Summary
The current prevention efforts for STIs, HIV and viral hepatitis in the WHO European Region, especially in the Central and Eastern subregions, are hindered by healthcare disparities, data gaps, and limited resources. In this comprehensive narrative review, we aim to highlight both achievements and persisting challenges while also exploring new developments that could significantly impact the prevention of these infections in the near future. While pre-exposure prophylaxis (PrEP) for HIV has been broadly approved and implemented in 38 out of 53 countries in the region, challenges remain, including cost, limited licensing, and incomplete adherence. We explore innovative approaches like on-demand PrEP, long-acting injectable cabotegravir, and intravaginal rings that have shown promising results, alongside the use of six-monthly lenacapavir, the outcomes of which are pending. Additionally, the potential of doxycycline post-exposure prophylaxis has been discussed, revealing efficacy in reducing chlamydia and syphilis risk, but effectiveness against gonorrhoea being contingent on tetracycline resistance rates, and the need of further data to determine potential resistance development in other bacteria and its impact on the gut microbiome. We examine successful vaccination campaigns against HBV and HPV, the ongoing development of vaccines for chlamydia, syphilis, herpesvirus, and gonorrhoea, and challenges in HIV vaccine research, including lines of research with significant potential like sequential immunization, T-cell responses, and mRNA technology. This review underscores the research endeavors that pave the way for a more resilient and robust approach to combating STIs, HIV, and viral hepatitis in the region.
Keywords: Pre-exposure prophylaxis, Post-exposure prophylaxis, Prevention vaccine, HIV, Gonorrhea, Syphilis, Chlamydia, Hepatitis B or C, HPV, sexually transmitted infections
Key messages.
Overview of HIV and STI prevention
-
•
In Europe, although HIV combination prevention has reduced transmission, the region falls short of the UNAIDS 90-90-90 goals for 2020, with late HIV diagnosis remaining a challenge in all sub-regions, and is likely to experience a major challenge in reaching the 95-95-95 for 2025.
-
•
Healthcare disparities, data gaps, and limited resources hinder prevention efforts in Europe, especially in Central and Eastern subregions, limiting the potential of strategies such as combination prevention or pre-exposure prophylaxis (PrEP) for HIV.
Successful interventions
-
•
Successful implementation of PrEP within the context of combination prevention approach resulted in significant reductions in new HIV diagnoses in France and the United Kingdom.
-
•
Ukraine has achieved significant progress in HIV prevention through strong government and community support which has led to a reduction in new infections, although progress is threatened by the ongoing war with Russia.
-
•
The Netherlands implemented a comprehensive action plan for sexual health, and Sweden's chlamydia action plan with a change in the testing strategy resulted in a decline in reported infections.
PrEP and PEP for HIV
-
•
The evidence showing the effectiveness of daily and on-demand PrEP with emtricitabine and tenofovir disoproxil fumarate (TDF-FTC) resulted in broad approval, implementation, and a reduction in HIV incidence. In 2022, it was rolled out in 38/55 countries in the WHO European region, with 130,000 people receiving PrEP prescriptions.
-
•
PrEP availability and implementation face regional disparities and inequalities, especially in Central and Eastern Europe, due to challenges such as cost, limited licensing and lack of national guidelines, incomplete adherence, and low self-perceived risk levels.
-
•
Innovative strategies such as long-acting cabotegravir injectables (every 2 months), have shown superior efficacy compared to daily TDF/FTC. Other new alternatives include dapivirine intravaginal rings, with reported lower efficacy compared to TDF/FTC, and lenacapavir subcutaneous injection (every 6 months) with pending results of ongoing trials.
-
•
Post-exposure prophylaxis for HIV has been available since 1997 in most European countries for occupational and non-occupational exposure, but barriers include cost, awareness, side effects, and stigma, especially for non-occupational exposure.
PrEP and PEP with doxycycline for bacterial STIs
-
•
Doxycycline post-exposure prophylaxis (PEP) reduces chlamydia and syphilis risk in men who have sex with men, but effectiveness against gonorrhea depends on tetracycline resistance rates in the population. Concerns include potential resistance development in other bacteria and impact on the gut microbiome.
-
•
Ongoing trials assess doxycycline PEP efficacy and safety for other key populations, including women, and therefore addressing equity concerns.
Vaccines
-
•
Hepatitis B virus vaccination effectively prevents transmission, cirrhosis, and liver cancer, with high coverage in Europe.
-
•
Human papillomavirus (HPV) vaccines effectively prevent cervical lesions and anogenital warts, and are included in most country vaccination programs, except for some Central and Eastern European countries.
-
•
Ongoing vaccine development targets chlamydia infection, syphilis, herpesvirus, and gonorrhoea. Chlamydia and syphilis vaccines are in the early phase of development. Prophylactic and therapeutic candidates for herpesvirus, and the efficacy of serogroup B meningococcal vaccines against gonorrhoea are currently being investigated in clinical trials.
-
•
The genetic diversity and immune evasion mechanisms of HIV hamper vaccine development, with 7 out of 8 trials failing to show protective effects, while the RV144 trial demonstrated reduced HIV acquisition. Current research focuses on sequential immunization, T-cell help/responses and use of mRNA technology.
Introduction
The transmission of HIV and sexually transmitted infections (STIs) are a pressing health issue in the European Region of the World Health Organization (WHO), which includes 53 countries in 3 sub-regions (Fig. 1).1,2 Although reported cases of newly diagnosed HIV have declined by 24% in 2021 compared to 2019, there are variations across sub-regions. The highest numbers and incidence are in the East (78% of the overall number and 32.4 per 100,000 population, Fig. 1), while the lowest numbers but the fastest increase (41% increase in 2021 compared to 2020) are observed in the Centre sub-region.1 Late HIV diagnosis, which is associated with poor outcomes such as severe disease and death, and an increased risk of ongoing transmission remains a challenge with 54% of people living with HIV being diagnosed late in Europe in 2021.1 By 2021, the overall performance of the WHO European region for the global 90-90-90 targets set by The Joint United Nations Programme on HIV/AIDS (UNAIDS) for 2020 was 82-85-92, which falls short of the overall target of 73% of all people living with HIV being virally suppressed. There were significant differences between sub-regions and lower rates in the East and Centre sub-regions compared to the West.3 Significant gaps in each pillar of the cascade such as the low testing rates reported for several key populations, and the challenges in accessing antiretroviral treatment and HIV care among specific groups such as mobile populations, people who inject drugs, incarcerated people, and people living in conflict regions, coupled with ongoing increase in new diagnoses in the Centre, and the persistently high rate of late presentations, suggest that Europe may experience a major challenge in reaching the 95-95-95 for 2025.3
Fig. 1.
Geographical areas, HIV incidence, and PrEP initiation in the WHO European Region. Legend: A. WHO European Region in accordance with the WHO HIV/AIDS surveillance report, which includes 53 countries categorized by sub-regions. The following list includes European Union (EU) members (marked with an asterisk) and non-EU countries. Western Europe (23 countries): Andorra, Austria∗, Belgium∗, Denmark∗, Finland∗, France∗, Germany∗, Greece∗, Iceland, Ireland∗, Israel, Italy∗, Luxembourg∗,Malta∗, Monaco, Netherlands∗, Norway, Portugal∗, San Marino, Spain∗, Sweden∗, Switzerland, and United Kingdom. Central Europe (15 countries): Albania, Bosnia and Herzegovina, Bulgaria∗, Croatia∗, Cyprus∗, the Czech Republic∗, Hungary∗, the former Yugoslav Republic (FYR) of Macedonia, Montenegro, Poland∗, Romania∗, Serbia, Slovak Republic∗, Slovenia∗, and Turkey. Eastern Europe (15 countries): Armenia, Azerbaijan, Belarus, Estonia∗, Georgia, Kazakhstan, Kyrgyz Republic, Latvia∗, Lithuania∗, Moldova, Russian Federation, Tajikistan, Turkmenistan, Ukraine, and Uzbekistan. Data from: European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe 2009. Stockholm:European Centre for Disease Prevention and Control; 2010. B. HIV incidence in Europe in the year 2021. Incidence expressed as population per 100,000 persons-year. Data from: European Centre for Disease Prevention and Control/WHO Regional Office for Europe. HIV/AIDS surveillance in Europe. 2021–2020 data. Stockholm: ECDC; 2021. C. Cumulative numbers of PrEP initiation in European countries up to Q2 2023. Data from: https://data.prepwatch.org/.
Bacterial STIs, including Chlamydia trachomatis (chlamydia), Treponema pallidum (syphilis), Neisseria gonorrhoeae (gonorrhea) infections, and lymphogranuloma venereum (LGV), have shown a rising trend since the 1990s, and increased significantly by 9%, 25%, 55%, and 75%, respectively, between 2015 and 2019 in the European Union (EU)/European Economic Area (EEA).4 LGV is largely underdiagnosed in Europe due to the lack of appropriate diagnostic tools, particularly in countries in the WHO Central and Eastern Europe (CEE) regions.5 Other STIs, including Mycoplasma genitalium and Trichomonas vaginalis, as well as emerging and re-emerging infectious diseases such as mpox, are becoming increasingly problematic in Europe. Data on STIs is very limited in non-EU regions of Europe and mostly in countries in CEE due to the heterogeneity in surveillance systems, underdiagnosis, and underreporting, lack of access to testing and treatment by key populations mentioned above who are most vulnerable to and disproportionately affected by STIs, and unavailability of modern testing technologies.4,6 This surge in STIs has significantly impacted sexual and reproductive health laying a heavy burden on national health systems.4,6
HIV prevention is based on a combination approach that includes health promotion interventions such as condoms, needle and syringe programs (NSP), opioid substitution therapy (OST), sex education, and national HIV strategies/guidelines, as well as HIV testing, antiretroviral treatment (ART), and pre-exposure prophylaxis (PrEP), all of which can be delivered through the healthcare settings or through community-based organisations and should be tailored to the risk profile of the recipient.7 While overall, 42/52 European countries could provide enough indicators of combination prevention, only five were categorised as having evidence of combination prevention implementation, 15 as having some evidence and 22 as having partial evidence.7 In recent years, HIV prevention strategies in Europe have been strengthened contributing to a reduction on the acquisition risk at the population level.7, 8, 9 UK reported that the number of new HIV diagnoses dropped by 39% in men who have sex with men and 24% in heterosexual men between 2015 and 2018.8 However, STI prevention to date has mainly focused on improving diagnostic capacity, treatment access, and quality of care, but with significant disparities between countries, and only 23/52 European countries, the majority being EU members reported on STI testing coverage as an indicator of combination prevention.7,10 Over the years, prevention interventions for STIs have faced numerous challenges, including limited testing opportunities, lack of effective treatment options for eliminating several viral STIs, inadequate condom use, and the increasing rates of antibiotic resistance to several bacterial pathogens. Moreover, testing and treatment coverage varies significantly between regions, countries and key populations resulting in inequitable access to preventive tools among key populations and among different regions, mainly CEE.
The UNAIDS and the Global Health Sector Strategies of the WHO on HIV, viral hepatitis, and STIs have set goals to be achieved by 2030 in Europe.2,11 However, the implementation of these strategies remains challenging for several countries. First, economic, cultural, social, and political diversity between countries leads to significant inequalities.3,12 Second, monitoring prevention strategies necessitates good quality epidemiological data and reporting, which is heterogeneous and inadequate or lacking in specific European regions, mainly CEE.13 Third, coverage of sexual health services also varies due to differences in healthcare systems across countries.4,13 Fourth, risk-based eligibility criteria for prevention interventions need careful consideration and assessment at the regional level. Fifth, barriers such as costs, limited public funding and reimbursement, adherence, and demand exceeding clinical capacity hinder the full potential of PrEP for HIV.12,14,15 Sixth, effective vaccines for the primary prevention of HIV and bacterial STIs are not yet available. Finally, antimicrobial resistance and emerging STIs also pose new challenges to prevention and control efforts.16 In addition, the 2022 mpox outbreak has represented a public health emergency of international concern, characterized by an unprecedented global spread of the infection in previously non-endemic countries, with transmission predominantly being through sexual contact among men who have sex with men, disproportionally affecting people living with HIV,17,18 and severe presentations in people with low CD4 counts.19 This recent outbreak highlights the global relevance of emerging and re-emerging infectious diseases with potential novel epidemiological and clinical characteristics, including new modes of transmission, as well as the importance of increasing surveillance and monitoring, strengthening prevention strategies, and investing in research of effective vaccines and broad-spectrum therapies.
The aim of this narrative review is to provide an overview of the current status of HIV and STI prevention in Europe, highlight successful interventions and case studies, identify existing gaps, and challenges, and explore new developments in the field that could significantly impact the prevention of new HIV and STI infections. This paper focuses on addressing the elements of HIV combination prevention, PrEP and PEP for HIV, PrEP and PEP with doxycycline for bacterial STIs, and vaccines for HIV and several STIs, and does not review the mechanism through which interventions are delivered. Other articles in the series cover additional topics, such as the epidemiology of STIs and inequalities in access to sexual health services in the article on epidemiology,20 the mitigation of antimicrobial resistance in M. genitalium and N. gonorrhoeae in the article on treatment,21 and the screening for T. vaginalis and C. trachomatis in the article on asymptomatic screening.22
Search strategy and selection criteria.
This is a non-systematic narrative review. We searched the PubMed for studies reporting PEP, PrEP and vaccines for HIV and STIs. Keywords used in the database search were (‘pre-exposure prophylaxis’ OR ‘PrEP’) OR (‘post-exposure prophylaxis’ OR ‘PEP’) OR (‘prevention’) OR (‘vaccine’ OR ‘vaccines’) AND (‘HIV’ OR ‘human immunodeficiency virus’ OR ‘gonorrhea’ OR ‘syphilis’ OR ‘herpes simplex virus’ OR ‘HSV’ OR ‘STI’ OR ‘STIs’ OR ‘sexually transmitted infections’ OR ‘hepatitis B’ OR ‘HBV’ OR ‘human papillomavirus’ OR ‘HPV’). The publication year was limited to the latest fifteen years (from 6th February 2008 to 6th February 2023). Hand searching from the reference lists of the retrieved articles was also included and individual screenings were run for specific topics as required. The final reference list was generated on the basis of originality and relevance to the broad scope of this review. Only the articles published in English were included.
Key areas of successful interventions and case studies
Since 2015, significant progress has been made in prevention strategies for STIs including HIV and hepatitis in the WHO European region23,24 with increased treatment coverage rates for HIV and HCV, and a higher rate of three-dose hepatitis B vaccination, with several countries already reaching the regional hepatitis B control targets.25 In addition, efforts to achieve or maintain the elimination of vertical transmission (EVT) for HIV and syphilis have resulted in the official confirmation by the WHO of Belarus for EVT of both HIV and syphilis, and the confirmation of Armenia and the Republic of Moldova for EVT of HIV and syphilis, respectively.26
France became the first country to roll out PrEP in Europe in January 2016, following the successful ANRS IPERGAY study which had started in 2012.27,28 Real-world data suggests a high adherence to HIV testing rates of over 81% among PrEP users, and a much lower estimated HIV incidence rate compared to non-users (incidence of 0.12 per 100 person-years compared to 1–2 per 100 person-years, respectively).29 In the United Kingdom (UK), the PROUD PrEP trial for men who have sex with men also started in 2012 and demonstrated the effectiveness of PrEP.30 The implementation of a combination prevention approach led to a 32% reduction in new HIV diagnoses among men who have sex with men attending selected sexual health clinics since 2013, compared to the previous year.31 The UK reported a 53% decline in new HIV diagnoses between 2015 and 2021, while France reported a 20% decline between 2019 and 2021.32, 33, 34, 35, 36
Ukraine achieved significant progress in HIV prevention before the conflict with Russia, with full support from the government and the community, resulting in around 70% coverage of harm reduction programs and a 21% reduction in new infections over the past decade.37,38 However, Russia's war in Ukraine since 2022 has severely impacted HIV services and jeopardized the progress made.38
In terms of STI prevention interventions in Europe, 15 out of 29 EU member countries had a national STI strategy in 2018, representing a 12% increase compared to 2012.10 The Netherlands developed a national action plan for STI, HIV, and sexual health for the period 2018–2022. The plan was based on the consideration that sexuality is a positive human experience and had two main goals, ensuring citizens have adequate knowledge and make informed choices for safe and pleasing sex and providing access to appropriate and affordable healthcare, advice, support, and protection for sexual health.10,39
In 2006, Sweden developed a strategy to limit the transmission of HIV infection, other blood-borne infections and other STIs for the period 2006–2016.40 The strategy included a universal surveillance system for chlamydia, with free testing, counselling, treatment, and partner notification as well as raising awareness on condom use and improving access to testing among young people, and improving sexual education in schools, which targeted to reduce the prevalence of C. trachomatis infection by 2014.41 A new mutant variant generating false negative test results caused a sharp increase in chlamydia in 2007,42 leading to the development of a chlamydia action plan for 2009–2014. This resulted in a decline in reported chlamydia infections and reduced the frequency of the new variant43 being an excellent example of an effective response.
Despite the progress made, additional efforts are necessary to achieve the UNAIDS and WHO targets for HIV and STI. A WHO survey revealed that in 2019 more than 60% of reporting countries globally had a national STI strategy with the European region having the lowest rate at 37%. Elimination of vertical transmission strategies for HIV and syphilis was reported by 70% of countries globally with lower rates (48%) in Europe compared to other regions. However, Europe had a higher rate of HIV surveillance and the highest rate (82%) of N. gonorrhoeae antimicrobial resistance surveillance.44
PrEP and PEP for HIV
A sustained focus on HIV prevention is required to reach the Sustainable Development Goals (SDGs) by 2030.45 PrEP and PEP are two key elements of the combination prevention strategy for HIV.7
Current strategies and regional uptake
The efficacy and safety of daily oral PrEP with emtricitabine (FTC) and tenofovir disoproxil fumarate (TDF) for HIV prevention were established over a decade ago. However, it was not until 2015, following the results of two European studies that the full potential of PrEP was recognized leading to its broad approval and use with effectiveness as high as 97% when taken daily or on demand.27,30,46 The WHO has recommended both daily and on-demand/event-driven PrEP with TDF-FTC since 2019.24,47,48 Cisgender men, and trans and gender diverse people assigned male at birth, who are not taking exogenous estradiol-based hormones, may be offered oral on demand PrEP or daily oral PrEP as options. Cisgender women, trans and gender diverse people assigned female at birth, people with potential exposure through injecting practices, and people taking exogenous estradiol-based hormones may use daily oral PrEP.
In 2022, PrEP was rolled out through the healthcare system in 38 out of 55 countries in the WHO European region, with approximately 130,000 people receiving a PrEP prescription that year.15 Targeted and high-coverage PrEP implementation has led to a significant reduction in HIV incidence at the population level (Fig. 1B and C).8,48
PEP for HIV has been widely accepted and used since 1997 when evidence emerged supporting its effectiveness. A case–control study demonstrated an 81% reduction in the risk of HIV seroconversion when zidovudine was administered after needlestick exposure.49 While limited evidence exists for PEP after sexual exposure or injection-drug use, its safety and feasibility have contributed to its widespread use and resulted in the recommendation of PEP in European guidelines for occupational and non-occupational exposure to HIV.50 PEP availability is very high in most European countries, with the CEE reporting a 95.5% and 86.4% availability for both occupational and non-occupational exposure, respectively.12
Challenges
Despite the increasing number of European countries where PrEP is licensed, its utilization remains low. There are substantial inequalities and regional disparities in availability and implementation within Europe, such as much delayed introduction in CEE countries (Fig. 1C), lower access among people who inject drugs, migrant populations and women, and hesitancy of healthcare providers to offer PrEP.12,15 As a result, overall coverage for people who are at the highest acquisition risk remains low. The 2017 European men who have sex with men Internet Survey (EMIS) revealed that 32% of participants had never heard of PrEP. In France, only 28% of men who have sex with men reported the use of PrEP for their last condomless anal intercourse with a casual partner.51 A study in 2019 showed a significant gap of PrEP use in the Europe, where more than 500,000 (range 420.000–610.000) men who have sex with men who wanted and needed PrEP were not accessing it (overall gap 17.4%), with the largest gap for non-EU and/or CEE countries with rates up to 45%.52 Barriers to scaling up PrEP in Europe include the cost of the drug itself and the associated costs of delivering PrEP in healthcare settings, lack of licensing and coverage, limited technical expertise, lack of national guidelines, incomplete adherence, and low levels of awareness and self-perceived risk.12,53
While most European countries fully reimburse occupational PEP (77.3% in CEE), only a few countries in Europe provide full reimbursement for non-occupational PEP (36.4% in CEE). The lack of cost coverage, limited awareness among healthcare workers and key populations, concerns about side effects, and stigma remain significant barriers to PEP uptake in Europe.12
New PrEP regimens
Medication adherence is a key determinant of PrEP efficacy and presents particular challenges in daily dosing regimens for some of the key populations, such as cisgender and transgender women, young individuals, racially and ethnically minoritized communities, and people who inject drugs.46 To address adherence challenges and offer more appealing regimens to all at-risk profiles, new PrEP agents are being developed, including novel oral agents, long-acting injectables, broadly neutralizing monoclonal antibodies (bNAbs), topical products, and new drug delivery systems like implants and transdermal devices.
One alternative to daily and on-demand PrEP is a daily combination of tenofovir alafenamide and FTC (TAF/FTC), which has demonstrated non-inferiority to TDF/FTC with an improvement in biomarkers of nephrotoxicity and bone mineral density loss.54
Long-acting agents and extended-release formulations show promise as novel PrEP options. The long-acting injectable cabotegravir (CAB-LA) is the first injectable ART approved for HIV PrEP in the United States (US) and several African countries.55 CAB-LA has demonstrated safety and superior effectiveness compared to daily TDF-FTC for preventing HIV infection among men who have sex with men, trans-gender, and cis-gender women when administered as a single 600-mg intramuscular injection every 2 months.56,57 While implementation challenges remain, CAB-LA is expected to increase PrEP uptake, adherence, and retention, and reduce stigma58 and is currently being evaluated by the European Medicines Agency. Lenacapavir (LEN), another long-acting antiretroviral drug, from the new class of HIV capsid inhibitors, has been approved for the treatment of multi-drug resistant HIV-1 infection. It is also being assessed as an injectable PrEP agent, in phase III trials in men who have sex with men, trans- and cis-gender women, and people who inject drugs.59 Long-acting LEN can be self-administered subcutaneously every 6 months and holds great potential for PrEP pending the results of effectiveness trials.
Long-acting bNAbs are another promising approach to preventing HIV acquisition. Two advanced and several early-stage clinical trials assessing bNAbs are underway.60
Topical agents or microbicides are other alternatives proposed as PrEP agents. Following mixed results from clinical trials with tenofovir vaginal gel, new topical products are under development, including vaginal rings, vaginal and rectal gels, and films.61 In 2021, the WHO recommended a monthly dapivirine intravaginal ring for HIV prevention in cis-gender women, based on the results of two phase-III randomized placebo-controlled clinical trials showing a 27% and 35% relative reduction in HIV incidence.62,63
Finally, innovative alternatives for PrEP include new drug delivery systems like subdermal implants and transdermal devices that could provide continuous protection from HIV over an extended period. Additionally, multipurpose prevention technologies are developed to offer combined prevention for HIV and other STIs, with or without contraception. Several of these products are at the early stages of development and/or entering early clinical trials.
Although these new PrEP agents have shown promise, some factors need to be considered before they can be routinely used. Some products may be associated with delays in HIV diagnosis and the emergence of HIV drug resistance. CAB-LA substantially alters the timing and patterns of HIV seroconversion, and although breakthrough infection is rare, diagnosis with antigen/antibody-based HIV tests can be delayed.58 Moreover, cost and logistic challenges, such as higher drug cost, the requirement for additional staff time for intramuscular injections, and more frequent laboratory monitoring, can limit implementation and accessibility in low- and middle-income countries.
PrEP and PEP with doxycycline for STIs
The use of doxycycline as prophylaxis (doxy-PEP and doxy-PrEP) for bacterial STIs is a relatively new concept with a historical background and has been assessed in studies involving individuals with high-risk sexual behavior. Doxycycline is generally well tolerated and approved for long-term use in acne treatment or primary prophylaxis against infectious diseases like malaria.64 In a pilot trial involving 30 men who have sex with men with HIV and a history of syphilis, daily PrEP with 100 mg of doxycycline decreased the risk of acquiring bacterial STIs, including T. pallidum, C. trachomatis or N. gonorrhoeae infections, at week 48.65
Doxy-PEP in Europe was first used in the ANRS IPERGAY sub-study in France where 232 men who have sex with men on HIV PrEP were randomized in an open-label study to receive 200 mg of doxy-PEP 24–72 hours after sexual contact and a control group without PEP.66 The doxy-PEP group showed a significant reduction in the risk of acquiring syphilis or chlamydia compared to the control group, but not for gonorrhea.66 A US randomized trial assessing the same dosing regimen in 501 men who have sex with men on HIV PrEP or people living with HIV with a history of bacterial STI reported a significant reduction in all three bacterial STIs, including gonorrhea.67 This difference in effectiveness may be attributed to the higher rate of tetracycline resistance of N. gonorrhoeae in France compared to the US.67 Finally, the preliminary results of the DOXYVAC trial, which was presented very recently showed a strong reduction (70–80%) of chlamydia and syphilis incidence among 502 participants with a median follow up of 9 months.68 While the data supporting the use of doxy-PEP for the prevention of bacterial STIs among men who have sex with men with the highest risk of STIs is promising and based on three large-scale, well-conducted randomized clinical trials, longer-term monitoring is still needed to weigh the risks and benefits. On the other hand, evidence on the efficacy of doxy-PrEP is very limited, based on a single small-scale study.
To date, no cases of resistance to doxycycline have been reported in C. trachomatis or T. pallidum,69 although these organisms are not routinely tested for antimicrobial resistance. Resistance has been reported to occur with high selective pressure on Chlamydia suis in pigs. T. pallidum was able to develop high-level resistance to azithromycin in humans by means of a single point mutation on the 23S rRNA gene, and a point mutation in the 16S rRNA gene is a plausible mechanism for the development of doxycycline resistance.70 Antibiotic resistance remains a major concern for N. gonorrhoeae and M. genitalium. The increase in doxycycline resistance rates after exposure in N. gonorrhoeae isolates is well-established. A study on PEP found a higher doxycycline resistance in N. gonorrhoeae isolates in the doxycycline group compared to the standard care group, but resistance testing in the cohort was limited; moreover, the number of doxycycline-resistant Staphylococcus aureus isolates increased.67 On the other hand, doxycycline plays a role in the treatment of M. genitalium, for which antimicrobial resistance continues to increase, partly due to the extensive use of azithromycin for STI syndromes. Although doxycycline has limited efficacy for M. genitalium, with microbiological cure rates between 30 and 40%, it has the added benefit of decreasing the bacterial load.71 Thus, current guidelines for the first-line treatment of M. genitalium recommend a 7-day regimen of doxycycline, followed by either an extended regimen of azithromycin or a 7-day regimen of moxifloxacin, for macrolide-susceptible and macrolide-resistant infections, respectively.71 Moreover, there is also a significant concern about the potential negative effect of doxycycline on the gut microbiome.67 A clinical trial is currently evaluating gut microbiota and S. aureus colonization in a cohort of doxycycline PrEP.72 Guidelines exercise caution in endorsing doxy-PEP and recommend its use on a case-by-case basis.73, 74, 75, 76
Studies have shown that individuals with high-risk sexual behavior and frequent STIs, have a strong interest in and willingness to take doxycycline prophylaxis. A US survey among men who have sex with men revealed that almost 70% would be willing to take doxycycline, preferably as a one-time dosing PEP (52%) compared to daily PrEP (40%).77 Users of a gay social networking app have reported significant interest in trying doxy-PEP78 as have participants in a study in Canada.79 However, the actual uptake of doxycycline prophylaxis varies among study cohorts ranging from 2.2% to 9.9%.80, 81, 82, 83, 84
Raising awareness among key populations about the risks and benefits of STI prophylaxis with doxycycline is crucial, and healthcare providers play a critical role in this respect. Currently, less than half of healthcare providers in the US report that they would be willing to prescribe doxycycline, but this percentage would rise to almost 90% upon recommendation by the Centers for Disease Control and Prevention (CDC).77 Studies indicate that the interest in STI prophylaxis is high and some people are already using it off-label.80,83,85
Research on doxy-PEP/PrEP has primarily focused on men who have sex with men and people living with HIV due to their high STI risk and ease of enrollment. However, concerns about equity are raised since targeting only men who have sex with men may not address adverse outcomes that predominantly affect women, such as pelvic inflammatory disease and infertility.64 Potential beneficiaries of STI prophylaxis include military recruits and officers, adolescents, female sex workers, ethnic minorities with limited sexual education, and people already taking HIV PrEP.86,87 Doxycycline's efficacy in these populations and in cisgender women is being assessed in clinical trials. In a preliminary report, doxy-PEP was not effective in cisgender women, which was attributed to anatomical characteristics of the endocervical tissue, low adherence, or resistance.88 A more recent analysis of the same study reported very low doxycycline levels in participants' hair samples (used to asses long term compliance) suggesting that poor adherence to doxycycline might have resulted in failure.89 A computational model suggested a 10% reduction in new syphilis cases over a decade with 20% uptake and 80% adherence among men who have sex with men, but higher doxycycline doses would be needed to prevent STIs in other populations, such as heterosexuals with behaviorally bisexual men serving as a bridge population.90,91
The randomized trials on doxy-PEP provide good evidence for the prevention of the most common bacterial STIs among men who have sex with men with the highest risk of STIs. However, major concerns on the development of antibiotic resistance in sexually transmitted and other pathogens require additional data to define the potential public health risks and benefits to ensure its safe and effective use. Therefore, close monitoring of doxycycline users in this context is critical. Currently, the International Antiviral Society (IAS)-US Guidelines on HIV Treatment and Prevention recommends doxycycline PEP in men who have sex with men on HIV PrEP on a case-to-case basis and the California Department of Public Health has implemented the recommendation for men who have sex with men or transgender women.92,93
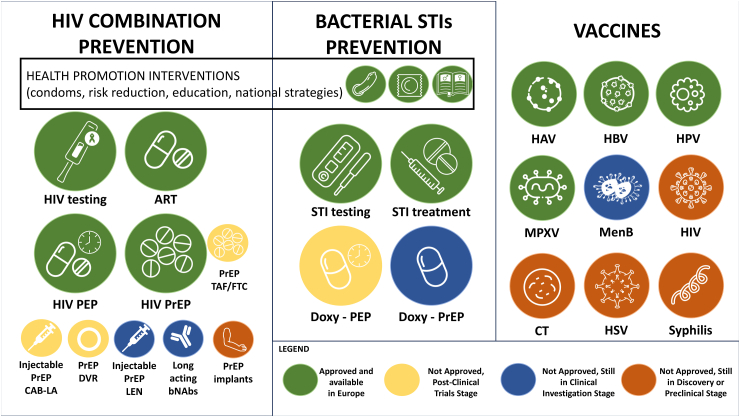
Vaccines
Vaccines are the ultimate game-changers in terms of infection prevention due to their potential to provide long-term and widespread protection in the community. The need for effective vaccines is underscored by the ongoing limitations of current efforts to control STIs which have proven inadequate in effectively reducing incident infections and associated morbidity as discussed in detail earlier. Consequently, developing effective vaccines is pivotal in the response to the HIV epidemic and to other STIs. This section includes working vaccines, which have proven to be highly effective in preventing STIs, and those that are in development and have a promising profile.
Working vaccines
To date, working vaccines for hepatitis A virus (HAV), hepatitis B virus (HBV), and human papillomavirus (HPV) have proved to be successful in decreasing the number of incident infections.
Hepatitis A vaccines have been available since the 1990s with well-documented effectiveness in the long-term and with the global decline in disease burden over the last three decades, they have become a tool to control the infection in endemic areas in specific key populations.94 Men who have sex with men are particularly at risk as large outbreaks within this key population have been reported across all continents.95 According to ECDC data, 22 EU/EEA countries have reported outbreak-confirmed cases in this group and specific HAV strains continue to spread.96,97 The CDC recommends universal vaccination of men who have sex with men with the HAV vaccine given as a 2-dose schedule six months apart.98
The HBV vaccine is highly effective in preventing vertical transmission when given as a full 3- or 4-dose schedule at birth and in early infancy. Vaccination during later childhood and adulthood prevents onward transmission, liver cirrhosis, and hepatocellular carcinoma.99,100 The current reduction in HBV-associated hepatocellular carcinoma rates is attributed to HBV vaccination.101 Global efforts have led to an increase in HBV vaccination coverage among WHO member states, reaching an estimated global coverage of 83% by the end of 2020.100 However, the coverage of birth dose vaccination varies significantly between regions from 6% to 84% with a global average of 42%.100 Overall, the 3-dose vaccination rate among infants is high (91%) in Europe with a more than 10% increase compared to 2009.100 The WHO's target for 2030 is to reduce the incidence of chronic HBV infections by 95% and HBV-associated deaths by 65%.99
The efficacy of HPV vaccination against cervical precancerous lesions is 96%.102, 103, 104 It is also effective in preventing male anogenital warts, and precancerous lesions.102, 103, 104 In countries where female vaccination coverage reached 50%, HPV vaccines were reported to decrease HPV type 16/18 and HPV 31/33/45 infections by 68% and 28%, respectively, among 13–19-year-old girls.105 Anogenital warts among boys aged <20 years decreased by 34% despite the unavailability of HPV vaccination for boys at the time of analysis, which strongly suggested a herd effect.105 Long-term follow-up did not reveal any serious safety signals.102 Despite the proven efficacy and safety of HPV vaccines, their uptake varies globally. By 2019, the majority of European countries had included HPV vaccines in their vaccination programs, initially targeting girls and later expanding the coverage to boys with some exceptions mainly from CEE.106 Governments and healthcare providers have a pivotal role in including HPV vaccination in their countries’ public health strategy and in boosting vaccine uptake. Table 1 includes the technical details of HBV and HPV vaccines and a summary of their efficacy.
Table 1.
Types, immunization schedules, and efficacy of HPV and HBV vaccines.
| Vaccine | Type & immunogen(s) | Adjuvant | Schedule | Efficacy |
|---|---|---|---|---|
| HPV yeast cell derived | ||||
| 2-valent (Cervarix®) | Virus like particle HPV 16 L1 HPV 18 L1 |
aluminum hydroxide, 3-O-deacylated-4′–monophosphoryl lipid A | 0-2-6 | In women: 92.9% against CIN2, 80% against CIN3107 |
| 4-valent (Gardasil®) | Virus like particle HPV 6 L1 HPV 11 L1 HPV 16 L1 HPV 18 L1 |
aluminum hydroxyphosphate sulfate | 0-2-6 | In women: 100%, against HPV 16/18-related CIN 2/3 & AIS(96) |
| 9-valent (Gardasil9®) | Virus like particle HPV 6 L1 HPV 8 L1 HPV 16 L1 HPV 18 L1 HPV 31 L1 HPV 33 L1 HPV 45 L1 HPV 52 L1 HPV 58 L1 |
aluminium hydroxyphosphate sulfate | ages 9–14: 0 & 6–12 months (2-dose) age >15.0 & 2 & 6 months (3-dose) |
Similar efficacy with Gardasil 9 plus, induction of antibodies for 5 additional HPV strains In girls: 98.2% against HPV16/18-related CIN 2/3, AIS, Cervical Cancer 96% against HPV 6/11/16/18 related disease 88.7% against HPV 6/11/16/18 related persistent infection, genital warts, vulvar vaginal lesions In boys: 74.9% against HPV 6/11/16/18 related disease, 100% against penile/perineal/perianal intraepithelial neoplasia 89.3% against genital warts102,108 |
| HBV Recombinant Yeast Derived109 | ||||
| Recombivax® | Virus like particle Small S protein |
aluminium hydroxyphosphate sulfate | 0,1, 6 0,1,2,12 |
|
| Engerix B® | Virus like particle Small S protein |
aluminium hydroxyphosphate | ||
| Heplisav® | Virus Like particle Small S protein |
cytidine phosphoguanosine (CpG) 1018 adjuvant | 0,1 | 95.7% seroprotection vs 79.5% with Engerix B®110 Effective in previous nonresponders110 |
| HBV Recombinant Mammalian Cell Derived | ||||
| Gen Hevac B® | Virus like particle S protein, PreS2 |
aluminum hydroxide | 0,1,6 (103) | |
| Sci-B-Vac® | Virus Like Particle Small S, Pre-S1, Pre S-2 |
aluminum hydroxide | 0,1,6 | Seroprotection against HBV was 91.4% vs 76.5% among EngerixB group Better protection in elderly, can also be considered as therapeutic vaccination, and effective in previous nonresponders111 |
Vaccines in development
Chlamydia trachomatis vaccines
As of the present date, no working vaccine for chlamydia has been presented since the early efforts in the 1960s. Early studies revealed that inactivated whole-cell immunization actually increased susceptibility to subsequent infection, which was attributed to the priming of regulatory T cells instead of effector T cells and tissue-resident memory T cell development.112 However, there have been some promising advancements in vaccine development. For instance, combining UV-inactivated whole bacteria with the TLR7/8 agonist resiquimod and packing into charge-switching synthetic particles, induced protective immunity.112 To induce strong immune responses against chlamydia at mucosal sites may require developing mucosal adjuvants and delivery systems to direct the immune response towards mucosal tissues.113,114 Both IFNγ-producing CD4+ T cells and chlamydia-targeted antibody responses are essential for achieving protective immunity. Certain subunits of C. trachomatis like major outer membrane proteins (MOMP) or polymorphic membrane proteins are promising vaccine candidates. For instance, the VD4 region of MOMP has demonstrated the ability to induce protective immunity in mice and pigs.115,116 In a phase I trial, a combination of CTH522 with CAF01 liposomes as an adjuvant was reported to elicit a strong IgG and cell-mediated IFNγ response and was well tolerated.117 However, chlamydia vaccines are still in the early phase of development.
Syphilis vaccines
Although immunization with 60 doses of inactivated T. pallidum was shown to protect against T. pallidum infection118 in the rabbit model, multiple vaccine prototypes have failed to confer full protection. Factors such as the unavailability of culture, the paucity of outer membrane proteins (OMPs), and the intrinsic difficulties associated with OMP characterization have hindered the development of a syphilis vaccine.118, 119, 120 In addition, T. pallidum employs several immune evasion mechanisms that can impede the development of protective antigen-specific immune responses.121, 122, 123, 124, 125, 126 While there is controversy, a few vaccine candidates such as TprK, TprC, and Tp0751 have shown encouraging results in accelerating lesion healing, reducing bacteria burden, or limiting spirochete dissemination.127,128 Only a limited number of studies have tested multiple recombinant antigens simultaneously129; thus, it is plausible that multi-antigen vaccines can further improve protective outcomes. Advances in bioinformatic tools and in vitro culturing techniques may aid in overcoming the challenges of identifying and characterizing antigens, particularly OMPs.119,130,131 The selected antigens should induce antibodies that promote opsonization and removal of bacteria by innate immune cells, and block bacteria dissemination to distant organs.132 Type I interferon-γ-producing CD4+ T cells have the potential to enhance the phagocytic capability of macrophages,132 contributing to this process. Consequently, surface-exposed antigens (i.e., OMPs) may be good vaccine candidates, as they can induce both opsonic antibodies and CD4+ T cell responses. An ideal syphilis vaccine should target several antigens to prevent T. pallidum infection, bacterial dissemination, disease progression, and/or transplacental invasion while covering bacterial genetic variability and protecting against possible reinfections with similar or different T. pallidum isolates.
Herpesvirus vaccines
No licensed vaccine exists against herpes simplex virus (HSV) infections but several prophylactic or therapeutic vaccine candidates are in development.133, 134, 135 (Table 2) Challenges in herpes vaccination include the absence of widely accepted endpoints for clinical trials, lack of industry interest, limited understanding of immunological correlates of prevention, and the modest effect of previous herpes exposure on vaccine effectiveness.134
Table 2.
Characteristic of developing HSV vaccines.
| Vaccine | Type & immunogen(s) | Adjuvant | Schedule | Efficacy |
|---|---|---|---|---|
| Herpes simplex vaccine | ||||
| GEN-003 | Recombinant insect cell HSV-s glycoprotein D2 + truncated form of infected cell polypeptide 4 |
Matrix M-2 | 0-3-6 weeks | Durable reductions in viral shedding among 60 μg antigen plus 50/75 μg adjuvant recipients135 |
| HerpeVac | Glycoprotein D (gD) | Alum/3-deacylated form of Monophosphoryl Lipid A (MPL) | 0, 1, 6 months | 35% against HSV1 disease133 |
Mpox vaccines
The recent mpox outbreak in humans represents a major global health problem, with a high predominance of men who have sex with men among affected individuals and frequency of genital lesions, implying the likelihood of sexual transmission. From January 1, 2022, to September 8, 2023, a total of 26,087 cases were reported from Europe, but the numbers have since declined which is attributed to combined measures such as timely diagnosis, isolation, partner notification, contact tracing, and vaccination.136,137 Currently, three vaccines containing attenuated strains of the vaccinia virus are available, developed based on the high degree of sequence similarity between orthopoxviruses, and the wide range of antibody response to membrane and structural proteins.138 (Table 3) Although preliminary studies have reported favorable outcomes, robust evidence is still lacking.138,139
Table 3.
| Vaccine | Type | Schedule | Use and efficacy | Recommended populations |
|---|---|---|---|---|
| ACAM2000® (US) | Second generation Replication-competent vaccinia virus |
Single dose | Authorized for use against smallpox. Available for use against mpox by an FDA Expanded Access Investigational New Drug (EA-IND) protocol Level and duration of protective efficacy unknown. |
Mass vaccination of the general population is not recommended Primary prevention: High risk groups including men who have sex with men, people with multiple partners, healthcare providers and laboratory staff at high risk. |
| LC16m18® (Japan) | Third-generation Attenuated, minimally replication-competent vaccinia virus | Single dose | Post-exposure preventive vaccination: Close contacts of cases within four days of exposure |
|
| Imvanex® (Europe) JYNNEOS® (US) Imvamune® (Canada) |
Third-generation Replication-deficient modified vaccinia Ankara virus |
Two doses 28 days apart | Authorized for use against smallpox and mpox. Effectiveness for mpox is 36%–86% for vaccination with a single dose and 66%–89% after two doses. |
∗ACAM2000 is not recommended for people for whom replicating vaccines are contraindicated (such as pregnant women, immunocompromised people, young children) |
HIV vaccines
The genetic diversity of HIV, both within and between individuals, makes it a challenging target for vaccine development. Moreover, its complex immune evasion mechanisms and ability to integrate into host immune cells further augment its capacity to counteract immune responses.140,141 To date, 7 out of 8 vaccine trials have failed to demonstrate a protective effect.141 Only the RV144 trial (CRFAE_01 canarypox/gp120 vaccine) demonstrated a reduction in HIV acquisition and was associated with higher levels of HIV-1 variable loop 2 (V2) antibodies and lower levels of IgA type antibodies binding to the envelope protein.142 In contrast, the HVTN 702 trial in Africa did not show any protective effect, despite participants developing higher anti-gp120 and gp140 antibodies, whereas the RV144 recipients had higher levels of V2-specific antibodies.143 The HVTN706 Mosaico trial was stopped early after an interim analysis that showed no benefit of vaccination with AD26 vector carrying the 4-valent T cell mosaic genes.144
Current research is focused on sequential immunization with antigenically different immunogens and the induction of T-cell help/responses at specific time points.141
The success of mRNA technology in COVID-19 vaccines has encouraged researchers to develop an HIV vaccine using a similar platform. The eOD-GT8 60mer mRNA vaccine (mRNA-1644) has recently been evaluated in a clinical trial (NCT05414786).145
Serogroup B meningococcal vaccine for gonorrhoea prevention
Developing a vaccine for N. gonorrhoeae has been challenging, mainly due to the high antigenic variability of the bacterial surface and a lack of suitable animal models. As of now, there is no effective gonorrhoea-specific vaccine available. However, observational studies have shown that serogroup B meningococcal vaccines, which are used to protect against Neisseria meningitidis infections, provide cross-protection against N. gonorrhoeae. In a retrospective case–control study, exposure to the outer membrane vesicle meningococcal B vaccine MeNZB was associated with a 31% reduction in gonorrhea diagnosis.146 More recently, the broader spectrum four-component serogroup B meningococcal vaccine 4CMenB has shown efficacy ranging from 30 to 46% in preventing gonorrhea in four observational studies, two of them in adolescents and young adults.147, 148, 149, 150 Modelling studies have suggested that even a partially effective vaccine could reduce the population prevalence of gonorrhoea by 40–60% and the incidence by up to 25% over 70 years.151, 152, 153
The efficacy of serogroup B meningococcal vaccine to prevent N. gonorrhoeae infections is currently under investigation in several clinical trials (NCT04597424, NCT04350138, NCT04415424, NCT05766904, NCT05294588).
Conclusions and future perspective
There has been great progress in HIV and STI prevention over the past few years (Fig. 2). The combination approach for HIV prevention contributed to the population-level reduction in transmission, antiretroviral treatment and HIV PrEP coverage have increased in Europe, and successful vaccines for HBV and HPV have led to a reduction in incident infections with rising coverage rates. International bodies have supported countries to improve their national strategies for HIV and STI prevention. However, these strategies have not yet reached their full potential in curtailing the HIV and STI epidemic in Europe. One in five people living with HIV in the European region are still unaware of their status, and 54% are diagnosed late. Although relatively small, an alarming fraction of people living with HIV are not receiving HIV treatment, especially in the Centre and East sub-regions. As a result, a proportion of people living with HIV have not reached viral suppression, thus limiting the effects of ‘treatment as prevention'. At each step of the HIV PrEP care continuum, several barriers persist that hinder the implementation of the new prevention strategies. Moreover, the increasing trend in bacterial STIs is ongoing. Prevention of STIs is a challenging issue considering that there is no consensus on a fully effective strategy to reduce the number of new infections. Antibiotic resistance in N. gonorrhoeae is becoming a major issue jeopardizing treatment success and whether or not the wider use of doxy-PEP will induce resistance in N. gonorrhoeae and M. genitalium isolates in the future is unknown.
Fig. 2.
Prevention strategies for STIs: HIV combination prevention, prevention for bacterial STIs and vaccines. ART: antiretroviral treatment; bNAbs: broadly neutralizing antibodies; CAB-LA: long-acting cabotegravir; CT: Chlamydia trachomatis; Doxy: doxycycline; DVR: dapivirine vaginal ring; HAV: hepatitis A virus; HBV: hepatitis B virus; HIV: human immunodeficiency virus; HPV: human papillomavirus; LEN: lenacapavir; MenB: serogroup B meningococcal vaccine; MPXV: monkeypox virus; PEP: post-exposure prophylaxis; PrEP: pre-exposure prophylaxis; STI: sexually transmitted infection; HSV: herpes simplex virus.
STI prevention including HIV, is an evolving field that accommodates new strategies. Next-generation HIV PrEP agents show promise in terms of uptake, adherence, and persistence. New approaches for STI prevention such as doxy-PrEP and -PEP and the use of meningococcal vaccines are being explored with promising results. Although with no solid evidence yet for high efficacy, studies on vaccines for other STIs are ongoing.
Although we have all the necessary tools for the effective prevention of HIV and STIs, none of the above-mentioned strategies alone will be sufficient to change the game at the regional level. A more ambitious and holistic approach with multilevel interventions is still required to address individual, structural, social, and political barriers. To achieve this goal, we need to “scale up interventions to sufficient levels to have the necessary impact on HIV and STI incidence, considering the technical, social, economic, and political structures of each country and the specific needs of each population. Special attention must be given to awareness and education, expanding the scope of prevention interventions to all eligible individuals, developing and updating national guidelines, and providing easy and free-of-charge access to all prevention tools while eliminating the stigma associated with these infections, and involving the community at all intervention levels. A firm commitment from European countries to HIV and STI prevention and full support from international organizations is the key to reaching global targets in Europe.
Contributors
DG conceptualized the review, developed the methodology, and oversaw the preparation of the manuscript. AA and CB conducted the literature search. DG, TN, AA, CB, GL, AÇ, JC, GS, KK, and JMM wrote the original draft. DG, TN, and JMM contributed to the visualization elements. OM contributed to editing the final document. All authors reviewed and edited the final version and approved the final manuscript.
Editor note
The Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Declaration of interests
Deniz Gökengin attends advisory board meetings for Gilead, GSK, and MSD, and receives grant from GSK. Jean Michel Molina reported receiving grants from Merck for a treatment trial funded by ANRS, payments for serving on advisory boards of ViiV, Gilead, and Merck, expert testimony payments from Merck and a payment from Aelix for participation on an advisory board. Geoffroy Liegeon has received drugs from Gilead Sciences and ViiV Healthcare for two academic-funded PrEP clinical trials.
The remaining authors have nothing to declare.
Acknowledgements
The authors would like to thank Otilia Mardh for her valuable contributions to the text.
References
- 1.ECDC. WHO . 2022. HIV/AIDS surveillance in Europe 2022: 2021 data.https://www.ecdc.europa.eu/sites/default/files/documents/2022-Annual_HIV_Report_final.pdf [Google Scholar]
- 2.Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. https://www.who.int/publications/i/item/9789240053779
- 3.Continuum of HIV care - monitoring implementation of the Dublin Declaration on partnership to fight HIV/AIDS in Europe and central Asia. European Centre for Disease Prevention and Control; 2021. progress report. [DOI] [Google Scholar]
- 4.Geretti A.M., Mardh O., de Vries H.J.C., et al. Sexual transmission of infections across Europe: appraising the present, scoping the future. Sex Transm Infect. 2022 doi: 10.1136/sextrans-2022-055455. [DOI] [PubMed] [Google Scholar]
- 5.Cole M.J., Field N., Pitt R., et al. Substantial underdiagnosis of lymphogranuloma venereum in men who have sex with men in Europe: preliminary findings from a multicentre surveillance pilot. Sex Transm Infect. 2020;96(2):137–142. doi: 10.1136/sextrans-2019-053972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization . World Health Organization; 2021. Global progress report on HIV, viral hepatitis and sexually transmitted infections, 2021: accountability for the global health sector strategies 2016–2021: actions for impact.https://apps.who.int/iris/handle/10665/341412. License: CC BY-NC-SA 3.0 IGO [Google Scholar]
- 7.HIV Combination prevention: monitoring implementation of the Dublin Declaration on partnership to fight HIV/AIDS in Europe and Central Asia (2018 progress report) European Centre for Disease Prevention and Control; 2020. https://www.ecdc.europa.eu/en/publications-data/hiv-combination-prevention-monitoring-implementation-dublin-declaration Available at: [Google Scholar]
- 8.New HIV diagnoses fall by a third in the UK since 2015 - GOV.UK. https://www.gov.uk/government/news/new-hiv-diagnoses-fall-by-a-third-in-the-uk-since-2015
- 9.Desai M., Field N., Grant R., McCormack S. Recent advances in pre-exposure prophylaxis for HIV. BMJ. 2017;359:j5011. doi: 10.1136/bmj.j5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Developing a national strategy for the prevention and control of sexually transmitted infections. European Centre for Disease Prevention and Control; 2019. https://www.ecdc.europa.eu/en/publications-data/developing-national-strategy-prevention-and-control-sexually-transmitted Available at: [Google Scholar]
- 11.UNAIDS. End inequalities. End AIDS. Global AIDS strategy 2021-2026. https://www.aidsdatahub.org/sites/default/files/resource/unaids-global-aids-strategy-2021-2026.pdf
- 12.Gokengin D., Bursa D., Skrzat-Klapaczynska A., et al. PrEP scale-up and PEP in central and eastern Europe: changes in time and the challenges we face with no expected HIV vaccine in the near future. Vaccines (Basel) 2023;11(1):122. doi: 10.3390/vaccines11010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gokengin D., Oprea C., Begovac J., et al. HIV care in Central and Eastern Europe: how close are we to the target? Int J Infect Dis. 2018;70:121–130. doi: 10.1016/j.ijid.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 14.ECDC HIV Pre-exposure prophylaxis in the EU/EEA and the UK: implementation, standards and monitoring operational guidance. https://www.ecdc.europa.eu/sites/default/files/documents/HIV-Pre-Exposure-Prophylaxis-in-the-EU-EEA-UK.pdf Available at:
- 15.ECDC Pre-exposure prophylaxis for HIV prevention in Europe and central Asia. https://www.ecdc.europa.eu/sites/default/files/documents/hiv-infection-prevention-pre-exposure-prophylaxis-dublin-declaration-feb-2023.pdf
- 16.Merrick R., Cole M., Pitt R., et al. Antimicrobial-resistant gonorrhoea: the national public health response, England, 2013 to 2020. Euro Surveill. 2022;27(40) doi: 10.2807/1560-7917.ES.2022.27.40.2200057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitjà O., Ogoina D., Titanji B.K., et al. Monkeypox. Lancet. 2023;401(10370):60–74. doi: 10.1016/S0140-6736(22)02075-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarín-Vicente E.J., Alemany A., Agud-Dios M., Ubals M., et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400(10353):661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mitjà O., Alemany A., Marks M., et al. Mpox in people with advanced HIV infection: a global case series. Lancet. 2023;401(10380):939–949. doi: 10.1016/S0140-6736(23)00273-8. [DOI] [PubMed] [Google Scholar]
- 20.Mitjà O., Padovese V., Folch C., et al. Epidemiology and determinants of reemerging bacterial sexually transmitted infections (STIs) and emerging STIs in Europe. Lancet Reg Health Eur. 2023;34:100742. doi: 10.1016/j.lanepe.2023.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitjà O., Suñer C., Giacani L., et al. Treatment of bacterial sexually transmitted infections in Europe : gonorrhoea, Mycoplasma genitalium, and syphilis. Lancet Reg Health Eur. 2023;34:100737. doi: 10.1016/j.lanepe.2023.100737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kenyon C., Herrmann B., Hughes G., et al. Management of asymptomatic sexually transmitted infections in Europe: towards a differentiated, evidence-based approach. Lancet Reg Health Eur. 2023;34:100743. doi: 10.1016/j.lanepe.2023.100743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Action plan for the health sector response to viral hepatitis in the WHO European Region. WHO Regional Office for Europe; Copenhagen: 2017. https://www.euro.who.int/__data/assets/pdf_file/0008/357236/Hepatitis-9789289052870-eng.pdf Available at: [Google Scholar]
- 24.Action plan for the health sector response to HIV in the WHO European Region. WHO Regional Office for Europe; Copenhagen: 2017. https://apps.who.int/iris/handle/10665/329423 Available at: [Google Scholar]
- 25.More countries reaching hepatitis B control targets brings the WHO European Region closer to eliminating viral hepatitis as a public health threat, WHO. https://www.who.int/europe/news/item/24-04-2023-more-countries-reaching-hepatitis-b-control-targets-brings-the-who-european-region-closer-to-eliminating-viral-hepatitis-as-a-public-health-threat Available at:
- 26.WHO validates countries' elimination of mother-to-child transmission of HIV and syphilis. https://www.who.int/news/item/08-06-2016-who-validates-countries-elimination-of-mother-to-child-transmission-of-hiv-and-syphilis Available at: [PMC free article] [PubMed]
- 27.Molina J.M., Capitant C., Spire B., et al. On-demand preexposure prophylaxis in men at high risk for HIV-1 infection. N Engl J Med. 2015;373(23):2237–2246. doi: 10.1056/NEJMoa1506273. [DOI] [PubMed] [Google Scholar]
- 28.First medicine for HIV pre-exposure prophylaxis recommended for approval in the EU, European Medicines agency science medical health. https://www.ema.europa.eu/en/news/first-medicine-hiv-pre-exposure-prophylaxis-recommended-approval-eu Available at:
- 29.Tassi M.F., Laurent E., Gras G., et al. PrEP monitoring and HIV incidence after PrEP initiation in France: 2016-18 nationwide cohort study. J Antimicrob Chemother. 2021;76(11):3002–3008. doi: 10.1093/jac/dkab263. [DOI] [PubMed] [Google Scholar]
- 30.McCormack S., Dunn D.T., Desai M., et al. Pre-exposure prophylaxis to prevent the acquisition of HIV-1 infection (PROUD): effectiveness results from the pilot phase of a pragmatic open-label randomised trial. Lancet. 2016;387(10013):53–60. doi: 10.1016/S0140-6736(15)00056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown A.E., Mohammed H., Ogaz D., et al. Fall in new HIV diagnoses among men who have sex with men (MSM) at selected London sexual health clinics since early 2015: testing or treatment or pre-exposure prophylaxis (PrEP)? Euro Surveill. 2017;22(25):30553. doi: 10.2807/1560-7917.ES.2017.22.25.30553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Estcourt C., Yeung A., Nandwani R., et al. Population-level effectiveness of a national HIV preexposure prophylaxis programme in MSM. AIDS. 2021;35(4):665–673. doi: 10.1097/QAD.0000000000002790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.HIV testing, PrEP, new HIV diagnoses, and care outcomes for people accessing HIV services: 2022 report - GOV.UK. https://www.gov.uk/government/statistics/hiv-annual-data-tables/hiv-testing-prep-new-hiv-diagnoses-and-care-outcomes-for-people-accessing-hiv-services-2022-report
- 34.Health England P . 2019. HIV in the UK: towards zero HIV transmissions by 2030.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/965765/HIV_in_the_UK_2019_towards_zero_HIV_transmissions_by_2030.pdf [Google Scholar]
- 35.Jourdain H., de Gage S.B., Desplas D., Dray-Spira R. Real-world effectiveness of pre-exposure prophylaxis in men at high risk of HIV infection in France: a nested case-control study. Lancet Public Health. 2022;7(6):e529–e536. doi: 10.1016/S2468-2667(22)00106-2. [DOI] [PubMed] [Google Scholar]
- 36.Bulletin de santé publique VIH/sida. 2019. https://www.santepubliquefrance.fr/maladies-et-traumatismes/infections-sexuellement-transmissibles/vih-sida/documents/bulletin-national/bulletin-de-sante-publique-vih-sida.-octobre-2019 Available at: [Google Scholar]
- 37.Global AIDS monitoring 2019: Ukraine. UNAIDS. https://www.unaids.org/sites/default/files/country/documents/UKR_2020_countryreport.pdf Available at:
- 38.The Lancet H Fate of people with HIV in jeopardy in Ukraine. Lancet HIV. 2022;9(4):e223. doi: 10.1016/S2352-3018(22)00074-1. [DOI] [PubMed] [Google Scholar]
- 39.National Institute for Public Health and the Environment, Netherlands . Bilthoven; Netherlands: 2018. National action plan on STIs, HIV and sexual health 2017–2022.https://www.rivm.nl/bibliotheek/rapporten/2017-0158.pdf Available at: [Google Scholar]
- 40.Regeringskansliet . Regeringskansliet; Stockholm: 2005. Nationell strategi mot hiv/aids och vissa andra smittsamma sjukdomar – prop.2005/06:60.http://www.regeringen.se/rattsdokument/proposition/2005/12/prop.-20050660/ Available at: [Google Scholar]
- 41.Veličko I., Ploner A., Sparén P., Herrmann B., Marions L., Kühlmann-Berenzon S. Changes in the trend of sexually acquired Chlamydia infections in Sweden and the role of testing: a time series analysis. Sex Transm Dis. 2021;48(5):329–334. doi: 10.1097/OLQ.0000000000001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herrmann B., Törner A., Low N., et al. Emergence and spread of Chlamydia trachomatis variant, Sweden. Emerg Infect Dis. 2008;14(9):1462–1465. doi: 10.3201/eid1409.080153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dahlberg J., Hadad R., Elfving K., et al. Ten years transmission of the new variant of Chlamydia trachomatis in Sweden: prevalence of infections and associated complications. Sex Transm Infect. 2018;94(2):100–104. doi: 10.1136/sextrans-2016-052992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor M.M., Wi T., Gerbase A., et al. Assessment of country implementation of the WHO global health sector strategy on sexually transmitted infections (2016-2021) PLoS One. 2022;17(5) doi: 10.1371/journal.pone.0263550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.2025 AIDS targets - UNAIDS. https://aidstargets2025.unaids.org/
- 46.Fonner V.A., Dalglish S.L., Kennedy C.E., et al. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS. 2016;30(12):1973–1983. doi: 10.1097/QAD.0000000000001145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Differentiated and simplified pre-exposure prophylaxis for HIV prevention: update to WHO implementation guidance. Technical brief. World Health Organization; Geneva: 2022. https://www.who.int/publications/i/item/9789240053694 Available at: [Google Scholar]
- 48.Molina J.M., Ghosn J., Assoumou L., et al. Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): a prospective observational cohort study. Lancet HIV. 2022;9(8):e554–e562. doi: 10.1016/S2352-3018(22)00133-3. [DOI] [PubMed] [Google Scholar]
- 49.Cardo D.M., Culver D.H., Ciesielski C.A., et al. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for disease control and prevention needlestick surveillance group. N Engl J Med. 1997;337(21):1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 50.Sultan B., Benn P., Waters L. Current perspectives in HIV post-exposure prophylaxis. HIV AIDS (Auckl) 2014;6:147–158. doi: 10.2147/HIV.S46585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velter A., Ousseine Y., Dupire P., Roux P., Mercier A. Trends in HIV protection methods among HIV-negative men who have sex with men: results from the Rapport au sexe survey 2017-2019-2021, France. Bull Epidemiol Hebd Santé Publique France. 2022;8:430. http://beh.santepubliquefrance.fr/beh/2022/24-25/2022_24-25_0.html Available at: [Google Scholar]
- 52.Hayes R., Schmidt A.J., Pharris A., et al. Estimating the 'PrEP Gap': how implementation and access to PrEP differ between countries in Europe and central asia in 2019. Euro Surveill. 2019;24(41) doi: 10.2807/1560-7917.ES.2019.24.41.1900598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rapid assessment on access to PrEP in EU/EEA countries. https://www.prepwatch.org/resources/rapid-assessment-on-access-to-prep-in-eu-eea-countries/
- 54.Mayer K.H., Molina J.M., Thompson M.A., et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet. 2020;396(10246):239–254. doi: 10.1016/S0140-6736(20)31065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.CDC . A Clinical Practice Guideline; 2021. US public health service: preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update. [Google Scholar]
- 56.Delany-Moretlwe S., Hughes J.P., Bock P., et al. Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. Lancet. 2022;399(10337):1779–1789. doi: 10.1016/S0140-6736(22)00538-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Landovitz R.J., Donnell D., Clement M.E., et al. Cabotegravir for HIV prevention in cisgender men and transgender women. N Engl J Med. 2021;385(7):595–608. doi: 10.1056/NEJMoa2101016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liegeon G., Ghosn J. Long-acting injectable cabotegravir for PrEP: a game-changer in HIV prevention? HIV Med. 2022;24(6):653–663. doi: 10.1111/hiv.13451. [DOI] [PubMed] [Google Scholar]
- 59.Segal-Maurer S., DeJesus E., Stellbrink H.J., et al. Capsid inhibition with lenacapavir in multidrug-resistant HIV-1 infection. N Engl J Med. 2022;386(19):1793–1803. doi: 10.1056/NEJMoa2115542. [DOI] [PubMed] [Google Scholar]
- 60.Long-acting HIV prevention tools | HIV.gov. https://www.hiv.gov/hiv-basics/hiv-prevention/potential-future-options/long-acting-prep/
- 61.Coelho L.E., Torres T.S., Veloso V.G., Landovitz R.J., Grinsztejn B. Pre-exposure prophylaxis 2.0: new drugs and technologies in the pipeline. Lancet HIV. 2019;6(11):e788–e799. doi: 10.1016/S2352-3018(19)30238-3. [DOI] [PubMed] [Google Scholar]
- 62.Nel A., van Niekerk N., Kapiga S., et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016;375(22):2133–2143. doi: 10.1056/NEJMoa1602046. [DOI] [PubMed] [Google Scholar]
- 63.Baeten J.M., Palanee-Phillips T., Brown E.R., et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016;375(22):2121–2132. doi: 10.1056/NEJMoa1506110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grant J.S., Stafylis C., Celum C., et al. Doxycycline prophylaxis for bacterial sexually transmitted infections. Clin Infect Dis. 2020;70(6):1247–1253. doi: 10.1093/cid/ciz866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bolan R.K., Beymer M.R., Weiss R.E., Flynn R.P., Leibowitz A.A., Klausner J.D. Doxycycline prophylaxis to reduce incident syphilis among HIV-infected men who have sex with men who continue to engage in high-risk sex: a randomized, controlled pilot study. Sex Transm Dis. 2015;42(2):98–103. doi: 10.1097/OLQ.0000000000000216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Molina J.M., Charreau I., Chidiac C., et al. Post-exposure prophylaxis with doxycycline to prevent sexually transmitted infections in men who have sex with men: an open-label randomised substudy of the ANRS IPERGAY trial. Lancet Infect Dis. 2018;18(3):308–317. doi: 10.1016/S1473-3099(17)30725-9. [DOI] [PubMed] [Google Scholar]
- 67.Luetkemeyer A.F., Donnell D., Dombrowski J.C., et al. Postexposure doxycycline to prevent bacterial sexually transmitted infections. N Engl J Med. 2023;388(14):1296–1306. doi: 10.1056/NEJMoa2211934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Molina J.M., Bercot B., Assoumou A., et al. 2023. ANRS 174 DOXYVAC: an open-label randomized trial to prevent STIs in MSM on PrEP. 30th CROI, conference on retroviruses and opportunistic infections.https://www.croiconference.org/abstract/anrs-174-doxyvac-an-open-label-randomized-trial-to-prevent-stis-in-msm-on-prep/ Seattle. Abstract 119. [Google Scholar]
- 69.Tantalo L., Lieberman N., Mañá C., et al. Antimicrobial susceptibility of Treponema pallidum subspecies pallidum: an in-vitro study. Lancet Microbe. 2023 doi: 10.1016/S2666-5247(23)00219-7. [DOI] [PubMed] [Google Scholar]
- 70.Lenart J., Andersen A.A., Rockey D.D. Growth and development of tetracycline-resistant Chlamydia suis. Antimicrob Agents Chemother. 2001;45(8):2198–2203. doi: 10.1128/AAC.45.8.2198-2203.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jensen J.S., Cusini M., Gomberg M., Moi H., Wilson J., Unemo M. 2021 European guideline on the management of Mycoplasma genitalium infections. J Eur Acad Dermatol Venereol. 2022;36(5):641–650. doi: 10.1111/jdv.17972. [DOI] [PubMed] [Google Scholar]
- 72.Impact of the daily doxycycline pre-exposure prophylaxis (PrEP) on the incidence of syphilis, gonorrhoea and Chlamydia (syphilaxis) https://clinicaltrials.gov/ct2/show/NCT03709459 Available at:
- 73.UKHSA . 2021. Position statement on doxycycline as prophylaxis for sexually transmitted infections 2021 update.https://www.bashhguidelines.org/position-statements/bashh-position-statement-on-doxycycline-as-prophylaxis-for-sexually-transmitted-infections-2021-update/ Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gandhi R.T., Bedimo R., Hoy J.F., et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the international antiviral society–USA Panel. JAMA. 2023;329(1):63–84. doi: 10.1001/jama.2022.22246. [DOI] [PubMed] [Google Scholar]
- 75.Cornelisse V.J., Ong J.J., Ryder N., et al. Interim position statement on doxycycline post-exposure prophylaxis (Doxy-PEP) for the prevention of bacterial sexually transmissible infections in Australia and Aotearoa New Zealand - the Australasian Society for HIV, Viral Hepatitis and Sexual Health Medicine (ASHM) Sex Health. 2023;20(2):99–104. doi: 10.1071/SH23011. [DOI] [PubMed] [Google Scholar]
- 76.Health SF department of public Health update doxycycline post-exposure prophylaxis reduces incidence of sexually transmitted infections. https://www.sfcityclinic.org/providers/guidelines/hiv-and-sti-prevention Available at:
- 77.Park J.J., Stafylis C., Pearce D.D., et al. Interest, concerns, and attitudes among men who have sex with men and health care providers toward prophylactic use of doxycycline against Chlamydia trachomatis infections and syphilis. Sex Transm Dis. 2021;48(9):615–619. doi: 10.1097/OLQ.0000000000001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spinelli M.A., Scott H.M., Vittinghoff E., Liu A.Y., Coleman K., Buchbinder S.P. High interest in doxycycline for sexually transmitted infection postexposure prophylaxis in a multicity survey of men who have sex with men using a social networking application. Sex Transm Dis. 2019;46(4):e32–e34. doi: 10.1097/OLQ.0000000000000942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fusca L., Hull M., Ross P., et al. High interest in syphilis pre-exposure and post-exposure prophylaxis among gay, bisexual and other men who have sex with men in vancouver and toronto. Sex Transm Dis. 2020;47(4):224–231. doi: 10.1097/OLQ.0000000000001130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carveth-Johnson T., Stingone C., Nwokolo N., Whitlock G. Doxycycline use in MSM taking PrEP. Lancet HIV. 2018;5(9):e482. doi: 10.1016/S2352-3018(18)30210-8. [DOI] [PubMed] [Google Scholar]
- 81.Chow E.P.F., Fairley C.K. Use of doxycycline prophylaxis among gay and bisexual men in Melbourne. Lancet HIV. 2019;6(9):e568–e569. doi: 10.1016/S2352-3018(19)30186-9. [DOI] [PubMed] [Google Scholar]
- 82.Vanbaelen T., Reyniers T., Rotsaert A., et al. Prophylactic use of antibiotics for sexually transmitted infections: awareness and use among HIV PrEP users in Belgium. Sex Transm Infect. 2022;98(8):625. doi: 10.1136/sextrans-2022-055511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Evers Y.J., van Liere G., Dukers-Muijrers N., Hoebe C. Use of doxycycline and other antibiotics to prevent STIs among men who have sex with men visiting sexual health clinics in the Netherlands. Sex Transm Infect. 2020;96(7):550–551. doi: 10.1136/sextrans-2019-054325. [DOI] [PubMed] [Google Scholar]
- 84.Ringshall M., Cooper R., Rawdah W., et al. Chemsex, sexual behaviour and STI-PrEP use among HIV-PrEP users during the COVID-19 pandemic in Brighton, UK. Sex Transm Infect. 2022;98(4):312. doi: 10.1136/sextrans-2021-055216. [DOI] [PubMed] [Google Scholar]
- 85.O'Halloran C., Croxford S., Mohammed H., et al. Factors associated with reporting antibiotic use as STI prophylaxis among HIV PrEP users: findings from a cross-sectional online community survey, May-July 2019, UK. Sex Transm Infect. 2021;97(6):429–433. doi: 10.1136/sextrans-2020-054592. [DOI] [PubMed] [Google Scholar]
- 86.Dubourg G., Raoult D. The challenges of preexposure prophylaxis for bacterial sexually transmitted infections. Clin Microbiol Infect. 2016;22(9):753–756. doi: 10.1016/j.cmi.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 87.Bertens M.G., Eiling E.M., van den Borne B., Schaalma H.P. Uma Tori! Evaluation of an STI/HIV-prevention intervention for afro-caribbean women in the Netherlands. Patient Educ Couns. 2009;75(1):77–83. doi: 10.1016/j.pec.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 88.Stewart J., Oware K., Donnell D., et al. CROI 2023; Seattle: 2023. Doxycycline postexposure prophylaxis for prevention of STIs among cisgender women. [Google Scholar]
- 89.Stewart J., Oware K., Donnell D., et al. STI & HIV 2023 World Congress; Chicago, IL USA: 2023. Adherence to doxycycline PostExposure prophylaxis for STI prevention among cisgender women; pp. 24–27. [Google Scholar]
- 90.Tran N.K., Goldstein N.D., Welles S.L. Countering the rise of syphilis: a role for doxycycline post-exposure prophylaxis? Int J STD AIDS. 2022;33(1):18–30. doi: 10.1177/09564624211042444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fairley C.K., Chow E.P.F. Doxycycline post-exposure prophylaxis: let the debate begin. Lancet Infect Dis. 2018;18(3):233–234. doi: 10.1016/S1473-3099(17)30726-0. [DOI] [PubMed] [Google Scholar]
- 92.Gandhi R.T., Bedimo R., Hoy J.F., et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the international antiviral society–USA Panel. JAMA. 2023;329(1):63–84. doi: 10.1001/jama.2022.22246. [DOI] [PubMed] [Google Scholar]
- 93.California Department of Public Health Doxycycline post-exposure prophylaxis (doxy-PEP) for the prevention of bacterial sexually transmitted infections (STIs) https://www.cdph.ca.gov/Programs/CID/DCDC/CDPH Document Library/CDPH-Doxy-PEP-Recommendations-for-Prevention-of-STIs.pdf Available at:
- 94.Herzog C., Van Herck K., Van Damme P. Hepatitis A vaccination and its immunological and epidemiological long-term effects - a review of the evidence. Hum Vaccin Immunother. 2021;17(5):1496–1519. doi: 10.1080/21645515.2020.1819742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ndumbi P., Freidl G.S., Williams C.J., et al. Hepatitis A outbreak disproportionately affecting men who have sex with men (MSM) in the European Union and European Economic Area, June 2016 to May 2017. Euro Surveill. 2018;23(33) doi: 10.2807/1560-7917.ES.2018.23.33.1700641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Spread of hepatitis a virus strains of genotype IB in several EU countries and the United Kingdom. https://www.ecdc.europa.eu/en/news-events/spread-hepatitis-virus-strains-genotype-ib-several-eu-countries-and-united-kingdom Available at:
- 97.Epidemiological update: hepatitis A outbreak in the EU/EEA mostly affecting men who have sex with men. https://www.ecdc.europa.eu/en/news-events/epidemiological-update-hepatitis-outbreak-eueea-mostly-affecting-men-who-have-sex-men-1 Available at:
- 98.Centers for Diseasae Control and Prevention Adult immunization schedule by age. https://www.cdc.gov/vaccines/schedules/hcp/imz/adult.html Available at:
- 99.Regional Committee for Europe, 72nd Session . World Health Organization. Regional Office for Europe; 2022. Seventy-second regional committee for Europe: tel Aviv, 12–14 September 2022: regional action plans for ending AIDS and the epidemics of viral hepatitis and sexually transmitted infections 2022–2030.https://apps.who.int/iris/handle/10665/361524 Available at: [Google Scholar]
- 100.Flores J.E., Thompson A.J., Ryan M., Howell J. The global impact of hepatitis B vaccination on hepatocellular carcinoma. Vaccines (Basel) 2022;10(5):793. doi: 10.3390/vaccines10050793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dasgupta P., Henshaw C., Youlden D.R., Clark P.J., Aitken J.F., Baade P.D. Global trends in incidence rates of primary adult liver cancers: a systematic review and meta-analysis. Front Oncol. 2020;10:171. doi: 10.3389/fonc.2020.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Markowitz L.E., Unger E.R. Human papillomavirus vaccination. N Engl J Med. 2023;388(19):1790–1798. doi: 10.1056/NEJMcp2108502. [DOI] [PubMed] [Google Scholar]
- 103.Paavonen J., Naud P., Salmerón J., et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374(9686):301–314. doi: 10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 104.Giuliano A.R., Palefsky J.M., Goldstone S., et al. Efficacy of quadrivalent HPV vaccine against HPV Infection and disease in males. N Engl J Med. 2011;364(5):401–411. doi: 10.1056/NEJMoa0909537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Palefsky J.M., Giuliano A.R., Goldstone S., et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med. 2011;365(17):1576–1585. doi: 10.1056/NEJMoa1010971. [DOI] [PubMed] [Google Scholar]
- 106.Spayne J., Hesketh T. Estimate of global human papillomavirus vaccination coverage: analysis of country-level indicators. BMJ Open. 2021;11(9) doi: 10.1136/bmjopen-2021-052016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Qiao Y.L., Wu T., Li R.C., et al. Efficacy, safety, and immunogenicity of an Escherichia coli-produced bivalent human papillomavirus vaccine: an interim analysis of a randomized clinical trial. J Natl Cancer Inst. 2020;112(2):145–153. doi: 10.1093/jnci/djz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Joura E.A., Giuliano A.R., Iversen O.E., et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372(8):711–723. doi: 10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 109.Pattyn J., Hendrickx G., Vorsters A., Van Damme P. Hepatitis B vaccines. J Infect Dis. 2021;224(12 Suppl 2):S343–S351. doi: 10.1093/infdis/jiaa668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Champion C.R. Heplisav-B: a hepatitis B vaccine with a novel adjuvant. Ann Pharmacother. 2021;55(6):783–791. doi: 10.1177/1060028020962050. [DOI] [PubMed] [Google Scholar]
- 111.Vesikari T., Finn A., van Damme P., et al. Immunogenicity and safety of a 3-antigen hepatitis B vaccine vs a single-antigen hepatitis B vaccine: a phase 3 randomized clinical trial. JAMA Netw Open. 2021;4(10) doi: 10.1001/jamanetworkopen.2021.28652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stary G., Olive A., Radovic-Moreno A.F., et al. VACCINES. A mucosal vaccine against Chlamydia trachomatis generates two waves of protective memory T cells. Science. 2015;348(6241) doi: 10.1126/science.aaa8205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yu H., Karunakaran K.P., Jiang X., Brunham R.C. Subunit vaccines for the prevention of mucosal infection with Chlamydia trachomatis. Expert Rev Vaccines. 2016;15(8):977–988. doi: 10.1586/14760584.2016.1161510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.de la Maza L.M., Darville T.L., Pal S. Chlamydia trachomatis vaccines for genital infections: where are we and how far is there to go? Expert Rev Vaccines. 2021;20(4):421–435. doi: 10.1080/14760584.2021.1899817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Olsen A.W., Follmann F., Erneholm K., Rosenkrands I., Andersen P. Protection against Chlamydia trachomatis infection and upper genital tract pathological changes by vaccine-promoted neutralizing antibodies directed to the VD4 of the major outer membrane protein. J Infect Dis. 2015;212(6):978–989. doi: 10.1093/infdis/jiv137. [DOI] [PubMed] [Google Scholar]
- 116.Bøje S., Olsen A.W., Erneholm K., et al. A multi-subunit Chlamydia vaccine inducing neutralizing antibodies and strong IFN-γ⁺ CMI responses protects against a genital infection in minipigs. Immunol Cell Biol. 2016;94(2):185–195. doi: 10.1038/icb.2015.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Abraham S., Juel H.B., Bang P., et al. Safety and immunogenicity of the chlamydia vaccine candidate CTH522 adjuvanted with CAF01 liposomes or aluminium hydroxide: a first-in-human, randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Infect Dis. 2019;19(10):1091–1100. doi: 10.1016/S1473-3099(19)30279-8. [DOI] [PubMed] [Google Scholar]
- 118.Miller J.N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973;110(5):1206–1215. [PubMed] [Google Scholar]
- 119.Edmondson D.G., Hu B., Norris S.J. Long-term in vitro culture of the syphilis spirochete Treponema pallidum subsp. pallidum. mBio. 2018;9(3):011533. doi: 10.1128/mBio.01153-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Radolf J.D., Kumar S. The Treponema pallidum outer membrane. Curr Top Microbiol Immunol. 2018;415:1–38. doi: 10.1007/82_2017_44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kumar S., Caimano M.J., Anand A., et al. Sequence variation of rare outer membrane protein β-barrel domains in clinical strains provides insights into the evolution of Treponema pallidum subsp. pallidum, the syphilis spirochete. mBio. 2018;9(3) doi: 10.1128/mBio.01006-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Deka R.K., Brautigam C.A., Liu W.Z., Tomchick D.R., Norgard M.V. Evidence for posttranslational protein flavinylation in the syphilis spirochete Treponema pallidum: structural and biochemical insights from the catalytic core of a periplasmic flavin-trafficking protein. mBio. 2015;6(3) doi: 10.1128/mBio.00519-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Centurion-Lara A., Giacani L., Godornes C., Molini B.J., Brinck Reid T., Lukehart S.A. Fine analysis of genetic diversity of the tpr gene family among treponemal species, subspecies and strains. PLoS Negl Trop Dis. 2013;7(5):e2222. doi: 10.1371/journal.pntd.0002222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lin M.J., Haynes A.M., Addetia A., et al. Longitudinal TprK profiling of in vivo and in vitro-propagated Treponema pallidum subsp. pallidum reveals accumulation of antigenic variants in absence of immune pressure. PLoS Negl Trop Dis. 2021;15(9) doi: 10.1371/journal.pntd.0009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grillová L., Oppelt J., Mikalová L., et al. Directly sequenced genomes of contemporary strains of syphilis reveal recombination-driven diversity in genes encoding predicted surface-exposed antigens. Front Microbiol. 2019;10:1691. doi: 10.3389/fmicb.2019.01691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lieberman N.A.P., Lin M.J., Xie H., et al. Treponema pallidum genome sequencing from six continents reveals variability in vaccine candidate genes and dominance of Nichols clade strains in Madagascar. PLoS Negl Trop Dis. 2021;15(12) doi: 10.1371/journal.pntd.0010063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ávila-Nieto C., Pedreño-López N., Mitjà O., Clotet B., Blanco J., Carrillo J. Syphilis vaccine: challenges, controversies and opportunities. Front Immunol. 2023;14 doi: 10.3389/fimmu.2023.1126170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lukehart S.A., Molini B., Gomez A., et al. Immunization with a tri-antigen syphilis vaccine significantly attenuates chancre development, reduces bacterial load, and inhibits dissemination of Treponema pallidum. Vaccine. 2022;40(52):7676–7692. doi: 10.1016/j.vaccine.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Lukehart S.A., Molini B., Gomez A., et al. Immunization with a tri-antigen syphilis vaccine significantly attenuates chancre development, reduces bacterial load, and inhibits dissemination of Treponema pallidum. Vaccine. 2022;40(52):7676–7692. doi: 10.1016/j.vaccine.2022.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hawley K.L., Montezuma-Rusca J.M., Delgado K.N., et al. Structural modeling of the Treponema pallidum outer membrane protein repertoire: a road map for deconvolution of syphilis pathogenesis and development of a syphilis vaccine. J Bacteriol. 2021;203(15) doi: 10.1128/JB.00082-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romeis E., Tantalo L., Lieberman N., Phung Q., Greninger A., Giacani L. Genetic engineering of Treponema pallidum subsp. pallidum, the Syphilis Spirochete. PLoS Pathog. 2021;17(7) doi: 10.1371/journal.ppat.1009612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lithgow K.V., Cameron C.E. Vaccine development for syphilis. Expert Rev Vaccines. 2017;16(1):37–44. doi: 10.1080/14760584.2016.1203262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Belshe R.B., Leone P.A., Bernstein D.I., et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366(1):34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Egan K., Hook L.M., LaTourette P., Desmond A., Awasthi S., Friedman H.M. Vaccines to prevent genital herpes. Transl Res. 2020;220:138–152. doi: 10.1016/j.trsl.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Van Wagoner N., Fife K., Leone P.A., et al. Effects of different doses of GEN-003, a therapeutic vaccine for genital herpes simplex virus-2, on viral shedding and lesions: results of a randomized placebo-controlled trial. J Infect Dis. 2018;218(12):1890–1899. doi: 10.1093/infdis/jiy415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Multi-country outbreak of mpox external situation report 20, published 13 April 2023. https://www.who.int/docs/default-source/coronaviruse/situation-reports/20230413_mpox-external-sitrep-20.pdf?sfvrsn=211415ea_3&download=true Available at:
- 137.Public health considerations for mpox in EU/EEA countries, European Centre for Disease Prevention and Control. https://www.ecdc.europa.eu/en/publications-data/public-health-considerations-mpox-eueea-countries Available at:
- 138.Poland G.A., Kennedy R.B., Tosh P.K. Prevention of monkeypox with vaccines: a rapid review. Lancet Infect Dis. 2022;22(12):e349–e358. doi: 10.1016/S1473-3099(22)00574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Hazra A., Zucker J., Bell E., et al. Mpox in people with past infection or a complete vaccination course: a global case series. Lancet Infect Dis. 2023 doi: 10.1016/S1473-3099(23)00492-9. [DOI] [PubMed] [Google Scholar]
- 140.Watanabe Y., Bowden T.A., Wilson I.A., Crispin M. Exploitation of glycosylation in enveloped virus pathobiology. Biochim Biophys Acta Gen Subj. 2019;1863(10):1480–1497. doi: 10.1016/j.bbagen.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Haynes B.F., Wiehe K., Borrow P., et al. Strategies for HIV-1 vaccines that induce broadly neutralizing antibodies. Nat Rev Immunol. 2023;23(3):142–158. doi: 10.1038/s41577-022-00753-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 143.Haynes B.F., Gilbert P.B., McElrath M.J., et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366(14):1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Gray G.E., Bekker L.G., Laher F., et al. Vaccine efficacy of ALVAC-HIV and bivalent subtype C gp120-MF59 in adults. N Engl J Med. 2021;384(12):1089–1100. doi: 10.1056/NEJMoa2031499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Clinical trials, a study to evaluate the safety and immunogenicity of eOD-GT8 60mer mRNA vaccine (mRNA-1644) https://clinicaltrials.gov/ct2/show/NCT05414786 Available at:
- 146.Petousis-Harris H., Paynter J., Morgan J., et al. Effectiveness of a group B outer membrane vesicle meningococcal vaccine against gonorrhoea in New Zealand: a retrospective case-control study. Lancet. 2017;390(10102):1603–1610. doi: 10.1016/S0140-6736(17)31449-6. [DOI] [PubMed] [Google Scholar]
- 147.Abara W.E., Bernstein K.T., Lewis F.M.T., et al. Effectiveness of a serogroup B outer membrane vesicle meningococcal vaccine against gonorrhoea: a retrospective observational study. Lancet Infect Dis. 2022;22(7):1021–1029. doi: 10.1016/S1473-3099(21)00812-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Wang B., Giles L., Andraweera P., et al. Effectiveness and impact of the 4CMenB vaccine against invasive serogroup B meningococcal disease and gonorrhoea in an infant, child, and adolescent programme: an observational cohort and case-control study. Lancet Infect Dis. 2022;22(7):1011–1020. doi: 10.1016/S1473-3099(21)00754-4. [DOI] [PubMed] [Google Scholar]
- 149.Bruxvoort K.J., Lewnard J.A., Chen L.H., et al. Prevention of Neisseria gonorrhoeae with meningococcal B vaccine: a matched cohort study in southern California. Clin Infect Dis. 2023;76(3):e1341–e1349. doi: 10.1093/cid/ciac436. [DOI] [PubMed] [Google Scholar]
- 150.Raccagni A.R., Galli L., Spagnuolo V., et al. Meningococcus B vaccination effectiveness against Neisseria gonorrhoeae infection in people living with HIV: a case-control study. Sex Transm Dis. 2023;50(5):247–251. doi: 10.1097/OLQ.0000000000001771. [DOI] [PubMed] [Google Scholar]
- 151.Hui B.B., Padeniya T.N., Rebuli N., et al. A gonococcal vaccine has the potential to rapidly reduce the incidence of Neisseria gonorrhoeae infection among urban men who have sex with men. J Infect Dis. 2022;225(6):983–993. doi: 10.1093/infdis/jiab581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Craig A.P., Gray R.T., Edwards J.L., et al. The potential impact of vaccination on the prevalence of gonorrhea. Vaccine. 2015;33(36):4520–4525. doi: 10.1016/j.vaccine.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Looker K.J., Booton R., Begum N., et al. The potential public health impact of adolescent 4CMenB vaccination on Neisseria gonorrhoeae infection in England: a modelling study. BMC Public Health. 2023;23(1):1. doi: 10.1186/s12889-022-14670-z. [DOI] [PMC free article] [PubMed] [Google Scholar]