaric et al./SSSU21/Krotik et al.22 |
| Southern Europe | ||||
| Albania | n/a | 0.3 | 2.4 | Barbaric et al. |
| Bosnia-Herzegovina | 3.6 | 0.3 | 0.8 | PIH16 |
| Montenegro | 3.05 | 0.48 | 0.32 | SOM17 |
| North Macedonia | 4.6 | 0.67 | 0.4 | Barbaric (CT, syphilis), SAID (NG) |
| Serbia | 10 | 1.54 | 2.3 | Barbaric (CT, syphilis), SAID (NG) |
| Central Asia | ||||
| Kazakhstan | 13.5 | 10.5 | 18.8 | Barbaric et al./BNS23 |
| Kyrgyzstan | 19.25 | 2.25 | 4.8 | ODPK15 |
| Tajikistan | n/a | 2.7 | 4 | Barbaric et al. |
| Turkmenistan | n/a | n/a | n/a | n/a |
| Uzbekistan | n/a | 10.7 | 8.7 | Barbaric et al. |
| Western Asia | ||||
| Armenia | 26.3 | 9.8 | 3.7 | Barbaric et al.9/SCRA24 |
| Azerbaijan | 19 | 3 | 8.2 | Barbaric et al./TSSCRA25 |
| Georgia | 39 | 18.5 | 26.5 | Barbaric et al./NSOG26 |
| Israel | 15.50 | 5.85 | 5.61 | MHI27 |
| Turkey | n/a | n/a | n/a | n/a |
Regarding the methods used to calculate notification rates, data for EU/EEA countries were extracted from the individual country notification rates in the ECDC Annual Epidemiological Reports for 2019,28, 29, 30 with a detailed description of the calculation methods available elsewhere.31 In short, the numerator comprises reported cases, while the denominator consists of population data obtained from Eurostat as of January 1st of each year. For non- EU/EEA countries our primary approach involved utilizing notification rates per 100,000 population as reported in the official national documents. In cases where this information was not available, we considered the absolute number of notified cases as the numerator, and the population of the respective year as the denominator.
The table includes data for 53 countries within the WHO European Region which were divided into two groups: the EU/EEA category and the non-EU/EEA category, and further categorized according to the United Nations geoscheme regions.
Countries that were part of the WHO European Region but were not in the EU/EEA region in 2019 (the UK was an EU member state in 2019). For the purpose of this review Switzerland and the European microstates—Andorra, Monaco, and San Marino—that are part of the WHO European Region but were not EU/EEA members in 2019 were allocated to their respective UN Geoscheme. Liechtenstein, an EEA member state, started to report to the European Surveillance system in 2020.
Data from 29 EU/EEA countries in 2019 were extracted from the Surveillance Atlas of Infectious Diseases (SAID),8 hosted by the European Centre for Disease Prevention and Control (ECDC). In addition to SAID, national statistical reports were utilized for CT in France (BSP)19 and for syphilis in Lithuania (LMH)18 Data for 3 countries: Andorra (GA),10 Austria (SA)11 and Switzerland (OFSP)12 were extracted from their respective national statistical reports. We could not find data for the years 2019 (or 2018) for 2 countries: Monaco and San Marino.
Data from 11 countries in the non- EU/EEA group were obtained from an assessment conducted by the WHO Regional Office for Europe (WHO/Europe), which included data extracted from the WHO Communicable Diseases Annual Reporting Forms (Babaric et al.)9 and complemented with national reports (NSCRB,20 SSSU,22 BNS,23 SCRA,24 TSSCRA25). The data corresponds to the year 2019, except for Albania, North Macedonia, Serbia and Tajikistan, which corresponds to the year 2018. Data from 6 other non- EU/EEA countries was obtained from national statistical reports, including notification rates for CT, NG and syphilis from Moldova (BSM),13 the Russian Federation (FSSS),14 Kyrgyzstan (ODPK),15 Bosnia-Herzegovina (PIH),16 Montenegro (SOM),17 and Israel (MHI)27 The data for Bosnia and Herzegovina are only from the Republic of Srpska. We could not find data for the years 2019 (or 2018) for 2 countries—Turkmenistan and Turkey. CT reports were not found or not reported in Albania, Austria, Germany, Czechia, the Russian Federation, Tajikistan and Uzbekistan. NG reports were not found for Germany.
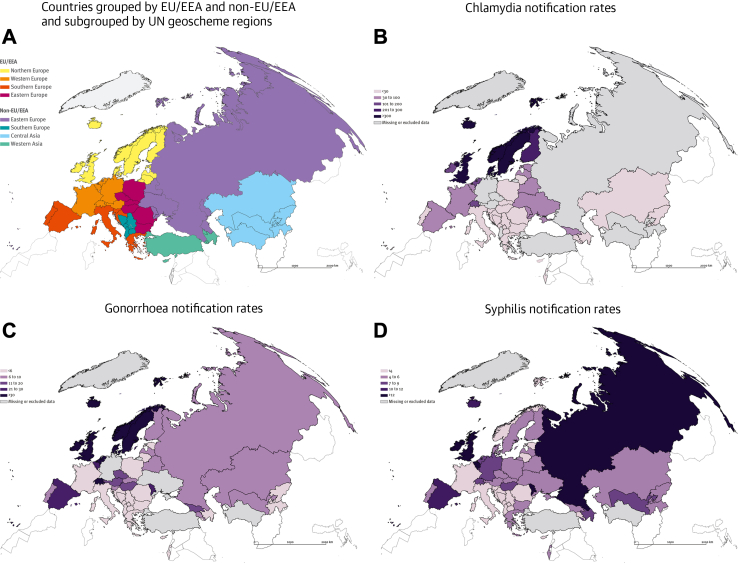
There are considerable regional variations in the bacterial STI notification rates among countries in the WHO European Region. For the purpose of comparison and considering the main data sources, we grouped countries as EU/EEA8 and non- EU/EEA,9 and further categorized according to the regions of the United Nations geoscheme (including European regions and Central and Western Asia). Countries with data originating from national reports were added to the respective geoscheme regions (Fig. 1, panel A). We primarily centred on 2019 data, available for a substantial number of countries.8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27
Fig. 1.
Geographical areas and case notification rates per 100,000 population ofChlamydia trachomatis(CT),Neisseria gonorrhoeae(NG),and Treponema pallidum(syphilis) infections in the WHO European Region in 2019. Legend. Panel A: Countries grouped by EU/EEA and non-EU/EEA and subgrouped by UN geoscheme regions (including European regions and Central and Western Asia). Panel B: Notification Rates per 100,000 population of Chlamydia trachomatis infections. Panel C: Notification Rates per 100,000 population of Neisseria gonorrhoeae infections. Panel D: Notification Rates per 100,000 population of Treponema pallidum infections. The UK was an EU member state in 2019. Switzerland and the European microstates – Andorra, Monaco, and San Marino – that are part of the WHO European Region but were not EU/EEA members in 2019 were allocated to their respective UN Geoscheme region.
In 2019, EU/EEA countries in Northern Europe had higher notification rates of CT, NG, and syphilis, than EU/EEA countries in other regions and non- EU/EEA countries. For CT, the majority of EU/EEA countries in Northern Europe had CT notification rates of >300 per 100,000 population (Table 1, Fig. 1 panel B), while EU/EEA countries in other regions reported rates ranging from 0.07 to 143.2 per 100,000.28 Epidemiological figures in non- EU/EEA countries, showed notification rates of CT ranging from 3.1 to 71.7/100,000, with the highest rates observed in the Republic of Moldova (71.7), Belarus (43.3), Georgia (39.0), and Ukraine (31.6).9
The overall case notification rate of NG infections in EU/EEA countries in 2019 was 31.3/100,000 with an outlying high rate recorded in the UK (116.1) (Table 1, Fig. 1 panel C).29 Historically, the incidence of NG in non-EU/EEA countries in Eastern Europe has been higher than the rest of the WHO European Region with estimates in some countries higher than 40/100,000,32 but the reported notification rates in 2019 were not as high (ranging from 7.4 to 25), and even lower in the rest of non-EU/EEA countries (0.3–10.5), with the exception of Georgia (18.5).9
Regarding syphilis, high rates (>8 per 100,000 population) were observed both in Northern Europe countries (e.g., Ireland [15.1], UK [13.1]), Western Europe (e.g., Netherlands [104.5], Belgium [72.0]), Southern Europe countries (e.g., Spain [10.4], Malta [19.2]),30 and some non-EU/EEA countries of Eastern Europe (e.g., the Russian Federation [15]) and Central and Western Asia (e.g., Georgia [26.5], Kazakhstan [18.8]) (Table 1, Fig. 1 panel D).9
LGV is largely underdiagnosed in Europe partly because of the lack of appropriate LGV diagnostics, particularly in Eastern Europe and Central/Western Asia.7 According to ECDC data, in 2019, 3112 cases of LGV were reported in EU/EEA countries, almost all among men who have sex with men.33 However, 87% of the reported cases were from only four countries (France, the Netherlands, Spain, and the UK), indicating variations in the occurrence or reporting of this infection across Europe. LGV is barely reported in non-EU/EEA countries, where only Belarus, Serbia, and Ukraine report LGV cases.9
Epidemiological data on STIs in the WHO European Region must be interpreted in the context of high heterogeneity in access to testing, testing policies, diagnostic techniques, surveillance systems, and reporting practice. Across country comparisons may be biased due to several factors: i) countries with higher testing rates and testing programmes for key populations will likely identify higher numbers as compared to countries with reduced access to testing, possibly due to stigma or discrimination,34 ii) infections that are routinely screened for at the population level in some countries (e.g., CT in the UK, or PrEP-associated screening in western countries)9,35,36 will result in a higher number of notifications; iii) countries with wider access to sensitive molecular testing will identify more infections.37 iv) countries with more consolidated surveillance (i.e., adequately implemented in routine practice) and more complete reporting will show higher rates than countries with less efficient systems.38, 39, 40, 41 This highlights the unequal availability and robustness of epidemiology data and its strong correlation with underperforming surveillance systems and unequal access to STI testing, diagnosis and care across Europe.
Temporal trends
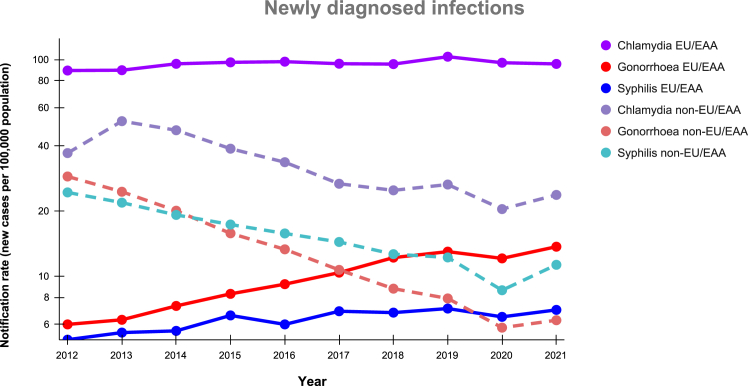
In the EU/EEA countries, overall notification rates per 100,000 population for all bacterial STIs reached an all-time high in 2019,42 but a modest decrease was observed in 2020 due to the impact of the COVID-19 pandemic followed by a rebound in 2021 (Fig. 2).43 It is challenging to discern the extent to which behaviour changes or changes in testing performance/policy have contributed to the recent rise in STI notifications. Among countries that reported consistently to ECDC for the ten year period from 2012 to 2021 (Supplementary appendix Table S1), the notification rate of CT has slightly increased from 89 in 2012 to 96/100,000 in 2021 (Fig. 2, Supplementary appendix Table S2), partly attributed to intensified screening efforts and expanded availability of sensitive diagnostic techniques.38,44 Notification rates of NG have shown a considerable increase from 6 in 2012 to 14/100,000 in 2021. A contributing factor is likely the enhanced screening (i.e., screening of asymptomatic patients and extragenital screening), particularly among men who have with men and especially those using pre-exposure prophylaxis for HIV (PrEP), resulting in a notable number of diagnoses among this population.41 Similarly, syphilis notifications have substantially increased from 5 in 2012 to 7/100,000 in 2021, with expansion of the population tested for syphilis and behaviour changes potentially playing a role.40 Finally, the increase in the number of cases of LGV (from 1780 in 2015 to 3112 cases in 2019) may be primarily attributed to changes in testing policies among men who have sex with men in countries reporting a large number of cases, along with rising number of cases among those with negative HIV status.8
Fig. 2.
Trend of newly diagnosed infections over time (rate per 100,000) from 2012 to 2021 in the WHO European Region, including EU/EEA∗ and non-EU/EEA countries∗∗. Legend: ∗For the calculation of trends in EU/EEA countries, data were considered from those countries that reported consistently to ECDC for the ten-year period, namely 19 countries for CT, 23 for NG and 23 for syphilis (Supplementary appendix, Table S2 and S3). The UK ceased contributing data to the European Surveillance System in 2020 following UK withdrawal from the EU, thus is not included in the calculation of a trend. Details on number of CT, NG and syphilis cases reported every year by each country and the corresponding national notification rates are available from ECDC Surveillance Atlas.8 ∗∗For the calculation of trends in non-EU/EEA, data was extracted from an assessment conducted by the WHO/Europe in 11 countries,9 and national statistical reports from 6 countries. (Supplementary Appendix, Tables S3 and S4).13, 14, 15, 16, 17,27
Availability of STI data in non-EU/EEA countries are more limited, with inconsistent collection of information on modes of transmission, hindering comparability with EU/EEA data and conclusions about STI epidemics. Data collated from national statistical reports indicates a steady decline in all three bacterial STIs in the past decade (Fig. 2 and Supplementary Appendix Tables S3 and S4).9,13, 14, 15, 16, 17,20, 21, 22, 23, 24, 25, 26, 27 In these countries, the notification rates of CT, NG, and syphilis have each declined per 100,000 population between 2012 and 2021, CT from 37.1 to 23.8, NG from 29.8 to 6.3, and syphilis from 24.4 to 11.3.
It is not clear if the steady decline in new diagnoses of all three bacterial STIs across the non-EU/EEA represents a true decline or reflects changing access to testing, changing testing practices or changing reporting practices. Therefore, notified cases may not reflect the true epidemiological situation and further in-depth exploration is needed. Despite the scarcity of evidence, several factors may have contributed to the observed decline, including reduced access to testing in some countries, possibly due to stigma or discrimination,34 particularly affecting key populations (with limited data reported for this group), as well as differences in STI screening policies,9,35 and access diagnostic methods.37 Furthermore, implementation of PrEP for HIV programmes is considerably less extended in Eastern Europe and Central Asia compared to Western Europe countries, thus enhanced STI screening of men who have sex with men that use PrEP is likely more limited in the non-EU/EEA context.36
Gender distribution, transmission category, age at diagnosis, and HIV coinfection in STI cases
Disaggregated demographic variables are available from the ECDC Surveillance Atlas for EU/EEA countries up to 2021, but they are more limited for non-EU/EEA countries, with the assessment published by WHO/Europe providing age and sex-disaggregated data available for CT and syphilis in 11 countries, and NG in 14 countries in 2019.9
In the EU/EEA among all countries that provided data to the ECDC in 2021 (Table 2, Supplementary appendix Table S5), 80% of CT notifications were among heterosexual females and males, with a slightly higher proportion among females (Table 2). NG notifications were five times higher in men compared to women, with 62% cases reported through homosexual transmission and 20% through heterosexual transmission in men. Syphilis diagnoses were predominantly reported among men, with 75% of cases attributed to homosexual transmission. Similarly, the non-EU/EEA countries reported a higher number of CT cases in women and more cases of syphilis and NG in men than in women.9 The male-to-female ratio of syphilis and NG cases in the non-EU/EEA countries were lower than in EU/EEA countries, 1.5 and 2.6, respectively. A small percentage of cases in the EU/EEA data were reported under “other” transmission category without further information about population groups such as non-heterosexual women, transgender and gender diverse individuals, highlighting the need for improved reporting of case characteristics at national and EU/EEA level.
Table 2.
Characteristic of bacterial STIs reported by EU/EEA countries to the European surveillance system, 2021.
| Characteristics of cases in 2021a | Chlamydia | Gonorrhoea | Syphilis |
|---|---|---|---|
| No. of reported cases | 184,542 | 46,723 | 25,270 |
| No. of reporting countriesb | 27 | 27 | 28 |
| Male-to-female ratio | 0.9:1 | 5:1 | 9:1 |
| Range of national notification rates per 100,000 population | <1–627 | 2–123 | <1 to 32 |
| Distribution by age group (years) | |||
| 0–14 | 0.3% | 0.2% | 0.1% |
| 15–24 | 59.6% | 27.4% | 12.0% |
| 25–34 | 26.7% | 38.6% | 31.9% |
| 35–44 | 8.3% | 20.4% | 25.8% |
| 45+ | 5.1% | 13.4% | 30.1% |
| Distribution by transmission category | |||
| No. of cases with info available | 175,557 | 42,776 | 16,497 |
| Heterosexual females | 47.6% | 16.6% | 8.8% |
| Heterosexual males | 31.5% | 20.2% | 15.2% |
| Men who have sex with men | 19.4% | 62.0% | 75.4% |
| Otherc | 1.4% | 1.3% | 0.6% |
| Median age in years by transmission category (IQR) | |||
| No. of cases with info available | 64,259 | 24,241 | 13,393 |
| Heterosexual females | 21 (19–25) | 23 (20–30) | 31 (23–42) |
| Heterosexual males | 23 (21–27) | 27 (22–35) | 37 (28–48) |
| Men who have sex with men | 32 (26–42) | 32 (26–40) | 35 (28–45) |
| Percentage of cases with HIV-positive status by transmission category | |||
| No. of cases with info available | 10,267 | 13,983 | 5279 |
| Heterosexual females | 3.1% | 2.6% | 1.7% |
| Heterosexual males | 4.8% | 5.1% | 4.9% |
| Men who have sex with men | 17.1% | 14.3% | 29.8% |
Includes cases from all countries that provided data for 2021 irrespective of consistency of reporting in the previous years and of surveillance system coverage.
Details regarding reporting countries, reported cases, and notification rates by country can be found in the Supplementary Appendix Table S5.
There is likely underreporting of STIs among non-heterosexual women, and transgender and gender diverse individuals, highlighting the need for better quality data at national an EU level.
Age-stratified analyses show higher proportion of most STIs among young age groups, although the contribution of these groups to the overall burden varies between countries and infections.9,32,38,40,41 In the EU/EEA dataset, the majority of CT cases (60%) were observed in people aged 15–24 years, in contrast only 12% of syphilis cases occurred in this age-group (Table 2). By transmission category, the median age for heterosexual individuals with CT/NG is younger (21–27 years), while for both homosexual and heterosexual men with syphilis, it is highest (35–37 years). Nonetheless, in 2022 and 2023, several Western European countries have noticed an increase of heterosexual transmission of NG among young people.45, 46
Completeness of information on HIV-serostatus varies in TESSy due to differences in surveillance data sources (e.g., clinical settings vs laboratory) and aggregated vs case-based reporting. Some publications indicate that people living with HIV are over-represented in bacterial STI cases,47 likely due to more frequent screenings and increased contact with healthcare and contributing behaviour factors such as risk compensation or high-risk behaviour among men who have sex with men.48
Sexually transmitted infections in key populations: multidimensional determinants and persistent inequities
As per the WHO, key populations—comprising men who have sex with men, transgender and gender diverse individuals, sex workers, people who inject drugs, and those in prisons and custodial settings—are defined as groups at increased risk for HIV, viral hepatitis, and STIs, emphasizing the need to prioritize prevention, diagnosis, and treatment.49 Their increased risk stems from biological factors (e.g., enhanced anal intercourse efficiency, acute effects of STIs), individual behaviour (e.g., substance use, condomless sex), sexual networks (e.g., partner numbers, sexual venues), and structural factors (e.g., societal and health system discrimination, punitive laws, poverty).50,51 STI prevention and control interventions are interconnected and need to address root causes, namely, the structural and social determinants of health.52
-
•
Men who have sex with men
Globally, evidence of rising STI cases among men who have sex with men has been observed over the past decade. A systematic review in 2019 (58 studies) identified high prevalence of CT (6.9% [5.4–8.6]), NG (8% [6.3–11.0]), and early syphilis (5.3% [3–8]) among men who have sex with men tested before enrolment in PrEP,53 and another review (34-point estimates) reported a syphilis prevalence of 3.4% [1.8–5.4].40 In Europe, specifically, two extensive online surveys conducted among men who have sex with men across 46 European countries (EMIS-2010, EMIS-2017) observed a significant 76% increase in prevalence of self-reported CT/NG and an 83% increase in syphilis between 2010 and 2017.39 The surveys identified factors associated with syphilis diagnosis, including living with HIV, having more frequent condomless anal intercourse with non-steady male partners, recent STI-screening, selling sex, and PrEP use.54 The same data also suggested that changes in testing-practices and/or frequency likely played a larger role in reported increases in gonorrhoea and chlamydia than did changes in behaviour.
Men who have sex with men face some syndemic issues, including chemsex and other mental health issues, and could have external factors influencing their sexual behaviour, such as risk compensation during PrEP use, all of which could increase their susceptibility to STIs. Chemsex (i.e., use of certain types of drugs before or during sex) and ‘slamming’ (engaging in chemsex via injection) may also increase the risk of STIs transmission.55 A recent study in Belgium developed and evaluated a mobile phone application (‘Budd-app’) for individuals engaging in chemsex, revealed behaviours such as prolonged sessions (mean of 17.5 h), polydrug use (95% of sessions), and unsafe dosing (49% of sessions).56
Regarding the impact of PrEP for HIV on STI cases, studies yield conflicting results. Some suggest higher STI prevalence among PrEP users related to enhanced screening, biological synergies and risk compensation behaviours,57, 58, 59 while others find no significant increase in incidence or prevalence of STIs.60,61 These results reinforce the interaction of preventive combination interventions and sexual behaviour and that PrEP programs should integrate high-quality STI and sexual health services.53,62 (see the Series article on prevention).2
-
•
Transgender and gender diverse people
Transgender and gender diverse people encounter structural barriers, including criminalization, stigma, and discrimination like other key populations, but experience violence at very high rates.63 They also face lack of legal recognition along with policy barriers to general and specialized healthcare,64 making them much more susceptible to various health issues like substance use disorders, depression, and self-harm, HIV, and bacterial STIs. A systematic review (25 studies, 11 countries) reported varying prevalence of HIV in transgender women (0%–49.6%) and transgender men (0%–8.3%) in different studies, as well as varying prevalence of CT (2.7%–24.7%), NG (2.1%–19.1%), and syphilis (1.4%–50.4%) in transgender women, with limited data on transgender men.65
-
•
Sex workers
Sex workers, including female, male, transgender, and gender diverse individuals, are highly susceptible to HIV and STI acquisition due to unsafe working conditions, sub-optimal access to health and prevention services, and barriers to consistent condom use.66 Social determinants such as the lack of social protection, housing, food insecurity, reduced education opportunities, and disability further compound their risks.
A study in Barcelona revealed high prevalence of HIV (25%) and bacterial STI (CT 10%, NG 19%) among cisgender men and transgender women sex workers.67 Syndemic conditions, alcohol, polydrug use, violence (affecting 40% of individuals), and lack of healthcare access were common impacting 79% of cisgender men and 68% of transgender women. Condomless anal sex (aOR 1.81) and frequent alcohol consumption (aOR 2.73) were associated with violence, and alcohol consumption (aOR 1.88) with having more clients.68 Another study in the US found a 40% HIV prevalence and high STI prevalence (CT 18%, NG 10%), with insertive anal sex with clients increasing the risk (RR 3.48).69
Solutions to address high STI prevalence among sex workers include improving sexual health, addressing violence, and considering sex work legislation.70,71 An ecological analysis (27 European countries) showed that legalising or decriminalizing aspects of sex work (n = 17 countries) is associated with lower HIV prevalence among sex workers compared to countries criminalising sex work (n = 10 countries).72 Multicomponent and peer-co-designed approaches have also proven effective in improving mental and physical health outcomes for female and street-based workers, with limited data for other sex workers.73
-
•
People who inject drugs and people in prisons
People who inject drugs are a recognized key target population for HIV and viral hepatitis prevention and to a lesser extent for bacterial STIs. Although the prevalence of STIs observed is low, scant condom use, higher risk to be victims of sexual violence and to exchanging sex for money, makes it necessary for prevention programs to include messages related to sexual risk practices, especially among young people and women.74 On the other hand, the prevalence of bacterial STIs among people in prisons is heterogeneous in selected studies (CT 1.0%–6.7%, NG 0.6%–7.8%), with recreational drug use, low educational levels, and sex without a condom as major risk factors.75
-
•
Migrants and refugees
Migrants and refugees are not classified as key population for STIs by WHO, yet structural inequities like sexism, racism, heterosexism, and poverty contribute to elevated STI cases within this group.52 A study conducted in Milan involving 537 undocumented migrant women from 39 countries found high STI prevalence (HPV 24%, CT 8%).76 In Malta, a study of 143 migrants from outside Europe attending a sexual health clinic revealed a 73% STI prevalence,77 along with recorded experiences of sexual violence leading to sexual health complications. Recognizing these risks during migration is essential for comprehensive STI prevention and care.
A scoping review (11 articles, 5 focused solely on Europe as the receiving region, and 6 were mixed but included Europe) identified six upstream social and structural determinants that undermine the sexual and reproductive health and rights of migrants and refugees in high income countries. These determinants are the economic crisis in Europe, legal entitlements barriers, inadequate resources for migrant health, poor living and working conditions (e.g., living in underserved areas, detention or reception centres, confiscation of passports, etc), cultural and linguistic barriers, and stigma/discrimination.78 Another review including experiences worldwide identified similar determinants.79 This highlights the need for targeted policy-making and planning to address structural challenges, and the importance of improving STI/HIV awareness, testing, and early service utilization among migrants.
New challenges beyond known determinants for STIs
-
•
Social and sexual networks
Social and sexual networks play an important role in STI transmission,52 and network epidemiology has advanced our understanding of “risk-potential networks” driving STI spread.80 Factors like the number of connections, being part of a core group, how different groups mix, the relationship between individuals, and having multiple concurrent sexual partners81 influence behaviours and infectious outcomes. For example, core groups with both a high STI prevalence and a high number of multiple inter-connected partners are believed to be particularly relevant for explaining increases of syphilis.54,82 Conversely these core groups are thought to be much less important in the overall increase in cases of CT and NG.83 Moreover, the role of networks in the transmission of Monkeypox virus (MPXV) during the early phase of the outbreak in Belgium has been recently described.84
-
•
Pandemics: COVID-19
Several studies have documented a decrease of STI consultations and/or diagnoses during the first COVID-19 wave in 2020, particularly with regards to asymptomatic infections. However, it remains challenging to ascertain whether these reductions are real or due to underdiagnoses and underreporting during lockdown periods.43
An impact assessment survey (EuroTEST COVID-19) collected 98 responses from secondary care clinics, community sites, and NGOs (in 23 EU/EEA and 11 non-EU/EEA countries). The survey compared 2020 data to 2019 and found 95% of respondents experienced testing volume decreases, with 64% facing severe disruptions to testing provisions.85 Volumes of community-based voluntary counselling and testing (CBVCT) dropped significantly (>50%) due to lockdown closures (69%), reduced attendance in sites that continued operating (66.2%), and limited staff (59.7%).85 No differences were observed between EU/EEA and non-EU/EEA countries.
The COVID-19 pandemic impacted sexual and reproductive health (SRH) services leading to unmet needs among the general population. Data from a UK study among 6654 participants aged 18–59 years found that 20.8% used SRH services, and 9.7% reported attempting but being unable to access the service they needed.86 In addition to disruptions in access to SRH services, members of populations in vulnerable situations were at increased risk of indirect impacts arising from responses to COVID-19, such as isolation, loss of income and residential instability.87,88
The challenges encountered throughout all stages of the STI care cascade during epidemics like COVID-19 should serve as lessons, informing the development of effective interventions to mitigating their impact and enhance future public health preparedness.
Molecular epidemiology: networks of transmission and clustering
Molecular tools are essential for understanding STI epidemiology, especially for NG, allowing the investigation of transmission networks and antimicrobial resistance (AMR) monitoring. Whole genome sequencing (WGS) enhances resolution in describing NG transmission networks compared to multi-locus sequence typing (MLST), yet the latter is more cost-effective and feasible with lower-volume DNA samples. On the other hand, sequencing of CT and T. pallidum has been limited because these are difficult-to-culture organisms. Nevertheless, recent developments in DNA enrichment techniques have enabled advances in the application of WGS to these bacteria.89
Tracking networks of transmission
Sequencing of NG isolates indicates a high molecular concordance among sexual contact pairs,90 with most genomically linked cases diagnosed within a short time (over half within 30 days, and three-quarters within 90 days).91 Moreover, NG cases are predominantly acquired locally, with a smaller proportion linked to distantly situated national or international transmission events. In a study in Brighton (2011–2015)91 almost three-quarters of cases were local, with a smaller proportion linked to other national (18%) or international (9%) populations. Travel-related partnerships involved a minority of unique sequence types acquired outside the area.90 The impact of COVID-19 lockdown was also assessed by WGS in the Netherlands, showing a marked increase in a single specific NG strain with a reduction in genetic diversity consistent with extensive local transmission but limited transnational transmission.92 Regarding NG networks of transmission in Europe, most studies have identified a difference in sequence types circulating between homosexual men and heterosexual individuals.93 A few clusters involve solely homosexual and heterosexual men, but not heterosexual women, suggestive of mixing between gay and heterosexual-identifying men who have sex with men. Sub-groups with sequence types that vary based on ethnicity, age, particular sexual behaviours or HIV status have all been described90,93,94 indicating distinct sexual networks.
In the case of syphilis, T. pallidum slow acquisition of single nucleotide polymorphisms hampers molecular transmission studies. Nevertheless, a UK study combining genomic data and meta-data demonstrated distinct homosexual and heterosexual transmission networks with little bridging between the two populations.95 Further evidence revealed the presence of two concurrent major lineages (SS14 and Nichols) circulating in Europe and globally,96 with most major sub-lineages detected across Europe consistent with international sexual network connections. Additionally, genotypic resistance to azithromycin has been shown to have emerged multiple times in different locations,89 suggesting no single newly emergent strain drives the recent macrolide-resistant syphilis epidemic in Europe.
Molecular epidemiology for CT has prominently involved monitoring diagnostic-escape variants, exemplified by the nvCT variant, initially identified in Sweden in 2006 with a plasmid DNA 77bp deletion. This deletion made a specific nucleic acid amplification test (NAAT) ineffective, leading to undiagnosed infections and subsequent nationwide dissemination of the variant.97 Modifications in testing protocols subsequently reduced infections caused by this strain from over 56% in 2007 to 6.5% in 2015.97 In February 2019, a diagnostic-escape CT mutant (23S rRNA, C1515T) emerged in Finland (10% in 2019, 2% in 2022) and spread to Norway (84% prevalence in 2020), while another escape mutant (23S rRNA, G1523A) became prevalent in Denmark (95% in 2020).98 Moreover, specific LGV CT strains, have been identified as the cause of recent epidemics among men who have sex with men, as demonstrated through genotyping of the outer-membrane-protein (OmpA).99 An LGV epidemic was caused by the L2b variant in 2004 in Europe, and new variants have been identified later, but all derive from the initial L2b strain.
NG antimicrobial resistance
Sequencing to identify drug-resistant NG can provide insights into the feasibility of resistance-guided treatment. If most resistant cases are linked to one or a few mutations, standard NAATs used in clinical practice could be adapted for detection, and allow diversification of treatment for NG100 (see the Series article on treatment).4 For instance, most quinolone and cefixime resistance results from few mutations,101 allowing genotypic diagnosis of resistance, faster than phenotypic methods. Notably, a test targeting the gyrA mutation which causes >90% of cases of quinolone resistance holds potential for use at individual level.100
Moreover, the integration of antimicrobial resistance surveillance for NG into existing monitoring platforms, like the European Gonococcal Antimicrobial Surveillance Programme (Euro-GASP), has proven advantageous. Euro-GASP provides a large-scale, representative sample of NG isolates in Europe,102 enabling the repeated assessment of this dataset for tracking of specific lineages. Notably, the NG-MAST G1407 lineage includes all cephalosporin-resistant isolates (5% cefixime, 1% ceftriaxone), despite it also includes a significant number of susceptible isolates.103 This lineage was highly prevalent from 2009 to 2013, representing 23% of isolates in 2009–10, and 17% of isolates in 2013. However, its prevalence decreased over time, shifting from predominantly affecting homosexual to heterosexual men. By 2018, the transmission of this lineage had declined to 2.1%. The overall decline in this strain has resulted in a higher proportion of circulating strains that are now susceptible to cephalosporins.104 Moreover, the 2018 EURO-GASP identified ciprofloxacin resistance remained high (>50%) nearly all (>99%) due to S91F mutation in gyrA, and a novel genogroup (NG-MAST G12302) with low-level azithromycin resistance (8%) emerging predominantly in pharyngeal isolates of men who have sex with men.105 Overall resistance patterns varied across countries (25%–75%); with azithromycin resistance from 0% to >50%, and cefixime resistance from <1% to 25%. Euro-GASP data showed similar levels of AMR between European-born and non-European-born individuals.106
Emerging STIs: zoonotic origins and shifts in transmission routes
Emerging STIs in Europe are characterized by an increasing number of infections that were previously uncommon or that are newly recognized, along with certain STIs that were once limited to other geographic regions becoming more common within specific European populations. These infections can be attributed to diverse factors such as zoonotic cross-species transmission (e.g., mpox), evolving routes of transmission resulting from changes in sexual behaviour (e.g., Hepatitis A, B, C, D or Shigella), global travel and migrations (e.g., Zika and Ebola), or geographical expansion to a new continent (e.g., LGV C. trachomatis) (Table 3).
Table 3.
Emergent Sexually Transmissible Pathogens in Europe.
| Pathogen | Traditional Transmission Mechanism | Sexual Transmission Mechanisms | Groups at increased risk of sexual acquisition | Frequency |
|---|---|---|---|---|
| Mpox | Zoonotic transmission | Direct contact with lesions, body fluids | Men who have sex with men | 2022 pandemic |
| Hepatitis A | Faeco-oral transmission | Oral-anal sex | Men who have sex with men | Periodic outbreaks |
| Hepatitis B | Percutaneous of permucosal contact with body fluids | Unprotected sex in unvaccinated individuals | Sexually active heterosexual individuals and men who have sex with men | Ongoing transmission |
| Hepatitis C | Specific sexual practices (i.e., fisting), injecting drug use, contaminated blood products | High-risk anal sex involving contact with blood | Men who have sex with men | Ongoing transmission |
| Hepatitis D | Injecting drug use, contaminated blood products | High-risk anal sex involving contact with blood. Super-infection in chronic hepatitis B or simultaneous co-infection with hepatitis B |
Men who have sex with men and sex-workers | Ongoing transmission |
| Shigella | Faeco-oral transmission | Oral-anal sex | Men who have sex with men | Ongoing transmission |
| Zika | Aedes mosquito bite | Transmission via genital fluids | Partners of individuals contracting Zika, including travel-related | Sporadic case reports |
| Ebola | Contact with infected body fluids | Transmission via genital fluids | Partners of individuals contracting Ebola | Sporadic case reports |
| LGV | Sexual contact with infected individuals in the tropics | Sexual contact in temperate climates | Men who have sex with men, core groups | Ongoing |
Zoonotic STI origins, cross-species transmission, and human adaptation
Several STIs have a zoonotic origin or are presumed to have originated from animals, with HIV being a prominent example of zoonotic STI that has transitioned to human-to-human transmission. Some emerging STIs, like mpox, are still classified as zoonoses, and there is a long list of pathogens with close related microbial species in other animals, including Haemophilus ducreyi,107 T. pallidum subsp. pertenue108 and others.
Determining zoonotic origins is complex due to confounding factors.109 HIV is a well described zoonotic STI, with its zoonotic source in chimpanzees discovered in 1999,110 but it was not immediately apparent when it was first reported in 1981.111,112 Zoonotic events involve host susceptibility, whilst the likelihood of these resulting in an epidemic is impacted by the ability to sustain human-to-human transmission,110,113 and mutation and recombination may play roles in overcoming barriers to cross-species transmission and sustaining transmission.114
Mpox is an STI with a zoonotic origin that caused a global epidemic in 2022, primarily affecting men who have sex with men.115 Human cases were first reported in the Democratic Republic of the Congo in 1970 with sporadic subsequent outbreaks in Africa linked to zoonotic transmission events from rodents.116 The outbreak in 2022 involved direct contact with lesions during sex as the major mode of transmission,117,118 and caused severe disease in people with advanced HIV.119 Genetic sequencing of 2022 revealed MPXV lineage 2b strains that had been circulating in Nigeria in 2017 with notable divergence due to APOBEC-related mutations.120 This indicates significant human-to-human transmission and possible host adaptation. A study of 1900 MPXV genomes found thirteen derived lineages with ten distinct non-synonymous mutations in genes related to immune evasion, virulence and host recognition that likely contribute to the rapid evolution and diversification of current MPXV lineages.121 Mpox growth was linked to sexual networks with many partners,122 with behaviour change and vaccination being potential control measures. This highlights the role of core groups in sustaining transmission.122
Sex as emerging route of transmission
Several pathogens, like Hepatitis A virus, Shigella, formerly transmitted primarily through the enteric route, have seen ongoing transmission among men who have sex with men due to sexual transmission mechanisms like oral-anal sex. In high-income countries Shigella spp, an enteric pathogen causing acute colitis, has been primarily acquired through travel. However, since the 1970s, cases of sexually transmitted shigella have emerged particularly among men who have sex with men. Certain lineages of both Shigella flexneri and Shigella sonnei,123 primarily transmitted within this population, have acquired antibiotic resistance to multiple antibiotics and spread rapidly through sexual networks spanning countries and continents.124 The emergence of Shigella in this population is linked to specific sexual practices such as rimming (oral-anal sex).125 In the UK sexual transmission amongst men who have sex with men now accounts for nearly 50% of reported cases. Multiple waves of transmission have occurred driven by strains with differing antibiotic resistance patterns. Initially, the most notable pattern was azithromycin resistance, associated with serotype 3A S. flexneri, but subsequent S. sonnei strains resistant to ciprofloxacin have also emerged and more recently plasmid-mediated extended beta-lactam resistance has been described, initially in S. sonnei and with emerging evidence of horizontal gene transfer to S. flexneri strains.126,127
Similarly, sustained sexual transmission of Hepatitis A virus among men who have sex with men is well-documented, leading to recommendations for vaccination in this population. However, the implementation of vaccination strategies has been inconsistent, leading to periodic outbreaks like a multi-country outbreak in Europe in 2016–17, with approximately 4100 confirmed cases.128 Subsequent outbreaks have been reported in Hungary, Croatia, and Romania in 2022–2023,129 as men who have sex with men in these and other Eastern Europe and Central Asia countries remain unvaccinated against hepatitis A.
In addition, other pathogens like Hepatitis C virus, formerly transmitted by percutaneous transmission, have also demonstrated instances of sexual transmission through contact with blood during sex. The risk is higher among men who have sex with men,130 particularly among those who also are living with HIV or those who are on PrEP for HIV, because of differences in sexual practice that incur a greater risk of trauma and blood exposure.131 Transmission risk is associated to condomless anal sex, traumatic sexual practices (such as fisting, group sex and chem-sex), injecting drugs, and recent diagnosis of an ulcerative STI.131,132 More recently widespread access to directly acting anti-viral agents which are highly effective in the treatment of hepatitis C has significantly reduced the burden of primary Hepatitis C virus infections in Europe with the majority of cases now due to re-infections amongst men who have sex with men. Similarly, the incidence of acute Hepatitis B virus infection in Europe has declined due to vaccination programmes. Traditionally, Hepatitis B virus has been transmitted through various body fluids, and among acute cases nowadays, acquisition is most commonly reported among both heterosexual individuals and men who have sex with men who are unvaccinated.133
Geographic extension of STIs
LGV is an example of geographical expansion, which was previously limited to heterosexual individuals in East and West Africa and has since 2003 expanded among networks of men who have sex with men in high-income countries due to high-risk sexual behaviours, leading to an endemic level of transmission.6 The reasons for the expansion of LGV cases from tropical to temperate regions are not fully understood. Independent risk factors for LGV in Europe include concurrent genital ulcerative disease, HIV, previous STIs, unprotected anal sex, recent travel, and meeting partners online. The rectal-urethral LGV ratio is 15:1, suggesting that brief urethral infections are sufficient for transmission but too short to manifest symptoms, in addition to an oro-anal transmission route.134 This implies that sustaining transmission requires frequent sexual encounters with multiple partners.
Zika virus and Ebola virus have also been classified as emerging STIs because of transmission via genital fluids. Several Ebola cases during the late phase of the 2014 to 2016 epidemic in West Africa were attributed to sexual transmission,135,136 with viral RNA detected in semen for an average of 19 months, but no cases have been reported in Europe. In 2015, Zika virus caused a large epidemic in the Americas, with imported cases in Europe, including instances of sexual transmission137,138 (infectivity in semen up to 30 days), but current cases are low (22 in 2020).
Conclusions
Looking forward, this report highlights several important aspects that require attention and action. First, given the important regional variations in reported STI notification rates across the WHO European Region, efforts should be made to improve and harmonize epidemiological surveillance systems, as well as monitoring and evaluation protocols of programs and services, including access to testing and screening in instances where public health benefit has been demonstrated. Regarding surveillance, disaggregation for men who have sex with men at high risk, non-heterosexual women and transgender and gender diverse individuals should be encouraged. Second, to address the community-transmission of STIs, especially among vulnerable populations, it is important to recognize the role played by dense sexual networks and a combination of social and structural factors. New data collection approaches and research methods should be put in place to describe these factors, measure how they contribute to STI-burden and integrate them in prevention strategies and programmes. Enhancing case-based reporting with programmatic, social, behavioural, and structural data on STI risk factors and determinants is crucial to better understand the dynamics of these epidemics to improve the design and effectiveness of multilevel preventive interventions. Finally, close monitoring and research are needed to identify of potential new emerging STIs, new antimicrobial resistance patterns, and identify and strengthen effective preparedness measures.
Contributors
VP and OMi conceived the review and coordinated its preparation. MAR and OMa conducted the search of surveillance data. OMa, OMi, and JC wrote the part on epidemiological overview. MM and OMi wrote the part on molecular epidemiology. CF, VP, IR, and KB wrote the part on key populations. MM, AT, AC and OMi wrote the part on emerging STIs. All authors reviewed and edited the final version and approved the final manuscript.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
The authors declare no competing interests.
Acknowledgments
We thank Gerard Carot-Sans for help in the preparation of the final document. OMi and MARA were supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant agreement No. 850450; grant holder: Oriol Mitjà).
Disclaimers: The views and opinions expressed herein are the authors' own and do not necessarily state or reflect those of ECDC. ECDC is not responsible for the data and information collation and analysis and cannot be held liable for conclusions or opinions drawn.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100742.
Contributor Information
Oriol Mitjà, Email: omitja@lluita.org.
Valeska Padovese, Email: valeska.padovese@gov.mt.
Appendix A. Supplementary data
References
- 1.World Health Organization (WHO) Regional action plans for ending AIDS and the epidemics of viral hepatitis and sexually transmitted infections 2022–2030. 2023. https://apps.who.int/iris/bitstream/handle/10665/369243/9789289058957-eng.pdf?sequence=1&isAllowed=y [cited 2023 Jul 27]. Available from:
- 2.Gökengin D., Noori T., Alemany A., et al. Prevention strategies for sexually transmitted infections, HIV, and viral hepatitis in Europe. Lancet Reg Health Eur. 2023 doi: 10.1016/j.lanepe.2023.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenyon C., Herrmann B., Hughes G., et al. Management of asymptomatic sexually transmitted infections in Europe: towards a differentiated, evidence-based approach. Lancet Reg Health Eur. 2023 doi: 10.1016/j.lanepe.2023.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitjà O., Suñer C., Giacani L., et al. Treatment of bacterial sexually transmitted infections in Europe : gonorrhoea, mycoplasma genitalium, and syphilis. Lancet Reg Health Eur. 2023 doi: 10.1016/j.lanepe.2023.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (WHO) Global progress report on HIV, viral hepatitis and sexually transmitted infections. 2021. https://www.who.int/publications/i/item/9789240027077 [cited 2023 Jul 26]. Available from:
- 6.Dal Conte I., Mistrangelo M., Cariti C., et al. Lymphogranuloma venereum: an old, forgotten re-emerging systemic disease. Panminerva Med. 2014;56:73–83. [PubMed] [Google Scholar]
- 7.Cole M.J., Field N., Pitt R., et al. Substantial underdiagnosis of lymphogranuloma venereum in men who have sex with men in Europe: preliminary findings from a multicentre surveillance pilot. Sex Transm Infect. 2020;96(2):137–142. doi: 10.1136/sextrans-2019-053972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Centre for Disease Prevention and Control . 2023. Surveillance Atlas of infectious diseases.https://www.ecdc.europa.eu/en/surveillance-atlas-infectious-diseases [cited 2023 Jun 9]. Available from: [Google Scholar]
- 9.Barbaric J., Kuchukhidze G., Seguy N., et al. Surveillance and epidemiology of syphilis, gonorrhoea and chlamydia in the non-European union countries of the world health organization European region, 2015 to 2020. Euro Surveill. 2022;27(8) doi: 10.2807/1560-7917.ES.2022.27.8.2100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Health Department (Andorra) Surveillance of mandatory notification diseases 2017-2021. https://www.salut.ad/images/stories/Salut/pdfs/Vigilancia_Malalties_Declaracio_obligatoria.pdf [cited 2023 Aug 5]. Available from:
- 11.Statistik Austria Jahrbuch der gesundheitsstatistik. 2021. https://www.statistik.at/fileadmin/user_upload/Gesundheitsstatistik-JB_2021_Web-barrierefrei.pdf [cited 2023 Aug 5]. p. ISBN 978-3-903264-42-B. Available from:
- 12.Federal Office of Public Health of the Swiss Confederation VIH, syphilis, gonorrhée et chlamydiose en Suisse en 2019: survol épidémiologique. Maladies Transmissibles. https://www.bag.admin.ch/dam/bag/fr/dokumente/mt/p-und-p/hiv-sti-statistiken-analysen-und-trends/hiv-sti-fallzahlen-2019.pdf.download.pdf/vih-ist-survol-epidemiologique-2019.pdf [cited 2023 Aug 5]. Available from:
- 13.National Bureau of Statistics of the Republic of Moldova Statistical databank “Statbank”. https://statistica.gov.md/en/statistical-databank-78.html [cited 2023 Aug 5]. Available from:
- 14.Federal state statistics service (Russia) Russian statistical yearbook. 2020. https://eng.rosstat.gov.ru/Publications/document/74811 [cited 2023 Aug 5]. Available from:
- 15.Government of Kyrgyzstan Open data portal of the Kyrgyz republic. https://data.gov.kg/ [cited 2023 Aug 5]. Available from:
- 16.Public Health Institute of Republic of Srpska . 2020. Analysis of the population health in the republic of srpska.https://www.phi.rs.ba/pdf/publikacije/Zdravstveno_stanje_stanovnistva_u_2020_godini-WEB.pdf [cited 2023 Aug 5]. Available from: [Google Scholar]
- 17.Montenegro statistical Office Statistical yearbook. 2020. http://www.monstat.org/uploads/files/publikacije/godisnjak 2020/GODISNJAK 2020 .pdf [cited 2023 Aug 5]. p. ISSN 0354-2076. Available from:
- 18.Higienos Institutas Health statistic of lithuania 2019. 2020. https://www.hi.lt/health-statistic-of-lithuania.html [cited 2023 Aug 5]. Available from:
- 19.Santé Publique - France Bulletin de santé publique VIH-IST. 2020. https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-sexuellement-transmissibles/vih-sida/documents/bulletin-national/bulletin-de-sante-publique-vih-ist.-decembre-2020 [cited 2023 Aug 5]. Available from:
- 20.National statistical committe of the Republic of Belarus Statistical yearbook of the republic of Belarus. https://www.belstat.gov.by/en/ofitsialnaya-statistika/publications/catalogues-of-statistical-publications/statistical-yearbook-of-the-republic-of-belarus/ [cited 2023 Aug 8]. Available from:
- 21.State Statistical Service of Ukraine Demographic and social statisitcs. https://ukrstat.gov.ua/ [cited 2023 Aug 8]. Available from:
- 22.Krotik O.I. Epidemiology of sexually transmitted infections in Ukraine. Reprod Endocrinol. 2020;(56):8–12. [Google Scholar]
- 23.National boureau of statistics of the republic of Kazakhstan Statistical yearbook of the republic of Kazakhstan. https://stat.gov.kz/publication/collections/?year=&name=16245&period=) [cited 2023 Aug 8]. Available from:
- 24.Government of Armenia Statistical committee of the republic of Armenia. https://armstat.am/en/?nid=586 [cited 2023 Aug 8]. Available from:
- 25.Government of Azerbaijan The state statistical committee of the republic of Azerbaijan. https://www.stat.gov.az/source/healthcare/?lang=en [cited 2023 Aug 8]. Available from:
- 26.National Statistical Office of Georgia Statistical yearbook of Georgia. https://www.geostat.ge/en/single-categories/95/statistical-yearbook [cited 2023 Aug 8]. Available from:
- 27.Ministry of Health (Israel) Weekly epidemiological reports. https://www.gov.il/he/Departments/DynamicCollectors/weekly-epidemiological-report?skip=0 [cited 2023 Aug 5]. Available from:
- 28.European Centre for Disease Prevention and Control Chlamydia infection - Annual epidemiological report for 2019. 2019. https://www.ecdc.europa.eu/en/publications-data/chlamydia-infection-annual-epidemiological-report-2019 [cited 2023 Jun 9]. Available from:
- 29.European Centre for Disease Prevention and Control Gonorrhoea - Annual epidemiological report for 2019. 2019. https://www.ecdc.europa.eu/en/publications-data/gonorrhoea-annual-epidemiological-report-2019 [cited 2023 Jun 9]. Available from:
- 30.European Centre for Disease Prevention and Control Syphilis - Annual epidemiological report for 2019. 2019. https://www.ecdc.europa.eu/en/publications-data/syphilis-annual-epidemiological-report-2019 [cited 2023 Jun 9]. Available from:
- 31.European Centre for Disease Prevention and Control Surveillance systems overview for 2019. 2019. https://www.ecdc.europa.eu/en/publications-data/surveillance-systems-overview-2019 [cited 2023 Jun 9]. Available from:
- 32.Unemo M., Ison C.A., Cole M., Spiteri G., Van De Laar M., Khotenashvili L. Gonorrhoea and gonococcal antimicrobial resistance surveillance networks in the WHO European Region, including the independent countries of the former Soviet Union. Sex Transm Infect. 2013;89(Suppl 4):iv42–i46. doi: 10.1136/sextrans-2012-050909. [DOI] [PubMed] [Google Scholar]
- 33.European Centre for Disease Prevention and Control Lymphogranuloma venereum - Annual Epidemiological report for 2019. 2022. https://www.ecdc.europa.eu/en/publications-data/lymphogranuloma-venereum-annual-epidemiological-report-2019 [cited 2023 Jul 26]. Available from:
- 34.ILGA Europe Annual review 2022. https://www.ilga-europe.org/report/annual-review-2022/ [cited 2023 Aug 8]. Available from:
- 35.Uusküla A., Puur A., Toompere K., DeHovitz J. Trends in the epidemiology of bacterial sexually transmitted infections in eastern Europe, 1995–2005. Sex Transm Infect. 2010;86(1):6–14. doi: 10.1136/sti.2009.037044. [DOI] [PubMed] [Google Scholar]
- 36.Gokengin D., Bursa D., Skrzat-Klapaczynska A., et al. PrEP scale-up and PEP in central and eastern Europe: changes in time and the challenges we face with No expected HIV vaccine in the near future. Vaccines. 2023;11(1):122. doi: 10.3390/vaccines11010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boiko I., Golparian D., Krynytska I., Unemo M. High prevalence of Chlamydia trachomatis, Neisseria gonorrhoeae and particularly Trichomonas vaginalis diagnosed using US FDA-approved Aptima molecular tests and evaluation of conventional routine diagnostic tests in Ternopil, Ukraine. APMIS. 2019 Sep;127(9):627–634. doi: 10.1111/apm.12975. [DOI] [PubMed] [Google Scholar]
- 38.Redmond S.M., Alexander-Kisslig K., Woodhall S.C., et al. Genital Chlamydia prevalence in Europe and non-European high income countries: systematic review and meta-analysis. PLoS One. 2015 Jan 23;10(1) doi: 10.1371/journal.pone.0115753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marcus U., Mirandola M., Schink S.B., Gios L., Schmidt A.J. Changes in the prevalence of self-reported sexually transmitted bacterial infections from 2010 and 2017 in two large European samples of men having sex with men-is it time to re-evaluate STI-screening as a control strategy? PLoS One. 2021;16(3) doi: 10.1371/journal.pone.0248582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsuboi M., Evans J., Davies E.P., et al. Prevalence of syphilis among men who have sex with men: a global systematic review and meta-analysis from 2000–20. Lancet Glob Heal. 2021;9(8):e1110–e1118. doi: 10.1016/S2214-109X(21)00221-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whelan J., Abbing-Karahagopian V., Serino L., Unemo M. Gonorrhoea: a systematic review of prevalence reporting globally. BMC Infect Dis. 2021;21(1):1–23. doi: 10.1186/s12879-021-06381-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Geretti A.M., Mardh O., de Vries H.J.C., et al. Sexual transmission of infections across Europe: appraising the present, scoping the future. Sex Transm Infect. 2022;98(6):451–457. doi: 10.1136/sextrans-2022-055455. [DOI] [PubMed] [Google Scholar]
- 43.Sentís A., Prats-Uribe A., López-Corbeto E., et al. The impact of the COVID-19 pandemic on Sexually Transmitted Infections surveillance data: incidence drop or artefact? BMC Public Health. 2021;21(1):1–7. doi: 10.1186/s12889-021-11630-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.European Centre for Disease Prevention and Control. Public health guidance on chlamydia control in Europe [Internet]. 2016 [cited 2023 Jun 9]. Available from: https://www.ecdc.europa.eu/en/publications-data/public-health-guidance-chlamydia-control-europe.
- 45.Vives N., Lugo R., López E., et al. Increase in gonorrhoea among very young adolescents in Catalonia Spain. Eurosurveillance. 2013;18(33):20560. doi: 10.2807/1560-7917.es2013.18.33.20560. [DOI] [PubMed] [Google Scholar]
- 46.Surveillance Report. Weekly Communicable Disease Threats Report, week 39, Week 25, 18–24 June 2023. 5. Increases in gonorrhoea notification in heterosexual populations in EU/EEA countries.
- 47.Kalichman S.C., Pellowski J., Turner C. Prevalence of sexually transmitted co-infections in people living with HIV/AIDS: systematic review with implications for using HIV treatments for prevention. Sex Transm Infect. 2011;87:183–190. doi: 10.1136/sti.2010.047514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pufall E.L., Kall M., Shahmanesh M., Nardone A., Gilson R., Delpech V., et al. Positive Voices Study Group Sexualized drug use (’chemsex’) and high-risk sexual behaviours in HIV-positive men who have sex with men. HIV Med. 2018;19(4):261–270. doi: 10.1111/hiv.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.World Health Organization (WHO) Consolidated guidelines on HIV, viral hepatitis and STI prevention, diagnosis, treatment and care for key populations. Guideline. 2022. https://www.who.int/publications/i/item/9789240052390 [cited 2023 Jul 26]. Available from: [PubMed]
- 50.Baral S., Logie C.H., Grosso A., Wirtz A.L., Beyrer C. Modified social ecological model: a tool to guide the assessment of the risks and risk contexts of HIV epidemics. BMC Public Health. 2013;13(1):1–8. doi: 10.1186/1471-2458-13-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mayer K.H., Allan-Blitz L. Similar, but different: drivers of the disproportionate HIV and sexually transmitted infection burden of key populations. J Int AIDS Soc. 2019;22(Suppl Suppl 6) doi: 10.1002/jia2.25344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Academies of Sciences Engeneering and Medicine . The National Academiess Press; 2021. Sexually transmitted infections: adopting a sexual health paradigm. [PubMed] [Google Scholar]
- 53.Ong J.J., Baggaley R.C., Wi T.E., et al. Global epidemiologic characteristics of sexually transmitted infections among individuals using preexposure prophylaxis for the prevention of HIV infection: a systematic review and meta-analysis. JAMA Netw Open. 2019;2(12) doi: 10.1001/jamanetworkopen.2019.17134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mendez-Lopez A., Stuckler D., Marcus U., et al. Social and behavioural determinants of syphilis: modelling based on repeated cross-sectional surveys from 2010 and 2017 among 278,256 men who have sex with men in 31 European countries. Lancet Reg Heal. 2022;22 doi: 10.1016/j.lanepe.2022.100483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Knoops L., van Amsterdam J., Albers T., Brunt T.M., van den Brink W. Slamsex in The Netherlands among men who have sex with men (MSM): use patterns, motives, and adverse effects. Sex Health. 2022;19(6):566–573. doi: 10.1071/SH22140. [DOI] [PubMed] [Google Scholar]
- 56.Platteau T., Herrijgers C., Florence E., et al. Drug behaviors, sexually transmitted infection prevention, and sexual consent during chemsex: insights generated in the Budd app after each chemsex session. Front Public Heal. 2023;11 doi: 10.3389/fpubh.2023.1160087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jansen K., Steffen G., Potthoff A., et al. STI in times of PrEP: high prevalence of chlamydia, gonorrhea, and mycoplasma at different anatomic sites in men who have sex with men in Germany. BMC Infect Dis. 2020;20:1–14. doi: 10.1186/s12879-020-4831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Powell V.E., Gibas K.M., DuBow J., Krakower D.S. Update on HIV preexposure prophylaxis: effectiveness, drug resistance, and risk compensation. Curr Infect Dis Rep. 2019;21:1–8. doi: 10.1007/s11908-019-0685-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marcus J.L., Katz K.A., Krakower D.S., Calabrese S.K. Risk compensation and clinical decision making—the case of HIV preexposure prophylaxis. N Engl J Med. 2019;380(6):510. doi: 10.1056/NEJMp1810743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoornenborg E., Coyer L., Achterbergh R.C.A., et al. Sexual behaviour and incidence of HIV and sexually transmitted infections among men who have sex with men using daily and event-driven pre-exposure prophylaxis in AMPrEP: 2 year results from a demonstration study. lancet HIV. 2019;6(7):e447–e455. doi: 10.1016/S2352-3018(19)30136-5. [DOI] [PubMed] [Google Scholar]
- 61.Streeck H., Jansen K., Crowell T.A., et al. HIV pre-exposure prophylaxis was associated with no impact on sexually transmitted infection prevalence in a high-prevalence population of predominantly men who have sex with men, Germany, 2018 to 2019. Euro Surveill. 2022;27(14) doi: 10.2807/1560-7917.ES.2022.27.14.2100591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zheng Y., Yu Q., Lin Y., et al. Global burden and trends of sexually transmitted infections from 1990 to 2019: an observational trend study. Lancet Infect Dis. 2022;22(4):541–551. doi: 10.1016/S1473-3099(21)00448-5. [DOI] [PubMed] [Google Scholar]
- 63.Blondeel K., de Vasconcelos S., García-Moreno C., Stephenson R., Temmerman M., Toskin I. Violence motivated by perception of sexual orientation and gender identity: a systematic review. Bull World Health Organ. 2018;96(1):29–41L. doi: 10.2471/BLT.17.197251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veale J.F., Deutsch M.B., Devor A.H., et al. Setting a research agenda in trans health: an expert assessment of priorities and issues by trans and nonbinary researchers. Int J Transgender Heal. 2022;23(4):392–408. doi: 10.1080/26895269.2022.2044425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Gerwen O.T., Jani A., Long D.M., Austin E.L., Musgrove K., Muzny C.A. Prevalence of sexually transmitted infections and human immunodeficiency virus in transgender persons: a systematic review. Transgender Heal. 2020;5(2):90–103. doi: 10.1089/trgh.2019.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Argento E., Goldenberg S., Shannon K. Preventing sexually transmitted and blood borne infections (STBBIs) among sex workers: a critical review of the evidence on determinants and interventions in high-income countries. BMC Infect Dis. 2019;19(1):1–19. doi: 10.1186/s12879-019-3694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferrer L., González V., Martró E., et al. High HIV/STI prevalence among cisgender men and transgender women sex workers attending community-based centres in Barcelona, Spain: the Sweetie Project. Int J STD AIDS. 2022;33(12):1045–1053. doi: 10.1177/09564624221116536. [DOI] [PubMed] [Google Scholar]
- 68.Mesías-Gazmuri J., Folch C., Ferrer L., et al. Syndemic conditions and their association with HIV/STI sexual risk behaviors among transgender women and cisgender men sex workers in catalonia: the SexCohort project. Int J Behav Med. 2022;1–12 doi: 10.1007/s12529-022-10138-x. [DOI] [PubMed] [Google Scholar]
- 69.Poteat T., White R.H., Footer K.H.A., et al. Characterising HIV and STIs among transgender female sex workers: a longitudinal analysis. Sex Transm Infect. 2021;97(3):226–231. doi: 10.1136/sextrans-2019-054414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brookfield S., Dean J., Forrest C., Jones J., Fitzgerald L. Barriers to accessing sexual health services for transgender and male sex workers: a systematic qualitative meta-summary. AIDS Behav. 2020;24:682–696. doi: 10.1007/s10461-019-02453-4. [DOI] [PubMed] [Google Scholar]
- 71.Platt L., Grenfell P., Meiksin R., et al. Associations between sex work laws and sex workers' health: a systematic review and meta-analysis of quantitative and qualitative studies. PLoS Med. 2018;15(12) doi: 10.1371/journal.pmed.1002680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reeves A., Steele S., Stuckler D., McKee M., Amato-Gauci A., Semenza J.C. National sex work policy and HIV prevalence among sex workers: an ecological regression analysis of 27 European countries. Lancet HIV. 2017;4(3):e134–e140. doi: 10.1016/S2352-3018(16)30217-X. [DOI] [PubMed] [Google Scholar]
- 73.Johnson L., Potter L.C., Beeching H., et al. Interventions to improve health and the determinants of health among sex workers in high-income countries: a systematic review. Lancet Public Heal. 2022;8(2):e141–e154. doi: 10.1016/S2468-2667(22)00252-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Folch C., Casabona J., Brugal M.T., et al. Sexually transmitted infections and sexual practices among injecting drug users in harm reduction centers in Catalonia. Eur Addict Res. 2011;17(5):271–278. doi: 10.1159/000329931. [DOI] [PubMed] [Google Scholar]
- 75.SeyedAlinaghi S., Pashaei Z., Rahimi E., et al. Prevalence of sexually transmitted infections and associated risk behaviors in prisoners: a systematic review. Heal Sci Reports. 2022;5(5):e819. doi: 10.1002/hsr2.819. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 76.Frati E.R., Fasoli E., Martinelli M., et al. Sexually transmitted infections: a novel screening strategy for improving women's health in vulnerable populations. Int J Mol Sci. 2017;18(6):1311. doi: 10.3390/ijms18061311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Padovese V., Farrugia A., Almabrok Ali Ghath S., Rossoni I. Sexually transmitted infections' epidemiology and knowledge, attitude and practice survey in a set of migrants attending the sexual health clinic in Malta. J Eur Acad Dermatol Venereol. 2021;35(2):509–516. doi: 10.1111/jdv.16949. [DOI] [PubMed] [Google Scholar]
- 78.Lebano A., Hamed S., Bradby H., et al. Migrants' and refugees' health status and healthcare in Europe: a scoping literature review. BMC Public Health. 2020;20:1–22. doi: 10.1186/s12889-020-08749-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davidson N., Hammarberg K., Romero L., Fisher J. Access to preventive sexual and reproductive health care for women from refugee-like backgrounds: a systematic review. BMC Public Health. 2022;22(1):1–37. doi: 10.1186/s12889-022-12576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kenyon C.R., Delva W. It's the network, stupid: a population's sexual network connectivity determines its STI prevalence. F1000Res. 2018;7:1880. doi: 10.12688/f1000research.17148.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Doherty I.A., Padian N.S., Marlow C., Aral S.O. Determinants and consequences of sexual networks as they affect the spread of sexually transmitted infections. J Infect Dis. 2005;191(Supplement_1):S42–S54. doi: 10.1086/425277. [DOI] [PubMed] [Google Scholar]
- 82.Gunn R.A., Klausner J.D. Enhancing the control of syphilis among men who have sex with men by focusing on acute infectious primary syphilis and core transmission groups. Sex Transm Dis. 2019;46(10):629. doi: 10.1097/OLQ.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stephens S.C., Fann C.K., Strona F.V., et al. Identifying syphilis risk networks through venue attendance in San Francisco. Sex Transm Dis. 2014;41(5):333. doi: 10.1097/OLQ.0000000000000116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanhamel J., Laisnez V., Liesenborghs L., Brosius I., Berens-Riha N., Vanbaelen T., et al. Understanding sexual transmission dynamics and transmission contexts of monkeypox virus: A mixed-methods study of the early outbreak in Belgium (May–June 2022) Sex Transm Infect. 2023;99(5):330–336. doi: 10.1136/sextrans-2022-055601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fernàndez-López L., Simões D., Casabona J. Impact of the COVID-19 pandemic on community-based testing for HIV, viral hepatitis and sexually transmitted infections in the WHO European Region, March to August 2020. Eur J Public Health. 2023;33(3):528–535. doi: 10.1093/eurpub/ckad010. ckad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dema E., Gibbs J., Clifton S., et al. Initial impacts of the COVID-19 pandemic on sexual and reproductive health service use and unmet need in Britain: findings from a quasi-representative survey (Natsal-COVID) Lancet Public Heal. 2022;7(1):e36–e47. doi: 10.1016/S2468-2667(21)00253-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Leyva-Moral J.M., Castro Ávila J., Villar M., et al. Impact of the COVID-19 health crisis on trans women and cis men sex workers in Spain. Arch Sex Behav. 2023;52(2):629–638. doi: 10.1007/s10508-022-02405-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.UNAIDS Strategic considerations for mitigating the impact of COVID-19 on key-population-focused HIV programs. 2022. https://www.unaids.org/en/resources/documents/2020/key-populations-strategic-considerations-covid19 [cited 2023 Jul 27]. Available from:
- 89.Beale M.A., Marks M., Sahi S.K., et al. Genomic epidemiology of syphilis reveals independent emergence of macrolide resistance across multiple circulating lineages. Nat Commun. 2019;10(1):3255. doi: 10.1038/s41467-019-11216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Town K., Bolt H., Croxford S., et al. Neisseria gonorrhoeae molecular typing for understanding sexual networks and antimicrobial resistance transmission: a systematic review. J Infect. 2018;76(6):507–514. doi: 10.1016/j.jinf.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Silva D., Peters J., Cole K., et al. Whole-genome sequencing to determine transmission of Neisseria gonorrhoeae: an observational study. Lancet Infect Dis. 2016;16(11):1295–1303. doi: 10.1016/S1473-3099(16)30157-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zondag H.C.A., de Korne-Elenbaas J., Bruisten S.M., de Vries H.J.C., van Dam A.P. Increased clonality among Neisseria gonorrhoeae isolates during the COVID-19 pandemic in Amsterdam, The Netherlands. Microb Genomics. 2023;9(4) doi: 10.1099/mgen.0.000975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Town K., Field N., Harris S.R., et al. Phylogenomic analysis of Neisseria gonorrhoeae transmission to assess sexual mixing and HIV transmission risk in England: a cross-sectional, observational, whole-genome sequencing study. Lancet Infect Dis. 2020;20(4):478–486. doi: 10.1016/S1473-3099(19)30610-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heymans R., Matser A A., Bruisten S.M., et al. Distinct Neisseria gonorrhoeae transmission networks among men who have sex with men in Amsterdam, The Netherlands. J Infect Dis. 2012;206(4):596–605. doi: 10.1093/infdis/jis399. [DOI] [PubMed] [Google Scholar]
- 95.Beale M.A., Thorn L., Cole M.J., et al. Genomic epidemiology of syphilis in England: a population-based study. Lancet Microbe. 2023 doi: 10.1016/S2666-5247(23)00154-4. pii: S2666-5247(23)00154-4. doi: 10.1016/S2666-5247(23)00154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beale M.A., Marks M., Cole M.J., et al. Global phylogeny of Treponema pallidum lineages reveals recent expansion and spread of contemporary syphilis. Nat Microbiol. 2021;6(12):1549–1560. doi: 10.1038/s41564-021-01000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dahlberg J., Hadad R., Elfving K., et al. Ten years transmission of the new variant of Chlamydia trachomatis in Sweden: prevalence of infections and associated complications. Sex Transm Infect. 2018;94(2):100–104. doi: 10.1136/sextrans-2016-052992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Puolakkainen M., Mannonen L., Unemo M. The diagnostic-escape Finnish new variant of Chlamydia trachomatis first described in 2019 remains present in Finland three years after the introduction of an updated Aptima Combo 2 assay. Clin Microbiol Infect. 2023;29(5):658–659. doi: 10.1016/j.cmi.2023.02.009. [DOI] [PubMed] [Google Scholar]
- 99.Seth-Smith H.M.B., Bénard A., Bruisten S.M., et al. Ongoing evolution of Chlamydia trachomatis lymphogranuloma venereum: exploring the genomic diversity of circulating strains. Microb Genomics. 2021;7(6) doi: 10.1099/mgen.0.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Marks M., Harding-Esch E. Antimicrobial Resistance in Gonorrhea: Diagnostics to the Rescue. Clin Infect Dis. 2021;73:304–305. doi: 10.1093/cid/ciaa591. [DOI] [PubMed] [Google Scholar]
- 101.Grad Y.H., Harris S.R., Kirkcaldy R.D., et al. Genomic epidemiology of gonococcal resistance to extended-spectrum cephalosporins, macrolides, and fluoroquinolones in the United States, 2000–2013. J Infect Dis. 2016;214(10):1579–1587. doi: 10.1093/infdis/jiw420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cole M.J., Quinten C., Jacobsson S., et al. The European gonococcal antimicrobial surveillance programme (Euro-GASP) appropriately reflects the antimicrobial resistance situation for Neisseria gonorrhoeae in the European Union/European Economic Area. BMC Infect Dis. 2019;19(1):1–12. doi: 10.1186/s12879-019-4631-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harris S.R., Cole M.J., Spiteri G., et al. Public health surveillance of multidrug-resistant clones of Neisseria gonorrhoeae in Europe: a genomic survey. Lancet Infect Dis. 2018;18(7):758–768. doi: 10.1016/S1473-3099(18)30225-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Day M.J., Jacobsson S., Spiteri G., et al. Significant increase in azithromycin “resistance” and susceptibility to ceftriaxone and cefixime in Neisseria gonorrhoeae isolates in 26 European countries, 2019. BMC Infect Dis. 2022;22(1):1–10. doi: 10.1186/s12879-022-07509-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sánchez-Busó L., Cole M.J., Spiteri G., et al. Europe-wide expansion and eradication of multidrug-resistant Neisseria gonorrhoeae lineages: a genomic surveillance study. Lancet Microbe. 2022;3(6):e452–e463. doi: 10.1016/S2666-5247(22)00044-1. [DOI] [PubMed] [Google Scholar]
- 106.Hernando Rovirola C., Spiteri G., Sabidó M., et al. Antimicrobial resistance in Neisseria gonorrhoeae isolates from foreign-born population in the European Gonococcal Antimicrobial Surveillance Programme. Sex Transm Infect. 2020;96(3):204–210. doi: 10.1136/sextrans-2018-053912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.González-Beiras C., Marks M., Chen C.Y., Roberts S., Mitjà O. Epidemiology of Haemophilus ducreyi infections. Emerg Infect Dis. 2016;22(1):1–8. doi: 10.3201/eid2201.150425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mitjà O., Marks M., Konan D.J.P., et al. Global epidemiology of yaws: a systematic review. Lancet Glob Heal. 2015;3(6):e324–e331. doi: 10.1016/S2214-109X(15)00011-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Marx P.A., Apetrei C., Drucker E. AIDS as a zoonosis? Confusion over the origin of the virus and the origin of the epidemics. J Med Primatol. 2004;33(5-6):220–226. doi: 10.1111/j.1600-0684.2004.00078.x. [DOI] [PubMed] [Google Scholar]
- 110.Gao F., Bailes E., Robertson D.L., et al. Origin of HIV-1 in the chimpanzee Pan troglodytes troglodytes. Nature. 1999;397(6718):436–441. doi: 10.1038/17130. [DOI] [PubMed] [Google Scholar]
- 111.Gottlieb M.S., Schroff R., Schanker H.M., et al. Pneumocystis carinii pneumonia and mucosal candidiasis in previously healthy homosexual men: evidence of a new acquired cellular immunodeficiency. N Engl J Med. 1981;305(24):1425–1431. doi: 10.1056/NEJM198112103052401. [DOI] [PubMed] [Google Scholar]
- 112.Siegal F.P., Lopez C., Hammer G.S., et al. Severe acquired immunodeficiency in male homosexuals, manifested by chronic perianal ulcerative herpes simplex lesions. N Engl J Med. 1981;305(24):1439–1444. doi: 10.1056/NEJM198112103052403. [DOI] [PubMed] [Google Scholar]
- 113.Kirchhoff F. Is the high virulence of HIV-1 an unfortunate coincidence of primate lentiviral evolution? Nat Rev Microbiol. 2009;7(6):467–476. doi: 10.1038/nrmicro2111. [DOI] [PubMed] [Google Scholar]
- 114.Sauter D., Kirchhoff F. Key viral adaptations preceding the AIDS pandemic. Cell Host Microbe. 2019;25(1):27–38. doi: 10.1016/j.chom.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 115.Mitjà O., Ogoina D., Titanji B.K., et al. Monkeypox. Lancet. 2023;401(10370):60–74. doi: 10.1016/S0140-6736(22)02075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Patrono L.V., Pléh K., Samuni L., et al. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat Microbiol. 2020;5(7):955–965. doi: 10.1038/s41564-020-0706-0. [DOI] [PubMed] [Google Scholar]
- 117.Tarín-Vicente E.J., Alemany A., Agud-Dios M., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suñer C., Ubals M., Tarín-Vicente E.J., et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect Dis. 2022;400(10353):661–669. doi: 10.1016/S1473-3099(22)00794-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitjà O., Alemany A., Marks M., et al. Mpox in people with advanced HIV infection: a global case series. Lancet. 2023;401(10380):939–949. doi: 10.1016/S0140-6736(23)00273-8. [DOI] [PubMed] [Google Scholar]
- 120.Isidro J., Borges V., Pinto M., et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28:1–4. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Luna N., Muñoz M., Bonilla-Aldana D.K., et al. Monkeypox virus (MPXV) genomics: a mutational and phylogenomic analyses of B. 1 lineages. Travel Med Infect Dis. 2023;52 doi: 10.1016/j.tmaid.2023.102551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Endo A., Murayama H., Abbott S., et al. Heavy-tailed sexual contact networks and monkeypox epidemiology in the global outbreak, 2022. Science. 2022;378(6615):90–94. doi: 10.1126/science.add4507. [DOI] [PubMed] [Google Scholar]
- 123.Mason L.C.E., Greig D.R., Cowley L.A., et al. The evolution and international spread of extensively drug resistant Shigella sonnei. Nat Commun. 2023;14(1):1983. doi: 10.1038/s41467-023-37672-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baker K.S., Dallman T.J., Ashton P.M., et al. Intercontinental dissemination of azithromycin-resistant shigellosis through sexual transmission: a cross-sectional study. Lancet Infect Dis. 2015;15(8):913–921. doi: 10.1016/S1473-3099(15)00002-X. [DOI] [PubMed] [Google Scholar]
- 125.Gilbart V.L., Simms I., Jenkins C., et al. Sex, drugs and smart phone applications: findings from semistructured interviews with men who have sex with men diagnosed with Shigella flexneri 3a in England and Wales. Sex Transm Infect. 2015;91(8):598–602. doi: 10.1136/sextrans-2015-052014. [DOI] [PubMed] [Google Scholar]
- 126.Thorley K., Charles H., Greig D.R., et al. Emergence of extensively drug-resistant and multidrug-resistant Shigella flexneri serotype 2a associated with sexual transmission among gay, bisexual, and other men who have sex with men, in England: a descriptive epidemiological study. Lancet Infect Dis. 2023;23(6):732–739. doi: 10.1016/S1473-3099(22)00807-6. [DOI] [PubMed] [Google Scholar]
- 127.Charles H., Prochazka M., Thorley K., et al. Outbreak of sexually transmitted, extensively drug-resistant Shigella sonnei in the UK, 2021–22: a descriptive epidemiological study. Lancet Infect Dis. 2022;22(10):1503–1510. doi: 10.1016/S1473-3099(22)00370-X. [DOI] [PubMed] [Google Scholar]
- 128.Ndumbi P., Freidl G.S., Williams C.J., et al. Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveill. 2018;23(33) doi: 10.2807/1560-7917.ES.2018.23.33.1700641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.European Centre for Disease Prevention and Control Spread of hepatitis A virus strains of genotype IB in several EU countries and the United Kingdom. https://www.ecdc.europa.eu/en/news-events/spread-hepatitis-virus-strains-genotype-ib-several-eu-countries-and-united-kingdom [cited 2023 Jul 27]. Available from:
- 130.Witt M.D., Seaberg E.C., Darilay A., et al. Incident hepatitis C virus infection in men who have sex with men: a prospective cohort analysis, 1984–2011. Clin Infect Dis. 2013;57(1):77–84. doi: 10.1093/cid/cit197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bradshaw D., Vasylyeva T.I., Davis C., et al. Transmission of hepatitis C virus in HIV-positive and PrEP-using MSM in England. J Viral Hepat. 2020;27(7):721–730. doi: 10.1111/jvh.13286. [DOI] [PubMed] [Google Scholar]
- 132.Vanhommerig J.W., Lambers F.A.E., Schinkel J., et al. Open forum infectious diseases. Oxford University Press; 2015. Risk factors for sexual transmission of hepatitis C virus among human immunodeficiency virus-infected men who have sex with men: a case-control study; p. ofv115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.European Centre for Disease Prevention and Controls Hepatitis B - Annual Epidemiological Report for 2021. https://www.ecdc.europa.eu/en/publications-data/hepatitis-b-annual-epidemiological-report-2021 [cited 2023 Sep 6]. Available from:
- 134.de Vrieze N.H.N., Versteeg B., Bruisten S.M., van Rooijen M.S., van der Helm J.J., de Vries H.J.C. Low prevalence of urethral lymphogranuloma venereum infections among men who have sex with men: a prospective observational study, sexually transmitted infection clinic in Amsterdam, The Netherlands. Sex Transm Dis. 2017;44(9):547. doi: 10.1097/OLQ.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Diallo B., Sissoko D., Loman N.J., et al. Resurgence of Ebola virus disease in Guinea linked to a survivor with virus persistence in seminal fluid for more than 500 days. Clin Infect Dis. 2016;63(10):1353–1356. doi: 10.1093/cid/ciw601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prevail I I I Study Group A longitudinal study of Ebola sequelae in Liberia. N Engl J Med. 2019;380(10):924–934. doi: 10.1056/NEJMoa1805435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Venturi G., Zammarchi L., Fortuna C., et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill. 2016;21(8) doi: 10.2807/1560-7917.ES.2016.21.8.30148. [DOI] [PubMed] [Google Scholar]
- 138.Mead P.S., Duggal N.K., Hook S.A., et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med. 2018;378(15):1377–1385. doi: 10.1056/NEJMoa1711038. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.