Summary
Background
Continuous glucose monitoring (CGM) has shown potential in improving maternal and neonatal outcomes in individuals with type 1/2 diabetes, but data in gestational diabetes mellitus (GDM) is limited. We aimed to explore the relationship between CGM-derived metrics during pregnancy and pregnancy outcomes among women with GDM.
Methods
We recruited 1302 pregnant women with GDM at a mean gestational age of 26.0 weeks and followed them until delivery. Participants underwent a 14-day CGM measurement upon recruitment. The primary outcome was any adverse pregnancy outcome, defined as having at least one of the outcomes: preterm birth, large-for-gestational-age (LGA) birth, fetal distress, premature rupture of membranes, and neonatal intensive care unit (NICU) admission. The individual outcomes included in the primary outcome were considered as secondary outcomes. We conducted multivariable logistic regression to evaluate the association of CGM-derived metrics with these outcomes.
Findings
Per 1-SD difference in time above range (TAR), glucose area under the curve (AUC), nighttime mean blood glucose (MBG), daytime MBG, and daily MBG was associated with higher risk of any adverse pregnancy outcome, with odds ratio: 1.22 (95% CI 1.08–1.36), 1.22 (95% CI 1.09–1.37), 1.18 (95% CI 1.05–1.32), 1.21 (95% CI 1.07–1.35), and 1.22 (95% CI 1.09–1.37), respectively. Time in range, TAR, AUC, nighttime MBG, daytime MBG, daily MBG, and mean amplitude of glucose excursions were positively associated, while time blow range was inversely associated with the risk of LGA. Additionally, higher value for TAR was associated with higher risk of NICU admission. We further summarized the potential thresholds of TAR (2.5%) and daily MBG (4.8 mmol/L) to distinguish individuals with and without any adverse pregnancy outcome.
Interpretation
The CGM-derived metrics may help identify individuals at higher risk of adverse pregnancy outcomes. These CGM biomarkers could serve as potential new intervention targets to maintain a healthy pregnancy status among women with GDM.
Funding
National Key R&D Program of China, National Natural Science Foundation of China, and Westlake Laboratory of Life Sciences and Biomedicine.
Keywords: Continuous glucose monitoring, Gestational diabetes mellitus, Adverse pregnancy outcomes, Prospective birth cohort
Research in context.
Evidence before this study
The usage of continuous glucose monitoring (CGM) may help improve maternal and neonatal outcomes among patients with type 1/2 diabetes. However, acceptable and achievable CGM targets for women with gestational diabetes mellitus (GDM) are lacking owing to the lack of corresponding evidence. We searched PubMed up-to January 5, 2023 for studies investigating associations between CGM-derived metrics and pregnancy outcomes among women with GDM, using the search terms “gestational diabetes mellitus”, “continuous glucose monitoring”, “CGM metrics”, and “pregnancy/birth outcomes”, without any applied restrictions. Only four related papers were found, with a sample size of less than 200 for all studies and relatively short duration of CGM recording.
Added value of this study
To the best of our knowledge, the present study is the largest study with 14-day CGM wearing among patients with GDM during their pregnancies, which shows significant association of diverse CGM-derived metrics with various pregnancy outcomes. Meanwhile, we illustrate potential thresholds of time above range and daily mean blood glucose for women with GDM to distinguish individuals with and without adverse pregnancy outcomes.
Implications of all the available evidence
These findings fill the gap of evidence from large prospective cohort, suggesting that CGM-derived metrics of hyperglycemia status and mean blood glucose are potential biomarkers of common adverse pregnancy outcomes among women participants with GDM.
Introduction
Gestational diabetes mellitus (GDM) prevalence is increasing globally, especially in Asian countries, where the prevalence has reached 20.8% in 2021.1 Patients with GDM tend to have higher risk of several adverse pregnancy and birth outcomes, such as hypertension (or preeclampsia),2 macrosomia,3 and preterm birth.4 Thus, it is essential for the patients to manage their glycemic status during pregnancy to minimize the adverse pregnancy consequences. Yet, our current knowledge about how the glycemic dynamics during pregnancy affect the pregnancy outcomes is still lacking, which prevents the development of precision intervention strategies to alleviate the influence of GDM status on the pregnancy or birth outcomes.
The emerging continuous glucose monitoring (CGM) technique makes it possible to capture dynamic glucose profiles, including postprandial glucose response, hyperglycemia, hypoglycemia, and glycemic variability, which is useful for blood glucose management.5 Several preliminary studies have explored the relationships between CGM-derived glycemic metrics and pregnancy outcomes.6, 7, 8 However, these studies had relatively small sample size (<200) and the CGM recording durations (24–72 h) were relatively short,6, 7, 8 which limited a comprehensive assessment of the relationship between glycemic metrics and pregnancy outcomes. Conducting a large cohort study is imperative to characterize the association of glycemic dynamics and variability with pregnancy outcomes, particularly among women with GDM, given its significant clinical implications.
Therefore, in the present study, we aimed to examine the association of CGM-derived diverse glycemic metrics over 14 days with any of the selected major adverse pregnancy outcomes among more than 1000 participants with GDM. We also tended to figure out optimal cut-offs of CGM-derived metrics for women with GDM to maintain a healthy pregnancy status and improve maternal and neonatal outcomes.
Methods
Study design and population
The present study was based on the Westlake Precision Birth Cohort study (WeBirth, ClinicalTrials.gov identifier: NCT04060056), which is an ongoing prospective birth cohort study, recruiting pregnant women with hyperglycemia from the Hangzhou Women's Hospital (Hangzhou Maternity and Child Health Care Hospital) in China since August 2019.9 Briefly, the WeBirth included pregnant women with the following conditions: i) aged ≥18 years and diagnosed with GDM, with a gestational age mainly ranging from 24 to 28 weeks; ii) intended to deliver at Hangzhou Women's Hospital and remain in Hangzhou with their child for ≥4 years. Meanwhile, pregnant women with cancers or other serious medical disorders were excluded. A standard 2-h 75 g oral glucose tolerance test was performed at 24–28 weeks’ gestation in all women. We adopted the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria for GDM diagnosis (fasting plasma glucose [FPG] ≥5.1 mmol/L, and/or 1-h plasma glucose ≥10.0 mmol/L, and/or 2-h plasma glucose ≥8.5 mmol/L).10 Overt diabetes in pregnancy (DIP) was considered as a subgroup of GDM, with more severe hyperglycemia (FPG ≥7.0 mmol/L, and/or glycated hemoglobin [HbA1c] ≥6.5%, and/or random plasma glucose ≥11.1 mmol/L + confirmation of FPG ≥7.0 mmol/L or HbA1c ≥6.5%).10 The study protocol was approved by the Ethics Committee of Westlake University (20190701ZJS0007). Written informed consent was obtained from each participant. Up to October 2022, 1709 participants were included in the cohort.
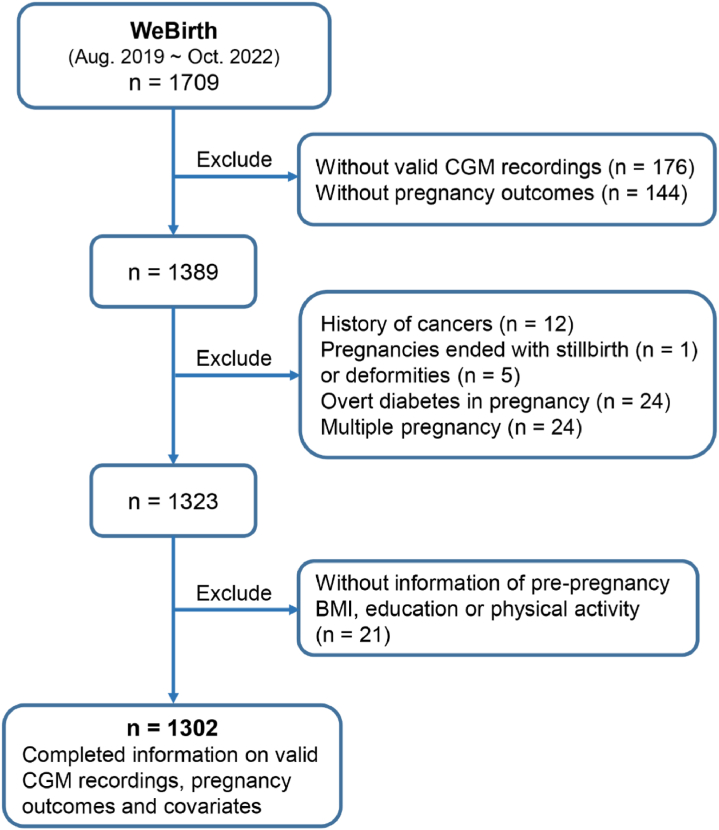
At enrollment, participants were asked to complete several interviewer-administered questionnaires to collect information on demographics, lifestyle, physical activity, medical histories, and health status before and during pregnancy. We collected the information on physical activity of the participants using the Pregnancy Physical Activity Questionnaire (PPAQ).11 According to the instruction of the PPAQ, the self-reported time spent in each activity was multiplied by its intensity to arrive at a measure of average daily energy expenditure (MET-h/day) attributable to each activity. We summed up energy expenditure of all activities for each participant to obtain the variable of physical activity (continuous). A CGM device (Freestyle Libre Pro; Abbott, Abbott Park, IL, USA) was used to monitor 14-day continuous glucose of each participant. The CGM sensor continuously monitors interstitial glucose concentrations and reads the values every 15 min. CGM readings were blind to participants during wearing. The CGM summary report was given to them after 14 days. In the present analysis, we excluded individuals with the following conditions: i) without valid CGM recordings (n = 176) or without follow-up data on pregnancy outcomes (n = 144); ii) with history of cancers (n = 12), pregnancies ended with stillbirth (n = 1) or deformities (n = 5), diagnosed with DIP (n = 24), or multiple pregnancy (n = 24); iii) without data of pre-pregnancy body mass index (BMI), education, or physical activity (n = 21). Finally, a total of 1302 participants were included in this study (Fig. 1). The definition of valid CGM data was described in the paragraph about CGM data preprocessing.
Fig. 1.
Flowchart of analysis sample selection. Abbreviations: WeBirth, Westlake Precision Birth Cohort; GDM, gestational diabetes mellitus; CGM, continuous glucose monitoring; BMI, body mass index.
Data collection of traditional glycemic traits
Blood samples were collected at baseline. Plasma glucose level was quantified using the Beckman Coulter AU5800 Clinical Chemistry Analyzer. HbA1c was measured by the Sebia CAPILLARYS 2 Flex Piercing (Cap 2FP; Sebia, Lisses, France).
CGM profiling and glycemic metrics extraction
Each participant was asked to wear a masked CGM device on the back of the upper arm to monitor 14-day interstitial fluid glucose. Individuals were excluded if their CGM recordings were <72 h. To rule out potential influence of unstable readings, we excluded CGM readings of: i) the first and last two incomplete days; ii) readings of the first 24 h in the remaining data after performing the previous step; and iii) days with extreme time spent below target range 3.5 mmol/L (TBR, >99th percentile). The remaining CGM recordings were considered as valid CGM data. Finally, we included more than 1.3 million glucose measurements among 1302 individuals for further analyses with an average of 1040 ± 219 measurements per person. The total valid person-time of CGM recordings was 14,101 ± 2968 person-days.
Time in range (TIR) was defined as the percentage of time that glucose levels fell within the target range of 3.5 mmol/L and 7.8 mmol/L over 24 h, with time above range (TAR) representing the percentage of time above the target range and TBR representing the percentage of time below the target range. Mean of continuous blood glucose (MBG) during nighttime (between 00:01 am and 06:00 am) and daytime (between 06:01 am and 24:00 pm) was considered as nighttime MBG and daytime MBG, respectively. Daily MBG was referred to the mean blood glucose level over a 24-h period. We calculated the total glucose area under the curve (AUC) to quantify glycaemia dynamics during the recording period. Mean amplitude of glucose excursions (MAGE) was an essential index for glycemic variability assessment. MAGE was characterized as arithmetic mean of the differences between consecutive amplitudes whose magnitudes were higher than one standard deviation from the mean in 24 h, which was calculated using the R package Continuous Glucose Monitoring Time Series Data Analysis (CGMTSA).12 Coefficient of variation (CV) was calculated as the ratio of the standard deviation (SD) to the mean of glucose measurements. To take the random errors into account, we calculated the CGM-derived metrics for each day and then obtained the average CGM-derived metrics for all days of each participant for further statistical analyses.
Pregnancy outcomes
The primary outcome of the present study was any of the selected major adverse pregnancy outcomes, defined as having at least one of the following conditions: preterm birth, large-for-gestational-age birth (LGA), fetal distress, premature rupture of membranes (PROM), and neonatal intensive care unit (NICU) admission. The secondary outcomes included aforementioned five individual adverse pregnancy outcomes. Preterm birth was referred to the condition of gestational age <37 weeks at birth, including spontaneous and medically indicated preterm birth. SGA and LGA were defined as gender- and gestational age-adjusted birth weight of newborns less than 10th percentile and greater than 90th percentile, respectively.13 The remained birth weight was appropriate for gestational age (AGA). Fetal distress was a syndrome of fetal hypoxia and/or acidosis in the womb, which was defined as having at least one of the following conditions: 1) non-reassuring patterns seen on cardiotocography, with tachycardia and bradycardia (especially during and after a contraction), late decelerations or decreased variability in the fetal heart rate (<110 times/min); 2) umbilical arterial blood gas analysis, PH < 7.2; 3) meconium contamination of the amniotic fluid in labour; and 4) a 1 min Apgar score <7.14 PROM was defined as rupture of the membranes (amniotic sac) before the onset of labour.15
Statistical analysis
We performed the Wilcoxon–Mann–Whitney test to compare differences in CGM-derived metrics between participants with and without any of our defined major adverse pregnancy outcomes. Generalized additive model (GAM) was employed to estimate the relationship between HbA1c and CGM-derived metrics, adjusted for maternal age (continuous), gestational age at baseline (continuous), pre-pregnancy BMI (continuous), and physical activity (continuous). For our primary analyses, we performed logistic regression analysis to estimate the odds ratio (OR, 95% confidence intervals [CI]) of any adverse pregnancy outcome per SD change in each of the CGM-derived biomarker (i.e., TIR, TAR, TBR, AUC, nighttime MBG, daytime MBG, daily MBG, MAGE, and CV), with model 1 as crude model, and model 2 adjusted for maternal age, pre-pregnancy BMI, gestational age at baseline, parity (primiparity, multiparity), education (≤high school or vocational school, university or professional school, >university), and physical activity. To account for multiple testing, false discovery rate (FDR) was calculated using Benjamini–Hochberg method. For secondary outcomes, we re-ran the above logistic regression analysis to examine the association of the aforementioned CGM-derived parameters with five individual outcomes (preterm birth, LGA, fetal distress, PROM, and NICU admission), adjusted for the same list of covariates of above model 2. FDR was calculated for the analysis of each secondary outcome.
For these identified outcomes with significant associations, we further evaluated the model performance by calculating the receiver operating characteristic (ROC) area under the curve (AUC). We displayed the cut-offs of CGM-derive metrics with the highest Youden's index. Furthermore, we compared the performance of CGM-derived metrics and traditional glycemic biomarkers for predicting these identified outcomes using two kinds of prediction models, logistic regression model and random forest model. The traditional glycemic biomarkers included HbA1c and OGTT glucose (i.e., fasting, 1-h and 2-h glucose). Considering the application and generalizability in clinical practice, we estimated the absolute risk for those adverse pregnancy outcomes showing significant relationships with daily MBG, as the percentage of women with adverse outcomes within each daily MBG categories (every 5 mg/dl). We also assessed associations of daily MBG in 5 mg/dl units with those adverse pregnancy outcomes using logistic regression adjusted for the same list of covariates of above model 2.
As sensitivity analysis, we additionally included gestational weight gain (continuous), diabetes treatment (yes/no), and HbA1c at baseline (continuous) to the logistic regression in addition to the covariates of above model 2, to estimate associations of CGM-derived metrics with adverse pregnancy outcomes. We also investigated relationships of different categories of CGM-derived metrics (by tertile, or by the clinically relevance threshold) with the outcomes. Due to the lack of evidence on CGM targets for women with GDM in pregnancy, here we tentatively applied the target values of TAR and TBR for type 2 diabetes to categorized these two metrics into binary variables: 5% for TAR, and 4% for TBR,16 conjoining the distributions of TAR and TBR among our participants (Table 1). Logistic regression analysis was performed to estimate ORs (95% CI) of adverse pregnancy outcomes (i.e., any adverse pregnancy outcome, preterm birth, LGA, fetal distress, PROM, and NICU admission) by tertile or by the above binary thresholds of TAR and TBR, adjusted for potential confounders including maternal age, pre-pregnancy BMI, gestational age at baseline, parity, education, and physical activity.
Table 1.
Characteristics of the study population: Westlake precision birth cohort study.a
| Total | Any adverse pregnancy outcome |
||
|---|---|---|---|
| No | Yes | ||
| No. of participants | 1302 (100%) | 790 (60.7%) | 512 (39.3%) |
| Age, years | 31.2 (3.7) | 31.3 (3.7) | 31.0 (3.6) |
| Gestational age at baseline, weeks | 22.1 (3.5) | 21.9 (3.6) | 22.3 (3.5) |
| Pre-pregnancy BMI, kg/m2 | 26.0 (1.9) | 26.0 (1.9) | 26.0 (1.8) |
| Primiparity | 866/1302 (66.5%) | 510/790 (64.6%) | 356/512 (69.5%) |
| Education | |||
| ≤High school or vocational school | 140/1302 (10.8%) | 81/790 (10.3%) | 59/512 (11.5%) |
| University or professional school | 968/1302 (74.3%) | 589/790 (74.6%) | 379/512 (74.0%) |
| >University | 194/1302 (14.9%) | 120/790 (15.2%) | 74/512 (14.5%) |
| Household income, ¥/year | |||
| <100,000 | 281/1302 (21.6%) | 186/790 (23.5%) | 95/512 (18.6%) |
| 100,000–200,000 | 465/1302 (35.7%) | 274/790 (34.7%) | 191/512 (37.3%) |
| >200,000 | 471/1302 (36.2%) | 279/790 (35.3%) | 192/512 (37.5%) |
| Unclear | 85/1302 (6.5%) | 51/790 (6.5%) | 34/512 (6.6%) |
| Smoking | |||
| Never smokers | 1249/1302 (96.1%) | 765/790 (97.0%) | 484/512 (94.7%) |
| Ex-smokers | 51/1302 (3.9%) | 24/790 (3.0%) | 27/512 (5.3%) |
| Current drinking | 35/1302 (2.7%) | 19/790 (2.4%) | 16/512 (3.1%) |
| Physical activity, MET-h/day | 19.5 (8.0) | 19.8 (7.9) | 19.1 (8.2) |
| OGTT glucose, mmol/L | |||
| Fasting | 4.8 (0.5) | 4.8 (0.5) | 4.9 (0.5) |
| 1-h | 9.9 (1.6) | 9.8 (1.6) | 9.9 (1.6) |
| 2-h | 8.4 (1.5) | 8.4 (1.4) | 8.5 (1.6) |
| HbA1c, % | 5.0 (0.3) | 5.0 (0.3) | 5.0 (0.3) |
| CGM-derived metrics | |||
| TIR, % | 92.8 (85.4, 96.7) | 92.8 (85.2, 96.7) | 92.8 (85.7, 96.7) |
| TAR, % | 1.1 (0.3, 2.6) | 1.0 (0.3, 2.5) | 1.2 (0.3, 3.0) |
| TBR, % | 4.0 (0.9, 12.3) | 4.5 (1.0, 12.8) | 3.4 (0.8, 11.3) |
| AUC, mmol/L·h | 112.8 (105.0, 120.6) | 111.9 (104.6, 119.6) | 114.2 (106.2, 122.7) |
| Nighttime MBG, mmol/L | 4.1 (3.7, 4.4) | 4.0 (3.7, 4.4) | 4.1 (3.8, 4.5) |
| Daytime MBG, mmol/L | 4.9 (4.6, 5.3) | 4.9 (4.6, 5.2) | 5.0 (4.6, 5.4) |
| Daily MBG, mmol/L | 4.7 (4.4, 5.1) | 4.7 (4.4, 5.0) | 4.8 (4.5, 5.2) |
| MAGE, mmol/L | 2.3 (1.9, 2.7) | 2.3 (1.9, 2.6) | 2.3 (1.9, 2.7) |
| CV, % | 20.1 (17.4, 23.0) | 20.2 (17.5, 22.8) | 20.0 (17.4, 23.3) |
Abbreviations: BMI, body mass index; MET, metabolic equivalent; OGTT, oral glucose tolerance test; HbA1c, glycated hemoglobin; CGM, continuous glucose monitoring; TIR, time in range; TAR, time above range; TBR, time below range; AUC, glucose area under the curve; MBG, mean blood glucose; MAGE, mean amplitude of glucose excursions; CV, coefficient of variation; SD, standard deviation; IQR, interquartile range; NICU, neonatal intensive care unit.
Data are mean (SD) for normally distributed continuous variables, medium (IQR) for skew distributed continuous variables, and n/total (%) for categorical variables. Any adverse pregnancy outcome was defined as the prevalence of at least one of the following conditions: preterm birth, large-for-gestational-age birth, fetal distress, premature rupture of membranes, and NICU admission.
According to birth weight, we further classified all the participants into three groups, AGA, LGA, and SGA. We performed multinomial logistic regression to explore association of CGM-derived metrics with LGA and SGA using the AGA group as the reference, adjusted for maternal age, gestational age at baseline, parity, education, and physical activity. As pregnant women may have different metabolic status, depending on pre-pregnancy overweight status,17 we categorized participants into three groups based on their pre-pregnancy BMI, as recommended by the Working Group on Obesity in China: ‘Underweight’ (pre-pregnancy BMI <18.5 kg/m2), ‘Normal’ (18.5 kg/m2≤ pre-pregnancy BMI <24 kg/m2), and ‘Overweight/obese’ (pre-pregnancy BMI ≥24 kg/m2).18 We did subgroup analysis to examine the association of CGM-derived biomarkers with our primary outcome (i.e., any adverse pregnancy outcome) stratified by the pre-pregnancy BMI categories using logistic regression models, adjusted for maternal age, gestational age at baseline, parity, education, and physical activity. To assess potential interaction of CGM-derived biomarkers with pre-pregnancy BMI, we also added a cross-product term in the above model, and nominal p for interaction <0.05 was considered as statistically significant. We did not perform further subgroup analysis for our secondary outcomes due to limited number of cases in the pre-pregnancy BMI stratified analysis. All statistical analyses were performed using Stata 15.0 or R version 4.0.4.
Role of the funding source
The funders were not involved in any part of the conduct of this study or in the decision to submit this work.
Results
Population characteristics
The mean age of our participants at recruitment was 31.2 ± 3.7 years, with gestational age of 26.0 ± 1.9 weeks and pre-pregnancy BMI of 22.1 ± 3.5 kg/m2 on average (Table 1). More than half of the women (66.5%) were primiparous. Our primary outcome of any adverse pregnancy outcome occurred in 39.2% (512/1302) of the participants, with the prevalence of individual outcomes: preterm birth, LGA, fetal distress, PROM, and NICU admission being 5.0%, 11.1%, 8.1%, 20.0%, and 9.1%, respectively. Individuals with at least one of the above pregnancy outcomes had higher levels of CGM-derived AUC, nighttime MBG, daily MBG, and daytime MBG (Fig. S1). We displayed the estimated CGM-derived metrics (including TIR, TBR, TAR, AUC, nighttime MBG, daytime MBG, daily MBG, MAGE, and CV) plotted against HbA1c using GAM model (Fig. S2). All CGM-derived metrics, except for TBR, exhibited a positive correlation with HbA1c.
Associations of CGM-derived glycemic features with adverse pregnancy outcomes
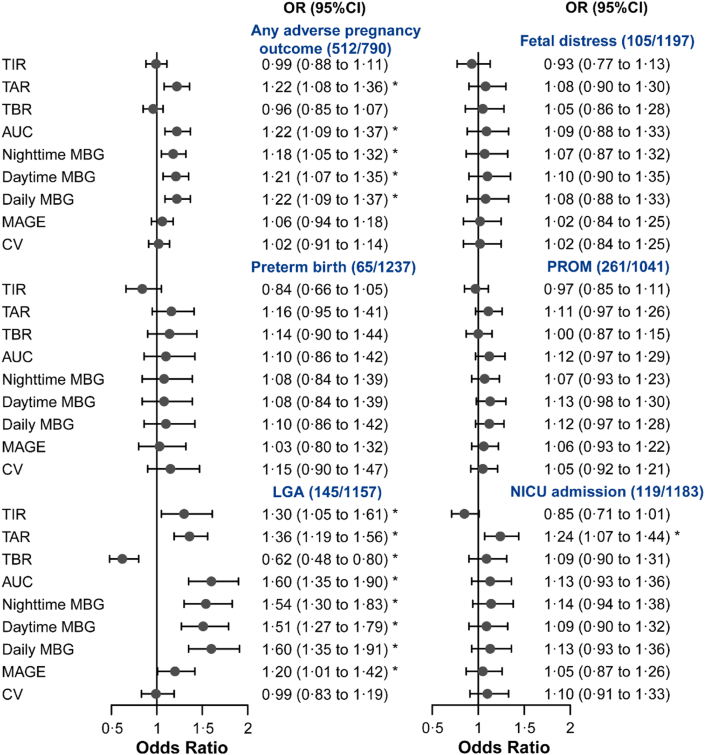
After adjusting for potential confounders and multiple testing correction, our results suggested that per 1-SD change in TAR, AUC, nighttime MBG, daytime MBG, and daily MBG was significantly (FDR <0.05) associated with increased risk of our primary outcome: any adverse pregnancy outcome, with OR: 1.22 (95% CI 1.08–1.36), 1.22 (95% CI 1.09–1.37), 1.18 (95% CI 1.05–1.32), 1.21 (95% CI 1.07–1.35), and 1.22 (1.09–1.37) in the model 2, respectively (Fig. 2). The results were consistent across model 1 and 2 (Table S1).
Fig. 2.
Associations of CGM-derived features with pregnancy outcomes. Forest plot presents the ORs (95% CI) of any adverse pregnancy outcome or each individual pregnancy outcome per standardized change of CGM-derived metrics (in SD unit). The case number of each outcome and the number of total participants were annotated as “(case/total)”. False discovery rate (FDR) was corrected based on Benjamini–Hochberg method. ∗FDR <0.05. Any adverse pregnancy outcome was defined as the prevalence of one or more of the following conditions: preterm birth, LGA, fetal distress, PROM, and NICU admission. Abbreviations: CGM, continuous glucose monitoring; OR, odds ratio; CI, confidence interval; TIR, time in range; TAR, time above range; TBR, time below range; AUC, glucose area under the curve; MBG, mean blood glucose; MAGE, mean amplitude of glucose excursions; CV, coefficient of variation; LGA, large for gestational age; PROM, premature rupture of fetal membranes; NICU, neonatal intensive care unit.
For the individual pregnancy outcomes, after adjusting for potential confounders and multiple testing correction, we found multiple significant associations between CGM-derived metrics and the risk of LGA and NICU admission (Fig. 2). Participants with higher level of TIR (per 1-SD, OR 1.30; 95% CI 1.05–1.61), TAR (OR 1.36; 95% CI 1.19–1.56), AUC (OR 1.60; 95% CI 1.35–1.90), nighttime MBG (OR 1.54; 95% CI 1.30–1.83), daytime MBG (OR 1.51; 95% CI 1.27–1.79), daily MBG (OR 1.60; 95% CI 1.35–1.91), and MAGE (OR 1.20; 95% CI 1.01–1.42) had a higher risk of LGA. Contrarily, per 1-SD change in TBR was associated with lower risk of LGA (OR 0.62; 95% CI 0.48–0.80). We also found that individuals with higher level of TAR were at greater risk of NICU admission (per 1-SD, OR 1.24; 95% CI 1.07–1.44).
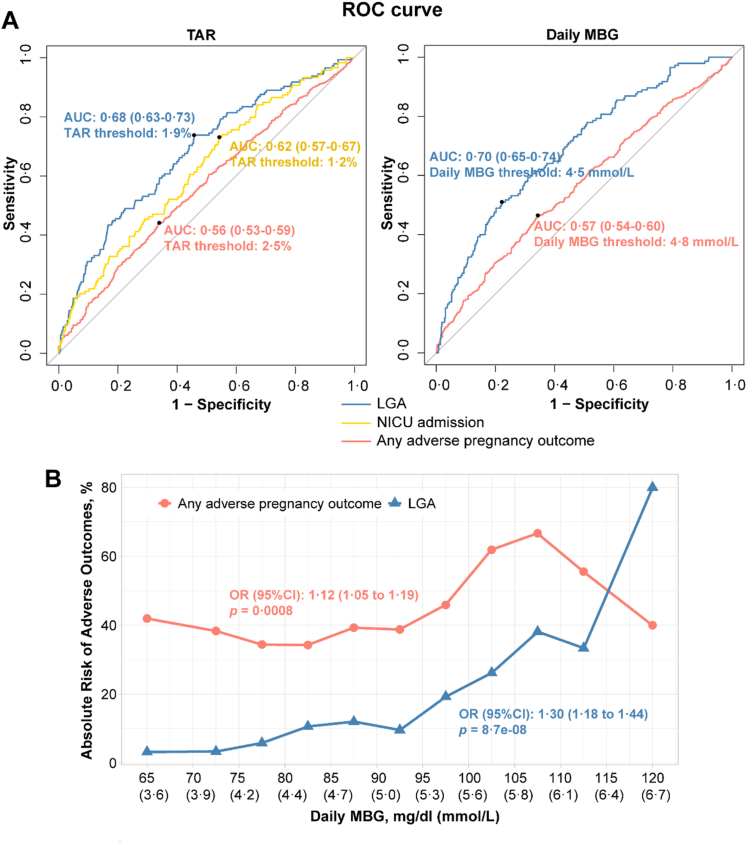
Potential thresholds of CGM-derived metrics for women with GDM
According to the significant results of logistic regression (Fig. 2), we further illustrated the performance of TAR and daily MBG for predicting adverse outcomes (i.e., any adverse pregnancy outcome, LGA, and NICU admission). The cut-off value of TAR with the best discrimination accuracy were 1.9% for LGA, 1.2% for NICU admission, and 2.5% for any adverse pregnancy outcome (Fig. 3A). The best threshold of daily MBG for preventing LGA and any adverse pregnancy outcome were ≤4.5 mmol/L and ≤4.8 mmol/L, respectively (Fig. 3A). Meanwhile, we found the performance of CGM-derived metrics were generally better than that of the traditional glycemic traits for predicting these three outcomes mentioned above (Table S2). As daily MBG was significantly associated with adverse pregnancy outcome and LGA, further analysis showed that the absolute risks for these two outcomes increased across the range of daily MBG (Fig. 3B). There was a 12% (95% CI 1.05–1.19) higher risk of any adverse pregnancy outcome for every 5 mg/dl rise in daily MBG. The absolute risk for any adverse pregnancy outcome ranged from 34.2% (100 of 292) to 66.7% (7 of 21). The lowest and highest absolute risks for any adverse pregnancy outcome were found among women with a daily MBG ranging from 4.4 mmol/L to 4.7 mmol/L and women with a daily MBG ranging from 5.8 mmol/L to 6.1 mmol/L, respectively. There was a 30% (95% CI 1.18–1.44) higher risk of LGA for every 5 mg/dl rise in daily MBG. The lowest absolute risk for LGA was 3.2% (1 of 31) for women with a daily MBG ranging from 3.3 mmol/L to 3.9 mmol/L. The highest absolute risk for LGA was 80.0% (2 of 3) for women with a daily MBG ranging from 6.4 mmol/L to 6.9 mmol/L.
Fig. 3.
Potential cutoffs of CGM-derived metrics for women with GDM. (A) ROC curves show the performance of TAR or daily MBG for predicting LGA, NICU admission or any adverse pregnancy outcome. AUC (95% CI) and optimal threshold of CGM-metric in each model were presented. The optimal threshold was determined by maximizing the Youden index. (B) Absolute risk was calculated as (No. of women with adverse outcome/No. of women in daily MBG category) × 100. The symbols represent the absolute risk for women in different daily MBG categories. Each daily MBG category was 5 mg/dl. The ORs (95% CI) were generated from the logistic regression to assessed associations of daily MBG in 5 mg/dl units with pregnancy outcomes. Any adverse pregnancy outcome was defined as the prevalence of one or more of the following conditions: preterm birth, LGA, fetal distress, premature rupture of fetal membranes, and NICU admission. Abbreviations: CGM, continuous glucose monitoring; ROC, receiver operating characteristic; TAR, time above range; MBG, mean blood glucose; LGA, large for gestational age; NICU, neonatal intensive care unit; AUC, the ROC area under the curve; OR, odds ratio; CI, confidence interval.
Sensitivity analysis
The result of the association between CGM-metrics and adverse pregnancy outcome was consistent when we additionally adjusted gestational weight gain, diabetes treatments, and HbA1c at baseline (Table S3). In the categorical analysis of CGM-derived metrics, positive associations of AUC, nighttime MBG, daytime MBG, and daily MBG with the risk of any adverse pregnancy outcome remained (FDR corrected, p for trend <0.05; Table S4). Compared with the lowest tertile, the highest tertile of AUC (OR 1.39; 95% CI 1.05–1.84), nighttime MBG (OR 1.40; 95% CI 1.06–1.85), daytime MBG (OR 1.45; 95% CI 1.10–1.92), and daily MBG (OR 1.39; 95% CI 1.05–1.83) was associated with higher risk of any adverse pregnancy outcome. Likewise, association between CGM-metrics and the risk of LGA was generally consistent with the primary per-SD analysis. Additionally, we observed positive association of daytime MBG with the risk of PROM (p for trend = 0.032). Women among the highest group of daytime MBG had a higher risk of PROM (OR 1.46; 95% CI 1.03–2.05; Table S4). Participants with TAR ≥5% (vs. <5%) was associated with higher risk of preterm birth (OR 2.17; 95% CI 1.11–4.24) and LGA (OR 2.18; 95% CI 1.35–3.52; Table S5). Conversely, participants with TBR ≥4% (vs. <4%) had a lower risk of LGA (OR 0.61; 95% CI 0.42–0.88).
Subgroup analyses
In the multinomial regression, we found similar results of LGA (Fig. S3A). However, there was no significant association of CGM-derived metrics with the risk of SGA. Our result revealed significant interaction between pre-pregnancy BMI and CGM-derived TAR, AUC, nighttime MBG, daytime MBG, and daily MBG on the risk of any adverse pregnancy outcome (p for interaction <0.05, Fig. S3B). The positive association between TAR and any adverse pregnancy outcome was significant among participants being overweight/obese before pregnancy (OR 1.47; 95% CI 1.18–1.83). Similarly, the positive association with any adverse pregnancy outcome for AUC, nighttime MBG, daytime MBG, and daily MBG was stronger among those with pre-pregnancy overweight/obese status compared with underweight or normal-weight participants (Fig. S3B).
Discussion
In the present study, we conducted a prospective cohort study among 1302 participants with GDM to investigate the associations of CGM-derived metrics with adverse pregnancy outcomes. Higher levels of TAR, AUC, nighttime MBG, daytime MBG, and daily MBG were associated with higher risk of any adverse pregnancy outcome (preterm birth, LGA, fetal distress, PROM, or NICU admission). We further summarized the potential thresholds of TAR and daily MBG for women with GDM to distinguish individuals with and without adverse pregnancy outcomes.
We demonstrated the dynamic blood glucose profiling of participants with GDM during the second trimester of pregnancy, and found that several CGM-derived biomarkers (e.g., TAR, AUC, nighttime MBG, daytime MBG, and MAGE) were associated with LGA. An early study involving 150 pregnant women with GDM and continuously monitoring the glucose for 72 h showed that mean glucose was positively associated with neonatal birth weight.19 Another two recent studies based on 162 and 97 pregnant women with GDM also reported that participants with higher nighttime glucose levels during the third trimester had higher risk of LGA birth.6,7 Similar association pattern was observed in pregnant women with type 1 diabetes.20 In line with our results, previous studies showed that higher MBG, TAR, and AUC, as well as more glycemic variability (MAGE and SD) in the second trimester were associated with increased risk of LGA.20, 21, 22 These findings suggested that hyperglycemia status and glycemic variability may play an important role in the relationship of GDM with excessive neonatal birth weight, which also suggested that CGM-derived targets may be effective markers for GDM management to prevent LGA or macrosomia.
Our present study was among the first to reveal that higher levels of TAR were associated with higher risk of preterm birth in participants with GDM. Several cohort studies from different countries reported that pregnancy hyperglycemia was positively associated with risk of spontaneous preterm birth.4,23 Our study substantially expanded the prior knowledge by characterizing the detailed pattern of hyperglycemia that was associated with preterm birth, which provided potential intervention targets for the prevention of preterm birth. Nevertheless, more research is needed to evaluate the translational potential of these CGM-derived metrics for the prediction of preterm birth in the future.
Previous studies revealed that women with GDM also experienced a higher risk of NICU admission.24,25 Maternal hyperglycemia can lead to fetal hyperinsulinemia, promoting development of insulin-sensitive tissues (i.g., liver, fat, and muscle) but not non-insulin-sensitive tissues (i.g., brain and kidney).26 The imbalance development of different fetal tissues may lead to poor tolerance to hypoxia in utero and poor adaptability to the external environment. This situation would result in increased adverse neonatal outcomes, such as respiratory distress, hypoglycemia, and macrosomia, which may in turn lead to an increase in NICU admission.24,26,27 Furthermore, we discovered that individuals with GDM who exhibited prolonged hyperglycemia (i.e., TAR) had a higher risk of NICU admission compared with those with shorter periods of hyperglycemia. PROM is also a common maternal complications in pregnant women with GDM,28 while few studies have revealed the metabolic factors which may influence the prevalence of PROM.29 Here, we discovered a positive correlation between daytime glucose level and the risk of PROM among women with GDM.
Women with obesity had an increased risk of adverse pregnancy outcome, such as macrosomia, preterm birth, LGA, and cesarean delivery.30 GDM combined with overweight and obesity may exaggerate the risk of developing adverse pregnancy outcomes.31 This was in line with our present finding that the association between maternal hyperglycemia status (e.g., TAR and daytime MBG) and adverse pregnancy outcome was stronger among overweight or obese women.
Long-term exposure to hyperglycemia in pregnant women has been correlated with fetal growth acceleration,32 which may help explain the discovered associations between maternal hyperglycemia and adverse pregnancy outcomes in the present study. Animal study revealed that hyperglycemia during pregnancy could induce placental structural and functional dysfunction.33 Meanwhile, evidence from human and mice suggested that high glucose level triggered DNA methylation epigenetic modifications in placenta34 and germ cells,35 and subsequently contributed to poor fetal outcomes. Taken together, these findings may conjointly explain the high correlations of mean glucose level and hyperglycemia ratio with adverse pregnancy outcomes in our study. More mechanistic research is needed to interpret our findings.
The present study has several strengths. First, we included a large number of participants with GDM who were connected to CGM for up to 14 days during the second trimester of pregnancy, and the CGM-derived metrics provided comprehensive profiling of the blood glucose homeostasis. Second, we have collected extensive clinical and lifestyle parameters, which enables us to explore associations of CGM-derived metrics with common and major adverse maternal and neonatal outcomes, adjusting for a wide range of potential confounders.
There are also several limitations. First, this study only included Chinese participants in a single center, and thus the findings may not be generalizable to other populations or ethnicities, although we have applied the wildly used IADPSG criteria for GDM diagnosis, which increases our comparability with other GDM populations. Furthermore, our study only included women with GDM (without overt diabetes in pregnancy) and did not have a normal control group, which may have contributed to the lack of statistical significance in some individual adverse outcomes. Second, the CGM data were collected during 24–28 weeks’ gestation, which may not represent glucose status throughout pregnancy. Third, the CGM devices were connected to participants after GDM diagnosis, which may potentially alter the patients’ dietary habits or lifestyle factors. Nevertheless, we have adjusted for potential confounders related to lifestyle factors, and the CGM recordings are masked from participants during measurements. Finally, the present results may not be causal due to the observational nature of our study, and therefore further randomized controlled trials and mechanism studies are necessary to validate our findings.
In conclusion, our study suggests that higher levels of CGM-derived metrics of hyperglycemia status and mean blood glucose are potential biomarkers of common adverse pregnancy outcomes among women participants with GDM. Meanwhile, the pre-pregnancy BMI may influence the association of the glycemic status with the pregnancy outcomes, which indicates a stratified prevention and management strategies for glycemic dynamics among patients with GDM. More studies with large sample size are warranted to replicate the present results to further establish an optimal glucose management strategy for GDM.
Contributors
J.-S.Z. and W.H. conceived the study concept and design. X.L., Y.F., S.L., M.S., Z.M., W.G., Y.L., F.X., Y.T., J.W., K.Z., C.X., Z.J., M.-Q.S., Y.-Y.W., and X.-H.W. were involved in data collection. X.L., J.-S.Z., and Y.F. analyzed the data and drafted the manuscript. J.-S.Z., X.L., and L.S. interpreted the data. All authors revised and approved the final version of the manuscript for publication. J.-S.Z. is the guarantor of this work and, as such, has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Data sharing statement
Aggregated data will be available upon request to the corresponding authors.
Declaration of interests
No potential conflicts of interest relevant to this article were reported.
Acknowledgements
The authors thank all the participants involved in the present study. We thank the High-Performance Computing Center and the Research Center for Industries of the Future at Westlake University for assistance in computing. This study was funded by the National Key R&D Program of China (2022YFA1303900), National Natural Science Foundation of China (82073529, 82103826, and 82173530), Zhejiang Provincial Natural Science Foundation of China (LQ21H260002, LQ21H040001), the Research Program (No. 202208012) of Westlake Laboratory of Life Sciences and Biomedicine, “Pioneer” and “Leading goose” R&D Program of Zhejiang (2022C03102), National Health Commission Scientific Research Fund--Major Science and Technology Program of Medicine and Health of Zhejiang Province (WKJ-ZJ-1911), and Science and Technology Program of Medicine and Health of Hangzhou (ZD20200035, OO2019054). The funder had no role in study design, data collection and analysis, decision to publish, or writing of the manuscript.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanwpc.2023.100823.
Contributor Information
Wen-Sheng Hu, Email: huws@zju.edu.cn.
Ju-Sheng Zheng, Email: zhengjusheng@westlake.edu.cn.
Appendix A. Supplementary data
References
- 1.Wang H., Li N., Chivese T., et al. IDF diabetes atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group's criteria. Diabetes Res Clin Pract. 2022;183 doi: 10.1016/j.diabres.2021.109050. [DOI] [PubMed] [Google Scholar]
- 2.Tobias D.K., Hu F.B., Forman J.P., Chavarro J., Zhang C. Increased risk of hypertension after gestational diabetes mellitus: findings from a large prospective cohort study. Diabetes Care. 2011;34(7):1582–1584. doi: 10.2337/dc11-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li M., Hinkle S.N., Grantz K.L., et al. Glycaemic status during pregnancy and longitudinal measures of fetal growth in a multi-racial US population: a prospective cohort study. Lancet Diabetes Endocrinol. 2020;8(4):292–300. doi: 10.1016/S2213-8587(20)30024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hedderson M.M., Ferrara A., Sacks D.A. Gestational diabetes mellitus and lesser degrees of pregnancy hyperglycemia: association with increased risk of spontaneous preterm birth. Obstet Gynecol. 2003;102(4):850–856. doi: 10.1016/s0029-7844(03)00661-6. [DOI] [PubMed] [Google Scholar]
- 5.Danne T., Nimri R., Battelino T., et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Law G.R., Alnaji A., Alrefaii L., et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care. 2019;42(5):810–815. doi: 10.2337/dc18-2212. [DOI] [PubMed] [Google Scholar]
- 7.Shen S.Y., Zurauskiene J., Wei D.M., et al. Identification of maternal continuous glucose monitoring metrics related to newborn birth weight in pregnant women with gestational diabetes. Endocrine. 2021;74(2):290–299. doi: 10.1007/s12020-021-02787-x. [DOI] [PubMed] [Google Scholar]
- 8.Panyakat W.S., Phatihattakorn C., Sriwijitkamol A., Sunsaneevithayakul P., Phaophan A., Phichitkanka A. Correlation between third trimester glycemic variability in non-insulin-dependent gestational diabetes mellitus and adverse pregnancy and fetal outcomes. J Diabetes Sci Technol. 2018;12(3):622–629. doi: 10.1177/1932296817752374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang X., Miao Z., Lu S., et al. Integration of multiomics with precision nutrition for gestational diabetes. Study protocol for the Westlake Precision Birth Cohort. iMeta. 2023;2(2) e96. [Google Scholar]
- 10.International Association of Diabetes and Pregnancy Study Groups Consensus Panel. Metzger B.E., Gabbe S.G., et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010;33(3):676–682. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chasan-Taber L., Schmidt M.D., Roberts D.E., Hosmer D., Markenson G., Freedson P.S. Development and validation of a pregnancy physical activity questionnaire. Med Sci Sports Exerc. 2004;36(10):1750–1760. doi: 10.1249/01.mss.0000142303.49306.0d. [DOI] [PubMed] [Google Scholar]
- 12.Shao J., Liu Z., Li S., et al. Continuous glucose monitoring time series data analysis: a time series analysis package for continuous glucose monitoring data. J Comput Biol. 2023;30(1):112–116. doi: 10.1089/cmb.2022.0100. [DOI] [PubMed] [Google Scholar]
- 13.Dai L., Deng C., Li Y., et al. Birth weight reference percentiles for Chinese. PLoS One. 2014;9(8) doi: 10.1371/journal.pone.0104779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie X., Kong B., Duan T. People’s Medical Publishing House; Beijing: 2018. Obstetrics and gynecology (the 9th version) pp. 138–140. [Google Scholar]
- 15.Prelabor rupture of membranes: ACOG Practice Bulletin, number 217. Obstet Gynecol. 2020;135(3):e80–e97. doi: 10.1097/AOG.0000000000003700. [DOI] [PubMed] [Google Scholar]
- 16.Battelino T., Danne T., Bergenstal R.M., et al. Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care. 2019;42(8):1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yong H.Y., Mohd Shariff Z., Mohd Yusof B.N., et al. Independent and combined effects of age, body mass index and gestational weight gain on the risk of gestational diabetes mellitus. Sci Rep. 2020;10(1):8486. doi: 10.1038/s41598-020-65251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou B.F., Cooperative Meta-Analysis Group of the Working Group on Obesity in China Predictive values of body mass index and waist circumference for risk factors of certain related diseases in Chinese adults--study on optimal cut-off points of body mass index and waist circumference in Chinese adults. Biomed Environ Sci. 2002;15(1):83–96. [PubMed] [Google Scholar]
- 19.Yu F., Lv L., Liang Z., et al. Continuous glucose monitoring effects on maternal glycemic control and pregnancy outcomes in patients with gestational diabetes mellitus: a prospective cohort study. J Clin Endocrinol Metab. 2014;99(12):4674–4682. doi: 10.1210/jc.2013-4332. [DOI] [PubMed] [Google Scholar]
- 20.Kristensen K., Ogge L.E., Sengpiel V., et al. Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia. 2019;62(7):1143–1153. doi: 10.1007/s00125-019-4850-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Law G.R., Ellison G.T., Secher A.L., et al. Analysis of continuous glucose monitoring in pregnant women with diabetes: distinct temporal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care. 2015;38(7):1319–1325. doi: 10.2337/dc15-0070. [DOI] [PubMed] [Google Scholar]
- 22.Sibiak R., Mrzewka-Rogacz B., Mantaj U., Gutaj P., Wender-Ozegowska E. 96-OR: Parameters of Glycemic Variability as Predictors of LGA in Pregnant Women with Well-Controlled type 1 Diabetes (T1D) Diabetes. 2021;70(Supplement_1) [Google Scholar]
- 23.Li M.F., Ma L., Yu T.P., et al. Adverse maternal and neonatal outcomes in pregnant women with abnormal glucose metabolism. Diabetes Res Clin Pract. 2020;161 doi: 10.1016/j.diabres.2020.108085. [DOI] [PubMed] [Google Scholar]
- 24.Wendland E.M., Torloni M.R., Falavigna M., et al. Gestational diabetes and pregnancy outcomes--a systematic review of the World Health Organization (WHO) and the International Association of Diabetes in Pregnancy Study Groups (IADPSG) diagnostic criteria. BMC Pregnancy Childbirth. 2012;12:23. doi: 10.1186/1471-2393-12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tian M.L., Du L.Y., Ma G.J., et al. Secular increase in the prevalence of gestational diabetes and its associated adverse pregnancy outcomes from 2014 to 2021 in Hebei province, China. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.1039051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barrett H.L., Dekker Nitert M., McIntyre H.D., Callaway L.K. Normalizing metabolism in diabetic pregnancy: is it time to target lipids? Diabetes Care. 2014;37(5):1484–1493. doi: 10.2337/dc13-1934. [DOI] [PubMed] [Google Scholar]
- 27.Mortier I., Blanc J., Tosello B., Gire C., Bretelle F., Carcopino X. Is gestational diabetes an independent risk factor of neonatal severe respiratory distress syndrome after 34 weeks of gestation? A prospective study. Arch Gynecol Obstet. 2017;296(6):1071–1077. doi: 10.1007/s00404-017-4505-7. [DOI] [PubMed] [Google Scholar]
- 28.Anthony N., Ahmad A., Bibi C., et al. Feto-maternal outcomes and treatment compliance in metformin versus insulin-treated gestational diabetic and non-diabetic patients at the Rehman Medical Institute, Peshawar. Cureus. 2021;13(8) doi: 10.7759/cureus.17424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guo M., Lu J., Yu X., Hu X., Hou W., Pang S. The protective role of serum uric acid against premature membrane rupture in gestational diabetes: a cross-sectional study. BMC Endocr Disord. 2021;21(1):95. doi: 10.1186/s12902-021-00736-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratnasiri A.W.G., Lee H.C., Lakshminrusimha S., et al. Trends in maternal prepregnancy body mass index (BMI) and its association with birth and maternal outcomes in California, 2007-2016: a retrospective cohort study. PLoS One. 2019;14(9) doi: 10.1371/journal.pone.0222458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linder T., Eder A., Monod C., et al. Impact of prepregnancy overweight and obesity on treatment modality and pregnancy outcome in women with gestational diabetes mellitus. Front Endocrinol. 2022;13 doi: 10.3389/fendo.2022.799625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zou J.J., Wei Q., Shi Y.Y., Wang K., Zhang Y.H., Shi H.J. Longitudinal associations between maternal glucose levels and ultrasonographic fetal biometrics in a Shanghai cohort. JAMA Netw Open. 2022;5(4) doi: 10.1001/jamanetworkopen.2022.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nteeba J., Varberg K.M., Scott R.L., Simon M.E., Iqbal K., Soares M.J. Poorly controlled diabetes mellitus alters placental structure, efficiency, and plasticity. BMJ Open Diabetes Res Care. 2020;8(1) doi: 10.1136/bmjdrc-2020-001243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reichetzeder C., Dwi Putra S.E., Pfab T., et al. Increased global placental DNA methylation levels are associated with gestational diabetes. Clin Epigenet. 2016;8:82. doi: 10.1186/s13148-016-0247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding G.L., Wang F.F., Shu J., et al. Transgenerational glucose intolerance with Igf2/H19 epigenetic alterations in mouse islet induced by intrauterine hyperglycemia. Diabetes. 2012;61(5):1133–1142. doi: 10.2337/db11-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.