Summary
This review explores the therapeutic challenges of sexually transmitted infections (STI) in Europe, which include increasing antimicrobial resistance and limited progress in drug discovery. We primarily focus on gonorrhoea, Mycoplasma genitalium, and syphilis infections. For gonorrhoea with escalating resistance rates we explore the possibility of combining ceftriaxone with another antibiotic or using alternative antibiotics to mitigate resistance emergence, and we provide insights on the ongoing evaluation of new antimicrobials, like gepotidacin and zoliflodacin. In the case of M. genitalium, which exhibits high resistance rates to first and second-line treatments, we emphasize the importance of resistance-guided therapy in regions with elevated resistance levels, and highlight the limited alternative options, such as pristinamycin and minocycline. Furthermore, we address the challenges posed by syphilis, where the primary treatment consists of penicillin or doxycycline, with challenges arising in neurosyphilis, allergy, pregnancy, and supply shortages and discuss the ongoing evaluation of alternative antimicrobials (e.g., ceftriaxone, cefixime, linezolid). Our findings identify priority actions and provide concrete solutions for long-term effective management of STIs and antimicrobial resistance mitigation.
Keywords: Gonorrhoea, Mycoplasma, Syphilis, Treatment
Key messages.
Gonorrhoea
-
•
The discovery of clinically resistant isolates of N. gonorrhoeae to first-line treatment with cephalosporins is a concerning development that demands immediate action, especially on the background of resistance rates of 10% for macrolides and 60% for fluoroquinolones.
-
•
Gradual dose escalation of ceftriaxone up to 1 g helped combat gonorrhoea resistance, but further escalation is unlikely to be effective.
-
•
Combining ceftriaxone with another antibiotic can potentially mitigate ceftriaxone resistance development, but it is crucial to identifying appropriate combinations that have a high level of efficacy and similar half-life.
-
•
Using specific older pre-existing antibiotics as alternatives to ceftriaxone could reduce exposure to ceftriaxone and limit the development of resistance; restricting their use just to gonorrhoea will help to preserve their effectiveness.
-
•
Molecular assays targeting resistance mutations can improve antibiotic stewardship and facilitate the use of recycled antibiotics, such as ciprofloxacin.
-
•
Gepotidacin, zoliflodacin, and lefamulin are new antimicrobials currently under clinical evaluation for the treatment of gonorrhoea.
-
•
Asymptomatic pharyngeal gonorrhoea is highly prevalent, and research on how to improve its detection and treatment is needed to prevent transmission of resistant strains.
-
•
Contact tracing remains crucial to breaking the chain of gonorrhoea transmission but adopting a test-and-wait approach for sexual partners (rather than routine empirical therapy) can be effective and will reduce the use of antibiotics.
-
•
Point-of-care tests and online postal self-sampling increase the accuracy, access, and acceptability of testing and may reduce infection and spread of resistance.
Mycoplasma genitalium
-
•
With global estimates for resistance to first-line macrolide antibiotics exceeding 50%, and second-line fluoroquinolone antibiotics approaching 10%, clinicians are increasingly encountering M. genitalium infections challenging to cure.
-
•
Azithromycin, given as an extended 5-day regimen, is recommended as the primary choice in Europe for treatment of uncomplicated M. genitalium infections without macrolide resistance mutations or resistance testing.
-
•
Macrolide resistance-guided therapy (RGT) uses molecular detection to identify genotypic resistance. In RGT, doxycycline is administered syndromically for a week while awaiting macrolide resistance testing results.
-
•
RGT algorithms incorporating assays to identify fluoroquinolone resistance markers can improve antibiotic precision and treatment efficacy in regions with prevalent fluoroquinolone resistance.
-
•
Pristinamycin and minocycline are options for infections failing moxifloxacin, or where resistance is detected, offering cure rates in the order of 70–75%.
-
•
The pipeline for new M. genitalium antimicrobials is very limited, with gepotidacin, zoliflodacin, solithromycin and a few other compounds demonstrating in vitro activity but no current clinical evaluation.
-
•
Community-level strategies to mitigate resistance development include enhanced antimicrobial resistance surveillance to guide antibiotic stewardship, reduce antibiotic overuse and inform regional clinical practice and policy. Indications for testing of M. genitalium infections have been narrowed to primarily involve symptomatic patients.
Syphilis
-
•
Penicillin is the primary treatment for syphilis, but there are challenges with its use, such as limited options for those allergic to β-lactams, especially pregnant women, challenges in managing neurosyphilis, and congenital syphilis, and reported shortages of penicillin G benzathine in European countries.
-
•
Doxycycline is a suitable treatment for syphilis based on current evidence but lacks evidence for neurosyphilis and is contraindicated in pregnant women, while its potential use as post-exposure prophylaxis (known as doxy-PEP) may contribute to selection of resistant strains.
-
•
Several antimicrobials, including ceftriaxone for neurosyphilis and cefixime and linezolid for early syphilis, are being evaluated to expand available options. Until controlled trials are completed it is important to follow internationally validated guidelines.
-
•
Linezolid has the potential to be an oral treatment for neurosyphilis due to its favorable pharmacokinetics and ability to penetrate the central nervous system, along with demonstrated in vitro anti-treponemal activity, but caution is advised due to concerns regarding a point mutation that may cause resistance in T. pallidum.
-
•
Amoxicillin and other widely used antibiotics for various infections, as well as zoliflodacin, and spectinomycin may exhibit therapeutic activity against treponemal infections, but further evidence is required.
-
•
Community-level strategies to interrupt transmission chains include case notification and contact tracing, and systematic screening of populations at risk. The development of new diagnostic tools that facilitate screening and treatment response monitorization, and novel notification and contact tracing software.
Search strategy and selection criteria.
References for this Review were identified through searches of PubMed with the search terms “gonorrhoea”, “mycoplasma”, “syphilis”, and “treatment” from 1995 until July 2023. Articles were also identified through searches of the authors’ own files. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this review.
Introduction
The therapeutic management of sexually transmitted infections (STIs) faces numerous challenges, necessitating a comprehensive revision of the current state of art in treatment approaches. In this review, we will focus on three specific bacterial STIs—gonorrhoea, Mycoplasma genitalium infection, and syphilis—chosen for their significant impact on sexual health and the multifaceted challenges they pose for treatment. Chlamydia trachomatis, despite being a major bacterial STI, is not included here because it was extensively reviewed in a previous review1 and no major therapeutic challenges or acquired antimicrobial resistance (AMR) have been identified since then.
Neisseria gonorrhoeae, C. trachomatis, and M. genitalium are associated with different STI syndromes, especially urethritis (Fig. 1). The frequency of each condition differs by geographic area and population (article on epidemiology in this series).2 Empirical presumptive treatment is usually initiated at the point-of-care for the initial management of these syndromes. The regimen chosen depends on the clinical evidence for gonococcal versus nongonococcal urethritis with a recommendation to pursue laboratory confirmation and optimize treatment to align with antibiotic stewardship thus minimizing the development of antibiotic-resistant bacteria. When immediate laboratory evaluations (e.g., gram stain) of urethral specimens are accessible, the empiric treatment for gonococcal urethritis involves ceftriaxone, while treatment for nongonococcal urethritis should be directed towards C. trachomatis with doxycycline. If rapid laboratory diagnosis is not available, then empirical treatment for gonococcal urethritis, and presumptive therapy for C. trachomatis is usually given (ceftriaxone and doxycycline). Nucleic acid amplification tests (NAATs) offer agent-specific results within hours to days; the time to results varies based on individual testing facility resources. When NAAT results are available to guide therapy, antimicrobial treatment can be directed towards a specific bacterial pathogen.
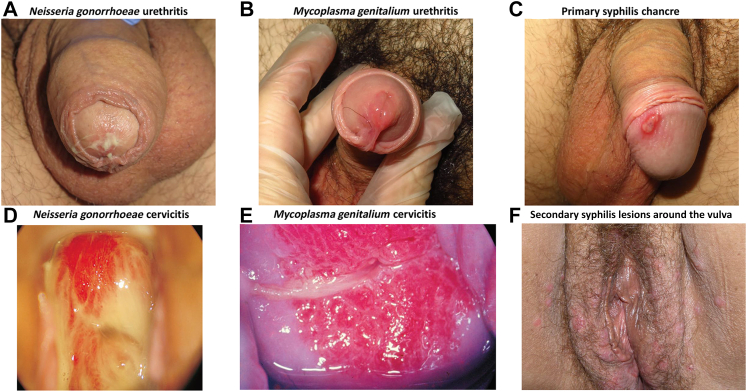
Fig. 1.
Common Clinical Presentation of Bacterial Sexually Transmitted Infections in male and female patients. Legend: (A) White - yellowish discharge characteristic of gonorrhea. (B) Image of urethritis caused by Mycoplasma genitalium infection with redness and inflammation in the meatus of the urethra and presence of transparent exudate. (C) Syphilis primary chancre on the glans of the penis. (D) Speculum exam shows a process of endocervicitis with purulent content. (E) Friability and congestion in a patient with Mycoplasma genitalium cervicitis. (F) Multiple secondary syphilis lesions around the vulva and inguinal area. Photo credits: Irene Fuertes (A–C), Marti Vall-Mayans (F).
Photos D and E, used with permission of the editor were adapted from ‘Atlas of Sexually Transmitted Diseases: Clinical Aspects and Differential Diagnosis’ available at https://doi.org/10.1007/978-3-319-57470-7.
Our aim is two-fold: to examine the current state of the art in bacterial STI treatment and to explore new approaches that can address emerging challenges and improve patient outcomes. We will address the critical aspects of treatment taking into consideration the impact of rising AMR, limitations of current regimens in certain situations or stages of the diseases, drug availability affected by shortages and access issues, and evolving trends in drug discovery or repurposing for STI therapeutics. By addressing these challenges head-on, we aim to promote the development of comprehensive and tailored approaches that are durable for the next five to 10 years.
Gonorrhoea
Gonorrhoea is an STI caused by infection with N. gonorrhoeae bacterium. Typical symptoms include vaginal or penile discharge, dysuria, and bleeding between periods for women (Fig. 1, panels A and D). If the infection ascends to the upper genital tract, it may cause pelvic inflammatory disease and epididymo-orchitis.3 In Europe, ceftriaxone administered as a single intramuscular injection (with or without azithromycin) is the current first-line treatment for N. gonorrhoeae infections of the urethra, cervix, rectum, and pharynx (Table 1).3
Table 1.
State-of-the-art in the approach to treatment of STIs in Europe.a
| Antibiotics with intrinsic proven activity in wild-type strains | Widespread antimicrobial resistant strains | Current first-line | Second line when allergy or treatment failure | Third line after treatment failure or resistance | Resistance guided therapy | Pregnancy | Empirical combination | |
|---|---|---|---|---|---|---|---|---|
| Gonorrhoea | Penicillin, cephalosporins, carbapenems, tetracyclines, macrolides, aminoglycosides, and fluoroquinolones | Penicillin, fluoroquinolones, tetracyclines, macrolides |
|
|
Guided by resistance testing |
Quinolone susceptibility confirmed by molecular tests:
|
|
|
| Mycoplasma genitalium | Tetracyclines, macrolides, fluoroquinolones, and streptogramins | Macrolides, fluoroquinolones |
|
|
|
Initial syndromic treatment:
|
|
|
| Syphilis | Penicillin, cephalosporins, macrolides, tetracyclines, oxazolidinones | Azithromycin |
Primary, secondary, and early latent syphilis:
|
Primary, secondary, and early latent syphilis with penicillin allergy:
|
First line is BPG, in case of penicillin allergy:
|
Treatment Guidelines (Europe): https://iusti.org/treatment-guidelines/-accessed 10 June 2023.
Similar Guidelines are available online: https://www.cdc.gov/std/treatment-guidelines/STI-Guidelines-2021.pdf (USA); https://sti.guidelines.org.au/ (Australia); https://www.bashh.org/guidelines (UK). BPG: Benzathine penicillin G.
The future management of individuals with gonorrhoea has several challenges. In 2020, the Euro-GASP surveillance project identified that, in Europe, 1% of gonococcal isolates had resistance to oral cefixime (MIC > 0.125 mg/L), 10% to azithromycin (MIC > 1 mg/L), and 58% to ciprofloxacin (MIC > 0.06 mg/L).4 Although current first-line therapy using cephalosporins remains highly effective,3 a small number of clinically resistant isolates (MIC > 0.125 mg/L) have been identified in Europe and relatively high rate of low-level resistance is found in the Asia–Pacific region.5,6 Our previous experience is of seeing the rapid loss of efficacy for penicillin, tetracyclines, and fluoroquinolones (and more recently increasing rates of resistance to macrolides)7 suggesting that it will be a question of ‘when’ rather than ‘if’ we need alternative treatments for gonorrhoea. This has been highlighted by the WHO which includes N. gonorrhoeae as one of its top 10 priority pathogens requiring research and development investment.8
Preserving cephalosporins
Cephalosporins are the last remaining highly effective treatment for gonorrhoea. Several strategies have been proposed to slow the emergence of resistance and prolong their efficacy for as long as possible (Table 2). The dose of intramuscular ceftriaxone has gradually been increased to its current level (500 mg in the US and Australia, or 1 g in Europe)3,9, 10, 11 to cover isolates with reduced susceptibility, but pharmacokinetic modelling suggests that further dose escalation beyond 1 g is unlikely to be effective in overcoming high-level resistance.12 The use of oral cefixime should be avoided because it results in lower drug levels and has been associated with the rapid emergence of resistance.13
Table 2.
Strategies to mitigate resistance development and spread.
| Improved use of current drugs | Drug discovery | Use of diagnostic tools | Community-level strategies to reduce transmission | |
|---|---|---|---|---|
| Gonorrhoea | ||||
| Mycoplasma genitalium | ||||
| Syphilis |
|
|
|
|
Combination therapy, involving ceftriaxone and a second agent, could inhibit the emergence of resistance, as has been used successfully in treating TB and HIV. However, for this approach to be effective, several criteria must be met:14 1) Availability of two effective antimicrobials with a high barrier for resistance development; 2) Independent mechanisms for developing antimicrobial resistance and 3) Preferably, similar pharmacokinetic properties because substantial differences in the half-lives of the two drugs could lead to a prolonged period of functional monotherapy with low drug levels, increasing the risk of resistant strains being selected if reinfection occurs. European and Australian guidelines (but not UK or USA guidelines)3,9, 10, 11 recommend the combination of azithromycin plus ceftriaxone, but the protective efficacy of azithromycin used in this way appears limited,16 and an increasing global prevalence of azithromycin resistance suggests that this combination is not optimal.54 Overall, combination therapy represents a potentially more robust and sustainable approach to gonorrhoea treatment, but only if suitable antibiotic combinations can be identified.
Several older pre-existing antibiotics might be effective to cure gonorrhoea and could potentially be used, reducing exposure of gonococcal isolates to ceftriaxone with the goal of keeping it ‘in reserve’ for those who need it. Spectinomycin, a broad-spectrum antibiotic registered in several European countries, has been recommended as an alternative treatment for gonorrhoea,15 reflecting the absence of resistance (MIC > 64 mg/L) in 2019 antimicrobial surveys,4 but production and availability of this agent is limited, resistance can develop rapidly and it has reduced efficacy in pharyngeal gonorrhoea.55 Gentamicin in combination with azithromycin is effective in clearing most genital infections and may be a useful treatment alternative but works less well for rectal and pharyngeal gonorrhoea (80–90% microbiological clearance).16,17 Ciprofloxacin, despite widespread fluoroquinolone resistance, can still be effective if patients with sensitive isolates of N. gonorrhoeae (∼40%) can be identified before treatment is given. Molecular AMR tests detecting wild type gyrA as a predictor of ciprofloxacin efficacy have the potential to achieve this and avoid the need for ceftriaxone, but cost and the potential for this approach to select ciprofloxacin resistant isolates also need to be considered.18,19 The use of such targeted approaches for other antibiotics may be more challenging as most lack a single sentinel gene mutation that closely correlates with effectiveness.56 Ertapenem is a potential alternative to ceftriaxone with similar treatment efficacy in one trial17 and it has also been used successfully as salvage therapy in a small number of patients with ceftriaxone resistance who have failed initial treatment. However, it needs to be used with care for treating bacterial STIs because it is also an important treatment for ESBL-producing enterobacterial infections. Finally, limiting the use of specific antibiotics to just treating gonorrhoea and preventing their more widespread use could reduce community exposure and limit the opportunity for resistance to develop.57
New antimicrobials
Gepotidacin and zoliflodacin, two promising antibiotics for the treatment of gonorrhoea, are currently in phase 3 development, and ongoing trial recruitment is set to be completed in 2023. Both are oral medications that act as topoisomerase inhibitors, although targeting different binding sites.20,21 Lefamulin, a pleuromutilin antibiotic, exhibits good in vitro activity against gonorrhoea and other sexually transmitted infections, such as Chlamydia trachomatis, but currently lacks clinical efficacy data for gonorrhoea.22
Community-level strategies to mitigate resistance development and spread
A number of approaches could be considered to achieve better control of gonorrhoea transmission, which has the potential to both reduce prevalence of disease and slow the spread of resistant isolates (Table 2).
Firstly, asymptomatic pharyngeal gonorrhoea is common and, despite its tendency to clear without treatment after around three months, it remains important in the evolution of resistance due to the easy transfer of genetic material between N. gonorrhoeae and commensal Neisseria species. There is increasing acceptance that gonorrhoea transmission may occur via saliva, and that not just fellatio but also kissing may pass on infection.24 More frequent testing may improve detection and treatment of missed infections, reducing onward transmission, but could also increase antibiotic usage and selective pressure for resistance. The optimal criteria for screening and the screening interval remain uncertain and should be a research priority (this topic is further discussed in the asymptomatic screening article of this series).58 Antibiotic efficacy is generally lower for pharyngeal gonorrhoea compared to genital infection, highlighting a need to ensure that new drugs are specifically evaluated for their effectiveness at this site.
Secondly, current partner management protocols need to be optimised. Identifying the sexual contacts of those with gonorrhoea is necessary to identify any transmitted infection, interrupt the chain of transmission, and reduce the risk of reinfection in the source patient. Novel approaches using digital automated partner notification have the potential to be more effective in achieving these aims and also be cost saving. Initial attempts had limited success, in part due to a lack of patient engagement, but recent results are encouraging as the technology and pathways are refined.25,26 In the past, offering empirical therapy to the sexual partners of those with gonorrhoea has been common, but the majority of such individuals (especially if asymptomatic) are not infected and a ‘test and only treat if positive’ approach reduces unnecessary antibiotic use and can be equally successful, while also reducing the risk of AMR in non-target pathogens.27
New technologies using molecular-based point-of-care tests for gonorrhoea offer a sensitive and specific way of making a rapid and accurate diagnosis.59 With these tests, patients can be diagnosed immediately, reducing the time to treatment and, therefore, shortening the period of infectivity and minimizing the risk of transmitting resistant isolates. The increasing use of online postal self-sampling also improves access to testing. Individuals request a test kit online, perform self-sampling at home, and send the specimens directly to the laboratory for testing. This approach can increase testing rates, particularly in those who might otherwise be reluctant or unable to access traditional face to face clinical services.23
Introducing new and costly drugs, implementing molecular assays for detecting resistance, controlling antibiotic availability and usage, and rapidly identifying sexual partners for testing and treatment are all resource-intensive approaches that may not be feasible or affordable in many healthcare systems. Management and monitoring pathways should be optimized to reflect available resources and identify the most cost-effective approaches while considering the high cost of future AMR.
Mycoplasma genitalium
M. genitalium is a prevalent STI that causes non-gonococcal urethritis in men, and cervicitis, pelvic inflammatory disease, and preterm birth in women (Fig. 1, panels B and E).38,45 Due to the lack of a cell wall, only a limited number of antimicrobial classes are efficacious against M. genitalium, including tetracyclines, macrolides, fluoroquinolones, and streptogramins (Table 1). However, several reasons preclude the use of some of these classes. A typical 7-day regimen of doxycycline has low efficacy for M. genitalium, with microbiological cure rates ranging from 30% to 40%. Azithromycin, in a single 1 g oral dose, was widely recommended for first-line treatment, demonstrating susceptibility in vitro and superiority to doxycycline in 2 of 3 randomised trials over a decade ago.60, 61, 62 However, due to high rates of selection of resistance mutations in the 23 S ribosomal RNA gene (>10%) following 1 g azithromycin,63,64 its efficacy declined from 85% prior to 2009 to 67% in studies up to 2014.65 The extensive use of this regimen globally for STI syndromes (and for C. trachomatis, M. genitalium and N. gonorrhoeae in combination with ceftriaxone), as well as the widespread use of josamycin in Russia and Central and Eastern European countries, are likely to have contributed to the accelerated selection of macrolide-resistant strains in M. genitalium. Azithromycin, given as an extended 5-day regimen, is currently recommended as the primary choice in Europe for treatment of uncomplicated M. genitalium infections without macrolide resistance mutations or resistance testing. Moxifloxacin, a fourth-generation fluoroquinolone, is the preferred second-line treatment when azithromycin fails or for macrolide-resistant infections. Sitafloxacin, from the same family and generation, is approved for use in M. genitalium infection in Japan and Australia, but not in most other countries, including Europe. Initially, when introduced in 2004, moxifloxacin had a cure rate approaching 100%.66 However, the first treatment failures emerged within few years, and pooled data showed a decline in efficacy from 100% prior to 2010 to 89% by 2016.67 As a result, clinicians opted to repurpose old drugs, such as pristinamycin and minocycline, to provide options for patients failing fluoroquinolones, or in whom they are contraindicated. Neither of these agents are highly effective: pristinamycin cures 75% of macrolide-resistant infections, and minocycline is slightly less effective at 70%.28,29 The increasing prevalence of AMR in M. genitalium has made it an emerging global health threat listed in the US CDC Watch List and with untreatable infections increasingly encountered by clinicians.
Prevalence of antimicrobial resistance
The prevalence and trends in AMR inform the use of antimicrobials for M. genitalium, particularly considering the rising resistance rates to recommended antibiotics. Culture-based drug susceptibility testing of M. genitalium is extremely difficult, as the organism is fastidious.68 However, PCR assays can detect certain genotypic markers, such as mutations associated with resistance or susceptibility to macrolides or fluoroquinolones. Macrolide resistance is mediated by single nucleotide polymorphisms mutations at positions 2058/2059/2062/2063 (E. coli numbering) in the 23 S rRNA gene, each resulting in the elevation of the mean inhibitory concentrations of macrolides, and leading to treatment failure.69,70 Specifically, mutations at position A2062G are linked to josamycin resistance in Russia.71 Fluoroquinolone resistance-associated mutations (QRAMs) are located in the parC and gyrA genes, with the S83I parC mutation being the most common and associated with elevation of minimum inhibitory concentrations (MICs) for moxifloxacin and clinical failure of moxifloxacin in 40% of cases.39,72, 73, 74, 75 GyrA mutations are less frequent, and tend to co-exist with parC mutations, particularly S83I, and this combination of mutations is associated with an increase in MICs to both moxifloxacin and sitafloxacin and moxifloxacin clinical failure exceeding 80%.39
A systematic review and meta-analysis described a marked increase globally in macrolide resistance from 10% before 2010 to 50% in 2016–2017.76 In samples collected in 2016–2017, the average prevalence of fluoroquinolone resistance and dual-resistant strains was 9% and 4%, respectively.39 All mutations were less common in the WHO European region than in the WHO Western Pacific and Americas.39 Macrolide resistance varied among European countries, possibly due to different national guidelines on the use of doxycycline for chlamydia treatment and different rates of chlamydia screening. For example, in Sweden, where doxycycline is used for chlamydia, the macrolide resistance rate for STIs in samples collected between 2003 and 2017 was low, at 15%.76 Additionally, low rates were observed in Central and Eastern European countries due to limited chlamydia screening and consequently reduced antimicrobial use. The global data meta-analysis is currently being updated to include publications from 2018 to 2023.77 Over the past four years, global macrolide resistance appears to have decreased slightly from 42% in 2015–2017 to 33% in 2018–2021, while key parC mutations have remained stable globally at 14% from 2015–2017 to 2018–2021. In the European region, macrolide resistance has risen from 30% in 2015–2017 to 43% in 2018–2021, and fluoroquinolone resistance from 7% to 12% in 2018–2021 (courtesy of Teck-Phui Chua; estimates from all studies include both women and men, without focusing on specific subpopulations).77
Resistance-guided therapy
To address concerns about rising macrolide resistance and reduce the empiric use of azithromycin, researchers in Australia developed a resistance-guided therapy (RGT) strategy for M. genitalium in 2013 involving a two-step approach with initial administration of doxycycline while awaiting resistance test results (Table 2). Patients with an STI syndrome are initially treated with doxycycline for 7 days rather than azithromycin to reduce its empiric use, and M. genitalium infections are tested at the time of diagnosis for macrolide-resistance. Doxycycline also has the added benefit of reducing the bacterial load of M. genitalium by 3 log10 (median six days) and is the treatment of choice for chlamydial infections.78 Clinicians then recall patients once M. genitalium and resistance results are available. For macrolide-susceptible infections, an extended azithromycin regimen (1 g on day one, followed by 500 mg on days 2–4) is recommended. This regimen has shown, in a prospective clinical evaluation, to be associated with less selection of resistance than 1 g SD (<4%) and high cure rates (96%),78 although no clinical trials have been conducted. For macrolide-resistant infections, a week of moxifloxacin (400 mg daily for 7 days) is recommended, with efficacy depending on the prevalence of quinolone-resistance mutations. Two prospective clinical studies evaluating sitafloxacin and moxifloxacin have demonstrated a 92% cure rate with this approach.78,79 In regions with increasing fluoroquinolone resistance, such as Asia and the Western Pacific, assays and algorithms that identify key fluoroquinolone resistance markers enable clinicians to avoid moxifloxacin when parC and gyrA mutations are present.38, 39, 40 Pristinamycin or minocycline can be chosen as alternative antibiotics, expected to cure around 75% of infections. This next generation of resistance-guided therapy may also reduce the need for test-of-cure visits in groups with a high predicted cure rate (>90%) and follow-up could be confidently recommended for symptomatic patients only.73
Successful implementation of RGT algorithms ultimately depends on the affordability and availability of resistance assays, not only in pathology laboratories and for high throughput platforms, but at point-of-care. Several point-of-care assays for M. genitalium macrolide resistance are currently available in most European countries, but these assays still have limitations in terms of costs and logistics, and their usage remains infrequent.80, 81, 82 Commercial molecular diagnostic assays for the detection of fluoroquinolone resistance-associated mutations in M. genitalium have undergone evaluation and are now also available.83
RGT is the recommended first-line treatment in the Australian, US, and UK M. genitalium guidelines (with the UK guidelines shortening the azithromycin regimen to 3 days: 1 g followed by 500 mg daily for 2 days).42, 43, 44 In Europe, current guidelines recommend using molecular assays to detect macrolide resistance mutations, but access to such tests is limited in most clinics in all regions.38 European guidelines recommend RGT if treatment of symptomatic patients is indicated before M. genitalium confirmatory results are available.38 However, the first-line treatment option following confirmation remains the extended azithromycin regimen.
Repurposed antimicrobials
Alternative treatments for patients unable to tolerate or contraindicated for fluoroquinolones, or in cases of fluoroquinolone clinical failure, are severely limited. Pristinamycin is the primary antimicrobial used in Europe, the UK and Australia for such cases (Table 1). It is registered in France and can be obtained with a special permit in most European countries,38 although global supply shortages have occurred intermittently.38,40 Pristinamycin can be used in combination with doxycycline (1 g three times daily and 100 mg twice daily, respectively) for ten days, or as monotherapy (1 g four times daily). Both regimens achieve 75% cure.28,29 Minocycline and doxycycline (both 100 mg twice daily) for 14 days are alternatives. Minocycline has showed higher activity compared to doxycycline and had a microbiological cure rate of 71% among 35 evaluable patients, and a recent series of 123 patients confirmed an overall cure rate of 68%.29,84 The use of an extended doxycycline regimen for 14 days has not been systematically evaluated but is also considered an option for third-line treatment.85
New antimicrobials
In addition to existing treatment options, lefamulin has shown high activity in vitro against M. genitalium with combined macrolide and fluoroquinolone resistance.30 However, there is limited and anecdotal experience of lefamulin in the treatment of patients with M. genitalium infection. A clinical trial of lefamulin in those with previous antibiotic treatment failure was recently suspended at the sponsor's request after the company dissolved.31 In a small series of patients in Australia and the US, lefamulin was not effective when administered as the sole agent and cure did not exceed 50% when administered following seven days of doxycycline.86 Several other antimicrobial agents, including gepotidacin, zoliflodacin, solithromycin, omadacycline, eravacycline, and tinidazole, have shown in vitro activity against M. genitalium, but there is not data on their clinical efficacy. Additionally, there is a published clinical case reporting the use of chloramphenicol (Table 1).32, 33, 34, 35, 36, 37
Community-level strategies to mitigate resistance development and spread
To mitigate the development of AMR at the community level, it is crucial to reduce the empirical or prophylactic use of antibiotics and establish coordinated molecular-based AMR surveillance programs that inform practice and policy. Unfortunately, AMR surveillance is not routinely performed in many countries or regions, including those within the European AMR Network.41 While systematic screening for M. genitalium is not supported by data or recommended in any international guidelines,38,42, 43, 44 the use of multiplex molecular assays has led to frequent screening in clinical practice. The benefits and risks of population screening for M. genitalium remain uncertain, due to limited data on its natural history and the impact of screening on AMR. However, countries with low rates of STI screening have demonstrated lower levels of antimicrobial consumption and AMR in STIs.87 Some subpopulations, such as high-risk pregnant women, may benefit from targeted screening to prevent preterm birth, but further studies are needed to evaluate its value due to limited current evidence.45,46
Syphilis
Syphilis, caused by the spirochete Treponema pallidum subsp. pallidum (T. pallidum), develops in consecutive stages over time: primary, secondary, and tertiary, with periods of asymptomatic latency in between (Fig. 1, panels C and F) Pathogen dissemination during the early stages can lead to neurosyphilis when invasion of the central nervous system (CNS) occurs, and in pregnant women, it can result in congenital syphilis and foetal loss. For therapeutic and management purposes, the European Centre for Disease Prevention and Control (ECDC) and US CDC define syphilis acquired <1 year as early syphilis and syphilis acquired ≥1 year as late syphilis; WHO considers syphilis acquired within 2 years as early syphilis.
Penicillin is the first-line therapy for all stages of syphilis with limited evidence available for alternative antibiotics (Table 1).88 The choice of penicillin form (benzathine, or aqueous crystalline), dose and duration depend on the stage and site of infection. Strong evidence from two large randomized clinical trials supports the efficacy of a single dose of long-acting benzathine penicillin G (BPG, 2.4 million units intramuscularly) for primary, secondary, and early latent syphilis, with serological cure rates of 79% (186/237),89 and 95% (157/165),90 at 6 and 9 months, respectively. Several studies have evaluated therapy with additional doses of BPG and found no additional benefit.91,92 People with late latent syphilis are treated with BPG intramuscularly once a week for three weeks (7.2 million units total). While this approach considers the likely possibility that T. pallidum might enter a resting state with organisms not dividing or multiplying very slowly and therefore less susceptible to antibiotics, there is a lack of clinical trial data. The recommended treatment for neurosyphilis is aqueous crystalline penicillin G (3–4 million units every 4 h intravenously for 10–14 days) because BPG does not result in treponemicidal levels of the antibiotic in the cerebrospinal fluid (CSF). Neonatal syphilis also requires intravenous infusions every 8 h or 12 h for 10 days.
Challenges of penicillin as first-line therapy
The management of syphilis presents the following three major challenges: 1) Treating neurosyphilis and neonatal syphilis requires a lengthy and intensive course of intravenous aqueous crystalline penicillin G which can be stressful for patients and results in high bed occupancy or reduced adherence in some settings. 2) No treatment options exist for pregnant women allergic to penicillin have, as doxycycline is contraindicated during pregnancy. Therefore, penicillin allergy testing, and desensitization are the only viable choices. 3) Global production and supply issues have led to treatment failures and increase in congenital syphilis cases, as health care providers turned to inefficacious antibiotics.93 A common reported error is the administration of aqueous crystalline penicillin intramuscularly, which is a short-acting form of penicillin.94 Shortages of BPG have been registered in various European countries, including France, Albania, Austria, Bulgaria and Czech Republic among others.95 The limited number of producers for penicillin's active ingredients, along with a rise on demand, increases the likelihood of future shortages. There are zero other options when there's no BPG to treat pregnant women and patients with neurosyphilis. Consequently, in the face of BPG shortages, several agencies have advised to treat uncomplicated non-pregnant patients with doxycycline to preserve the supply of BPG and maintain stock for high-priority patients.96
Another issue related to BPG is the reporting of a few cases of penicillin treatment failure,97, 98, 99 especially in people living with HIV but differentiating between reinfection and relapse remains challenging. While penicillin resistant T. pallidum has not been documented, the historical inability to culture the organism limits drug susceptibility testing. The emergence of resistance to penicillin in T. pallidum is considered very unlikely as it would require complex mutational processes, that do not seem to have occurred after over 70 years of continuous use of this antibiotic. However, there is a possibility of penicillin-resistant strains, as genomic analysis indicates potential targets like penicillin-binding proteins (PBPs) that could undergo mutations reducing their affinity for penicillin.100
Research is necessary to expand therapeutic options and develop future treatment strategies for challenging stages and situations in syphilis management.
Alternative antibiotics for syphilis treatment
A major obstacle to testing alternative antibiotics for syphilis was the long-standing inability to culture the causative agent, T. pallidum. However, a system for culturing T. pallidum was established in 2018, allowing for the determination of MICs of a broad repertoire of antimicrobial agents. In vitro studies have shown that the following antibiotics have anti-treponemal activity: penicillin G (MIC ≤ 0.003 mg/L), doxycycline (MIC 0.10 mg/L), ceftriaxone (MIC 0.0025 mg/L), several oral cephalosporins (cefixime, cefetamet, cefuroxime; MIC 0.01–0.03 mg/L), amoxicillin (MIC 0.02 mg/L), linezolid (MIC 0.5 mg/L), dalbavancin (MIC 0.125 mg/L), and spectinomycin (MIC 0.1 mg/L).101, 102, 103 Conversely, some antibiotics, such as moxifloxacin, balofloxacin, ertapenem, doripenem, metronidazole, isoniazid, pyrazinamide, clofazimine, and ivermectin, have no anti-treponemal activity.
Alternative antibiotics for syphilis treatment include ceftriaxone and doxycycline. Azithromycin, which was previously used, is no longer recommended, while amoxicillin is used in certain cases in a few countries (Table 1).
Azithromycin (2 g single oral dose) was previously recommended for early syphilis after showing similar effectiveness to BPG in randomized clinical trials.89,90 However, its use is no longer recommended due to high levels of resistance caused by either of two single nucleotide polymorphisms in the 23 S ribosomal RNA gene (A2058G or A2059G, E. coli numbering system).104 In laboratory studies, azithromycin is highly effective for wild-type strains (MIC < 0.03 mg/L), but ineffective against resistant strains at concentrations up to 2.0 mg/L.103 Global reports indicate high prevalence of macrolide resistance (ranging from 64% to 100%) in countries such as Australia, China, the US, and some European cities between 2004 and 2020.105, 106, 107, 108, 109 Macrolide use for unrelated infections (e.g., oral, skin, respiratory, and genital infections) shortly before the T. pallidum infection, creates selective pressure leading to the selection of resistant strains. Genomic studies have shown that several resistant variants evolved in parallel and then widely disseminated.110 Once resistance evolved in a lineage, it persists in descendants without reverting to a wild-type state, presumably because it has little to no effect on bacterial fitness.
Ceftriaxone is a second-line treatment for early syphilis but is less convenient than BPG because it requires multiple daily parenteral doses. Several observational studies have shown positive results,111 and a randomized trial in China reported a serological cure rate of 90% (101/112) at six months.112 The use of ceftriaxone for neurosyphilis remains controversial. An observational retrospective study in France, involving patients with neurosyphilis, reported a serological cure of 88% (21/24).113 However, CSF was not re-evaluated as a test for cure and the sample size was insufficient for drawing definitive conclusions. Further research is warranted considering the potential for achieving sufficient concentrations in the CNS, as despite the poor blood-brain barrier penetration of B-lactams (∼5%), a daily dose of 1–2 g of ceftriaxone achieves concentrations in CNS of 0.4 mg/L, which is more than 160-fold higher than the reported MIC value (MIC 0.0025 mg/L).103 A multicentre randomized controlled trial to test the efficacy of ceftriaxone in patients with neurosyphilis is planned in China.114
Doxycycline has been extensively evaluated for treating early and late syphilis in observational studies with reported success rates ranging from 96% (90/94) to 100% (34/34) in early syphilis, 79% (19/24) in late syphilis, and 65–75% in people living with HIV.105,115, 116, 117 In an observational study on neurosyphilis, doxycycline (200 mg/twice daily for 28 days) demonstrated a positive serological and clinical response in all 14/14 patients. However, the study had limitations, including the absence of CSF collection via lumbar puncture in 9 cases, and abnormal CSF findings proven in only 1 out of 4 cases tested.118 In vitro results showing a MIC for doxycycline of 0.10 mg/L suggest that a 100 mg dose can reach the clinical efficacy target in vivo, while a dose of 200 mg twice daily could not easily attain sufficient concentrations in CSF based on a 26% penetration rate. Doxycycline treatment failure in early syphilis is rare, and there have been no reported cases of resistant bacteria.119 Several studies (over 600 specimens from three continents) examining two mutations in the 16 S ribosomal RNA genes of T. pallidum that could result in resistance, as observed in other pathogens,120 have not identified any strains with these mutations. However, alternative resistance mechanisms or undetected mutations may exist that are not being identified and there is a concern that penicillin shortages and broader use of post-exposure prophylaxis for bacterial STIs with doxycycline (known as Doxy-PEP) could result in increased use of doxycycline and provide selective pressure for resistant mutants (advantages and disadvantages of Doxy-PEP are discussed in the prevention article of this series).121 If tetracycline-resistant T. pallidum were to emerge and spread, it would undoubtedly have a detrimental effect on syphilis management and control.
Amoxicillin has been assessed for early syphilis treatment with mixed results. A Japanese observational study found that oral amoxicillin (3 g/day, combined with probenecid for 14 days) in individuals with HIV achieved a 96% (273/286) serological cure rate, while another study using oral amoxicillin (1.5 g/day, without probenecid for 4 weeks) demonstrated a 95% (131/138) cure rate.47,48 A retrospective study conducted in Japan revealed that, among pregnant women treated with 1.5 g/day oral amoxicillin, 15/45 (33%) cases of late latent syphilis resulted in congenital syphilis, while no instances of congenital syphilis were observed among 26 cases of early syphilis.49 Oral amoxicillin is currently not recommended for the treatment of early syphilis, and should be specifically avoided in cases of late syphilis and during pregnancy. Moreover, it should not be used to treat neurosyphilis, since even very high doses do not attain treponemicidal levels in cerebrospinal fluid.
Repurposed and new antimicrobials
Oral cefixime (400 mg orally twice daily for 10 days) has been evaluated in a pilot randomized trial for the treatment of early syphilis, resulting in a serological cure of 87% (13/15),122 but the small sample size limits the generalizability of the findings. A phase 2 randomized trial is currently enrolling non-pregnant women in Brazil to further evaluate cefixime.50
Linezolid shows promise for the treatment of syphilis based on in vitro studies indicating anti-treponemal activity at concentrations ≥0.25 mg/L.101,103 It has a favourable pharmacokinetic profile for CNS penetration (38% in rabbit model) and has been successful in treating CNS infections caused by other bacterial species.51 However, due to the rapid spread of macrolide resistance in T. pallidum worldwide and the potential impact of single point mutations on the same target gene as azithromycin, caution is advised against the widespread use of linezolid. Nonetheless, it may be highly valuable in treating neurosyphilis. An ongoing randomized trial is testing its efficacy for primary and secondary syphilis.52
Finally, zoliflodacin is an effective antibiotic against N. gonorrhoeae, and it may also have activity for syphilis with a MIC of 2 mg/dL.103 The anti-treponemal activity of zoliflodacin could be explained by the conservation of key amino acid residues in the T. pallidum DNA gyrase subunit B protein, similar to that of N. gonorrhoeae GyrB protein.53
Priorities in drug susceptibility testing, antibiotic efficacy evaluation, and innovative strategies
To address the challenges in syphilis management and expand treatment options, future actions should prioritize comprehensive drug susceptibility testing of multiple strains, particularly in cases of treatment failure to first- and second-line antibiotics. This testing should encompass both culture-based and genotypic analysis. However, the collection of suitable samples for culture may face limitations due to the organism's limited viability, which requires immediate freezing for preservation. Genotypic analysis is also constrained by limitations in bacterial cultivation, but recent advancements in DNA enrichment techniques have overcome these challenges.
Additional clinical research is needed to evaluate the efficacy of ceftriaxone for neurosyphilis, establish the efficacy of oral antibiotics like amoxicillin or cefixime, and to explore the potential of linezolid in both early syphilis and neurosyphilis. Innovative developments in BPG formulations, specifically the use of smaller oblong-shaped crystals with a median particle size < 12.5 μm, aim to ensure a consistent release rate and potentially prevent the common issue of needle blockages during BPG administration. Deep learning-guided discovery of antibiotics may expedite the identification of new anti-T. pallidum molecules, but it requires substantial research efforts and economic interest dedicated to this field.
Implementing community-level strategies to reduce transmission may also prevent the potential emergence and dissemination of resistant strains (Table 2), including the clinical development and widespread use of a syphilis vaccine (addressed in a different article of this Series).121 Until the refinement of these techniques and completion of clinical trials, the recommendation remains to follow the guidelines based on the excellent response of BPG as the first-line treatment for syphilis.
Conclusions and Future Perspectives
In conclusion, the challenge of antibiotic resistance in STIs needs immediate action and innovative treatment approaches (Fig. 2).
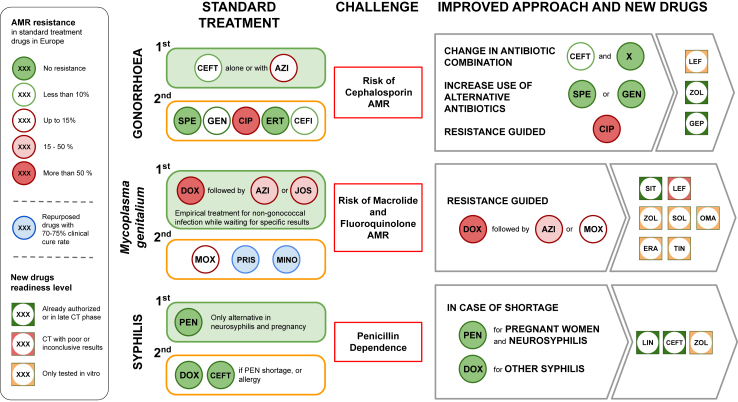
Fig. 2.
Summary Figure. Legend. CEFT, Ceftriaxone; AZI, Azithromycin; SPE, Spectinomycin; GEN, Gentamicin; CIP, Ciprofloxacin; ERT, Ertapenem; CEFI, Cefixime; JOS, Josamycin; MOX, Moxifloxacin; PRIS, Pristinamycin; MINO, Minocycline; LEF, Lefamulin; ZOL, Zoliflodacin; GEP, Gepotidacin; SIT, Sitafloxacin, SOL, Solithromycin; OMA, Omadacycline; ERA, Eravacycline; TIN, Tinidazole; LIN, Linezolid; CT, Clinical Trial; AMR, Antimicrobial resistance.
For gonorrhea, careful antibiotic selection for use in combinations is needed to combat resistance. Molecular assays and the clinical development of new antimicrobials offer promising avenues.
The high prevalence of resistance in M. genitalium requires individually tailored treatment strategies. The limited antimicrobial pipeline underscores the importance of community-level strategies and judicious antibiotic use.
For syphilis, penicillin challenges call for alternative treatments. Ceftriaxone shows promise, and other antimicrobials are being evaluated.
Looking ahead, new diagnostic tools and notification and contact-tracing software hold the potential for improved STI management. Ongoing research is vital to effectively address antibiotic resistance in STIs.
Contributors
OM conceived the review and coordinated its preparation. JDR wrote the part on gonorrhoea. CSB and CS wrote the part on mycoplasma. OM wrote the part on syphilis. CS prepared the tables and managed the bibliography. LG, MV, GST reviewed and edited the final version. All authors approved the final manuscript.
Declaration of interests
JR reports personal fees from GSK Pharma and Bayer Consumer Care; ownership of shares in GSK Pharma and AstraZeneca Pharma; lead author of the UK and European Guidelines on Pelvic Inflammatory Disease; Member of the European Sexually Transmitted Infections Guidelines Editorial Board. He is treasurer for the International Union against Sexually Transmitted Infections and chair of charity trustees for the Sexually Transmitted Infections Research Foundation charity.
CB reports to be recipient of Federal research funding support from Australian NHMRC (L1 Investigator grant) and the ARC (ARC Industrial Research Transformation Hub), payment of honoraria for drafting of guidelines from Abbott, and receipt of equipment from Speedx and Cepheid. She is a board member of ISSTDR.
The other authors declare no competing interests.
Acknowledgements
We thank Gerard Carot-Sans for help in the preparation of the final document. Salary wages of CS, MV, LG, and OM were supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (Grant agreement No. 850450; grant holder: Oriol Mitjà).
References
- 1.Unemo M., Bradshaw C.S., Hocking J.S., et al. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017;17:e235–e279. doi: 10.1016/S1473-3099(17)30310-9. [DOI] [PubMed] [Google Scholar]
- 2.Mitjà O., Padovese V., Folch C., et al. Epidemiology and determinants of reemerging bacterial sexually transmitted infections (STIs) and emerging STIs in Europe. Lancet Reg Health Eur. 2023;34:100742. doi: 10.1016/j.lanepe.2023.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Unemo M., Ross J.D.C., Serwin A.B., Gomberg M., Cusini M., Jensen J.S. 2020 European guideline for the diagnosis and treatment of gonorrhoea in adults. Int J STD AIDS. 2020 doi: 10.1177/0956462420949126. [DOI] [PubMed] [Google Scholar]
- 4.European Centre for Disease Prevention and Control . 2021. Gonococcal antimicrobial susceptibility surveillance in the European Union/summary of results for 2019. [Google Scholar]
- 5.Day M.J., Jacobsson S., Spiteri G., et al. Significant increase in azithromycin “resistance” and susceptibility to ceftriaxone and cefixime in Neisseria gonorrhoeae isolates in 26 European countries, 2019. BMC Infect Dis. 2022;22 doi: 10.1186/s12879-022-07509-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Unemo M., Lahra M.M., Cole M., et al. World Health Organization Global Gonococcal Antimicrobial Surveillance Program (WHO GASP): review of new data and evidence to inform international collaborative actions and research efforts. Sex Health. 2019;16:412–425. doi: 10.1071/SH19023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Unemo M., Shafer W.M. Antimicrobial resistance in Neisseria gonorrhoeae in the 21st Century: past, evolution, and future. Clin Microbiol Rev. 2014;27:587–613. doi: 10.1128/CMR.00010-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organisation . 2017. WHO publishes list of bacteria for which new antibiotics are urgently needed.https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed [Google Scholar]
- 9.Fifer H., Saunders J., Soni S., Sadiq T., Fitzgerald M. 2019. British Association for Sexual Health and HIV national guideline for the management of infection with Neisseria gonorrhoeae (2019)https://www.bashh.org/bashh [DOI] [PubMed] [Google Scholar]
- 10.Australasian Society for HIV VH and SHM (ASHM) 2021. Australia STI management guidelines gonorrhoea.https://sti.guidelines.org.au/sexually-transmissible-infections/gonorrhoea/ [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC) 2021. Sexually transmitted infections treatment guidelines, 2021, gonococcal infections among adolescents and adults.https://www.cdc.gov/std/treatment-guidelines/gonorrhea-adults.htm [Google Scholar]
- 12.Connolly K.L., Eakin A.E., Gomez C., Osborn B.L., Unemo M., Jerse A.E. Pharmacokinetic data are predictive of in vivo efficacy for cefixime and ceftriaxone against susceptible and resistant Neisseria gonorrhoeae strains in the gonorrhea mouse model. Antimicrob Agents Chemother. 2019;63 doi: 10.1128/AAC.01644-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen V.G., Mitterni L., Seah C., et al. Neisseria gonorrhoeae treatment failure and susceptibility to cefixime in Toronto, Canada. JAMA. 2013;309:163. doi: 10.1001/jama.2012.176575. [DOI] [PubMed] [Google Scholar]
- 14.Rice L.B. Will use of combination cephalosporin/azithromycin therapy forestall resistance to cephalosporins in Neisseria gonorrhoeae? Sex Transm Infect. 2015;91:238–240. doi: 10.1136/sextrans-2014-051730. [DOI] [PubMed] [Google Scholar]
- 15.Heller-Vitouch C., Tiplica G.-S., Hiltunen-Back E., et al. 2021. IUSTI-Europe statement on spectinomycin. [DOI] [Google Scholar]
- 16.Ross J.D.C., Brittain C., Cole M., et al. Gentamicin compared with ceftriaxone for the treatment of gonorrhoea (G-ToG): a randomised non-inferiority trial. Lancet. 2019;393:2511–2520. doi: 10.1016/S0140-6736(18)32817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries H.J.C., de Laat M., Jongen V.W., et al. Efficacy of ertapenem, gentamicin, fosfomycin, and ceftriaxone for the treatment of anogenital gonorrhoea (NABOGO): a randomised, non-inferiority trial. Lancet Infect Dis. 2022;22:706–717. doi: 10.1016/S1473-3099(21)00625-3. [DOI] [PubMed] [Google Scholar]
- 18.Klausner J.D., Bristow C.C., Soge O.O., et al. Resistance-guided treatment of gonorrhea: a prospective clinical study. Clin Infect Dis. 2021;73:298–303. doi: 10.1093/cid/ciaa596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herbst De Cortina S., Bristow C.C., Joseph Davey D., Klausner J.D. A systematic review of point of care testing for Chlamydia trachomatis, Neisseria gonorrhoeae, and trichomonas vaginalis. Infect Dis Obstet Gynecol. 2016;2016 doi: 10.1155/2016/4386127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor S.N., Morris D.H., Avery A.K., et al. Gepotidacin for the treatment of uncomplicated urogenital gonorrhea: a phase 2, randomized, doseranging, single-oral dose evaluation. Clin Infect Dis. 2018;67:504–512. doi: 10.1093/cid/ciy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bradford P.A., Miller A.A., O'Donnell J., Mueller J.P. Zoliflodacin: an oral spiropyrimidinetrione antibiotic for the treatment of Neisseria gonorrheae, including multi-drug-resistant isolates. ACS Infect Dis. 2020;6:1332–1345. doi: 10.1021/acsinfecdis.0c00021. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsson S., Golparian D., Oxelbark J., et al. Pharmacodynamic evaluation of lefamulin in the treatment of gonorrhea using a hollow fiber infection model simulating Neisseria gonorrhoeae infections. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.1035841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wellings K., Mehl G.L., Free C.J. eSexual health interventions: promising, but more evidence needed. Lancet Public Health. 2017;2:e162–e163. doi: 10.1016/S2468-2667(17)30051-8. [DOI] [PubMed] [Google Scholar]
- 24.Charleson F., Tran J., Kolobaric A., et al. A systematic review of kissing as a risk factor for oropharyngeal gonorrhoea or chlamydia. Sex Transm Dis. 2023 doi: 10.1097/OLQ.0000000000001777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folke T., Menon-Johansson A.S. An evaluation of digital partner notification tool engagement and impact for patients diagnosed with gonorrhea and syphilis. Sex Transm Dis. 2022;49:815–821. doi: 10.1097/OLQ.0000000000001707. [DOI] [PubMed] [Google Scholar]
- 26.Estcourt C. Improving care for people with sexually transmitted infections in a digital NHS. https://fundingawards.nihr.ac.uk/award/NIHR200856
- 27.Rasul R., McIver R., Patel P., Foster R., McNulty A. Non-empirical management of asymptomatic chlamydia and gonorrhoea reduces unnecessary antibiotic use fivefold: a before and after study. Sex Transm Infect. 2023;99:30–34. doi: 10.1136/sextrans-2021-055382. [DOI] [PubMed] [Google Scholar]
- 28.Read T.R.H., Jensen J.S., Fairley C.K., et al. Use of pristinamycin for Macrolide-Resistant mycoplasma genitalium infection. Emerg Infect Dis. 2018;24:328–335. doi: 10.3201/eid2402.170902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doyle M., Vodstrcil L.A., Plummer E.L., Aguirre I., Fairley C.K., Bradshaw C.S. Nonquinolone options for the treatment of Mycoplasma genitalium in the era of increased resistance. Open Forum Infect Dis. 2020;7 doi: 10.1093/ofid/ofaa291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paukner S., Gruss A., Jensen J.S. In vitro activity of lefamulin against sexually transmitted bacterial pathogens. Antimicrob Agents Chemother. 2018;62 doi: 10.1128/AAC.02380-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lefamulin for M Genitalium treatment failures. https://clinicaltrials.gov/ct2/show/study/NCT05111002
- 32.Jensen J.S., Fernandes P., Unemo M. In vitro activity of the new fluoroketolide solithromycin (cem-101) against macrolide-resistant and -susceptible mycoplasma genitalium strains. Antimicrob Agents Chemother. 2014;58:3151–3156. doi: 10.1128/AAC.02411-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damião Gouveia A.C., Unemo M., Jensen J.S. In vitro activity of zoliflodacin (ETX0914) against macrolide-resistant, fluoroquinolone-resistant and antimicrobial-susceptible Mycoplasma genitalium strains. J Antimicrob Chemother. 2018;73:1291–1294. doi: 10.1093/jac/dky022. [DOI] [PubMed] [Google Scholar]
- 34.Jensen J.S., Nørgaard C., Scangarella-Oman N., Unemo M. In vitro activity of the first-in-class triazaacenaphthylene gepotidacin alone and in combination with doxycycline against drug-resistant and -susceptible Mycoplasma genitalium. Emerg Microbes Infect. 2020;9:1388–1392. doi: 10.1080/22221751.2020.1775498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood G.E., Kim C.M., Aguila L.K.T., Cichewicz R.H. In vitro susceptibility and resistance of Mycoplasma genitalium to nitroimidazoles. Antimicrob Agents Chemother. 2023;67 doi: 10.1128/aac.00006-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waites K.B., Crabb D.M., Xiao L., Duffy L.B., Leal S.M. In vitro activities of eravacycline and other antimicrobial agents against human mycoplasmas and ureaplasmas. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00698-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waites K.B., Crabb D.M., Atkinson T.P., Geisler W.M., Xiao L. Omadacycline is highly active in vitro against Mycoplasma genitalium. Microbiol Spectr. 2022;10 doi: 10.1128/spectrum.03654-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen J.S., Cusini M., Gomberg M., Moi H., Wilson J., Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022;36:641–650. doi: 10.1111/jdv.17972. [DOI] [PubMed] [Google Scholar]
- 39.Murray G.L., Plummer E.L., Bodiyabadu K., et al. gyrA mutations in mycoplasma genitalium and their contribution to moxifloxacin failure: time for the next generation of resistance-guided therapy. Clin Infect Dis. 2023 doi: 10.1093/cid/ciad057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bissessor M., Tabrizi S.N., Twin J., et al. Macrolide resistance and azithromycin failure in a mycoplasma genitalium-infected cohort and response of azithromycin failures to alternative antibiotic regimens. Clin Infect Dis. 2015;60:1228–1236. doi: 10.1093/cid/ciu1162. [DOI] [PubMed] [Google Scholar]
- 41.European Antimicrobial Resistance Surveillance Network (EARS-Net) https://www.ecdc.europa.eu/en/about-us/networks/disease-networks-and-laboratory-networks/ears-net-data
- 42.Soni S., Horner P., Rayment M., et al. British Association for Sexual Health and HIV national guideline for the management of infection with Mycoplasma genitalium (2018) Int J STD AIDS. 2019;30:938–950. doi: 10.1177/0956462419825948. [DOI] [PubMed] [Google Scholar]
- 43.Australasian Society for HIV VH and SHM (ASHM) 2021. Australian STI Management guideline, Mycoplasma genitalium. [Google Scholar]
- 44.Centers for Disease Control and Prevention . Mycoplasma Genitalium; 2021. Sexually transmitted infections treatment guidelines, 2021.https://www.cdc.gov/std/treatment-guidelines/mycoplasmagenitalium.htm [Google Scholar]
- 45.Lis R., Rowhani-Rahbar A., Manhart L.E. Mycoplasma genitalium infection and female reproductive tract disease: a meta-analysis. Clin Infect Dis. 2015;61:418–426. doi: 10.1093/cid/civ312. [DOI] [PubMed] [Google Scholar]
- 46.Frenzer C., Egli-Gany D., Vallely L.M., Vallely A.J., Low N. Adverse pregnancy and perinatal outcomes associated with Mycoplasma genitalium: systematic review and meta-analysis. Sex Transm Infect. 2022;98:222–227. doi: 10.1136/sextrans-2021-055352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanizaki R., Nishijima T., Aoki T., et al. High-dose oral amoxicillin plus probenecid is highly effective for syphilis in patients with HIV infection. Clin Infect Dis. 2015;61:177–183. doi: 10.1093/cid/civ270. [DOI] [PubMed] [Google Scholar]
- 48.Ikeuchi K., Fukushima K., Tanaka M., Yajima K., Imamura A. Clinical efficacy and tolerability of 1.5 g/day oral amoxicillin therapy without probenecid for the treatment of syphilis. Sex Transm Infect. 2022;98:173–177. doi: 10.1136/sextrans-2020-054823. [DOI] [PubMed] [Google Scholar]
- 49.Nishijima T., Kawana K., Fukasawa I., et al. Effectiveness and tolerability of oral amoxicillin in pregnant women with active syphilis, Japan, 2010-2018. Emerg Infect Dis. 2020;26:1192–1200. doi: 10.3201/eid2606.191300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Taylor M.M., Taylor M.M., Kara E.O., et al. Phase II trial evaluating the clinical efficacy of cefixime for treatment of active syphilis in non-pregnant women in Brazil (CeBra) BMC Infect Dis. 2020;20 doi: 10.1186/s12879-020-04980-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cottagnoud P., Gerber C.M., Acosta F., Cottagnoud M., Neftel K., Täuber M.G. Linezolid against penicillin-sensitive and -resistant pneumococci in therabbit meningitis model. J Antimicrob Chemother. 2000 doi: 10.1093/jac/46.6.981. [DOI] [PubMed] [Google Scholar]
- 52.Alternative antibiotics for syphilis. https://clinicaltrials.gov/ct2/show/NCT05069974
- 53.Alm R.A., Lahiri S.D., Kutschke A., et al. Characterization of the novel DNA gyrase inhibitor AZD0914: low resistance potential and lack of cross-resistance in Neisseria gonorrhoeae. Antimicrob Agents Chemother. 2015;59:1478–1486. doi: 10.1128/AAC.04456-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu Z., Tadi D.A., Fu J., Azizian K., Kouhsari E. Global status of Azithromycin and Erythromycin Resistance Rates in Neisseria gonorrhoeae: a systematic review and meta-analysis. Yale J Biol Med. 2022;95:465–478. [PMC free article] [PubMed] [Google Scholar]
- 55.Moran J.S., Levine W.C. Drugs of choice for the treatment of uncomplicated gonococcal infections. Clin Infect Dis. 1995;20:S47–S65. doi: 10.1093/clinids/20.supplement_1.s47. [DOI] [PubMed] [Google Scholar]
- 56.Allan-Blitz L.T., Adamson P.C., Klausner J.D. Resistance-guided therapy for Neisseria gonorrhoeae. Clin Infect Dis. 2022;75:1655–1660. doi: 10.1093/cid/ciac371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Olesen S.W., Torrone E.A., Papp J.R., Kirkcaldy R.D., Lipsitch M., Grad Y.H. Azithromycin susceptibility among Neisseria gonorrhoeae isolates and seasonal macrolide use. J Infect Dis. 2019;219:619–623. doi: 10.1093/infdis/jiy551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kenyon C., Herrmann B., Hughes G., et al. Management of asymptomatic sexually transmitted infections in Europe: towards a differentiated, evidence-based approach. Lancet Reg Health Eur. 2023;34:100743. doi: 10.1016/j.lanepe.2023.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris S.R., Bristow C.C., Wierzbicki M.R., et al. Performance of a single-use, rapid, point-of-care PCR device for the detection of Neisseria gonorrhoeae, Chlamydia trachomatis, and Trichomonas vaginalis: a cross-sectional study. Lancet Infect Dis. 2021;21:668–676. doi: 10.1016/S1473-3099(20)30734-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Manhart L.E., Gillespie C.W., Lowens M.S., et al. Standard treatment regimens for nongonococcal urethritis have similar but declining cure rates: a randomized controlled trial. Clin Infect Dis. 2013;56:934–942. doi: 10.1093/cid/cis1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mena L.A., Mroczkowski T.F., Nsuami M., Martin D.H. A randomized comparison of azithromycin and doxycycline for the treatment of mycoplasma genitalium-positive urethritis in men. Clin Infect Dis. 2009;48:1649–1654. doi: 10.1086/599033. [DOI] [PubMed] [Google Scholar]
- 62.Schwebke J.R., Rompalo A., Taylor S., et al. Re-evaluating the treatment of nongonococcal urethritis: emphasizing emerging pathogens–a randomized clinical trial. Clin Infect Dis. 2011;52:163–170. doi: 10.1093/cid/ciq074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Read T.R.H., Fairley C.K., Tabrizi S.N., et al. Azithromycin 1.5 g over 5 days compared to 1 g single dose in urethral mycoplasma genitalium: impact on treatment outcome and resistance. Clin Infect Dis. 2017;64:250–256. doi: 10.1093/cid/ciw719. [DOI] [PubMed] [Google Scholar]
- 64.Horner P., Ingle S.M., Garrett F., et al. Which azithromycin regimen should be used for treating Mycoplasma genitalium? A meta-analysis. Sex Transm Infect. 2018;94:14–20. doi: 10.1136/sextrans-2016-053060. [DOI] [PubMed] [Google Scholar]
- 65.Lau A., Bradshaw C.S., Lewis D., et al. The efficacy of azithromycin for the treatment of genital mycoplasma genitalium: a systematic review and meta-analysis. Clin Infect Dis. 2015;61:1389–1399. doi: 10.1093/cid/civ644. [DOI] [PubMed] [Google Scholar]
- 66.Bradshaw C.S., Jensen J.S., Tabrizi S.N., et al. Azithromycin failure in Mycoplasma genitalium urethritis. Emerg Infect Dis. 2006;12:1149–1152. doi: 10.3201/eid1207.051558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Y., Le W.-J., Li S., Cao Y.-P., Su X.-H. Meta-analysis of the efficacy of moxifloxacin in treating Mycoplasma genitalium infection. Int J STD AIDS. 2017;28:1106–1114. doi: 10.1177/0956462416688562. [DOI] [PubMed] [Google Scholar]
- 68.Pitt R., Boampong D., Day M., Jensen J.S., Cole M. Challenges of in vitro propagation and antimicrobial susceptibility testing of Mycoplasma genitalium. J Antimicrob Chemother. 2022;77:2901–2907. doi: 10.1093/jac/dkac281. [DOI] [PubMed] [Google Scholar]
- 69.Jensen J.S., Bradshaw C.S., Tabrizi S.N., Fairley C.K., Hamasuna R. Azithromycin treatment failure in Mycoplasma genitalium-positive patients with nongonococcal urethritis is associated with induced macrolide resistance. Clin Infect Dis. 2008;47:1546–1553. doi: 10.1086/593188. [DOI] [PubMed] [Google Scholar]
- 70.Chrisment D., Charron A., Cazanave C., Pereyre S., Bébéar C. Detection of macrolide resistance in Mycoplasma genitalium in France. J Antimicrob Chemother. 2012;67:2598–2601. doi: 10.1093/jac/dks263. [DOI] [PubMed] [Google Scholar]
- 71.Guschin A., Ryzhikh P., Rumyantseva T., Gomberg M., Unemo M. Treatment efficacy, treatment failures and selection of macrolide resistance in patients with high load of Mycoplasma genitalium during treatment of male urethritis with josamycin. BMC Infect Dis. 2015;15:40. doi: 10.1186/s12879-015-0781-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murray G.L., Bodiyabadu K., Vodstrcil L.A., et al. parC variants in Mycoplasma genitalium: trends over time and association with moxifloxacin failure. Antimicrob Agents Chemother. 2022;66:1–5. doi: 10.1128/aac.00278-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sweeney E.L., Bradshaw C.S., Murray G.L., Whiley D.M. Individualised treatment of Mycoplasma genitalium infection—incorporation of fluoroquinolone resistance testing into clinical care. Lancet Infect Dis. 2022;22:e267–e270. doi: 10.1016/S1473-3099(21)00629-0. [DOI] [PubMed] [Google Scholar]
- 74.Vodstrcil L.A., Plummer E.L., Doyle M., et al. Combination therapy for mycoplasma genitalium, and new insights into the utility of parC mutant detection to improve cure. Clin Infect Dis. 2022;75:813–823. doi: 10.1093/cid/ciab1058. [DOI] [PubMed] [Google Scholar]
- 75.Hamasuna R., Le P.T., Kutsuna S., et al. Mutations in parc and gyra of moxifloxacin-resistant and susceptible mycoplasma genitalium strains. PLoS One. 2018;13 doi: 10.1371/journal.pone.0198355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Machalek D.A., Tao Y., Shilling H., et al. Prevalence of mutations associated with resistance to macrolides and fluoroquinolones in Mycoplasma genitalium: a systematic review and meta-analysis. Lancet Infect Dis. 2020;20:1302–1314. doi: 10.1016/S1473-3099(20)30154-7. [DOI] [PubMed] [Google Scholar]
- 77.Chua . World STI and HIV Congress; 2023. Evolving antimicrobial resistance in Mycoplasma genitalium: an updated global systematic review and meta-analysis. Late breaker abstract. [Google Scholar]
- 78.Read T.R.H., Fairley C.K., Murray G.L., et al. Outcomes of resistance-guided sequential treatment of mycoplasma genitalium infections: a prospective evaluation. Clin Infect Dis. 2019;68:554–560. doi: 10.1093/cid/ciy477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Durukan D., Read T.R.H., Murray G., et al. Resistance-guided antimicrobial therapy using doxycycline-moxifloxacin and doxycycline-2.5 g azithromycin for the treatment of Mycoplasma genitalium infection: efficacy and tolerability. Clin Infect Dis. 2020;71:1461–1468. doi: 10.1093/cid/ciz1031. [DOI] [PubMed] [Google Scholar]
- 80.Fernández-Huerta M., Salmerón P., Silgado A., et al. Clinical evaluation of the ResistancePlus MG FleXible test on the GeneXpert Infinity-48 s instrument: a near-patient assay for simultaneous detection of Mycoplasma genitalium and macrolide resistance. Diagn Microbiol Infect Dis. 2020;97 doi: 10.1016/j.diagmicrobio.2020.115062. [DOI] [PubMed] [Google Scholar]
- 81.Sweeney E.L., Mhango L.P., Ebeyan S., et al. Evaluation of the ResistancePlus MG FleXible cartridge for near-point-of-care testing of Mycoplasma genitalium and associated macrolide resistance mutations. J Clin Microbiol. 2020;58:2278–2283. doi: 10.1128/JCM.01897-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le Roy C., Bébéar C., Pereyre S. Performance of three commercial molecular diagnostic assays for the simultaneous detection of Mycoplasma genitalium and macrolide resistance. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.00020-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gardette M., Hénin N., Le Roy C., et al. Clinical performance of three commercial molecular diagnostic assays for the detection of fluoroquinolone resistance-associated mutations in Mycoplasma genitalium. J Clin Microbiol. 2022;60 doi: 10.1128/jcm.01135-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Clarke E.J., Vodstrcil L.A., Plummer E.L., et al. 2023. Efficacy of minocycline for the treatment of macrolide resistant Mycoplasma genitalium. Late breaker abstract. STI and HIV world congress. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gossé M., Nordbø S.A., Pukstad B. Evaluation of treatment with two weeks of doxycycline on macrolide-resistant strains of Mycoplasma genitalium: a retrospective observational study. BMC Infect Dis. 2021;21 doi: 10.1186/s12879-021-06910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manhart . 2023. Effectiveness of Lefamulin for Mycoplasma genitalium Treatment Failures in Australia and the United States (US). Late breaker abstract STI and HIV world congress. [Google Scholar]
- 87.Kenyon C., Laumen J., Van DIjck C. Could intensive screening for gonorrhea/Chlamydia in preexposure prophylaxis cohorts select for resistance? Historical lessons from a mass treatment campaign in Greenland. Sex Transm Dis. 2020;47:24–27. doi: 10.1097/OLQ.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 88.Janier M., Unemo M., Dupin N., Tiplica G.S., Potočnik M., Patel R. 2020 European guideline on the management of syphilis. J Eur Acad Dermatol Venereol. 2021;35:574–588. doi: 10.1111/jdv.16946. [DOI] [PubMed] [Google Scholar]
- 89.Hook E.W., Behets F., Van Damme K., et al. A Phase III equivalence trial of azithromycin versus benzathine penicillin for treatment of early syphilis. J Infect Dis. 2010;201:1729–1735. doi: 10.1086/652239. [DOI] [PubMed] [Google Scholar]
- 90.Riedner G., Rusizoka M., Todd J., et al. Single-dose azithromycin versus penicillin G benzathine for the treatment of early syphilis. N Engl J Med. 2005;353:1236–1244. doi: 10.1056/NEJMoa044284. [DOI] [PubMed] [Google Scholar]
- 91.Ganesan A., Mesner O., Okulicz J.F., et al. A single dose of benzathine penicillin G is as effective as multiple doses of benzathine penicillin G for the treatment of HIV-infected persons with early syphilis. Clin Infect Dis. 2015;60:653–660. doi: 10.1093/cid/ciu888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Andrade R., Rodriguez-Barradas M.C., Yasukawa K., Villarreal E., Ross M., Serpa J.A. Single dose versus 3 doses of intramuscular benzathine penicillin for early syphilis in HIV: a randomized clinical trial. Clin Infect Dis. 2017;64:759–764. doi: 10.1093/cid/ciw862. [DOI] [PubMed] [Google Scholar]
- 93.Nurse-Findlay S., Taylor M.M., Savage M., et al. Shortages of benzathine penicillin for prevention of mother-to-child transmission of syphilis: an evaluation from multi-country surveys and stakeholder interviews. PLoS Med. 2017;14 doi: 10.1371/journal.pmed.1002473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nieuwenburg S., Rietbergen N., van Zuylen D., Vergunst C., de Vries H. Erroneous treatment of syphilis with benzyl penicillin in an era with benzathine benzylpenicillin shortages. Sex Transm Infect. 2020;96:552. doi: 10.1136/sextrans-2019-054380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.World Health Organization Global shortages of penicillin. https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/stis/treatment/shortages-of-penicillin
- 96.Nelson R. Syphilis rates soar in the USA amid penicillin shortage. Lancet. 2023;402:515. doi: 10.1016/S0140-6736(23)01665-3. [DOI] [PubMed] [Google Scholar]
- 97.Fowler V.G., Maxwell G.L., Myers S.A., et al. Failure of benzathine penicillin in a case of seronegative secondary syphilis in a patient with acquired immunodeficiency syndrome: case report and review of the literature. Arch Dermatol. 2001;137:1374–1376. [PubMed] [Google Scholar]
- 98.Rolfs R.T., Joesoef M.R., Hendershot E.F., et al. A randomized trial of enhanced therapy for early syphilis in patients with and without human immunodeficiency virus infection. N Engl J Med. 1997;337:307–314. doi: 10.1056/NEJM199707313370504. [DOI] [PubMed] [Google Scholar]
- 99.Yoon J., Haam I.B., Chung K.Y., Lee M.G., Lee J.B. Relapsing syphilis. J Dermatol. 1993;20:436–440. doi: 10.1111/j.1346-8138.1993.tb01314.x. [DOI] [PubMed] [Google Scholar]
- 100.Cha J.Y., Ishiwata A., Mobashery S. A novel β-lactamase activity from a penicillin-binding protein of Treponema pallidum and why syphilis is still treatable with penicillin. J Biol Chem. 2004;279:14917–14921. doi: 10.1074/jbc.M400666200. [DOI] [PubMed] [Google Scholar]
- 101.Haynes A.M., Giacani L., Mayans M.V., et al. Efficacy of linezolid on Treponema pallidum, the syphilis agent: a preclinical study. eBioMedicine. 2021;65 doi: 10.1016/j.ebiom.2021.103281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Edmondson D.G., Wormser G.P., Norris S.J. In vitro susceptibility of Treponema pallidum subsp. pallidum to doxycycline. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.00979-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tantalo L., Lieberman N., Pérez-Mañà C., et al. In vitro antimicrobial susceptibility of Treponema pallidum subsp. pallidum. Lancet Microbe. 2023 doi: 10.1016/S2666-5247(23)00219-7. [DOI] [PubMed] [Google Scholar]
- 104.Lukehart S.A., Godornes C., Molini B.J., et al. Macrolide resistance in Treponema pallidum in the United States and Ireland. N Engl J Med. 2004;351:154–158. doi: 10.1056/NEJMoa040216. [DOI] [PubMed] [Google Scholar]
- 105.Fernández-Naval C., Arando M., Espasa M., et al. Multilocus sequence typing of Treponema pallidum subsp. pallidum in Barcelona. Future Microbiol. 2021;16:967–976. doi: 10.2217/fmb-2021-0037. [DOI] [PubMed] [Google Scholar]
- 106.Chen X.S., Yin Y.P., Wei W.H., et al. High prevalence of azithromycin resistance to Treponema pallidum in geographically different areas in China. Clin Microbiol Infection. 2013;19:975–979. doi: 10.1111/1469-0691.12098. [DOI] [PubMed] [Google Scholar]
- 107.Su J.R., Pillay A., Hook E.W., et al. Prevalence of the 23S rRNA A2058G point mutation and molecular subtypes in Treponema pallidum in the United States, 2007 to 2009. Sex Transm Dis. 2012;39:794–798. doi: 10.1097/OLQ.0b013e31826f36de. [DOI] [PubMed] [Google Scholar]
- 108.Taouk M.L., Taiaroa G., Pasricha S., et al. Characterisation of Treponema pallidum lineages within the contemporary syphilis outbreak in Australia: a genomic epidemiological analysis. Lancet Microbe. 2022;3:e417–e426. doi: 10.1016/S2666-5247(22)00035-0. [DOI] [PubMed] [Google Scholar]
- 109.Grillová L., Pĕtrošová H., Mikalová L., et al. Molecular typing of Treponema pallidum in the Czech republic during 2011 to 2013: increased prevalence of identified genotypes and of isolates with macrolide resistance. J Clin Microbiol. 2014;52:3693–3700. doi: 10.1128/JCM.01292-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beale M.A., Marks M., Sahi S.K., et al. Genomic epidemiology of syphilis reveals independent emergence of macrolide resistance across multiple circulating lineages. Nat Commun. 2019;10 doi: 10.1038/s41467-019-11216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Psomas K.C., Brun M., Causse A., Atoui N., Reynes J., Le Moing V. Efficacy of ceftriaxone and doxycycline in the treatment of early syphilis. Med Mal Infect. 2012;42:15–19. doi: 10.1016/j.medmal.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 112.Cao Y., Su X., Wang Q., et al. A multicenter study evaluating ceftriaxone and benzathine penicillin G as treatment agents for early syphilis in Jiangsu, China. Clin Infect Dis. 2017;65:1683–1688. doi: 10.1093/cid/cix611. [DOI] [PubMed] [Google Scholar]
- 113.Bettuzzi T., Jourdes A., Robineau O., et al. Ceftriaxone compared with benzylpenicillin in the treatment of neurosyphilis in France: a retrospective multicentre study. Lancet Infect Dis. 2021;21:1441–1447. doi: 10.1016/S1473-3099(20)30857-4. [DOI] [PubMed] [Google Scholar]
- 114.Du F.Z., Wu M.Z., Zhang X., Zhang R.L., Wang Q.Q. Ceftriaxone compared with penicillin G for the treatment of neurosyphilis: study protocol for a multicenter randomized controlled trial. Trials. 2022;23 doi: 10.1186/s13063-022-06769-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tsai J.C., Lin Y.H., Lu P.L., et al. Comparison of serological response to doxycycline versus benzathine penicillin G in the treatment of early syphilis in HIV-infected patients: a multi-center observational study. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ghanem K.G., Erbelding E.J., Cheng W.W., Rompalo A.M. Doxycycline compared with benzathine penicillin for the treatment of early syphilis. Clin Infect Dis. 2006;42:e45–e49. doi: 10.1086/500406. [DOI] [PubMed] [Google Scholar]
- 117.Dai T., Qu R., Liu J., Zhou P., Wang Q. Efficacy of doxycycline in the treatment of syphilis. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01092-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Girometti N., Junejo M.H., Nugent D., McOwan A., Whitlock G. Clinical and serological outcomes in patients treated with oral doxycycline for early neurosyphilis. J Antimicrob Chemother. 2021;76:1916–1919. doi: 10.1093/jac/dkab100. [DOI] [PubMed] [Google Scholar]
- 119.Xiao Y., Liu S., Liu Z., et al. Molecular subtyping and surveillance of resistance genes in Treponema pallidum DNA from patients with secondary and latent syphilis in Hunan, China. Sex Transm Dis. 2016;43:310–316. doi: 10.1097/OLQ.0000000000000445. [DOI] [PubMed] [Google Scholar]
- 120.Stamm L.V. Global challenge of antibiotic-resistant Treponema pallidum. Antimicrob Agents Chemother. 2010;54:583–589. doi: 10.1128/AAC.01095-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gökengin D., Noori T., Alemany A., et al. Prevention strategies for sexually transmitted infections, HIV, and viral hepatitis in Europe. Lancet Reg Health Eur. 2023;34:100738. doi: 10.1016/j.lanepe.2023.100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stafylis C., Keith K., Mehta S., et al. Clinical efficacy of cefixime for the treatment of early syphilis. Clin Infect Dis. 2021;73:907–910. doi: 10.1093/cid/ciab187. [DOI] [PMC free article] [PubMed] [Google Scholar]