Introduction
In this chapter we review approaches to studying the epidemiology of NTM pulmonary disease (NTM-PD), and update advances in the field since the last review published in 2015 1.
The importance of epidemiology: surveillance and research
Defining the NTM-PD disease burden is critical for justifying the need for resource allocation, both for clinical resources such as health care, as well as for the development of new therapeutics. A fuller understanding of the risk factors which contribute to changes in disease frequency is important for guiding and evaluating interventions, at either the individual-level through modification of behaviors, or through structural interventions beyond the individual. Epidemiologic surveillance is key for estimating the burden of disease (prevalence) as well as the frequency of new cases (incidence) in a given population 2, which in turn can lead to developing hypotheses regarding individual or general environmental risk factors for infection and disease 3, 4.
We here present a new approach for understanding the external and internal exposures which may lead to increased risk of NTM disease 5 (Figure 1). This paradigm is adapted from the fields of environmental epidemiology 5 and cancer epidemiology 6, whereby cumulative exposures to a variety of types of exposures will increase the risk of infection and disease. With respect to NTM-PD, specific environmental factors include those in an individual’s environment, such as those exposures from high-risk activities such as gardening, with aerosolization of soil. General environmental exposures include those beyond the control of a single individual which impact an entire population. Both factors interact with the human host, and host susceptibility, including biologic response, will influence the risk of developing NTM-PD 5 (Figure 1).
Figure 1.
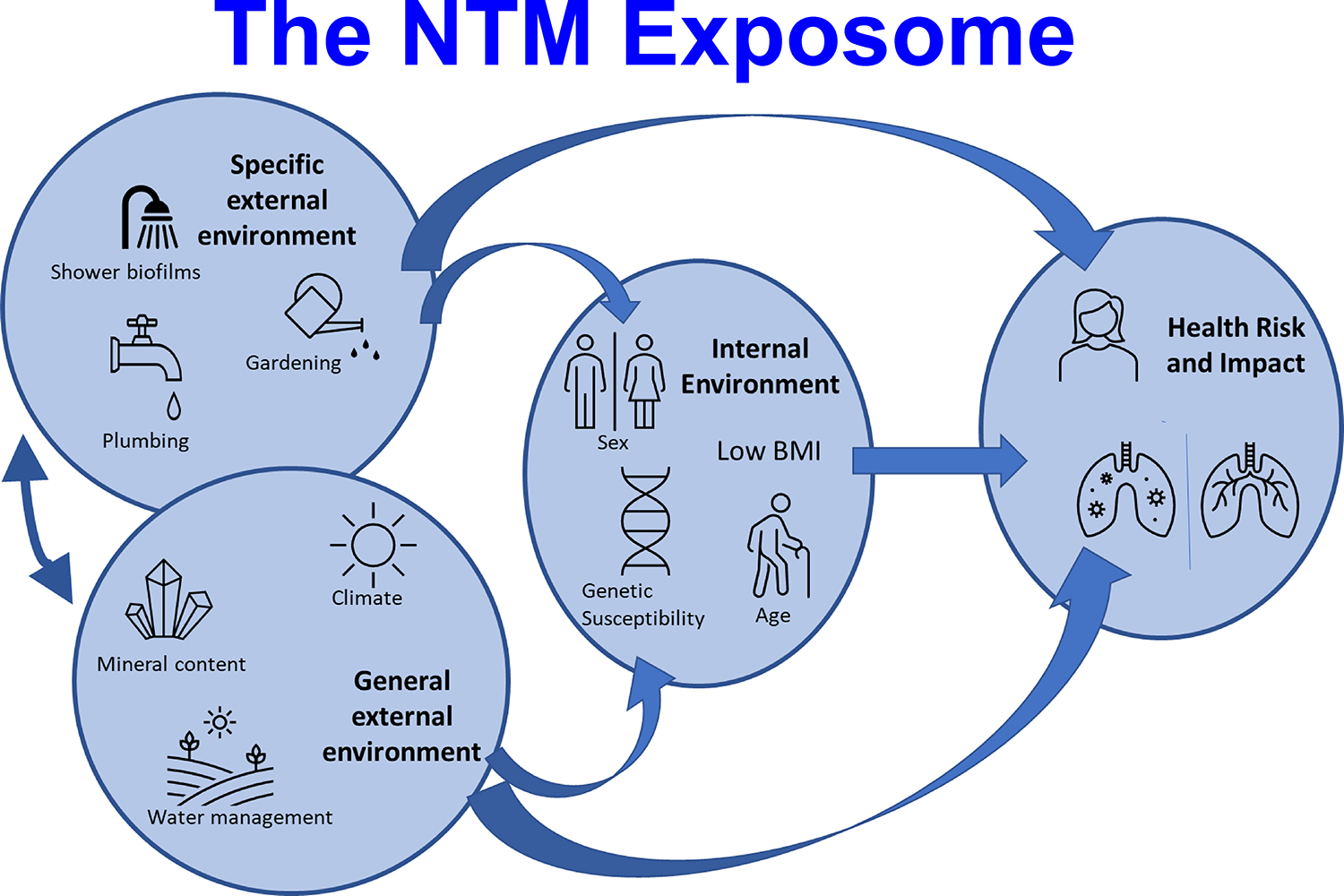
NTM exosome framework. Adapted from The exposome: a new paradigm to study the impact of environment on health, Vrijheid M., 69, 876–8, 2014 with permission from BMJ Publishing Group Ltd.
Methodologic challenges
A key methodologic challenge is the lack of a global surveillance system with standard case definitions that would allow comparison within and across countries and regions. The NTM-PD case definition which includes microbiologic, radiographic, and clinical criteria, was developed by ATS/IDSA in 2007 for diagnostic and treatment purposes 7, and endorsed in the more recent ATS/ERS/ESCMID/IDSA guidelines 8. In this review we will use the simplified term “ATS-criteria” acknowledging the equal contribution of the other societies. However, different case definitions may be used for epidemiologic purposes of monitoring infection rates and risk factor identification, as the decision about whether and when to treat is different from the surveillance goal of identifying patterns of infection and disease.
Ascertaining the radiographic and clinical criteria for NTM-PD is generally time consuming and costly, and therefore not feasible at the scale needed to study national and regional patterns, particularly in areas with a higher disease burden. Therefore, microbiology data from centralized public health laboratory systems, as well as administrative claims data with International Classification of Disease (ICD) codes have been increasingly used to better understand the epidemiology of NTM-PD. Each approach has strengths and limitations. Microbiologic data provide an indicator of exposure from the environment, but will overestimate the true disease prevalence, as not all isolates from respiratory secretions are clinically significant. In this review we use the term “isolation” or “isolates” to refer to culture positivity of respiratory specimens for NTM without necessarily attributing these isolates to a disease status in a specific patient. We therefore also avoid the term “colonization” which suggests “no disease”, a status which has not been well studied. Although the term “NTM infection” has often been used to refer to cases which satisfy the ATS microbiologic criteria 9–14 we generally avoid use of this term as the definition of this term has not been standardized.
The microbiologic component of the ATS criteria, as well as ICD codes (9 or 10) have been evaluated and found to have a high predictive value for NTM-PD (meeting the full ATS diagnostic criteria). In a large, population-based cohort from a centralized provincial laboratory in Ontario, Canada, 46% of those with an isolate of NTM met the microbiologic component of the ATS disease diagnostic criteria 14. Within the subset of patients being followed in a clinic setting, 73% of the patients who fulfilled the ATS microbiologic criteria met the full ATS disease criteria, indicating a high predictive value 14. Similarly, in a large national study using NTM isolates from the national referral laboratory in Denmark, among a small subset with isolates meeting ATS microbiologic criteria (termed “possible NTM disease”), 90% met the full ATS disease criteria 13. In a study in North Carolina, USA, based on mycobacterial isolation and full chart review, 55.7% of those with an isolate met the ATS microbiologic criteria, and 60.9% of these met the full criteria including microbiologic, clinical, and radiographic criteria 15. Thus, although analysis of isolates is important for understanding patterns and trends, case definitions based on 1 or 2 isolates will usually overestimate the burden of ATS defined NTM-PD.
ICD codes provide a more useful indicator of true disease, although they will tend to underestimate true disease prevalence, as administrative codes tend to lack sensitivity for rare disease. The sensitivity of the ICD codes relative to the ATS microbiologic criteria has been found to range from 27% 16 in a general population to 50% among persons with rheumatoid arthritis 17 and up to 69% among persons with bronchiectasis 18; the positive predictive value across various studies has ranged from 77% to 100% 17–19. Although these codes may underestimate true disease rates, the direction of this bias is unlikely to change substantially across time and populations, such that these codes are useful tools for epidemiologic purposes.
Two noteworthy examples of implementation of NTM-PD surveillance are from Queensland, Australia, and the state of Oregon, USA. In Queensland, laboratory-based notification of NTM isolates has been mandated since the inception of the Tuberculosis control program in the 1950’s, and all cases of NTM isolation are notifiable under the Public Health Act 3. Data from this surveillance system has since facilitated the characterization of NTM-PD epidemiology, particularly with respect to geographic distribution, environmental risk factors, and increasing burden 3. In the state of Oregon, USA, a pilot surveillance program was implemented from 2005–2006 and provided important insights regarding NTM epidemiology 4, 19. Currently, in the United States, only 4 states have mandated notification of NTM-PD, as part of a Centers for Disease Control and Prevention (CDC) through an Emerging Infections Network (EIN) pilot program 20, 21.
Despite the known limitations of death certificate data, in the United States, recent data have shown an increase in non-HIV associated NTM-related deaths from 1999–2014, during a period in which both HIV-associated NTM-related mortality decreased, and TB-associated mortality also decreased significantly 22. In Japan, in the absence of nationally representative data, mortality data were useful for providing insight into patterns by age, sex, and region, and for estimating prevalence 23. Several studies have identified the increased risk of mortality associated with NTM-PD 24, 25 In one study, persons with NTM-PD had a fourfold increased risk of death after adjusting for all other factors (HR=3.64) 26.
Risk factors: Environment
General and specific environmental risk factors identified to date are summarized in Table 1. Specific exposures refer to those estimated from studies which associate individual behaviors or household factors with human NTM pulmonary isolation or disease. General exposures refer to those not unique to a single individual but rather which affect the broader population.
Table 1:
Host and Environmental Risk factors for NTM infection and disease
| Risk Factor | Relative risk, Odds ratio, Relative Prevalence, or other measure | Case Definition |
|---|---|---|
|
| ||
| Environmental: specific individual exposures | ||
|
| ||
| NTM isolation from shower aerosols, Oregon, USA | 4.0 [OR]27 | MAC-PD, case-control study |
|
| ||
| Use of spray bottle to spray house plants, Oregon, USA | 2.7 [OR]29 | MAC-PD, case-control study |
|
| ||
| Use of Public Baths ≥ 1/week, S Korea | 4.0 [OR]30 | MAC-PD, case-control study |
|
| ||
| Indoor swimming pool use (in the past four months), USA | 5.9 [OR]31 | Incident pulmonary isolation persons with CF (pwCF) |
|
| ||
| Soil exposure, Japan (bronchiectasis with MAC vs bronchiectasis with no MAC) | 5.9 [OR]33 | MAC-PD, case-control study |
|
| ||
| Environment- general exposures, measured at population level: climate, soil, and water- related | ||
|
| ||
| Relative abundance of NTM in showerhead biofilm and population prevalence | Significant correlation, p<0.000135 | NTM-PD and NTM pulmonary isolation (Medicare with ICD codes; CF ≥ 1 pulmonary isolate) |
|
| ||
| Higher average annual precipitation, FL, USA | 1.34130 | Incident pulmonary isolation (≥ 1 isolate) among persons with CF |
|
| ||
| Increased soil sodium, FL, USA | 1.92130 | Incident pulmonary isolation (≥ 1 isolate) among persons with CF |
|
| ||
| Increased soil manganese | 0.59130 | Incident pulmonary isolation (≥ 1 isolate) among persons with CF |
|
| ||
| Increased molybdenum concentrations in source water | 1.4112 | M. abscessus incident pulmonary isolation (≥2 isolates), non-CF |
| 1.7911 | M. abscessus pulmonary isolation in persons with CF (≥1 isolate) | |
|
| ||
| Increased vanadium concentrations in source water | 1.4912 | Incident MAC isolation (≥2 isolates), non-CF |
| 1.2236 | Incident MAC pulmonary isolation, non-CF (≥2 isolates) | |
|
| ||
| Percent hydric soil in census blocks, NC, USA | 26.838 | NTM pulmonary isolation (≥ 1 isolate), non-CF |
|
| ||
| Proportion of area as surface water | 4.639 | NTM-PD (ICD codes) |
|
| ||
| Mean daily potential evapotranspiration | 4.039 | NTM-PD (ICD codes) |
|
| ||
| Copper soil levels, per 1 ppm increase | 1.239 | NTM-PD (ICD codes) |
|
| ||
| Sodium soil levels, per 0.1 ppm increase | 1.939 | NTM-PD (ICD codes) |
|
| ||
| Manganese soil levels, per 100 ppm increase | 0.739 | NTM-PD (ICD codes) |
|
| ||
| Increased average topsoil depth | 0.87 (M. intracellulare)42 | NTM isolation (≥ 1 isolate), non-CF |
|
| ||
| Soil bulk density | 1.8 (M. kansasii)42 | NTM isolation (≥ 1 isolate), non-CF |
Estimated from data in paper
Hazard ratio, fully adjusted for age, sex, income, rurality, and comorbidities for NTM (HIV, COPD, asthma, and GERD)
Comparison of patients with NTM/TB with uninfected anti-TNF users
risk decreased with increasing duration since use; see full paper for specific estimates
Case-control studies in the United States, South Korea, and Japan have identified individual factors related to water and soil exposure. A recent study in Oregon, United States, found that the NTM isolation in the household shower aerosols was significantly associated with NTM-PD 27, but that other home water and soil exposure were not. This finding is supported by a study in Queensland, Australia which found genetic matches between patient isolates and species identified from shower aerosols and household water supplies 28. In the initial Oregon study, the only household-level factors significantly associated with NTM-PD risk was spraying plants with a spray bottle [OR=2.7] 29. In South Korea, use of public baths at least weekly was associated with a fourfold increased risk of NTM-PD 30. In the United States, a national study of NTM-PD among persons with CF found that indoor swimming pool use at least monthly was significantly associated with incident NTM isolation 31 (Table 1), and that other behavioral factors, including showering frequency, were not. A study among children with CF in Florida found that those who lived in households which were within 500 meters of a body of water had a significant 9.4-fold increased odds of having NTM pulmonary isolation 32. In Japan, exposure to soil more than twice weekly was significantly associated with the NTM-PD 33. The high risk associated with frequent soil exposure is consistent with a population-based study of agriculture workers in Florida, which found that cumulative occupational exposure was significantly associated with infection, as defined by M. avium skin test sensitivity 34. The risk associated with any given behavior will depend on the intensity of the exposure and the NTM abundance in environmental exposure source–generally soil or water. The risk associated with household NTM isolation from shower aerosols in the NTM-PD study in Oregon is supported by a national study of shower biofilms, which found a significant correlation between the relative abundance of NTM in showerhead biofilms and state-level NTM-PD prevalence in both the CF population and the US Medicare beneficiary population aged ≥65 years 35.
Ecologic epidemiologic studies have found water, soil, and climate factors associated with an increased risk of NTM-PD; these studies have been conducted primarily in the United States and Australia (Table 1). In several studies conducted in the United States from 2020–2022, a consistently significant and strong association has been found between concentrations of molybdenum and M. abscessus infection, and vanadium and MAC infections in natural water sources (ground or surface) supplying municipal water systems: for every log increase in molybdenum concentrations the risk of M. abscessus isolation increased by 41% 12 to 79% 11; for every log increase in the concentration of vanadium, the risk of MAC isolation (≥ 2 isolates) increased 22% 36 to 49% 12 (Table 1). Interestingly, a case-control study in South Korea found NTM-PD patients had significantly higher median blood serum concentrations of molybdenum than controls 37.
Several other studies have identified risks related to soil, land use, and other climate factors. In North Carolina, USA, the percent hydric soil in census blocks was significantly associated with NTM isolation: census blocks with >20% hydric soils had a significant 26.8% increased adjusted mean patient count relative to those with ≤20% hydric soils 38. In Florida, in the CF population, increased soil sodium in the zip code of residence was associated with an increased risk of incident NTM isolation, whereas increased soil manganese was protective 39. In the same study, persons living in counties with above average annual levels of precipitation were also at increased risk of incident infection 39. In two national studies in the United States, vapor pressure (a measure of the water in the atmosphere at a given temperature) was predictive of disease prevalence among CF patients 31 as well as Medicare beneficiaries aged ≥65 years 31, 40. In addition, in the national Medicare study, evapotranspiration (the potential of the atmosphere to absorb water), and the proportion of the area as surface water were predictive of high-risk areas 39. In the Medicare study, higher manganese soil levels in the county were associated with lower incidence of NTM-PD, whereas higher copper and sodium levels in soil were associated with increased risk 39. In Queensland, Australia, an effect of temperature and rainfall was observed, but this varied by region: cyclic incidence patterns were associated with temperature and rainfall 41. In a prior study in Australia, increased average topsoil depth was protective against M. intracellulare, while increased soil bulk density was positively associated with M. kansasii disease 42. In Japan, NTM-PD mortality, a surrogate measure for disease prevalence and distribution, was higher in the warmer and more humid coastal areas and in an area with a large amount of surface water (lake) 23. A recent study found dust bioaerosols as a source of NTM, with higher relative abundance in East Asian inland cities in Japan and China than in desert areas 43.
Epidemiology of NTM Isolation and Disease by Global Region
In our review of epidemiological data of NTM-PD, we strive to include the highest quality data published since 2014. Ideal studies comprise population-based investigations including both clinical and microbiologic data and encompassing an adequate temporal period to best capture the burden of infection in the population. However, because these types of data are often not available, other data sources are used as described previously.
Systematic Review and Meta-analysis – Global
A systematic review and meta-analysis was recently conducted for global culture-based microbiologic data, indicating an overall increase worldwide in the frequency of isolation, including that which satisfies the ATS microbiologic criteria9. This review included only those studies with culture-based data for at least three years, and with at least 200 samples, representing findings from 47 publications in more than 18 countries. Overall, 82% of studies reported increasing isolation, and 66.7% reported increasing disease, using either the adapted ATS microbiologic criteria or the full radiographic and clinical criteria. The overall rate of increase was 4% (3.2–4.8) per year for isolation and 4.1% (3.2–5) per year for disease, which was most often defined using the ATS microbiologic criteria. Most of these studies focused on MAC, the predominant species, and to a lesser degree the M. abscessus group9 (Figure 2).
Figure 2.
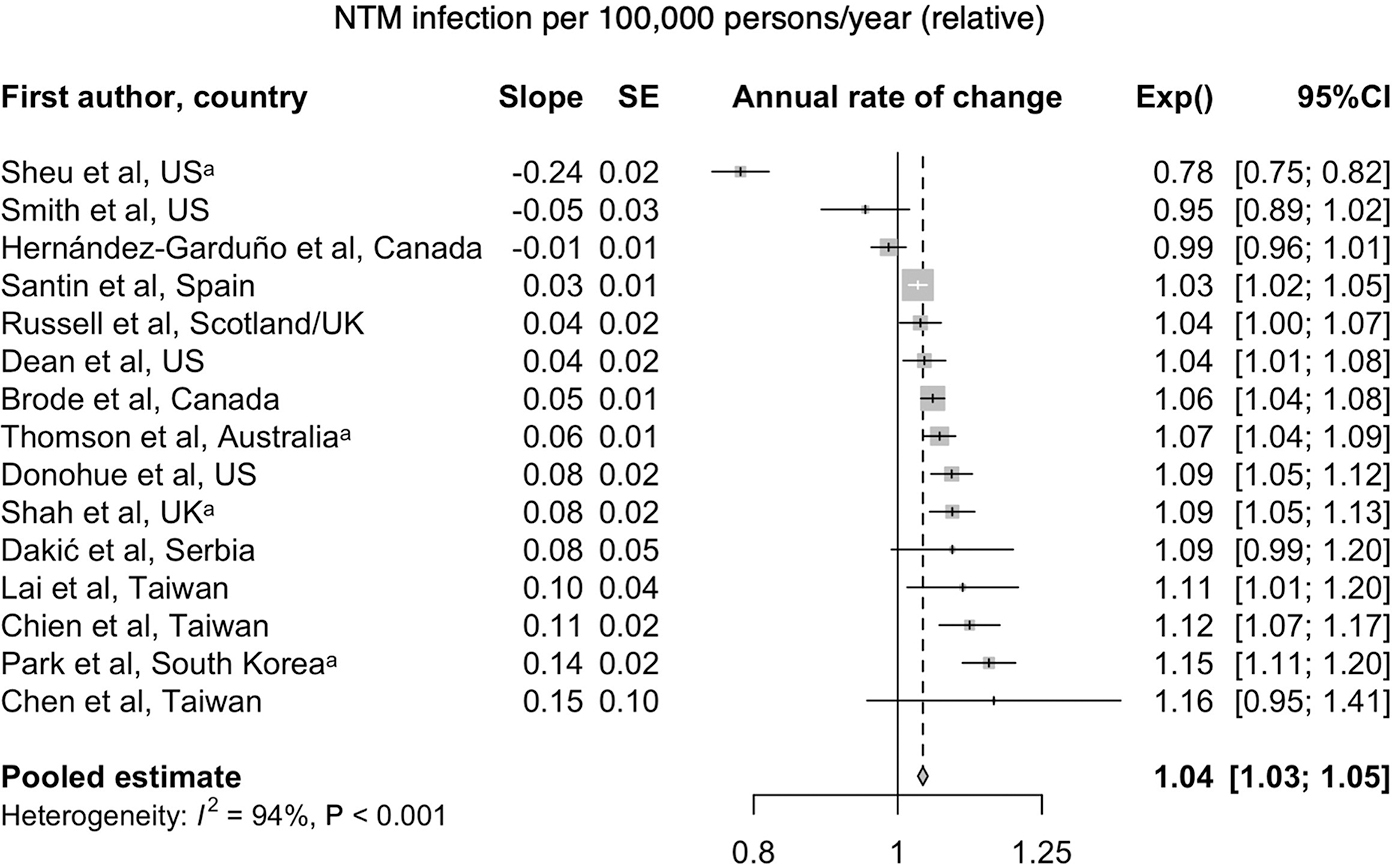
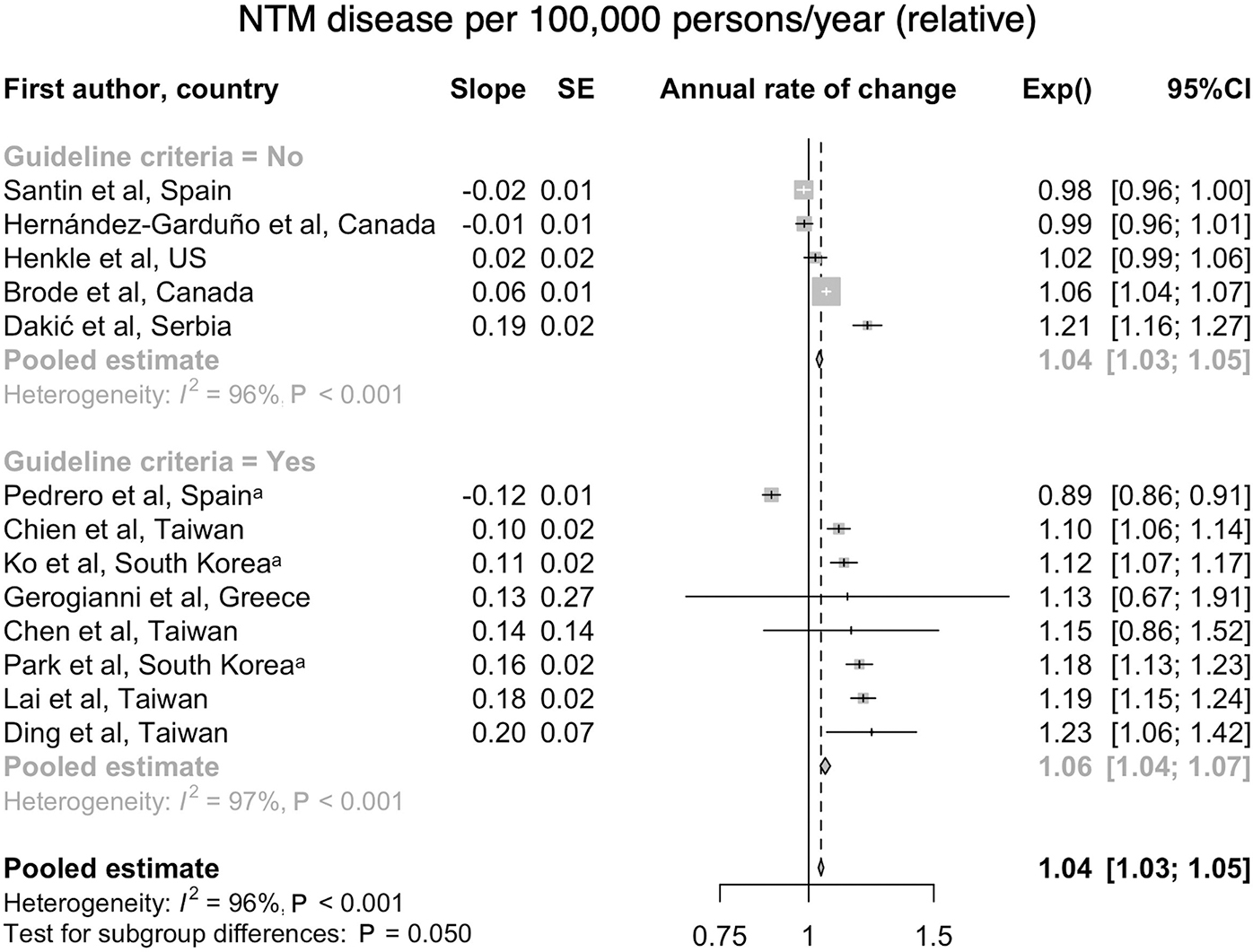
a. Forest plot of annual change of NTM infection per 100,000 persons/year. From Dahl VN, Mølhave M, Fløe A, et al. Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int J Infect Dis. Oct 13 2022;125:120–131. doi:10.1016/j.ijid.2022.10.013; with permission. (Figure 3 in original) b. Forest plot of annual change of NTM disease per 100,000 persons/year (Dahl et al., 2022); with permission. (Figure 6 in original)
A recent Delphi survey including a physician survey as well as chart review allowed comparison of prevalence rates across 4 European countries (France, Germany, Spain, UK) and Japan, and found a remarkably similar prevalence of NTM-PD of 6.1/100,000 to 6.6/100 000 across European countries, but with a fourfold increased prevalence in Japan, suggesting true differences in prevalence 44.
North America
In North America, national and subnational studies indicate increasing prevalence and incidence, and continue to demonstrate geographic heterogeneity in NTM isolation and disease. This section is based on five national studies from the United States, one provincial level study from Ontario, and nine state or territorial level studies in the United States. The national studies include three based on ICD codes to define disease, and two based on microbiologic data. The heterogeneity of methodologic approaches makes comparisons difficult, but overall, these studies indicate a picture of increasing prevalence dominated by MAC infections.
In the United States, a national study to estimate prevalence and incidence of NTM-PD defined this as two claims with NTM-PD ICD codes separated by 30 days. This study estimated a prevalence of 6.8/100,000 in 2008, increasing to 11.7/100,000 in 2015, with an estimated annual incidence from 3.1/100,000 to 4.7/100,000 during the same time period 45 (Figure 3). A separate study which estimated prevalence using a single ICD code for NTM to define a case estimated a prevalence of 27.9/100,000 in 2010, with a projected estimate of 181,037 cases in 2014, assuming a continued 8% annual prevalence increase 46. The discrepancy in estimates between the two studies for a similar year (2014/2015) is due to the more specific case definition in the former study. The geographic heterogeneity across states was consistent with prior reports and historic patterns, with the highest prevalence in the warm, humid areas in the Southeast and Southwest 46. A study in a high risk population, among veterans with COPD, also used two ICD codes for NTM-PD to define disease and found an increasing incidence and prevalence NTM-PD from 2001 to 2015, with incidence increasing from 34.2/100,000 to 70.3/100,000 and prevalence from 93.1/100,000 to 277.6/100,000 patients 47.
Figure 3.
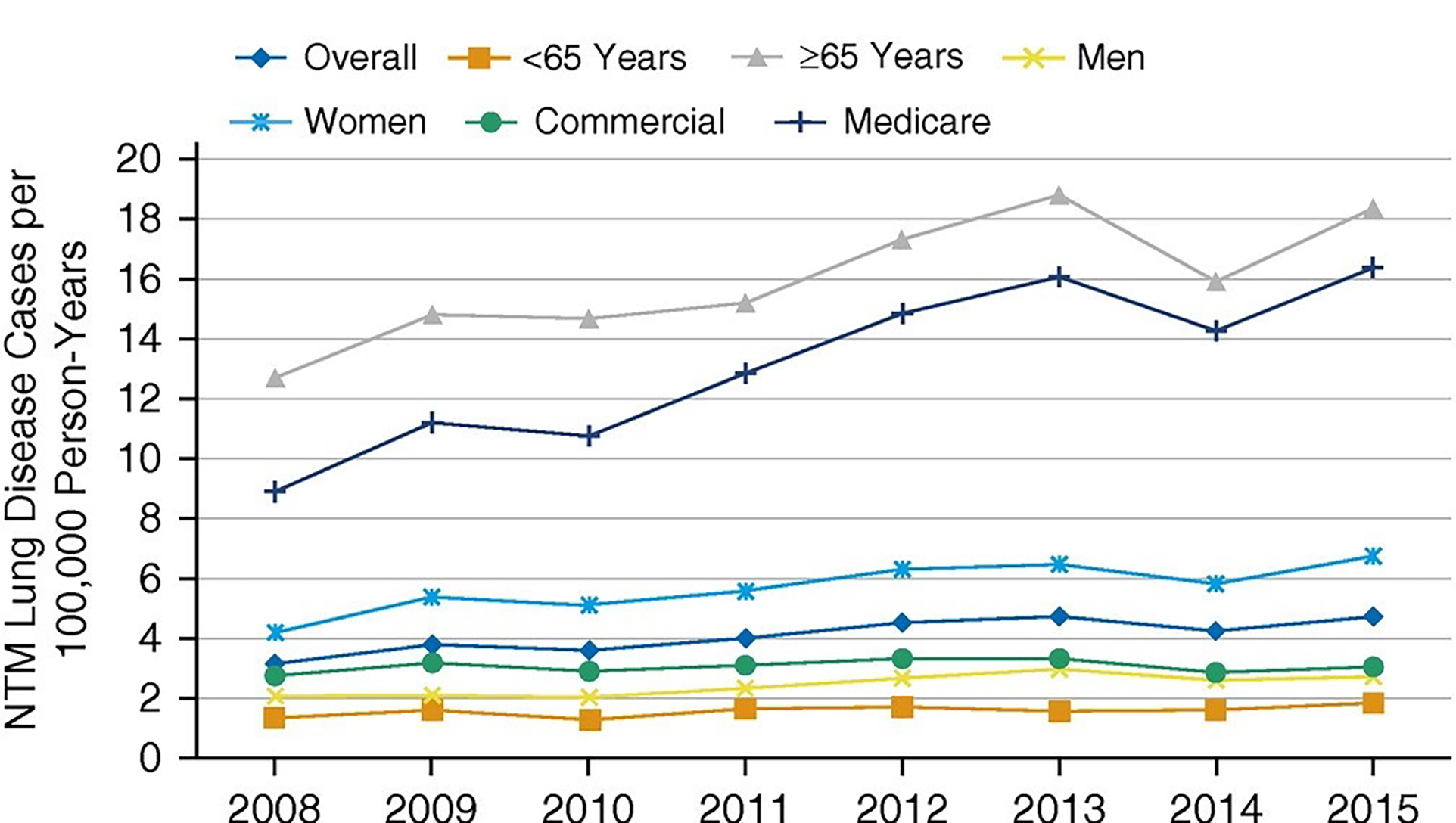
Annual incidence of NTM-PD in national U.S. health insurance plan (Optum EHR database) 2008–2015. Reprinted with permission of the American Thoracic Society. Copyright © 2023 American Thoracic Society. All rights reserved. Cite: Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q./2020/Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008–2015/Ann Am Thorac Soc./17/178–185. Annals of the American Thoracic Society is an official journal of the American Thoracic Society. (Figure 2 in original)
Two national microbiologic studies based on electronic health records demonstrated geographic variation in NTM species as well as increasing trends in AFB testing and NTM isolation prevalence in high-risk groups 48, 49. Among 5 million unique patients of whom 7,812 had at least one isolate, MAC was the most common species, ranging from 61 to 91% of isolates, and was most frequent in the South and Northeast regions; M. abscessus/M. chelonae ranged from 2% to 18% of isolates and were most frequent in the West. A separate national study using microbiology data from a different large EHR system to study frequency of AFB testing and pulmonary NTM isolation during 2009–2015 found an overall AFB testing rate of 45/10,000 population with an increasing annual percent change (APC) of 3.2% 49. The isolation rate for pathogenic NTM also increased with an APC of 4.5% and was highest among persons with CF and those with bronchiectasis 49.
One subnational study used population-based surveillance data from 2014 in four states (Mississippi, Missouri, Ohio, and Wisconsin) to describe NTM isolation. Overall prevalence was 13.3/100,000 population (after excluding M. gordonae), with a predominance of MAC, at a prevalence of 8.5/100,000 50. An earlier study which estimated trends from 2008–2013 in the same four states plus Maryland found an increasing trend, with an APC of 9.9% 51.
Hawaii and Florida have been identified as a ‘hotspots’ for NTM in the United States. In Hawaii, microbiologic data from a large, linked health care system were used to associate microbiologic data with demographic and clinical factors for the period 2005 and 2013. Isolation was based on at least one pulmonary isolate, and the ATS microbiologic criteria were used to define NTM-PD 52. Isolation prevalence increased significantly during the study period, from 20/100,000 in 2005 to 44/100,00 in 2013, with an APC of 6% and a period prevalence of 122/100,000; MAC was the most commonly isolated species, comprising 64% of isolates. NTM-PD (ATS microbiologic criteria) increased from 9/100,000 in 2005 to 19/100,000 in 2013 (Table 2). NTM isolation prevalence varied by ethnic group, with NTM isolation rates approximately twofold higher among persons who identified as Chinese, Korean, Japanese, and Vietnamese relative to those who identified as white, and the prevalence was twofold lower among Native Hawaiian and other Pacific Islander (NHOPI) relative to whites 52 (Table 3). Another study used KPH data to estimate NTM infection incidence by ethnic group during the period 2005–2019 (Table 2) 53. Cases were defined as ≥ 1 pulmonary isolate. Average annual isolation incidence was 44.8 cases/100,000 beneficiaries, with a cumulative incidence of 247 cases/100,000 over the study period. Beneficiaries who self-identified with only Asian ethnic groups had the highest NTM pulmonary isolation incidence (46 cases/100,000 person-years) and had a 30% increased risk after controlling for all other clinical and demographic factors 53. A separate study from the US Affiliated Pacific Islands (USAPI), based on samples submitted to the Diagnostic Laboratory Services in Hawaii, typically for evaluation of suspected TB, found a significantly increasing rate of NTM isolation, increasing from 0.5% of isolates screened in 2007 to 11.3% in 2011, corresponding to an NTM isolation prevalence increase of 2/100,000 to 48/100,000. MTB isolation remained stable during the same period 54.
Table 2:
Studies of Rates of Pulmonary NTM Isolation and Disease by Region
| North America | |||||||
|---|---|---|---|---|---|---|---|
| Location (dates) | Case Definition | Data Source/Cohort | Annual Prevalence (per 100,000 population) | Period Prevalence | Incidence (per 100,000 population) | ||
| Isolation (dates) | Disease (dates) | Period Duration (years) | Prevalence (per 100,000 population) | ||||
| Canada | |||||||
| Ontario, Canada (1998–2010)131 | Isolation: ≥ 1 isolate Disease: ATS microbiologic criteria |
Public Health Ontario Laboratory Database | 11.4–22.2 (1998–2010) (APC: 6.3%); Annual Change (MAC: 0.291, M. xenopi: 0.059, M. abscessus: 0.019) | 4.65 – 9.08 (1998–2010) (APC: 8.0%)+** | - | - | - |
| United States | |||||||
| U.S. (2008–2015)45 | ICD-9/ICD-10 | Optum Clinformatics Data Mart Care Claims database | - | 6.78–11.70 (APC: 7.5%) (2008–2015) | 8 | - | 3.13–4.73 (2008–2015) (APC: 5.2%) (D) |
| Florida (2012–2018)55 | ICD-9-CM/ICD-10 | OneFlorida Clinical Research Consortium database | - | 14.3–22.6 (2012–2018) | 7 | - | 6.5–5.4 (2012–2018) (D) |
| Hawaii (2005–2013)52 | Isolation: ≥ 1 isolate Disease: ATS microbiologic criteria |
Kaiser Permanente Hawaii databases | 20–44 (2005–2013) (APC: 6%)** | 9–19 (2005–2013)+** | 9 | Range by Ethnic group: 50–300 (I)** | - |
| Hawaii (2005–2019)53 | ≥ 1 isolate | Kaiser Permanente Hawaii EHR databases | - | - | 15 | - | Overall Annualized: 44.8 (I)**, Range by Ethnic group: 17–63 (I)**, Cumulative: 247 (I)** |
| Maryland, Mississippi, Missouri, Ohio, Wisconsin (2008–2013)51 | ≥ 1 isolate | Surveillance data from Maryland, Mississippi, Missouri, Ohio, Wisconsin | Overall: 8.7–13.9 (2008–2013)§# (APC: 9.9%)§#, Maryland: 9.5–9.29 (2008–2013)§# Mississippi:10.2–13.69 (2008–2013)§#, Missouri: 5.5–13.39 (2008–2013)§#, Ohio: 5.8–11.39 (2008–2013)§#, Wisconsin: 15.4–24.69 (2008–2013)§# | - | - | - | - |
| North Carolina (2006–2010)58 | ≥ 1 isolate | All labs that test for NTM in 3 counties (hospital, commercial, public health) | Overall: 9.4** | - | - | - | - |
| North Carolina (2006–2010)38 | ≥ 1 isolate | All labs that test for NTM in 3 counties (hospital, commercial, publica health | - | - | 5 | - | Cumulative: 8.8 (I) |
| Oregon (2007–2012)10 | ATS microbiologic criteria | All laboratories that test for NTM in Oregon | - | 5.9 (2011–2012)+ | 6 | - | 5.0 (APC: 2.2%) (D)+; 3.8 (D)§+ |
| U.S.–Affiliated Pacific Island Jurisdictions (2007–2011)54 | ≥ 1 isolate | Diagnostic Laboratory Services data | 2–48 (2007–2011) (adjusted rate ratio 1.65) | - | 4 | Overall: 106 (I); American Samoa (22) (I), Federated States of Micronesia (164) (I) | - |
| Central and South America | |||||||
| Location (dates) | Case Definition | Data Source/Cohort | Annual Prevalence (per 100,000 population) | Period Prevalence | Incidence (per 100,000 population) | ||
| Isolation (dates) | Disease (dates) | Period Duration (years) | Prevalence (per 100,000 population) | ||||
| French Guiana (2008–2018)61 | Isolation: ≥ 1 isolate Disease: Full ATS criteria |
Three general hospitals EHR data | - | - | 11 | - | 6.17 (I); 1.07 (D) |
| Mexico City, Mexico (2001–2017)62 | Full ATS criteria | Tertiary care referral medical center EHR data | - | - | 17 | - | SGM: 0.6/1000 admissions (2001–2011)–1.9 (2012–2017) (D)#; RGM: 0.3/1000 (2001–2011)−1.1/1000 (2012–2017) (D)# |
| Uruguay (2006–2018)67 | ≥ 1 isolate | National tuberculosis reference laboratory EHR data | 0.33 – 1.57 (2006–2018) (4.79-fold increase)# | - | - | - | - |
| Europe | |||||||
| Location (dates) | Case Definition | Data Source/Cohort | Annual Prevalence (per 100,000 population) | Period Prevalence | Incidence (per 100,000 population) | ||
| Isolation (dates) | Disease (dates) | Period Duration (years) | Prevalence (per 100,000 population) | ||||
| Czech Republic (2012–2018)81 | Full ATS criteria | Public Health Institute Ostrava database for Moravian and Silesian Regions | - | - | 7 | - | 1.1 (D)# |
| Denmark (1991–2015)68 | Isolation: ≥ 1 isolate Disease: Modified ATS microbiologic criteria |
International Reference Laboratory data | - | - | 25 | - | 2.14 (I); Definite and Possible NTM-PD: 1.10 (D)+# |
| England, Wales and Northern Ireland (January 2007–July 2012)77 | ≥ 1 isolate | Public Health England database | - | - | 6 | - | 3.4–5.0 (2007–2012) (I)** |
| France (2010–2017)69 | ICD-10 or suggestive medication | National Health System database | - | - | 8 | 5.92 (D) | 1.025–1.096 (2010–2017) (D) |
| Germany (2009–2014)72 | ICD-10 | Health Risk Institute health services research database | - | 2.3–3.3 (2009–2014)§ | - | - | - |
| Germany (2010–2011)26 | ICD-10 | German statutory health insurances claims database | - | - | 2 | - | 1.12–1.48 (2010–2011) (D); Cumulative: 2.6 (D) |
| Germany (2011–2016)71 | ICD-10 (German modification) | InGef research database of statutory health insurances claims | - | ICD-10 coded: 3.79§; Predicted: 19.05§ | 5 | - | ICD-10 coded: 1.56 (D)§; Predicted: 15.33 (D)§ |
| Greece (January 2007 - May 2013)84 | Isolation: ≥ 1 isolate Disease: Full (12 pts) ATS criteria and ATS microbiologic criteria (56 pts) |
Sismanoglio-A. Fleming General Hospital of Attiki EHR data | - | - | 7 | - | Cumulative:18.9 (I); Cumulative: 8.8 (D)++ |
| Portugal (2002–2012)73 | Treatment for NTM-PD | National Tuberculosis Surveillance System database | - | - | 11 | - | 0.54 (D)# |
| Scotland (2000–2010)86 | ATS microbiologic criteria | Scottish Mycobacteria Reference Laboratory data | - | - | 11 | - | 2.43 (D)+**# |
| Serbia (2010–2015)74 | Isolation: 1 isolate Disease: ATS microbiologic criteria |
The National Reference Laboratory of Serbia database | - | 0.31 (2011–2012)–0.47 (2014–2015)+** | 6 | - | 1.3 (I); 0.29 (D)+** |
| Spain (1994 –2014)75 | Isolation: ≥ 1 isolate Disease: ≥2 positive cultures or antimicrobial chemotherapy |
Referral Hospital (Bellvitge University Hospital) Laboratory data | - | - | 21 | 113.2 (I)**; 42.8 (D)+++ ** | - |
| Spain (1994–2015)76 | Isolation: ≥ 1 isolate Disease: Full ATS criteria (only RGM) |
Referral Hospital (Bellvitge University Hospital) Laboratory data | - | - | 22 | - | 0.34–1.73 (2003–2015) (APC: 8.3%) (I)# |
| Spain (1997–2016)78 | Full ATS criteria | Microbiology Laboratory of Cruces University Hospital data | - | - | 20 | - | 10.6–1.8 (2016) (APC: 3.3% (MAC), −6.5% (M. kansasii)) (D) |
| The United Kingdom (2006–2016)79 | ‘Strict Cohort’, highly likely to have NTM-PD; ‘Expanded Cohort’ possible NTM-PD++++ | Clinical Practice Research Datalink | - | Strict Cohort: 7.68–4.70 (2006–2016)# | 10 | Strict Cohort: 6.38 (D)# | Strict Cohort: 3.85–1.28 (2006–2016) (D)#; Expanded Cohort: 22.9–40.9 (2006–2016) (D)# |
| The Netherlands (2012–2019)70 | Drug combination in drug dispensing database, ICD-10 | IQVIA’s Real-World Data Longitudinal Prescription database, Outpatient Pharmacy Database of the PHARMO Database Network, IQVIA’s health insurance claims database, Hospitalization Database of the PHARMO Database Network | - | 2.3–5.9 (databases); 6.2–9.9 (pulmonologist survey) | - | - | - |
| East Asia | |||||||
| Location (dates) | Case Definition | Data Source/Cohort | Annual Prevalence (per 100,000 population) | Period Prevalence | Incidence (per 100,000 population) | ||
| Isolation (dates) | Disease (dates) | Period Duration (years) | Prevalence (per 100,000 population) | ||||
| Japan (2001–2009)132 | Full ATS criteria | Microbiological database of 11 hospitals in Nagasaki prefecture | - | - | 9 | - | 4.6–10.1 (2001–2009) (D) |
| Japan (2004–2006)23 | ICD-8–10, estimated from Death Statistics | Vital Statistics of Japan database | - | 33–65 (2005)§ | - | - | - |
| Japan (2009–2014) 107 | ICD-10 | The Japanese National Database of Health Insurance Claims | - | 29 (2011) | 6 | - | 8.6 (2011) (D) |
| Japan (2012–2013)105 | ATS microbiologic criteria | 3 major laboratories (SRL, Inc., LSI Medience Corporation, BML, Inc.) | - | 12 (2012–2013)+** | 2 | 24 (D)+** | - |
| South Korea (2001–2015)104 | Full ATS criteria | NTM Registry of Tertiary Referral Hospital (Samsung Medical Center) | - | - | 15 | - | 7.0–55.6 (2001–2015) (D) |
| South Korea (2003–2016)98 | ICD-10 | National Health Insurance Service database | - | 1.2–33.3§ | 14 | - | 1.0–17.9 (2003–2019) (D)§ |
| South Korea (2006–2016)100 | Full ATS criteria | Tertiary Referral Hospital (Severance Hospital) EHR data | - | - | 11 | - | 4.6–19.6 (2006–2016) (I); 1.2–4.8 (2006–2016) (APC: 14%) (D) |
| South Korea (2007–2018)99 | ICD-10 | Health Insurance Review and Assessment Service database | - | 5.3–41.7 | 12 | - | Diagnostic-based: 3.5–18.0 (2008–2018) (APC: 16.7%) (D); Clinically-refined: 2.9–12.3 (2008–2018) (APC: 13.2%) (D) |
| South Korea (2009–2015)133 | ICD-10 | Health Insurance Review and Assessment service database | - | - | 7 | - | 6.6–26.6 (2009–2015) (D) |
| South Korea (2009–2015)103 | Full ATS criteria | Tertiary care Hospitals (Pusan National University, Pusan National University Yangsan) EHR data | - | - | 7 | - | 6.8–12.9 (2009–2015) (D)# |
| Taiwan (2000–2012)115 | Full ATS criteria | Tertiary Medical Center (National Taiwan University) data | - | - | - | - | 3.4–13 (D) |
| Taiwan (2003–2018)113 | ICD-9-CM, treatment | National Health Insurance Research database | - | 0.68–7.17# (Treatment Case) | - | - | 0.54–3.35 (2003–2018) (D) (Treatment Case)# |
| Taiwan (2005–2013)112 | ICD-9-CM, ≥ 2 cultures, treatment | National Health Insurance Research database | - | - | - | - | 5.3–14.8 (2005–2013) (D)§# |
| Taiwan (2007–2010)114 | ≥ 1 isolate | National TB Registry of Taiwan CDC | - | - | - | - | 8.6 (I) |
| Taiwan (2010–2014)116 | Full ATS criteria | 6-Hospital EHR database | - | - | - | - | 46 (D) |
| West and Central Asia | |||||||
| Location (dates) | Case Definition | Data Source/Cohort | Annual Prevalence (per 100,000 population) | Period Prevalence | Incidence (per 100,000 population) | ||
| Isolation (dates) | Disease (dates) | Period Duration (years) | Prevalence (per 100,000 population) | ||||
| Iran (1990–2014)134 | - | - | - | - | 25 | 9.6–10.6 (D)# | - |
| Oceania | |||||||
| Location (dates) | Case Definition | Data Source/Cohort | Annual Prevalence (per 100,000 population) | Period Prevalence | Incidence (per 100,000 population) | ||
| Isolation (dates) | Disease (dates) | Period Duration (years) | Prevalence (per 100,000 population) | ||||
| Australia (2001–2016)41 | ≥ 1 isolate | Queensland Notifiable Conditions database | - | - | 16 | - | 11.1–25.88 (I)# |
| Australia (2012–2015)3 | ≥ 1 isolate | Queensland Notifiable Conditions database | - | - | 4 | - | 25.9 (I)# |
Rates expressed as annual rates per 100,000 population, averaged over study period unless otherwise specified
Studies which excluded M. gordonae
ICD-9 – International diagnostic classification of diseases – ninth revision
ICD-10 – International diagnostic classification of diseases – tenth revision
SGM – slow-growing mycobacteria
RGM – rapid-growing mycobacteria
EHR – electronic health records
APC – annual percent change
NTM-PD is defined using ATS microbiologic criteria
NTM-PD is defined using ATS microbiologic criteria and full ATS criteria
NTM-PD is defined ≥2 positive cultures or antimicrobial chemotherapy
‘Strict Cohort’ is defined using evidence of treatment and/or monitoring of NTM-PD and ‘expanded cohort’ is defined using NTM disease clinical terminology codes
Included patients with NTM isolated from extrapulmonary sites/specimens from extrapulmonary sites
Adjusted
Table 3:
NTM isolations from respiratory specimen (I) and NTM pulmonary disease (D) by species and region
| North America | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| Canada | ||||||
|
| ||||||
| Ontario, Canada (1998–2010)131 | I: 2631 | MAC (50.5) | M. xenopi (21.6) | M. gordonae (12.8) | M. fortuitum (5.0) |
M. abscessus (2.2) M. chelonae (1.5) |
|
| ||||||
| Toronto, Ontario, Canada (2003–2019)60 | D: 252 | M. avium (87.3) | M. xenopi (12.7) | - | - | - |
|
| ||||||
| United States | ||||||
|
| ||||||
| Florida (2011–2017)56 | I: 396 |
M. abscessus subsp. abscessus (31.8), M. abscessus subsp. bolletii (<1) M. abscessus subsp. massiliense (4.5) M. chelonae (1.8) |
M. gordonae (18.7) |
M. avium (1.6) M. chimaera (4.7) M. intracellulare (1.8) |
M. kansasii (4.7) | M. szulgai (3.9) |
|
| ||||||
| Hawaii (2005–2013)52 | 455 (I: 201, D: 254+) | MAC (63.7) | M. fortuitum (24.0) | M. abscessus (19.1) | - | - |
|
| ||||||
| Hawaii (2005–2019)53 | I: 739 | MAC (69) | M. fortuitum group (24) | M. abscessus (21) | M. kansasii (2) | - |
|
| ||||||
| Illinois, U.S. (2000–2012)135 | I: 448 |
M. avium (54) M. chimera (28) M. intracellulare (18) |
- | - | - | - |
|
| ||||||
| Iowa (1996–2017)136 | D: 185 | MAC (68.6) | M. kansasii (8.1) |
M. abscessus (7.6) M. chelonae (5.4) |
M. fortuitum (2.2); M. xenopi (2.2) | - |
|
| ||||||
| North Carolina (2006–2010)58 | I: 750 | MAC (50.9) | M. gordonae (20.4) | M. abscessus complex (13.6) | M. fortuitum (5) | M. mucogenicum (3.9) |
|
| ||||||
| Oregon (2007–2012)10 | I: 806 | M. avium/intracellulare complex (82.8) |
M. abscessus/chelonae complex (4.1) M. chelonae (<1) |
M. fortuitum complex (2.9) | M. lentiflavum (1.0) | M. kansasii (<1) |
| D: 1146+ | M. avium/intracellulare complex (85.7) |
M. abscessus/chelonae complex (5.9) M. chelonae (1.2) |
M. kansasii (1.2) | M. lentiflavum (1.0) | M. fortuitum complex (<1) | |
|
| ||||||
| U.S. (2009–2013)48 | I: 487 | MAC (77) | M. abscessus/M. chelonae (9) | M. kansasii (6) | M. fortuitum (5) | - |
|
| ||||||
| US-Affiliated Pacific Island Jurisdictions (2007–2011)54 | I: 35‡ | MAC (31.4) | M. fortuitum (20.0) | M. gordonae (14.3) | M. abscessus/ chelonae (5.7); M. parascrofulace um/M. fortuitum (5.7) | M. florentinum (2.9); M. kansasii (2.9); M. mucogenicum (2.9); M. paraffinicum (2.9); M. simiae (2.9); M. terrae (2.9) |
|
| ||||||
| Central and South America | ||||||
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| Ceará, Brazil (2005–2016)65 | I: 42 | M. abscessus (4.8); M. avium (4.8); M. fortuitum (4.8) | M. kansasii (2.4); M. szulgai (2.4) | - | - | - |
|
| ||||||
| French Guiana (2008–2018)61 | I: 147 |
M. fortuitum (23) M. avium (52) |
M. avium (12) M. intracellulare (17) |
M. gordonae (5); M. scrofulaceum (5) | M. abscessus (3); M. smegmatis (3) | M. interjectum (2); M. kansasii (2) |
| D: 31 | M. intracellulare (29) | M. abscessus (16) | M. genavense (3) | - | - | |
|
| ||||||
| Mexico City, Mexico (2001–2017)62 | D: 66 | MAC (47) | M. abscessus (27.3); M. fortuitum (27.3) | M. kansasii (7.6); M. scrofulaceum (7.6) | - | - |
|
| ||||||
| Panama (2012–2014)66 | I: 7 | M. avium (57.1) | M. haemophilum (28.6) | M. tusciae (14.3) | - | - |
|
| ||||||
| Rio Grande do Sul, Brazil (2003–2013)63 | D: 100 |
M. avium (26) M. intracellulare (9) |
M. kansasii (17) | M. abscessus (12) | M. fortuitum (4) | M. gordonae (3) |
|
| ||||||
| São Paulo, Brazil (2011–2014)64 | I: 2843 |
M. avium (18.3) M. intracellulare (14.9) |
M. kansasii (15.9) | M. gordonae (13.2) | M. fortuitum (10.9) |
M. abscessus (7.7) M. chelonae (3.4) |
| D: 448+ | M. kansasii (29.2) |
M. avium (20.8) M. intracellulare (19.6) |
M. abscessus (17.2) M. chelonae (<1) |
M. gordonae (4.5) | M. fortuitum (3.6) | |
|
| ||||||
| Uruguay (2006–2018)67 | I: 255† |
M. avium (23.9) M. intracellulare (33.7) |
M. kansasii (8.2) | M. gordonae (5.9) | M. peregrinum (4.7) |
M. abscessus (1.2) M. chelonae (3.1); M. fortuitum (3.1) |
|
| ||||||
| Europe | ||||||
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| Belgium (2015–2018)137 | I: 264 |
M. avium (12.1) M. chimaera/M. intracellulare (20.8) |
M. abscessus (12.9) M. chelonae (6.1) |
M. gordonae (3.4) | M. fortuitum (1.9); M. xenopi (1.9) | M. paragordonae (1.5) |
|
| ||||||
| Belgium (2010–2017)138 | I: 384 |
M. avium (25) M. chimaera (4.4) M. intracellulare (16.7) M. marseillense (<1) |
M. gordonae (14.6) | M. xenopi (7.6) | M. fortuitum (3.9) |
M. abscessus (3.1) M. chelonae (2.9) |
| D: 165 |
M. avium (31.5) M. chimaera (4.2) M. intracellulare (24.8) M. marseillense (<1) |
M. xenopi (8.5) |
M. abscessus (6.7) M. chelonae (<1) |
M. kansasii (4.2) | M. malmoense (3.6) | |
|
| ||||||
| I: 1926 | M. gordonae (42.6) | M. xenopi (15.3) | M. fortuitum (11.4) | M. terrae (6.6) | M. abscessus (2.1) | |
|
| ||||||
| Croatia (2006–2015)139 | D: 137 | M. xenopi (39.4) |
M. avium (22.6) M. chimaera (1.5) M. intracellulare (14.6) |
M. kansasii (5.8) | M. abscessus (4.4) |
M. chelonae (5.0) M. fortuitum (1.5); M. gordonae (1.5) |
|
| ||||||
| Czech Republic (2012–2018)81 | I: 2176† | M. xenopi (36.4) | M. avium-intracellulare complex (17.7) | M. gordonae (15.7) | M. fortuitum (9.7) | M. kansasii (6.2) |
| D: 303† | M. avium-intracellulare complex (47.2) | M. kansasii (23.8) | M. xenopi (18.2) |
M. abscessus (1.3) M. chelonae (2.3) |
M. fortuitum (2); M. malmoense (2) | |
|
| ||||||
| England, Wales and Northern Ireland (January 2007-July 2012)77 | I: 16294 | MAC (35.6) | M. gordonae (16.7) |
M. abscessus (5.0) M. chelonae (9.7) |
M. fortuitum (8.2) | M. kansasii (5.9); M. xenopi (5.9) |
|
| ||||||
| France (2002–2013)140 | D: 92 |
M. avium (29.3) M. intracellulare (29.3) |
M. kansasii (17.4) | M. xenopi (16.3) | M. abscessus (2.2) | M. fortuitum (1.1) |
|
| ||||||
| France (2009–2014)141 | D: 477 |
M. avium (31.2) M. intracellulare (28.1) |
M. xenopi (19.7) | M. kansasii (5.7) |
M. abscessus complex (3.8) M. chelonae (<1) |
M. fortuitum (2.7) |
|
| ||||||
| Germany (2006–2016) 142 | I: 216 | MAC (33.3) | M. gordonae (23.6) | M. xenopi (15.2) | M. abscessus complex (9.3) | - |
|
| ||||||
| Greece (January 2007-May 2013)84 | I: 122 | M. gordonae (13.9) |
M. avium (13.1) M. intracellulare (9.8) |
M. fortuitum (12.2) | M. lentiflavum (4.9); M. peregrinum (4.9) |
M. abscessus (1.6) M. chelonae (2.4); M. xenopi (2.4) |
| D: 12 |
M. avium (25) M. intracellulare (25) |
M. abscessus (8.3); M. fortuitum (8.3); M. gordonae (8.3); M. xenopi (8.3) | - | - | - | |
|
| ||||||
| Ireland (January 2007-July 2012)143 | I: 37 |
M. avium (59.5) M. intracellulare (10.8) |
M. gordonae (10.8) |
M. abscessus (5.4) M. chelonae (5.4); M. szulgai (5.4) |
M. malmoense (2.7) | - |
|
| ||||||
| The Netherlands (2008 – 2013)144 | D: 63 |
M. avium (36.5) M. chimera (4.8) M. intracellulare (12.7) |
M. malmoense (11.1) | M. kansasii (9.5) | M. abscessus (6.3) | M. simiae (4.8) |
|
| ||||||
| Poland (2010–2015)145 | I: 73 |
M. avium (22) M. intracellulare (8); M. kansasii (22) |
M. gordonae (19) | M. xenopi (11) | M. fortuitum (6) | M. abscessus (3) |
| D: 36 | M. kansasii (36) |
M. avium (28) M. intracellulare (11) |
M. xenopi (8) | M. abscessus (6) | M. gordonae (3) | |
|
| ||||||
| Poland (2013–2017)146 | I: 2799† | M. kansasii (27.2) |
M. avium (22) M. intracellulare (6.2) |
M. xenopi (16) | M. gordonae (14.5) | M. fortuitum (5.9) |
|
| ||||||
| Portugal (2005–2014)147 | I: 365 | MAC (48.2) | M. gordonae (13.7) | M. peregrinum (11) |
M. abscessus (1.6) M. chelonae (7.9) |
M. kansasii (5.8) |
|
| ||||||
| Scotland (2000–2010)86 | I: 933 | MAC (44.8) | M. malmoense (21.7) |
M. abscessus (13.7) M. chelonae (2.6) |
M. xenopi (4.5) | M. kansasii (3.9) |
|
| ||||||
| Serbia (2009–2016)148 | I: 296† | M. gordonae (22.3) | M. fortuitum (21.3) | M. xenopi (13.5) | M. peregrinum (12.2) | MAC (9.8)) |
| D: 83 | MAC (30.1) | M. xenopi (24.1) | M. kansasii (18.1) | - | - | |
|
| ||||||
| Serbia (2010–2015)74 | I: 565 | M. xenopi (17.3) | M. gordonae (12.9) | M. fortuitum (11.3) |
M. abscessus (4.6) M. chelonae (4.1) |
M. kansasii (4.2) |
| D: 126+ | M. xenopi (28.6) |
M. abscessus (15.1) M. chelonae (2.4) |
M. kansasii (11.1) | M. fortuitum (10.3) |
M. avium (7.9) M. intracellulare (6.3) |
|
|
| ||||||
| Spain (1994–2014)75 | I: 680 | M. kansasii (28.5) | MAC (20.4) | M. xenopi (14.1) | M. abscessus (2.5) | - |
| D: 257** | M. kansasii (59.9) | MAC (26.1) | M. xenopi (6.2) | M. abscessus (4.3) | - | |
|
| ||||||
| Spain (1994–2015)76 (only RGM) | I:116 | M. fortuitum (45.7) |
M. abscessus (5.2) M. chelonae (33.6) |
M. mucogenicum (8.6) | M. mageritense (1.7); M. peregrinum (1.7) | - |
| D: 15 |
M. abscessus (40) M. chelonae (26.7) |
M. fortuitum (26.7) | M. mucogenicum (6.7) | - | - | |
|
| ||||||
| Spain (1997–2016)78 | D: 327 | M. kansasii (83.8) | MAC (13.1) | - | - | - |
|
| ||||||
| Spain (2007–2013)149 | I: 156 D: 34+ |
MAC (64.1) MAC (79.4) |
M. gordonae (12.2) M. genavense (8.8) |
M. abscessus (1.9) M. chelonae (8.3) M. abscessus (2.9) M. chelonae (5.9) |
M. fortuitum (5.8) M. malmoense (2.9); M. xenopi (2.9) |
M. lentiflavum (4.5); M. scrofulaceum (4.5) - |
|
| ||||||
| Spain (2013–2017)150 | I: 314 |
M. avium (48.4) M. intracellulare (16.6) |
M. fortuitum (12.1) | M. gordonae (8.0) | M. lentiflavum (4.1) |
M. abscessus (<1) M. chelonae (3.2) |
|
| ||||||
| Switzerland (2015–2020)151 | I: 236† | M. gordonae (24.6) |
M. avium (23.3) M. chimera (3.4) M. intracellulare (6.8) |
M. xenopi (11.9) |
M. abscessus subsp. abscessus (6.4) M. chelonae (4.2) |
M. fortuitum (4.2) |
|
| ||||||
| The United Kingdom (2007–2014)152 | I: 853 |
M. avium (21.2) M. intracellulare (31.3) |
M. gordonae (15.2) |
M. abscessus (8.4) M. chelonae (8.4) |
M. xenopi (7.9) | M. malmoense (4.7) |
|
| ||||||
| Africa | ||||||
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| Botswana (Aug 2012–Nov 2014) (HIV–infected)89 | I: 228 |
M. avium (2.2) M. intracellulare (47.8) |
M. gordonae (7) | M. malmoense (3.9) | M. simiae (3.5) | M. asiaticum (2.6); M. fortuitum (2.6); M. scrofulaceum (2.6) |
|
| ||||||
| Ethiopia (2017)93 | I: 35 | M. simiae (42.9) | M. abscessus complex (14.3) | M. fortuitum (11.4) |
M. avium complex (5.7) M. intracellulare (8.6); M. gordonae (8.6) |
M. kansasii (2.9); M. scrofulaceum (2.9); M. szulgai (2.9) |
|
| ||||||
| Gabon (Jan 2018–Dec 2020)97 | I: 137 |
M. avium (5.1) M. intracellulare (54) |
M. fortuitum (21.9) |
M. abscessus (6.6) M. chelonae (2.2) |
M. kansasii (4.4) | M. mucogenicum (1.5) |
|
| ||||||
| Ghana (2012–2014)90 | I: 43 |
M. avium subsp. paratuberculosis (30.2); M. colombiense (7); M. intracellulare (41.9) |
M. abscessus (11.3) | M. mucogenicum (7) | M. simiae (2.3) | - |
|
| ||||||
| Ghana (Jan 2013– Mar 2014)91 |
I: 38 | MAC (23.7) | M. chelonae complex (7.9); M. simiae (7.9) | M. fortuitum complex (5.3) | M. arupense (2.6); M. flavescens (2.6); M. kansasii (2.6); M. terrae (2.6) | - |
|
| ||||||
| Kenya (2020)94 | I: 146 | MAC (31) | M. fortuitum complex (20) | M. abscessus complex (14) | - | - |
|
| ||||||
| Mali (2006–2013)92 | I:41 | M. avium (24.4) | M. specie (7.3) | M. simiae (4.9) | - | - |
|
| ||||||
| Nigeria, Cameroon, Ghana (Jan-Dec 2017)153 | I: 14 | M. fortuitum (35.7) |
M. engbaekii (14.3); M. avium (7.1) M. colombiense (7.1) M. intracellulare (14.3) |
M. gordonae (7.1); M. paraense (7.1); M. peregrinum (7.1) | - | - |
|
| ||||||
| Tanzania (Nov 2012–Jan 2013)95 | I: 36 | M. gordonae (16.7); M. interjectum (16.7) |
M. avium (5.5) M. colombiense (2.8) M. intracellulare (11.1) |
M. scrofulaceum (8.3) | M. fortuitum (5.5); M. kumamotonense (5.5) | - |
|
| ||||||
| Tanzania (Nov 2019 - Aug 2020)154 | I: 24 |
M. avium (8.3) M. intracellulare (16.7) |
M. abscessus subsp. abscessus (12.5) M. abscessus subsp. bolletii (4.2) |
M. fortuitum group (8.3) |
M. kansasii (4.2); M. simiae (4.2); M. szulgai (4.2) | - |
|
| ||||||
| Tunisia (2002–2016)88 | I: 30 | M. kansasii (23.3) | M. fortuitum (16.7); M. novocastrense (16.7) | M. chelonae (10) | M. gadium (6. 6); M. gordonae (6. 6) | M. flavescens (3.3); M. peregrinum (3.3); M. porcinum (3.3) |
|
| ||||||
| East Asia | ||||||
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| China (2008–2012)155 | I: 616 | M. kansasii (45) |
M. avium (3.6) M. intracellulare (20.8) |
M. chelonae-abscessus complex (14.9) | M. fortuitum (4.5) | - |
|
| ||||||
| China (2009–2019)156 | I: 1102 |
M. avium (13.2) M. intracellulare (54.8) |
M. chelonae-abscessus complex (16.5) | M. kansasii (8.2) | M. gordonae (3.3) | M. fortuitum (<1) |
|
| ||||||
| China (2010–2015)120 | I: 232 |
M. avium (4.7) M. intracellulare (40.5) |
M. abscessus (28.4) | M. kansasii (9.9) | M. fortuitum (8.6) | M. gordonae (4.3) |
| D: 72* |
M. avium (2.8) M. intracellulare (52.8) |
M. abscessus (33.3) | M. kansasii (9.7) | M. szulgai (1.4) | - | |
|
| ||||||
| China (2013–2016)157 | D: 607 |
M. avium (16.3) M. intracellulare (28.2) |
M. abscessus subsp. abscessus (23.9) M. abscessus subsp. massiliense (16.6) |
M. kansasii (10.0) | M. fortuitum (2.8) | M. gordonae (1.0) |
|
| ||||||
| China (2014–2021)119 | I: 1755 |
M. avium (7.8) M. intracellulare (51.6) |
M. abscessus (22.2) | M. kansasii (8.3) | M. fortuitum (2.1); M. gordonae (2.1) | M. paragordonae (1.3) |
|
| ||||||
| China (2015–2020)118 | I: 789 | M. abscessus (35.1) |
M. avium (6.8) M. intracellulare (12.8) |
M. fortuitum (10.1) | M. kansasii (10.0) | M. gordonae (9.4) |
|
| ||||||
| China (2017–2018)158 | D: 87 |
M. avium (11.5) M. intracellulare (70.1) |
M. chelonae-abscessus complex (11.5) | M. kansasii (7.5) | M. gordonae (1.1) | - |
|
| ||||||
| China (2018)159 | I: 24 |
M. avium (4.2) M. intracellulare (66.7) |
M. abscessus (12.5) | M. kansasii (8.3) | M. fortuitum (4.2); M. szulgai (4.2) | - |
|
| ||||||
| China (2019–2020)117 | D: 458 |
M. avium (8.5) M. intracellulare (52.6) |
M. abscessus complex (23.1) | M. kansasii (8.1) | M. szulgai (2.6) | - |
|
| ||||||
| Japan (2000–2013)108 | D: 592+ |
M. avium (70.6) M. intracellulare (22.3) |
- | - | - | - |
|
| ||||||
| Japan (2001–2009)132 | D: 975 |
M. avium (42.6) M. intracellulare (44.3) |
M. abscessus (3.1) M. chelonae (<1) |
M. gordonae (2.1); M. kansasii (2.1) | - | - |
|
| ||||||
| Japan (2006–2016)109 | I: 3620 | MAC (82.2) |
M. abscessus complex (4.5) M. chelonae (<1) |
M. kansasii (3.7) | M. fortuitum (2.1) | M. peregrinum (<1) |
| D: 2155+ | MAC (87.2) | M. abscessus complex (5.5) | M. kansasii (3.9) | M. fortuitum (1.3) | - | |
|
| ||||||
| Japan (2009–2015)160 | I: 416 |
M. abscessus (31) M. chelonae (11) |
M. avium (5) M. intracellulare (20) |
M. gordonae (18) | M. fortuitum (11) | - |
| D: 114 |
M. abscessus (36) M. chelonae (10) |
M. avium (6) M. intracellulare (27) |
M. fortuitum (10) | M. gordonae (6) | - | |
|
| ||||||
| Japan (2012–2013)105 | I: 26059 |
M. avium (61.8) M. intracellulare (31.1) |
M. kansasii (2.1) |
M. abscessus (2.0) M. chelonae (<1) |
M. fortuitum (1.1) | M. terrae (<1) |
| D: 7167+ |
M. avium (65.1) M. intracellulare (32.4) |
M. kansasii (3.4) | M. abscessus (2.7) | - | - | |
|
| ||||||
| South Korea (2001–2015)104 | D: 2329 | MAC (75) | M. abscessus (22) | M. kansasii (3) | - | - |
|
| ||||||
| South Korea (2006–2016)100 | D: 1017 | MAC (63.6) | M. abscessus complex (10.8) | M. fortuitum (2.1); M. kansasii (2.1) | - | - |
|
| ||||||
| South Korea (2007–2019)102 | I: 2807 |
M. avium (19.2) M. intracellulare (50.6) |
M. fortuitum complex (4.7) |
M. abscessus (4.5) M. chelonae (1.0) |
M. gordonae (3.5) | M. kansasii (1.1) |
|
| ||||||
| South Korea (2009–2015)103 | I: 5558 |
M. avium (23.1) M. intracellulare (38.9) |
M. abscessus (8.4) | M. kansasii (7.7) | - | - |
|
| ||||||
| South Korea (2014–2019)101 | I: 4962 |
M. avium (17.5) M. intracellulare (42.3) |
M. abscessus (7.0) M. chelonae (3.3) |
M. fortuitum (5.5) | M. kansasii (3.6) | M. mucogenicum (2.7) |
|
| ||||||
| Taiwan (2000–2012)115 | D: 3317 | MAC (41.5) |
M. abscessus (21.4) M. chelonae (9.4) |
M. fortuitum (13.4) | M. kansasii (7.1) | M. gordonae (5.0) |
|
| ||||||
| Taiwan (2007–2010)114 | I: 894 | MAC (32.4) |
M. abscessus complex (9.2) M. chelonae complex (17.6) |
M. fortuitum complex (17) | M. kansasii (9.8) | M. gordonae (7.0) |
|
| ||||||
| Taiwan (2010–2014)116 | D: 1674 | MAC (34.4) | M. abscessus (24.3) | M. kansasii (11) | M. fortuitum (9.8) | M. gordonae (4.7) |
|
| ||||||
| South & Southeast Asia | ||||||
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| India (1981–2020)161 | I: 2071 | MAC (18.9) |
M. abscessus (8.8) M. chelonae (10.3) |
M. fortuitum (9.8) | M. gordonae (6.9) | M. terrae (6.3) |
|
| ||||||
| India (2013–2015)126 | I: 209 |
M. abscessus (31.1) M. chelonae (8.1) |
M. fortuitum (20.6) |
M. avium (8.1) M. intracellulare (13.4) |
M. interjectum (4.3); M. simiae (4.3) | M. gordonae (3.3) |
|
| ||||||
| India (2014–2015)127 | I: 164 | M. chelonae (26.8) | M. fortuitum (12.8) | M. gordonae (9.1) | M. kansasii (6.1) | M. simiae (4.9) |
|
| ||||||
| India (2015–2020)125 | I: 43 |
M. abscessus complex (37.2) M. chelonae (2.3) |
M. fortuitum (23.3) |
M. chimaera (2.3) M. colombiense (2.3) M. intracellulare (7.0) M. marseillense (2.3) |
M. parascrofulace um (4.7); M. simiae (4.7) | - |
|
| ||||||
| Pakistan (2016–2019)122 | I: 169 | MAC (61) | M. abscessus (24) | M. fortuitum (5.5); M. kansasii (5.5) | M. gordonae (1.8); M. szulgai (1.8) | - |
|
| ||||||
| Singapore (2011–2012)162 | I: 511 |
M. abscessus (39.5) M. chelonae (1.8) |
M. fortuitum (16.6) | M. kansasii (15.5) | M. avium (15.1) | M. gordonae (7.0) |
|
| ||||||
| Singapore (2012–2016)163 | I: 2026† | M. chelonae-abscessus complex (49.9) | M. fortuitum (17) | MAC (15.4) | M. kansasii (11.5) | M. haemophilum (2.0) |
| D: 352 | M. chelonae-abscessus complex (56) | MAC (28.1) | M. fortuitum group (25.9) | M. kansasii (20.2) | M. scrofulaceum (2.3) | |
|
| ||||||
| West and Central Asia | ||||||
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| Iran (2011–2017)124 | I: 236 | M. abscessus (19.1) | - | - | - | - |
|
| ||||||
| Iran (2016–2018)123 | I: 95 | M. fortuitum (48.4) | M. simiae (16.8) | M. kansasii (15.7) | M. abscessus (7.3) | M. thermoresistibile (4.2) |
|
| ||||||
| Turkey (2015–2019)121 | I: 45† | M. fortuitum (24.4) |
M. abscessus (17.7) M. chelonae (8.9) |
M. lentiflavum (11.1); M. simiae (11.1) |
M. avium (4.4) M. intracellulare (8.9) |
M. gordonae (6.7) |
|
| ||||||
| Saudi Arabia (2006–2012)164 | I: 142 | MAC (35) | M. fortuitum (24) | M. chelonae-abscessus complex (17) | M. gordonae (6) | M. kansasii (4) |
| D: 40 | MAC (47.5) | M. abscessus (25) | M. kansasii (10) | M. fortuitum (7.5) | M. szulgai (5) | |
|
| ||||||
| Oceania | ||||||
|
| ||||||
| Location (dates) | N | Most Common Species (%) | ||||
|
| ||||||
| Australia (2001–2016)41 | I: 12219† |
M. avium (9.8) M. intracellulare (39.1) |
M. abscessus (8.5) M. chelonae (3.3) |
M. fortuitum (8.3) | M. kansasii (2.4) | - |
|
| ||||||
| French Polynesia (2008–2013)128 | I: 87† |
M. fortuitum (11.5) M. porcinum (18.4) M. senegalense (13.8) |
M. abscessus (29.9) M. bolletii (1.1) M. massiliense (1.1) |
M. mucogenicum complex (9.2) |
M. avium (1.1) M. chimaera (3.4) M.intracellular e (1.1) |
- |
|
| ||||||
| Papua New Guinea (2010–2012)129 | I: 9 |
M. avium (33.3) M. intracellulare (22.2); M. fortuitum (33.3) |
M. terrae (22.2) | - | - | - |
|
| ||||||
MAC – Mycobacterium avium complex
RGM – rapidly growing nontuberculous mycobacterial species
Included only patients whose radiographic disease pattern could be classified as cavitary, bronochiectatic, or consolidative
≥2 positive cultures or antimicrobial chemotherapy
M. avium and M. intracellulare concurrently isolated
Included patients with NTM isolated from extrapulmonary sites/specimens from extrapulmonary sites
Sub-analysis of study population
NTM-PD defined using ATS microbiologic criteria
In Florida, a study using the statewide clinical network and defining cases based on a single ICD code found an increasing disease prevalence, from 14.3/100,000 in 2012 to 22.6/100,000 in 2018 55. A second study in Florida at a single large academic medical center with NTM defined by pulmonary isolation found that M. abscessus was the predominant species, representing 39.1% of pulmonary isolates 56 (Table 3). The finding of a predominance of M. abscessus in Florida is consistent with a recent study showing that the South had the highest proportion of M. abscessus isolates, and Florida had the highest 5-year period prevalence of M. abscessus (17%) 57.
A population-based study in three counties of North Carolina (Durham, Wake, and Orange) estimated the prevalence of NTM isolation from 2006 through 2010 using primarily laboratory reports, for an average annual isolation prevalence of 9.4/100,000 (excluding M. gordonae; 11.5 including M. gordonae)58. MAC comprised 50.9% of isolates, followed by M gordonae (20.4%) and M. abscessus complex (13.6) (Table 3) 58. Cumulative incidence of NTM isolation was 8.8/100,000 across Durham County, Orange County, and Wake County (Table 2) 38. A statewide study in Oregon from 2007 to 2012 used the ATS microbiologic criteria to define NTM-PD and found an average annual incidence of 5/100,000, ranging from 4.8/100,000 in 2007 to 5.6/100,000 in 2012 10. The predominant species were MAC (85.7%), followed by rapidly growing mycobacteria (8.1%: M. abscessus/chelonae complex; M. chelonae; M. fortuitum complex) 10 (Table 2, Table 3).
Ontario, Canada
A prior report59 showed a significant increase in NTM isolation and disease (ATS microbiologic criteria) from 1998–2010, based on analysis of isolates from the Public Health Ontario Laboratory, which represents approximately 95% of NTM isolates in Ontario. A more recent analysis in a subset of patients being treated for NTM-PD in a tertiary care center and who met the full ATS NTM disease diagnostic criteria found a significant increase in M. avium-PD between the periods 2009–2012 and 2015–2018 and no significant change in non-M. avium species in the same period, demonstrating that the increase in NTM-PD is being driven by M. avium 60.
Central and South America
We identified six studies published between 2014 and 2022 from French Guiana 61, Mexico 62, Brazil 63–65, and Panama 66. All studies, apart from one, were single-center studies. The results of these studies may be impacted by referral bias. In addition, many studies lack a denominator in a defined population, which the limits the comparability of results.
In French Guiana, a retrospective, observational study of three hospitals during the period 2008–2018, identified 178 patients with NTM positive sputum cultures and 31 patients with NTM-PD. Incidence of NTM isolation and disease was 6.17/100,000 and 1.07/1000,000. This study defined disease using the full ATS diagnostic criteria. M. avium (52%) was the most common species among patients with NTM-PD, followed by M. intracellulare (29%), and M. abscessus (16%). Among patients with NTM isolates, M. fortuitum (23%) was most commonly identified, followed by M. intracellulare (17%), and M. avium (12%) 61.
A single center, retrospective study in Mexico City identified 158 patients that met the ATS criteria. The average annual isolation incidence increased for slow growing species from 0.6/1,000 admissions (2001–2011) to 1.9/1,000 admissions (2012–2017) and for rapidly growing species from 0.3/1,000 admissions (2001–2011) to 1.1/1,000 admissions (2012–2017) (Table 2). From these patients, the most common species were MAC (47%), M. abscessus (27.3%), and M. fortuitum (27.3%) (Table 3) 62.
Many studies on NTM in Brazil occur at the state-level and evaluate patient records at large, state referral hospitals that treat TB and NTM-related diseases. A retrospective, single institution study of patients in the state of Rio Grande do Sul identified 100 patients that met the full ATS criteria for NTM-PD (2003– 2013). The most common NTM species were M. avium (26%), M. kansasii (17%), M. abscessus (12%), and M. intracellulare (9%) (Table 3) 63. In the state of São Paulo, 448 patients were identified as NTM-PD using the ATS microbiologic criteria. From these patients, M. kansasii (29.2%), M. avium (20.8%), M. intracellulare (19.6%), and M. abscessus (17.2%) were the most common species (Table 3) 64. In the state of Ceará, NTM was isolated from pulmonary samples from 42 patients between 2005 and 2016. A high proportion of isolates were not sent to the National Reference Laboratory for identification (81%). Of the species that were identified, the most common were M. avium (4.8%), M. fortuitum (4.8%), and M. abscessus (4.8%) (Table 3) 65.
A study at the national tuberculous laboratory in Montevideo, Uruguay identified 255 NTM isolates from 204 TB suspects during 2006–2018; 210 were collected from pulmonary samples. Most NTM isolates were identified as MAC species (57.6%), followed by M. kansasii (8.2%), M. gordonae (5.9%), and M. peregrinum (4.7%) (Table 3) 67.
Europe
Studies from ten European countries published between 2014 and 2022 have calculated the incidence or prevalence of NTM isolation and/or NTM-PD (Table 2). Data sets, study populations and identification methods of NTM-PD patients differed significantly, thus comparison of the incidence or prevalence is difficult. Some of these studies have also calculated trends over the respective study period: either a stable (Denmark 68, France 69, The Netherlands 70) or increasing NTM-isolation or NTM-PD incidence or prevalence (Germany 26, 71, 72, Portugal 73, Serbia 74, Spain 75, 76, UK 77) was seen. Decreasing incidences for NTM isolation as reported in one study from Spain 78 and one from UK 79 are explained by the dominating decrease in M. kansasii isolation and PD in the Bilbao region 78 and by the selection of the data source, e.g. primary care records, where the decrease of the incidence and prevalence of the strictly defined NTM-PD cases probably represented a shift of management of NTM-PD towards secondary care 79. Species isolated from respiratory secretions or causing NTM-PD varies widely among countries and even within countries (Table 3); of note are the high percentages of NTM-PD caused by M. xenopi in Croatia, Czech Republic and Serbia, by M. kansasii in Poland and some Spanish regions, and by M. malmoense in Scotland and The Netherlands, resembling isolation data published by the NTM-NET 80. A changing epidemiology of species causing NTM-PD over time is also noted in some regions 78, such that analysis of aggregate trends may obscure species-specific changes. In Europe COPD often is a concomitant disease, with a mean age around 60 years and a generally similar overall male: female sex distribution with some exceptions (Table 4).
Table 4:
Age and Sex distribution of NTM isolations from respiratory specimen (I) and NTM pulmonary disease (D) by Region
| North America | |||||
|---|---|---|---|---|---|
|
| |||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/History of TB (%) | Other Pulmonary Disease (%) |
|
| |||||
| Florida, U.S. (2011–2017)56 | I: 271 | 60.5 | 28.5 | 20.8/2.2/2.2 | Asthma (2.8), CF (11.1),ILD (3.6), Lung Cancer (7.5) |
|
| |||||
| Florida, U.S. (2012–2018)55 | D: 7963 (ICD-9/10) | - | 54 | 27.7/ 30.7/10.6 | CF (4.1) |
|
| |||||
| Hawaii, U.S. (2005– 2013)52 | I: 201 | 65.2 | 50 | 39.8/27.9/- | Lung Cancer (12) |
| D: 254+ | 66 | 57 | 43/44/- | Lung Cancer (11) | |
|
| |||||
| Hawaii, U.S. (2005–2019)53 | I: 739 | Median: 63 | 54 | -/-/- | - |
|
| |||||
| Illinois, U.S. (2000–2012)135 | I: 448 | 63.0 | 63 | 11–22#/-/5–9# | - |
|
| |||||
| Iowa, U.S. (1996–2017)136 | D: 185 | Median: 63 | 55 | 30.3/26.5/- | CF (7.6), ILD (5.9), Structural Lung Disease (60.5) |
|
| |||||
| North Carolina, U.S. (2006– 2010)58 | I: 750 | - | 41.2 | -/-/- | - |
|
| |||||
| North Carolina, U.S. (2006– 2010)38 | I: 507 | 60 | 51.8 | -/-/- | - |
|
| |||||
| Oregon, U.S. (2007–2012)10 | I: 806 | Median: 66 | 49.6 | -/-/- | - |
| D: 1146+ | Median: 69 | 55.7 | -/-/- | - | |
|
| |||||
| U.S. (2001– 2015)47 | I: 4676 (ICD-9/ICD-10) | - | 0.13 | 100/2.5/2.6 | Asthma (12.2), ILD (12.8), Lung Cancer (3.1) |
|
| |||||
| U.S. (2008– 2015)45 | D: 6280 (ICD-9/ICD-10) | 69 | 67.6 | 52.6/37/7 | Aspergillosis (3.0), Asthma (23.2), CF (1.7) |
|
| |||||
| U.S.-Affiliated Pacific Island Jurisdictions (2007–2011)54 | I: 35‡ | Median: 34.8 | 49.2 | 27.6/-/44.8 | - |
|
| |||||
|
| |||||
| Central & South America | |||||
|
| |||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/History of TB (%) | Other Pulmonary Disease (%) |
|
| |||||
| Ceará, Brazil (2005–2016)65 | I: 69† | 38.6 | 26.1 | 11.9/-/73.8 | All (13)* |
|
| |||||
| French Guiana (2008–2018)61 | 178 (D: 31, I: 147) | 49 | 39.3 | 17/5/16 | Chronic Pulmonary Disease (33) |
|
| |||||
| Mexico City, Mexico (2001–2017)62 | D: 67 | Median: 52–61## | 48–50## | -/-/- | Any (13)* |
|
| |||||
| Rio Grande do Sul, Brazil (2003–2013)63 | D: 100 | 54.6 | 49 | 17/22/85 | CF (1), Silicosis (1) |
|
| |||||
|
| |||||
| Europe | |||||
|
| |||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/History of TB (%) | Other Pulmonary Disease (%) |
|
| |||||
| Belgium (2010–2017)138 | D: 165 | Median: 64 | 43 | 41.8/20/7.3 | Aspergillosis (4.2), CF (6.1), Prior NTM-PD (13.9) |
|
| |||||
| Croatia (2006–2015)139 | D: 137 | Median: 66 | 47.5 | 45.3/29.2/27.7 | Asthma (2.2), Prior NTM-PD (5.1) |
|
| |||||
| France (2002–2013)140 | D: 92 | Median: 61.5 | 42.4 | 14.1/17.4/12 | All (46.7)* |
|
| |||||
| France (2009–2014)141 | D: 477 | Median: 65 | 29.6–56.2# | -/-/31.4 | All (68)* |
|
| |||||
| France (2010–2017)69 | D: 5628 (ICD–10 or suggestive medication) | 60.9 | 47.1 | 18.8/10.6/14.1 | CF (3.2), Lung Cancer (5.7) |
|
| |||||
| Germany (2009–2014)72 | D: 85–126 per year (ICD-10) (2009–2014) | 55–61 (2009–2014) | 43.4–52.9 (2009–2014) | 62.4–79.2 (2009–2014)/6.6–18.3 (2009–2014)/13.5–24 (2009–2014) | Asthma (20.8–29.2) (2009–2014), Lung Cancer (3.5–10.3) (2009–2014) |
|
| |||||
| Germany (2010–2011)26 | D: 125 (ICD–10) | 49.8 | 50.4 | - | - |
|
| |||||
| Germany (2011–2016)71 | D: 218 (ICD-10, German modification) | 61.4 | 41.3 | 46.3/10.1/17 | Asthma (25.2). ILD (3.7) |
|
| |||||
| Greece (January 2007–May 2013)84 | I: 62 | – | – | 42/35/13 | Asthma (6.4) CF (1.6) |
| D: 12 | - | - | 50/25/41 | Asthma (8.3) | |
|
| |||||
| The Netherlands (2008–2013)144 | D: 63 | 60.8 | 50.8 | 66.7–71.4#/-/10.5–14.3# | Asthma (1.6–15.8#), Lung Cancer (5.3–14.3#) |
|
| |||||
| Poland (2010–2015)145 | D: 36 | 58.5 | 83.3 | 19/33/28 | Asthma (6), CF (8), ILD (28), Lung Cancer (16.7) |
|
| |||||
| Portugal (2002–2012)73 | D: 632 (NTM–PD treatment)† | Median: 54 | 39.7 | 6.3/-/- | ILD (3.3) |
|
| |||||
| Serbia (2009–2016)148 | D: 85† | 59.2 | 34.1 | 40/10/13 | - |
|
| |||||
| Serbia (2010–2015)74 | D: 126+ | 65.4 | 47.6 | - | - |
|
| |||||
| Spain (1997–2016)78 | D: 327 | 56.8 | 28.8 | 30.1/29.3/- | Any (56)* |
|
| |||||
| The United Kingdom (2007–2014)152 | D: 112 | 65 | 51.8 | 44.5/38.4/9.8 | Asthma (16.9), ILD (5.4) |
|
| |||||
|
| |||||
| East Asia | |||||
|
| |||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/History of TB (%) | Other Pulmonary Disease (%) |
|
| |||||
| China (2010–2015)120 | D: 72 | 54.1 | 47.2 | 8.3/6.9/15.3 | Silicosis (2.8) |
|
| |||||
| China (2013–2016)157 | D: 607 | - | 56.2 | 4.1/58.6/59.6 | - |
|
| |||||
| China (2014–2021)119 | I: 1755 | - | 53.6 | -/-/- | - |
|
| |||||
| China (2015–2020)118 | I: 789 | Median: 36 | 39.0 | -/-/22.4 | - |
|
| |||||
| China (2017–2018)158 | D: 87 | Median: 60 | 41.4 | 6.9/19.5/64.4 | - |
|
| |||||
| China (2019–2020)117 | D: 458 | - | 34.9 | 9.2/26.2/45.6 | Asthma (2.2) |
|
| |||||
| Japan (2004–2006)23 | D: 309 (ICD-8–10, estimated from Death Statistics) | 67 | 64.7 | 3.2/4.2/19.1 | Asthma (3.2), ILD (1.3), Lung Cancer (1.3) |
|
| |||||
| Japan (1999–2010)165 | D: 782 | 68.1 | 68.5 | 4.3/-/11.6 | ILD (3.5) |
|
| |||||
| Japan (2000–2013)108 | D: 592+ | 66.0 | 61.1 | 6.4/-/9.5 | Asthma (6.3), ILD (6.6) |
|
| |||||
| Japan (2001–2009)132 | D: 975 | 71.2 | 69.7 | 5.8/0.3/13.5 | Asthma (1.8), ILD (4.0), Lung Cancer (5.5), Silicosis (1.3) |
|
| |||||
| Japan (2006–2016)109 | D: 2155+ | Median: 69 | 66.5 | -/-/- | - |
|
| |||||
| Japan (2009–2014)107 | D (Incident):11034 (2011) (ICD-10) | 69.3 | 69.6 | 6.9/23.5 /6.3 | Aspergillosis (6.1), ILD (9.9), Lung Cancer (5.3) |
|
| |||||
| Japan (2009–2015)160 | D: 114 | Median: 74–78.5# | 59–68# | (5–24)#/(11–15)#/(16–17)# | Asthma (8–17)#, ILD (5–8)# |
|
| |||||
| Japan (2013–2015)166 | D: 184++ | 69.5 | 75.5 | -/5.4/8.2 | - |
|
| |||||
| Japan (2012–2013)105 | D: 7167+ | 73.6** | 65.5** | -/-/- | - |
|
| |||||
| Japan (2014)167 | D: 419 (ICD–10) | Median: 59 | 68 | 3.1/-/- | Aspergillosis (1.9), Asthma (18.3), DPB (1.6), ILD (6.0), Lung Cancer (4.5) |
|
| |||||
| South Korea (2001–2015)104 | D: 2329 | Median: 60 | 59 | -/-/- | - |
|
| |||||
| South Korea (2003–2016)98 | D: 46194 (ICD–10) | 55.8 | 61.1 | -/-/- | - |
|
| |||||
| South Korea (2006–2016)100 | D: 1017 | 62.7 | 58.8 | 13.5/83.9/48.3 | Asthma (7.3), Lung Cancer (4.0) |
|
| |||||
| South Korea (2007–2018)99 | D: 45321 (ICD–10) | - | 56.9 | -/-/- | - |
|
| |||||
| South Korea (2007–2019)102 | I: 2984† | - | 42.3 | -/-/- | - |
|
| |||||
| South Korea (2009–2015)133 | D: 52551 (ICD-10)† | 53.0 | 57.2 | 25.6/21.9/33.7 | Asthma (33.2), ILD (2.6), Lung Cancer (5.8) |
|
| |||||
| Taiwan (2000–2012)115 | D: 3317 | - | 42.3 | -/-/- | - |
|
| |||||
| Taiwan (2003–2018)113 | D(treatment case): 558 (ICD-9-CM)† | 62.5 | 42.5 | -/-/- | Chronic Lung Disease (42.8) |
|
| |||||
| Taiwan (2005–2013)112 | D: 450 (ICD-9-CM)† | - | 38 | -/-/- | - |
|
| |||||
| Taiwan (2007–2010)114 | I: 894 | - | 44.4 | -/-/- | - |
|
| |||||
| Taiwan (2010–2014)116 | D: 1674 | - | 44.3 | 23.4/15.6/25.4 | Asthma (5.2), ILD (6) |
|
| |||||
|
| |||||
| South-Southeast Asia | |||||
|
| |||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/History of TB (%) | Other Pulmonary Disease (%) |
|
| |||||
| India (1981–2020)161 | D: 365 | - | 44 | -/3/65 | - |
|
| |||||
| India (2013–2015)126 | I: 263† | Median: 48 | 39.5 | -/-/- | - |
|
| |||||
| India (2015–2020)125 | I: 105† | - | 50.5 | 5.7/-/16.2 | - |
|
| |||||
| Pakistan (2016–2019)122 | I: 169 | Median: 45 | 39 | -/-/65 | - |
|
| |||||
| Singapore (2011–2012)162 | I: 485 | Median: 70 | 37.9 | 14.2/28.7/34.4 | Asthma (6.6); ILD (3.7) |
|
| |||||
| Singapore (2012–2016)163 | D: 352 | Median: 67 | 48.3 | 13.1/36.4/27.6 | Asthma (4.0) |
|
| |||||
|
| |||||
| Central & West Asia | |||||
|
| |||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/History of TB (%) | Other Pulmonary Disease (%) |
|
| |||||
| Iran (2016–2018)123 | I: 95 | 47.4 | 42.1 | -/-/- | - |
|
| |||||
| Saudi Arabia (2006–2012)164 | D: 40 | 54 | 42 | 7.5/15/12 | Asthma (15), CF (2.5), ILD (10) |
|
| |||||
|
| |||||
| Oceania | |||||
|
| |||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/History of TB (%) | Other Pulmonary Disease (%) |
|
| |||||
| Australia (2001–2016)41 | I: 12219† | Median: 66 | 50.1 | -/-/- | - |
|
| |||||
| Australia (2012–2015)3 | I: 1222† | Median: 66 | 51 | -/-/- | - |
|
| |||||
| Papua New Guinea (2010–2012)129 | I: 9 | 35.7 | 88.9 | -/-/- | - |
ICD-9 – International diagnostic classification of diseases – ninth revision
ICD-10 – International diagnostic classification of diseases – tenth revision
COPD – Chronic Obstructive Pulmonary Disease
TB – Tuberculosis
SGM – slow-growing nontuberculous mycobacteria
RGM – rapid-growing nontuberculous mycobacteria
ILD – Interstitial lung disease
DPB – Diffuse panbrochiolitis
CF – Cystic Fibrosis
All pulmonary comorbidities, including COPD, Bronchiectasis, History of TB
MAC patients only
Included patients with NTM isolated from extrapulmonary sites/specimens from extrapulmonary sites
NTM-PD defined using ATS microbiologic criteria
NTM-PD defined using full ATS criteria or 2008 Japanese Society for Tuberculosis/Japanese Respiratory Society Guidelines for the diagnosis of NTM pulmonary infection
Range by NTM species
Range by RGM and SGM
Sub-analysis of study population
Czech Republic
NTM isolates from the Public Health Institute Ostrava representing the Moravian and Silesian Regions from eastern Czech Republic were classified according to ATS diagnostic criteria and the incidence of each NTM species presented as average annual incidence in the studied period 2012–2018: The average incidence of NTM-PD patients was 1.10/100,000 (1.33/100,000 in men and 0.88/100,000 in women) during the study period, with MAC-PD patients predominantly women and M. kansasii-PD and M. xenopi-PD predominantly men, with distinct epidemiology in local districts 81.
Denmark
A retrospective nationwide cohort study including microbiological data from the Danish International Reference Laboratory (Staten Serum Institute) in Denmark from 1991–2015 reported an annual (isolation) incidence rate of NTM isolation from a pulmonary site of 2.14/100,000 in adults aged 15 and above and (using ATS microbiologic criteria only) an incidence of 1.10/100,000 for cases with definite and possible NTM disease. No significant trend towards an increase or decrease for definite NTM disease incidence (that included patients with definite NTM-PD as defined by the authors, which were not reported separately) was found 68. However, a more recent study using ICD codes and representing the Central region of Denmark found an increasing trend in NTM-PD, suggesting a potential increase in clinically relevant disease 82
France
The National Health System Database (Système National des Données de Santé -SNDS) which covers more than 99% of the French population was used to detect newly diagnosed patients with NTM-PD not previously treated (using outpatient drug consumptions) or hospitalized for NTM-PD in the last 3 years, using ICD-10 codes. A stable incidence of NTM-PD between 1.025/100,000 in 2010 and 1.096/100,000 in 2017 was reported. The NTM-PD prevalence was estimated at 5.92/100,000 inhabitants over 8 years 69. The dataset did not include untreated NTM-PD patients (incidence and prevalence probably underestimated); however, the stable incidence is likely valid for France from 2010 through 2017 (Figure 4).
Figure 4.
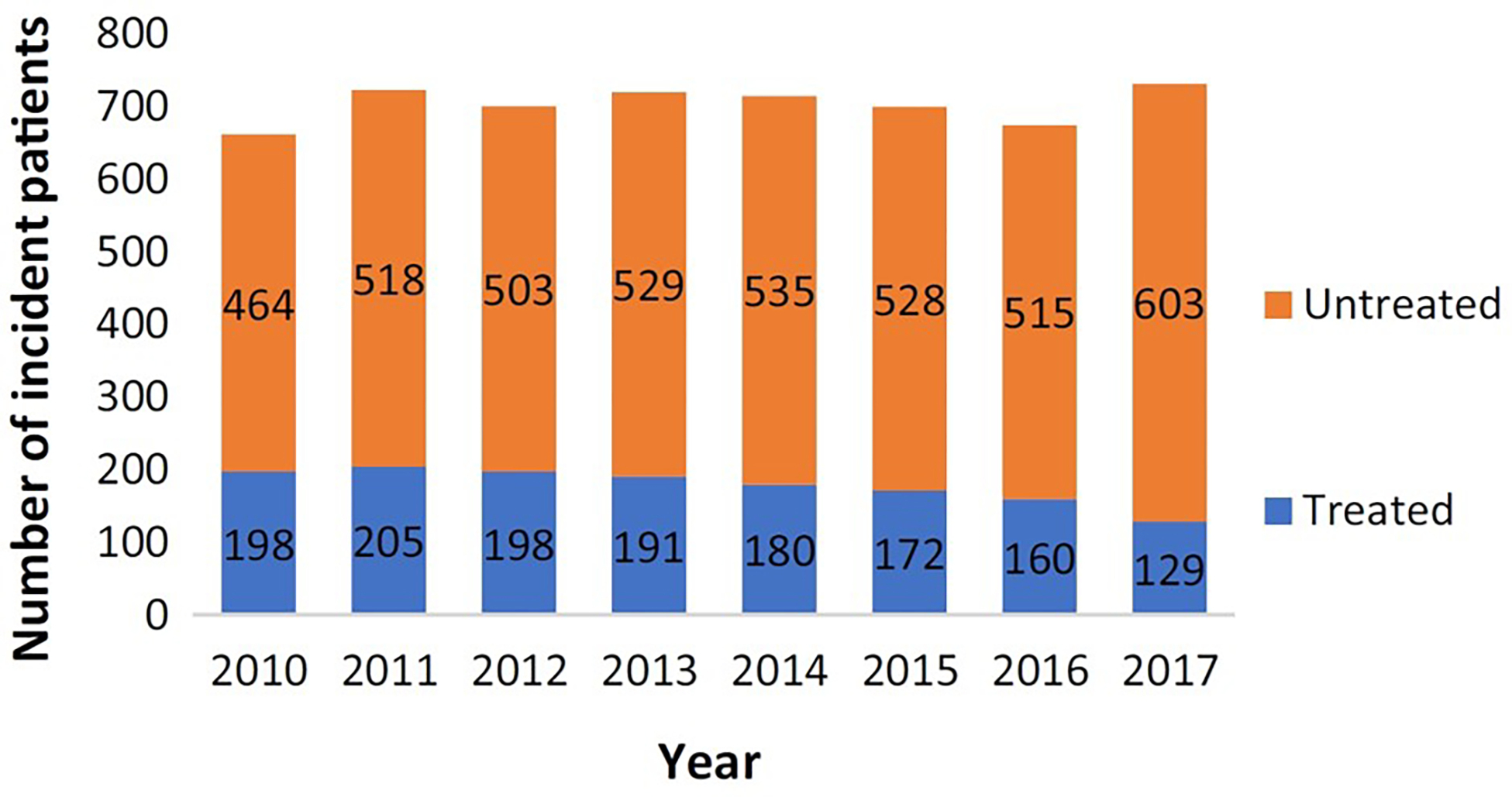
Incidence of NTM-PD in France by treatment status from 2010–2017. From Veziris N, Andréjak C, Bouée S, Emery C, Obradovic M, Chiron R. Non-tuberculous mycobacterial pulmonary diseases in France: an 8 years nationwide study. BMC Infect Dis. Nov 17 2021;21(1):1165. doi:10.1186/s12879-021-06825-x; with permission. (Figure 1 in original)
Germany
In Germany, a population-based cohort study with a nested case–control design used ICD-10 codes from > 80 German company statutory health insurance datasets for the years 2010–2011 to define 125 patients with NTM-PD and to calculate yearly incidence rates of 1.12/100,000 in 2010 and 1.48/100,000 in 2011 26. Similarly, an anonymized German health claims database with an ICD-10 code 2011–2016, was analyzed to find 218 incident NTM-PD patients; prevalence of 3.79/100,000 was estimated. Using a prediction model; the prevalence of NTM-PD for both ICD-10-coded and non-coded individuals was five-fold higher at 19.05/100,000 71. A third German study based on the Health Risk Institute health services research database (subset of ≈7 million persons) used ICD-10 codes to identify NTM-PD patients from 2009 to 2014. The data showed an increase in the annual overall prevalence rate from 2.3/100,000 to 3.3/100,000 72. Using ICD-10 codes, it is probable that these studies underestimate the incidence and prevalence of NTM-PD as agreement of ICD-10 codes with the ATS diagnostic criteria has not been studied in Germany. The positive predictive value of ICD-9-CM codes ranged from 57–64%, and sensitivity for the diagnosis of NTM-PD to be low ranging from 21% to 26.9% 83.
Greece
Adult in- and outpatients of the second largest tertiary referral hospital for patients with respiratory diseases in Athens, were retrospectively assessed for the presence of NTM isolation and NTM-PD using the ATS criteria. The reported incidence of pulmonary isolation (18.9/100,000 patients) and disease (8.8/100,000 patients) during the study period (2007–2013) was calculated as the total number of patients with respective isolation and disease divided by the total number of patients who attended this hospital as in- or outpatients, e.g. do not reflect the population of the area 84.
Portugal
All 632 NTM patients treated at tuberculosis outpatient centres in Portugal (defined as NTM-PD patients) that were recorded in the electronic database (structured questionnaire and stored in the National Tuberculosis Surveillance System (SVIG-TB), Lisbon, Portugal) between 2002–2012 were retrospectively analyzed. The estimated annual incidence of treated NTM-PD was 0.54/100,000, during the study period, an increase of NTM-PD, mostly due to an increase in MAC-PD, was described 73. The inclusion of only treated NTM-PD patients may have led to an underestimation; however, the fact that 41 of the treated patients had M. gordonae isolates leaves questions regarding the accuracy of the classification of NTM-PD in the patient cohort 85.
Serbia
In Serbia, a retrospective and noninterventional study was performed by the national TB laboratory network representative for the whole country. Overall, 565 patients with 777 pulmonary NTM isolates collected between 2010 and 2015 were analysed for the presence of NTM-PD using the ATS microbiologic criteria. The annual isolation incidence of NTM increased from 0.9/100,000 to 1.6/100,000 without reaching statistical significance. In contrast, annual incidence rates of NTM-PD increased from 0.18/100,000 to 0.47/100,000. The most frequent NTM species in this country was M. xenopi (with annual incidence rates of 0.04/100,000–0.11/100,000), the annual incidence rate for MAC-PD was low (between 0.01/100,000 and 0.07/100,000) 74.
Spain
In the Basque region in northern Spain, incidence rates of NTM-PD (as defined by ATS criteria) were calculated using the data from the Microbiology Laboratory and clinical records. Incidence of NTM-PD decreased from 1997–2016 significantly over the years from 10.6/100,000 and 8.8/100,000 in 2000 and 2001, respectively, to 1.8/100,000 in 2016. This decrease was solely explained by an annual decrease of 6.5% in M. kansasii-PD, whereas M. avium-PD had an annual rise of 3.3% 78. In 13 municipalities in the Barcelona-South Health Region of Catalonia, a similar trend for the annual prevalence rates per 100,000 population for both M. kansasii and MAC isolation and pulmonary disease has been calculated for the period between 1994–2014, with similar high decreases of 9% for isolation of, and 11% for disease due to M. kansasii and increases of 10% for isolation of and 13% for disease due to MAC. Here overall pulmonary disease prevalence was calculated at 42.8/100,000 75. Rapid-growing mycobacteria isolated at a reference mycobacterial laboratory of the Catalan region similarly increased 5-fold from 2003 (0.34/100,000) to 2015 (1.73/100,000) 76. The three studies performed in Spain during 1997 and 2016 show an increase of the incidence of MAC-isolation, of MAC-PD and of isolation of rapid growing mycobacteria, but a decrease in M. kansasii-isolation and M. kansasii–PD, supporting the conclusion that epidemiology of NTM should consider the different mycobacterial species separately.
The Netherlands
A study in the Netherlands used four separate databases, including two drug dispensing databases, an ICD-10 code database and a hospitalization database to estimate the prevalence of NTM-PD for the whole country between 2012 and 2019 by assigning the patients to “probable” NTM-PD patients and “possible” NTM-PD patients depending on the drug regimen used in the drug prescription databases, and to “confirmed NTM-PD patients” when the ICD-10 code A31.0 had been used. Prevalence estimates of the ICD-10 codes were corrected for the limited sensitivity (50%) for NTM-PD seen in previous studies and ranged in the different databases between 2.3/100,000 and 5.9/100,000 inhabitants, whereas a simultaneously performed survey amongst pulmonologists (likely over-) estimated the annual NTM-PD prevalence between 6.2/100,000 and 9.9/100,000. No increase was seen during the study period, in any of the study populations 70 (Figure 5)
Figure 5.
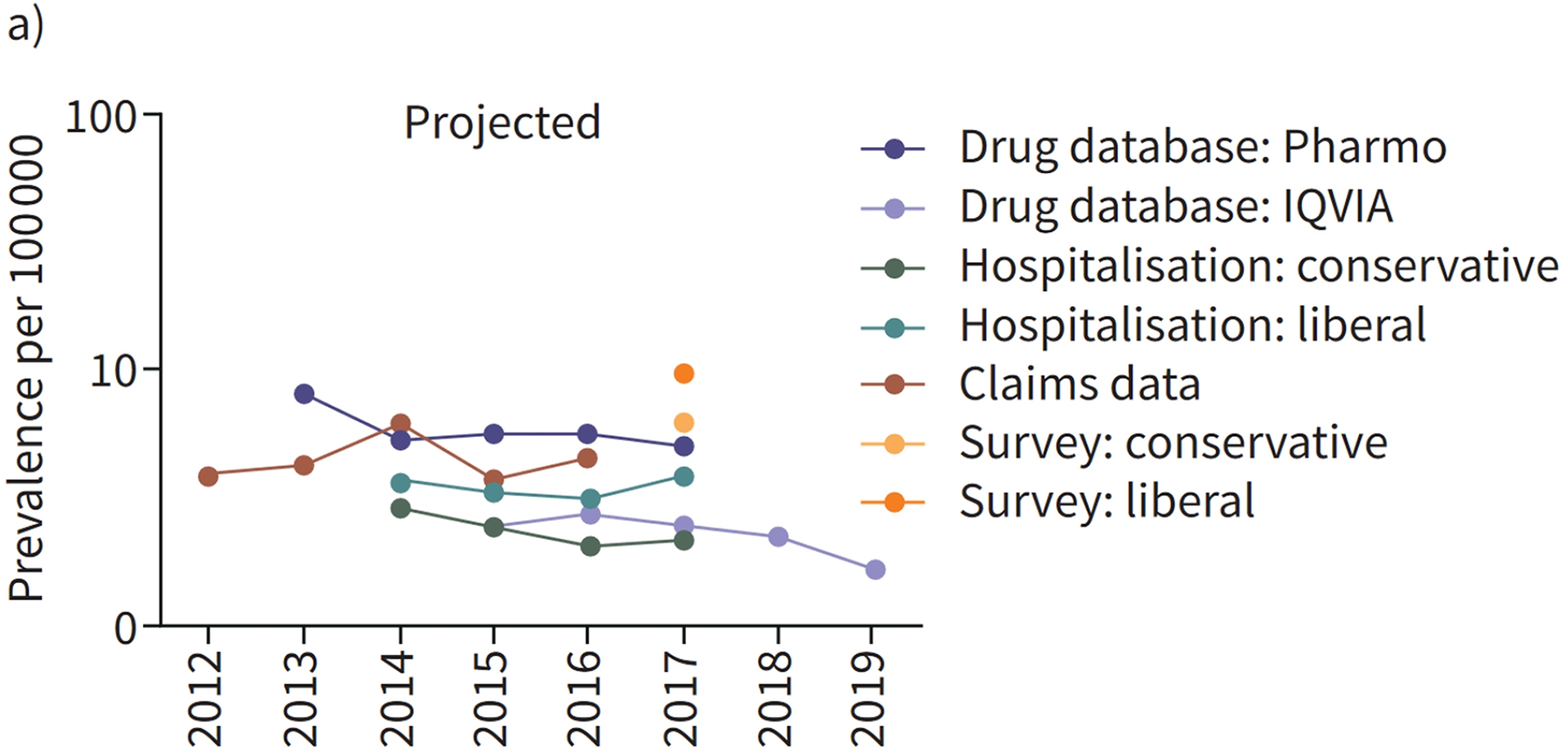
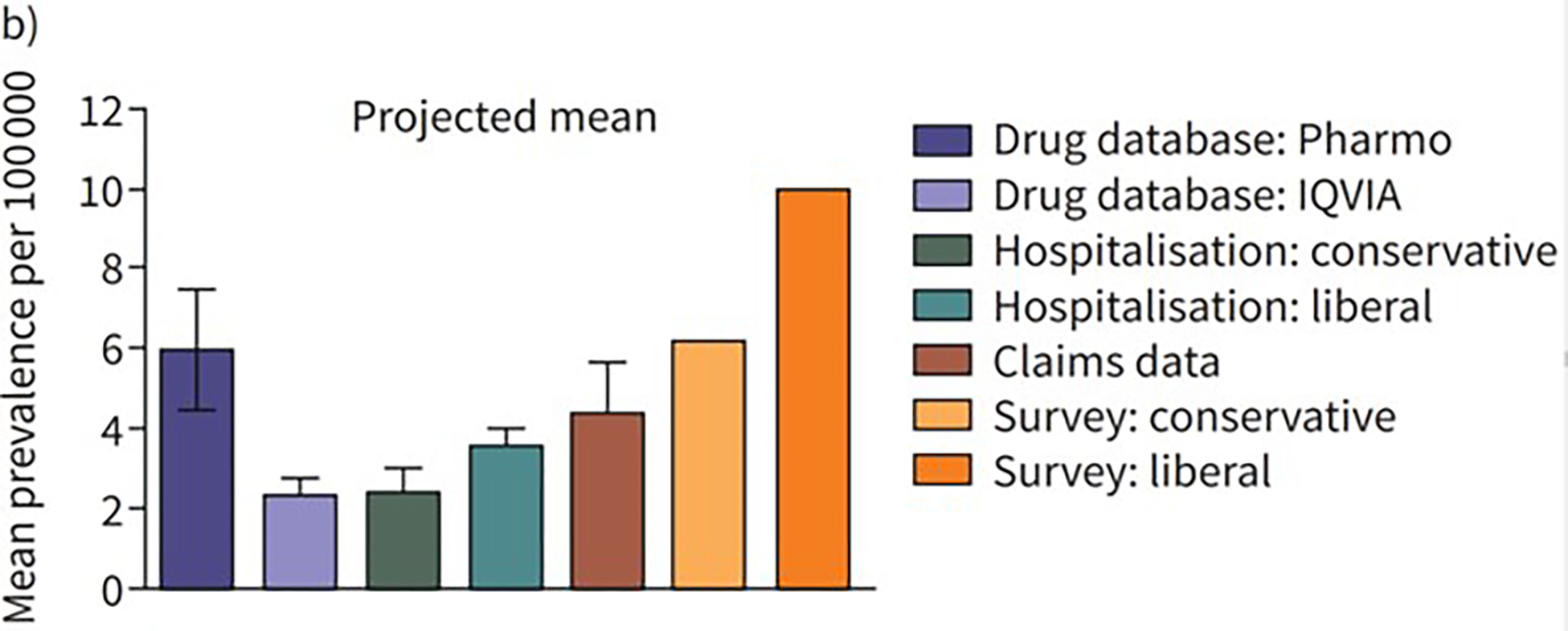
a. Annual prevalence per 100,000 of NTM-PD in the Netherlands by database (2012–2019). From Schildkraut JA, Zweijpfenning SMH, Nap M, et al. The epidemiology of nontuberculous mycobacterial pulmonary disease in the Netherlands. ERJ Open Res. Jul 2021;7(3)doi:10.1183/23120541.00207-2021; with permission. 5b. Mean prevalence per 100,000 (2012–2019) of NTM-PD by database (Schildkraut et al., 2021); with permission. (Figure 1 in original). Reproduced with permission of the ERS 2023. ERJ Open Res 7: 00207–2021; DOI: 10.1183/23120541.00207-2021 Published 12 July 2021.
The United Kingdom
Primary care electronic healthcare records (Clinical Practice Research Datalink) representing 6.8% of the UK population were used to extract data for patients with NTM isolates during 2006 and 2016 and to classify patients as highly likely to have NTM-PD (‘strict cohort’, 85% of these being treated) and possible NTM-PD (‘expanded cohort’). A significant decrease in the incidence in the strict cohort from 2006 (3.85/100,000 person-years) to 2016 (1.28/100,000 person-years) was paralleled by a decrease of NTM-PD prevalence from 7.68/100,000 to 4.7/100,000 in primary care patients. This trend probably represents a shift of care of these (treated) patients towards secondary care 79. This assessment is supported by a study from England, Wales and Northern Ireland that included all culture positive NTM isolates between 2007 and 2012 reported to Public Health England by five mycobacterial reference laboratories. The NTM isolation incidence in people with pulmonary isolates rose significantly from 4.0/100,000 to 6.1/100,000, driven by the MAC isolation incidence increasing from 1.3/100,000 in 2007 to 2.2/100,000 in 2012 77. Scottish isolates of NTM that had been submitted between 2000 and 2010 to the Scottish Mycobacteria Reference Laboratory were used to assign NTM-PD status to the respective patients using the ATS microbiologic criteria. A mean rate of 2.43 episodes of infection (that included extrapulmonary infections)/100,000 (range 2.06–2.71) was calculated, without significant trend over the study period. The incidence of NTM-PD was not calculated separately 86.
African Region
Cohorts in African studies from 2014–2022 comprise patients with presumptive tuberculosis and/or patients with “chronic pulmonary TB” that were retrospectively analyzed. Data on NTM-PD were still rare and are mostly from studies from South Africa published before 2014 87, and thus not included in this review. Most of the studies have not performed chest imaging and seldom repetitive sputum isolation, so criteria for NTM-PD could not be applied. The data still suggest that a potentially significant proportion of African TB suspects may have NTM disease but may not be detected in routine care. The increasing availability of rapid TB-identification tests has decreased the number of studies reporting patients as being treated as MDR-TB while actually having NTM-PD. Good population-based studies to determine the incidence of NTM-PD in African countries are lacking; isolation prevalence in TB-suspects is reported here (Table 5). Similar to previous studies summarized by Okoi 87 most studies report an isolation prevalence of below 10%. The ratio of growth of NTM to all mycobacterial growth varied significantly between 1% (Nigeria), 12% (Mali), 54% (Botswana) and 78% (Zambia), which may not be explained solely by different patient populations and detection methods (Table 5). The species isolated from respiratory secretions differ significantly within the African continent with MAC being the most frequent isolated species in most studies (Table 3). Maps of the continent with the distribution of the most frequent isolates from respiratory diseases and species causing NTM-PD have been published in a review covering African publications from 1940–2016 87.
Table 5:
Studies of Rates of Pulmonary NTM Isolates in Africa
| Prevalence of Sputum Isolates | ||||||||
|---|---|---|---|---|---|---|---|---|
| Location (dates) | Patient Cohort | Isolation Method | Ratio NTM/ Mycobacterial Growth (%) | Period Prevalence of NTM Isolation | ||||
| Period Duration (years) | Prevalence | |||||||
| Botswana (Aug 2012–Nov 2014)89 | 228 of 1940 symptomatic HIV infected patients with culture result | negative SD-Bioline Ag MPT64 assay (Abbott); GenoType CM and AS assays (Hain Lifescience) | 53.4 (3.5 with two positive sputum cultures) | 3 | 53.4% among culture positive specimen | |||
| Mali (2006–2013)92 | 41 of 439 patients suspected of having TB | negative MTBc Gen-Probe (AccuProbe); Nucleic acid probes for MAC, M. gordonae, M. kansasii (AccuProbe); gene sequencing | 12.34 | 8 | 9.34% among individuals suspected of having TB | |||
| Nigeria, Cameroon, Ghana (Jan-Dec 2017)153 | 16 of 503 mycobacterial isolates from new and | negative IS6110 PCR; hsp65 PCR with sequencing | Nigeria: 1 Cameroon: 1.3 Ghana: 8.4 |
1 | 3.2% among isolates | |||
| previously treated pulmonary TB patients | ||||||||
| Sub-Saharan Africa (1940–2016)87 | ATS criteria (systematic review) in 37 relevant studies | molecular techniques (n = 26), biochemical testing identification tools (n = 9); immunochromatographic assays (n = 2) | 77 | 7.5% (isolation prevalence from all 37 papers reviewed) | ||||
| Tunisia (2002–2016)88 | 60 of 1863 specimen from HIV-negative patients with presumptive clinical pulmonary TB with culture result | biochemical tests: niacin, nitrate, heat-resistant catalase and para-nitro benzoic acid; PCR targeting the recA intein. | 3.2 | 15 | isolation prevalence of 0.2/100,000 population of northern Tunisia. | |||
| Zambia (Aug 2013-July 2014)96 | 923 symptomatic patients with NTM-isolates among 6123 TB survey participants (1188 with mycobacterial growth) | negative capilia (TAUNS) lateral flow assay | 77.7 | 2 | 1477/100,000 symptomatic NTM-patients among TB survey participants aged 15 years and above | |||
| Age and Sex Distribution and Pre-existing Pulmonary Diseases | ||||||||
| Location (dates) | N | Mean Age (years) | Female (%) | COPD (%)/ Bronchiectasis (%)/TB (%) | Pre-existing Pulmonary Disease (%) | |||
| Botswana (Aug 2012 - Nov 2014)89 | I: 228 | NA; 45 patients > 50 years | 53.90 | -/-/12.4 | - | |||
| Ethiopia (2017)93 | I: 51 | 32.4 (of all TB-aNA NTM-pts) | 51 | -/-/- | - | |||
| Gabon (2018–2020)97 | I: 1363 | 40 | 38.70 | -/-/23.3 | - | |||
| Ghana (2012–2014)90 | I: 43 | 39.4 | 37.2 | -/-/- | - | |||
| Ghana (Jan 2013-Mar 2014) 91 | I: 473 | 39 | 68.4 | -/-/- | - | |||
| Kenya (2020)94 | I: 166 | 39 | 27 | -/-/- | - | |||
| Mali (2006–2013)92 | I: 41 | 35.8 years of all 439 pts | 19.5 | -/-/- | - | |||
| Nigeria, Cameroon, Ghana (Jan-Dec 2017)153 | I: 503 | 45.14 | 35.30 | -/-/- | - | |||
| Subsaharan Africa (1940–2016)87 | D: 266 (from 37 studies) | 35 years based on 17 of 37 studies with age data (isolation) | - | -/-/- | - | |||
| Tanziana (Nov 2019–Aug 2020)154 | I: 24 | 47 | 33 | -/-/- | Retreatment cases (60.9) | |||
| Tunisia (2002–2016)88 | I: 60 | - | 30 | -/-/- | - | |||
| Zambia (Aug 2013–July 2014)96 | I: 923 | NA; age groups 15– 24: 8.7%; 65+: 28.6% | 51.70 | -/7.15/- | Chest X-Ray: Nodules (22), Cavitation ( 13.8), Fibrosis (15) | |||
Sub-Saharan Africa
A systematic review published in 2017 87 retrieved 37 out of 373 articles published from 1940–2016 from which data on pulmonary NTM isolation in patient cohorts from sub-Saharan Africa were extracted. The calculated prevalence of pulmonary NTM isolation was 7.5% and 16.5% (512 of 3,096) in those previously treated for tuberculosis. MAC was the most frequently isolated NTM in 19 of 37 studies with a large variation of MAC isolation prevalence from 15.0% in Tanzania to 57.8% in Mali. NTM-PD as defined in the ATS criteria was described in 7 of the 37 studies: 266 (27.7%) of 962 patients with sufficient data had NTM-PD. M. kansasii (69.2%) was the most common species amongst the isolates 87.
North Africa
A study over a long period (2002–2016) looked retrospectively at sputum collected from HIV-negative Tunisian patients with presumptive pulmonary TB. 60 of 1,864 (3.2%) specimens grew NTM; from 30 isolates 7 were M. kansasii, none belonged to the MAC group, possibly indicating problems in the selection and identification process. The estimated NTM isolation prevalence of 0.2/100,000 population calculated for the regions of Bizerte and Zaghouan during the period 2002–2011, should thus be quoted with caution 88.
Southern Africa
In Botswana, a 2012–2014 substudy of the Xpert evaluation study for TB detection performed in 22 clinical sites (representing 12 out of the 28 districts and included HIV patients). 228/427 (53.4%) of patients with mycobacterial growth had NTM (114 MAC, (109 M. intracellulare), 16 M. gordonae, 9 M. malmoense, 8 M. simiae ), only 8 of these (3.5%) had two positive sputum cultures and presumptive NTM-disease 89.
West Africa
Two studies from 2012–2014 were reported from Ghana looking at 1,755 mycobacterial isolates from smear positive sputum samples 90 in a HIV-positive cohort of 571 patients 91. The NTM isolation rate in smear positive sputum was 2.5%, identified by hsp65 gene PCR as M. avium subs paratuberculosis (30.2%), M. intracellulare (41.9), and M. abscessus (11.3%) 90. In the HIV cohort, the NTM isolation rate was 51% (50/98 patients with mycobacterial growth). 38 NTM isolates were speciated: nine were MAC (23.7%), 3 were M. simiae (7.9%) and 3 were M. chelonae complex (7.9%). Sixty-five percent of the patients with NTM isolates had a CD4-count <100, 24% had died during the 6 months of follow up, similar to 30% of TB patients 91. In a retrospective single-center study from Mali 2006–2013, NTM were detected in 41/439 (9.34%) TB suspects and 41/332 patients with mycobacterial isolations (12.3% isolation). Of the 20 species identified from patients; 50% were M. avium 92.
East Africa
In Ethiopia in 2017, 51 of 697 (7.3%) patients with presumptive pulmonary TB had NTM isolates, 34 had been misclassified as TB (14 new cases, 12 relapses, 7 treatment failures); species were identified in 35 isolates (42.9% M. simiae, 14.3% M. abscessus) 93. A study from Kenya in symptomatic patients detected 166 sputum isolates that were TB negative; of 146 identified, 122 were NTM, mostly MAC (31%), M. fortuitum complex (20%) and M. abscessus complex (14%) 94. In 2012–2013 in 744 sputum samples from 372 TB suspects in Tanzania, mycobacterial cultures were positive in 121 (32.5%) patients, 36 (9.7%) were NTM. The most frequent species of the 28 identified were M. gordonae and M. interjectum (each 6/28) 95. Our search retrieved only one population-based Zambian study 96. 923 participants in a 1-year (Aug 2013-July 2014) national TB prevalence survey, NTM positive sputum cultures and negative TB test were defined as NTM patients without further species differentiation. In this cohort, 923/1,188 (78%) of positive cultures were defined as NTM; 265/1,188 (12%) were MTB. Of the participants with NTM, 71% were symptomatic. The prevalence of symptomatic NTM was estimated at 1,477/100,00096.
Central Africa
In 1,363 sputum samples collected from presumptive TB patients in Gabon (2018–2020) in a mixed retro- and prospective cross-sectional study, 26.6% (137/515) patients with mycobacterial isolates had NTM, most were MAC (61.4%, 81/132) with M. intracellulare dominating (74/81). The next most common species were M. fortuitum (21.9%) and M. abscessus (6.6%)97.
Asian Region
Population-based epidemiological data have been reported from Taiwan, Korea, and Japan, using primarily insurance claims data since 2014. These reports showed a consistent upward trend in these countries. Importantly, the incidence has surpassed that of TB in Japan, similar to Western countries, and the estimated prevalence reached approximately 150/100,000 in 2020. The species distribution shows M. intracellulare predominance in China and Korea, but M. avium is common in Japan. Interestingly, the proportions of cases with M. abscessus species are higher in the southern regions of China, Taiwan, and Japan. The proportion of NTM is also increasing in culture-positive specimens in China. Middle-aged female predominance is reported in most countries. However, patients with underlying lung diseases, such as COPD and previous TB, seem more common in Taiwan and other countries than in Korea and Japan. More epidemiological reports from Asian countries are warranted.
South Korea
During 2014–2022, population-based data on the incidence and prevalence of NTM-PD using the National Health Insurance Service (NHIS) database were reported in South Korea 98, 99. Park et al reported that the incidence rate increased from 1.0/100,000 in 2002 to 17.9/100,000 in 2016; the prevalence rate was 1.2/100,000 in 2003 and increased to 33.3/100,000 in 2016 98 (Figure 6). The 5-year mortality rate was 17.8% (2003–2016), higher among men than among women (28.3% vs. 9.9%). Summarizing population-based and single-center studies, the proportion of NTM-PD patients who were women in South Korea was 56.9–61.1%, with a mean age of 53.0–62.7 years 98–100. Species in the M. avium complex (63.6–75%) were most commonly identified, followed by subspecies of M. abscessus (10.8–22%). M. intracellulare (38.3–50.6%) was isolated more frequently than M. avium (17.5–23.1%), in most regions, although there were exceptions 100–104.
Figure 6.
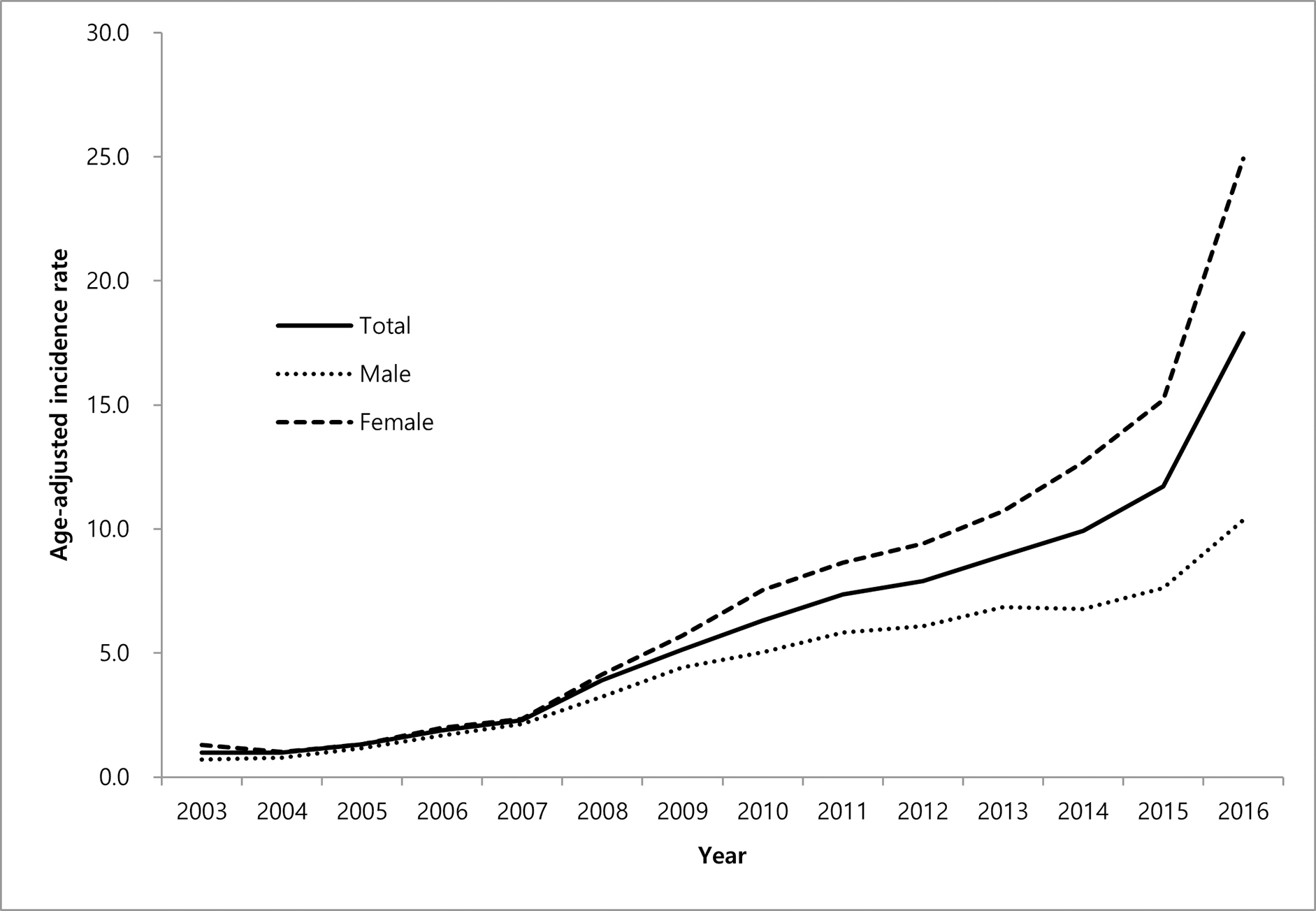
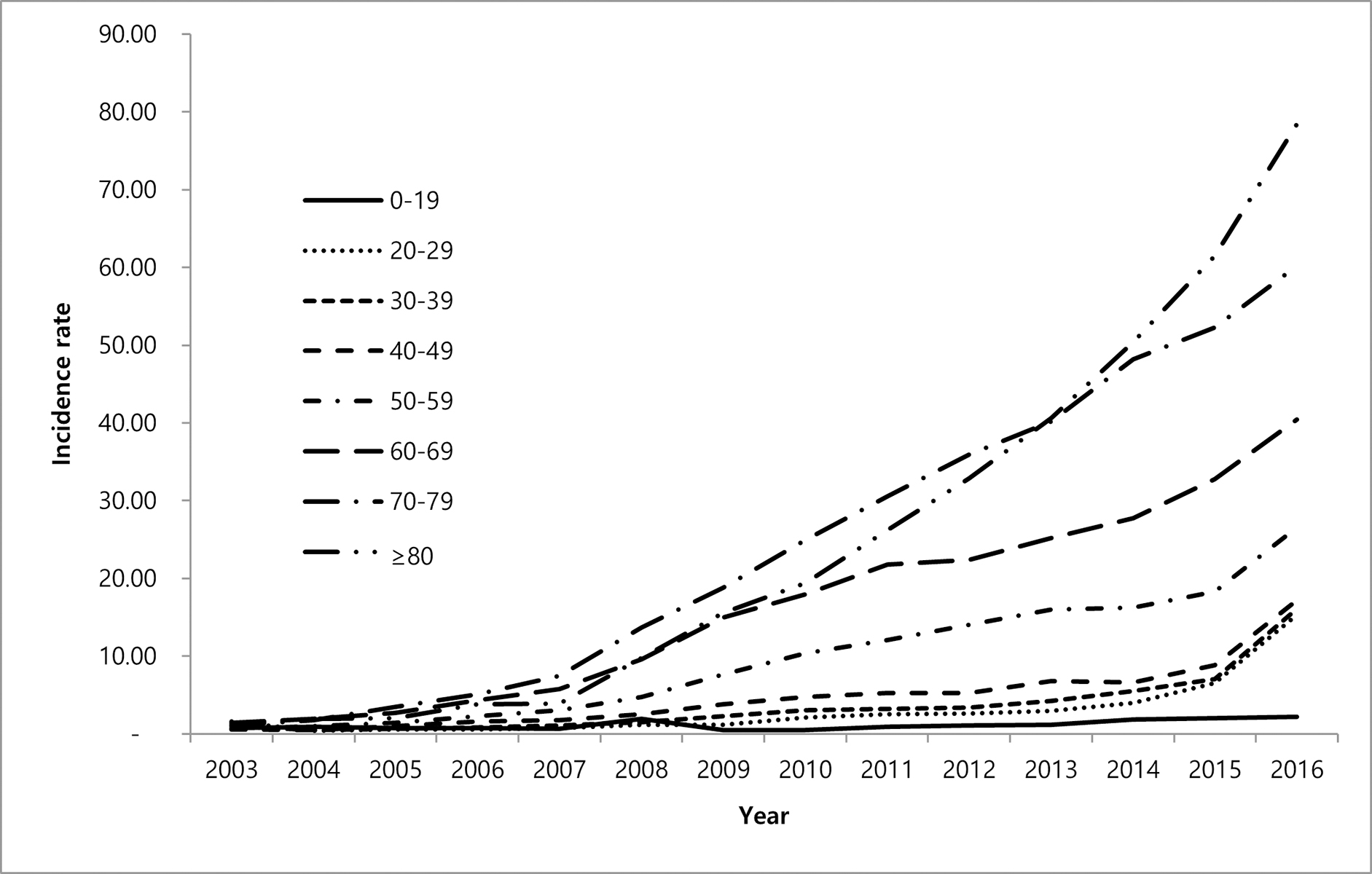
a. Age-adjusted incidence of NTM infection per 100,000 population by sex (2003–2016). From Park SC, Kang MJ, Han CH, et al. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: a nationwide population-based study. BMC Pulm Med. Aug 1 2019;19(1):140. doi:10.1186/s12890-019-0901-z; with permission. 6b. Incidence of NTM infection per 100,000 population by age group (2003–2016) (Park et al., 2019); with permission. (Figure 2 in original)
Japan
A nationwide laboratory data-based study analyzing 7,167 NTM-PD using the ATS microbiologic criteria found a period prevalence of 24 during 2012–2013 105. The most prevalent species was MAC (97.5%), followed by M. kansasii and M. abscessus (2–3%). In contrast to South Korea, the proportion of M. avium was higher than that of M. intracellulare (65.1% vs. 32.4%). Interestingly, the proportion of M. intracellulare gradually increased from the eastern to the western part of Japan and further increased to South Korea 106(Figure 7). Furthermore, the proportion of M. abscessus was higher in the Kyushu-Okinawa regions close to Korea and Taiwan. An insurance claims data analysis showed that the incidence and prevalence rates were 8.6/100,000 treated individuals and 29.0/100,0000 treated individuals, respectively107. Because this study used ICD codes to define disease, prevalence and incidence are likely underestimated. Patient characteristics were similar in South Korea, with an older female predominance105, 108, 109, but lung disease complications were less common compared to South Korea. However, although bronchiectasis was common in females, COPD, sequelae of TB, and interstitial pneumonia were higher in males with age. Analysis of mortality data found that the crude mortality rate increased from 0.003/100,000 to 1.93/100,000 from 1970 to 2016 23, 110. In Japan, annual health exams with regular chest imaging are optional for citizens over 40 and mandated for all workers over age 35. In South Korea, these exams are biannual for persons over age 40, with high compliance rates. These annual exams may lead to detection of milder cases, but are unlikely to explain recent increasing trends, as they have been in place for more than 30 years 111.
Figure 7.
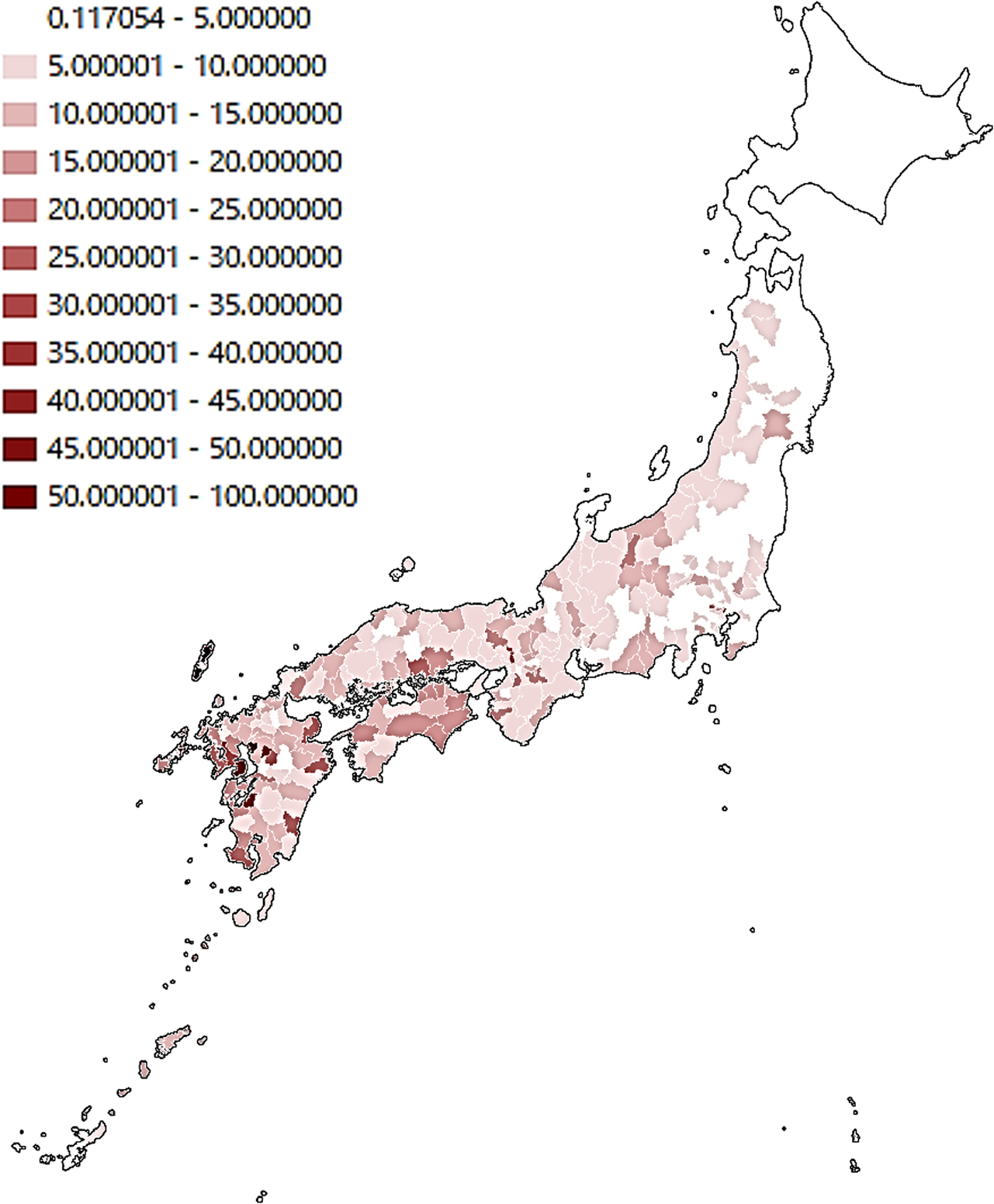
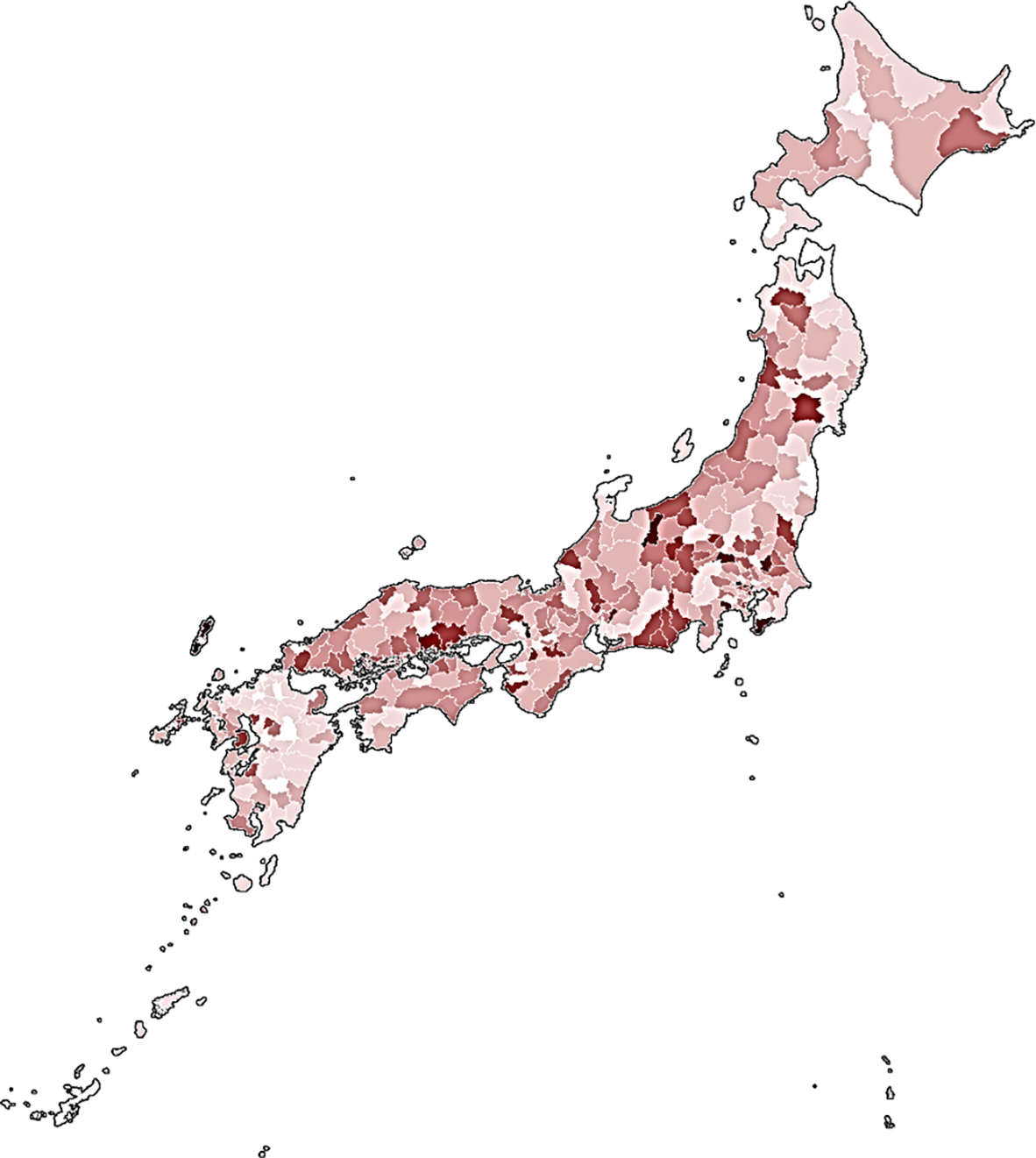
a. Incidence of M. intracellulare-PD per 100,000 population in 344 medical regions of Japan (2011–2013). From Morimoto K, Ato M, Hasegawa N, Mitarai S. Population-Based Distribution of Mycobacterium avium and Mycobacterium intracellulare in Japan. Microbiology Research. 2021;12(3):739–743; with permission. 7b. Incidence of M. avium-PD per 100,000 population in 344 medical regions of Japan (2011–2013) (Morimoto et al., 2021); with permission. (Figure 1 in original)
Taiwan
Well-analyzed studies using several methodologies have been published 112–116. The age-adjusted incidence rate increased from 5.3/100,000 in 2005 to 14.8/100,000 in 2013 112. The data among treated cases, an incidence and prevalence of 0.54/100,000–3.35/100,000 and 0.68/100,000–7.17/100,000, respectively, during the study period from 2003 to 2018 113. MAC was predominant (34.4–41.5%) 114–116, but the proportion of M. abscessus-chelonae complex species (24.3–30.8%) was higher than that for South Korea and Japan. A multicenter study reported that the proportion of M. abscessus was higher in southern Taiwan, while MAC was predominant in the northern part of the country 116. Male predominance was reported in most of the reports, which differed from South Korea and Japan 112–116.
China
No population-based data were found in China or other Asian regions. NTM represented between 4.8–8.5% of all mycobacteria isolated and between 6.6–15.5% of all pulmonary mycobacterial disease cases., with an upward trend 117–119. The isolates and disease proportion were higher in the south coastal area. MAC was predominant, followed by M. abscessus and M. kansasii. The proportion of M. intracellulare (28.2–70.1-%) was higher than that of M. avium (2.8–16.3%) 120, similar to South Korea. The proportion of RGM was higher in the southern region than in the other regions.
Southeast to Western Asia
The proportion of NTM in mycobacterial isolates in Turkey and Pakistan was 5.5–16.2% 121, 122. Single-center studies were reported in Iran123, 124 and India125–127, but the clinical trends and species need to be clarified in the future.
Oceania
In Australia, epidemiologic data are based primarily on notifications from the state of Queensland, where notification of mycobacterial infections is mandated. The number of reported cases in 2015 was 1,222 (25.9/100,000)3, showing a consistently upward trend. Nearly 40% of cases were M. intracellulare, followed by M. avium at 10% and M. abscessus at 8.5%. An isolated strain analysis was reported from French Polynesia and Papua New Guinea 128, 129.
Summary and Research Gaps
NTM-PD and NTM pulmonary isolation are increasing in most countries and regions globally. Isolate-based analysis in several countries indicate distinct trends by NTM species, highlighting the need for more species-specific analyses in NTM epidemiology. Available data regarding species indicates that MAC continues to predominate in most regions, although in Ontario, Canada and distinct European countries M. kansasii, M. xenopi, and M. malmoense are found with greater frequency. Variation in methods and case definitions limit comparisons across countries and regions. Population-based data including incidence and/or prevalence are available from North America, Europe, East Asia (Japan, South Korea, and Taiwan), and Queensland, Australia. Studies from Central and South America, Africa, China, the Middle East, and South Asia (India) among patients with suspected TB and MDR-TB are important to document the burden of undiagnosed NTM in this population and ensure appropriate treatment. This highlights the need for enhanced mycobacterial laboratory capacity to discriminate between cases of TB and NTM by routine speciation of mycobacterial isolates. Clinical data available from several regions have allowed better characterization of the affected populations. Across regions, the majority of cases occur among persons aged >50 years. In North America and East Asia, the proportion with COPD is typically less than 20%, whereas in Europe the proportion with COPD is higher, above 30% in 9 of 13 studies with this information.
Implementation of surveillance systems with standard case definitions is needed to allow for global comparison of prevalence and trends. Current data where NTM-PD is a notifiable condition have shown the importance and utility of these surveillance data. In particular, timely notification data have allowed inference of the incubation period for infection and disease and have further allowed identification of specific high risk environmental niches and environmental risk factors.
Key points.
Population-based data from North America, Europe, and East Asia (primarily Japan, South Korea, and Taiwan) demonstrate continued increases in NTM isolation and disease across regions and in most countries.
In countries and regions where population-based data are not available, including countries within the African continent, regions in China, as well as most of Central or South America, NTM species identification among persons screened for TB provides a measure of the unrecognized burden of NTM.
NTM-PD incidence and prevalence remain generally higher in East Asia (based on data primarily from South Korea, Japan, and Taiwan), North America, and Australia than in Europe
Species-specific differences exist in prevalence, trends, and distribution within countries and regions, and should be considered when analyzing trends data: in most geographic areas globally, increases are driven by MAC or M. avium disease.
Cumulative exposure to soil and water aerosols increases the risk of infection and disease in high-risk populations.
Water quality and composition influences the risk for NTM infection, as has been shown in a study analyzing the concentrations of vanadium and molybdenum in source water for municipal water systems which are associated with an increased risk of MAC and M. abscessus complex in the US.
Synopsis.
NTM isolation and pulmonary disease (NTM-PD) have continued to increase in most regions of the world, driven mainly by M. avium. Single-center studies also support increasing trends as well as a persistent burden of undiagnosed NTM among persons suspected of having tuberculosis (TB), in countries with moderate to high TB prevalence. Cumulative exposure to water and soil presents an increased risk to susceptible hosts, and trace metals in water supply are recently recognised risk factors. Establishing standard case definitions for subnational and national surveillance systems with mandatory notification of NTM-PD are needed to allow comparisons within and across countries and regions.
Clinics Care Points:
When physiicians or other health care providers suspect NTM pumonary infection or disease, they should collect appropriate samples including 3 samples for AFB culture, in addition to chest X rays or High Resolution CT (HRCT).
Acknowledgements
This work was supported in part by the Division of Intramural Research, National Institutes of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, MD.
Footnotes
Disclosures: D Rebecca Prevots, Julia Marshall, and Dirk Wagner have no disclosures. Kozo Morimoto has received funding from INSMED, Japan Agency for Medical Research and Development (AMED), Japan Society for the Promotion of Science, KAKENHI, Boehringer Ingelheim, and Asahi Kasei Pharma Corp
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevots DR, Marras TK. Epidemiology of human pulmonary infection with nontuberculous mycobacteria: a review. Clin Chest Med. Mar 2015;36(1):13–34. doi: 10.1016/j.ccm.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shih DC, Cassidy PM, Perkins KM, Crist MB, Cieslak PR, Leman RL. Extrapulmonary Nontuberculous Mycobacterial Disease Surveillance - Oregon, 2014–2016. MMWR Morb Mortal Wkly Rep. Aug 10 2018;67(31):854–857. doi: 10.15585/mmwr.mm6731a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomson R, Donnan E, Konstantinos A. Notification of Nontuberculous Mycobacteria: An Australian Perspective. Ann Am Thorac Soc. Mar 2017;14(3):318–323. doi: 10.1513/AnnalsATS.201612-994OI [DOI] [PubMed] [Google Scholar]
- 4.Winthrop KL, Henkle E, Walker A, Cassidy M, Hedberg K, Schafer S. On the Reportability of Nontuberculous Mycobacterial Disease to Public Health Authorities. Ann Am Thorac Soc. Mar 2017;14(3):314–317. doi: 10.1513/AnnalsATS.201610o [DOI] [PubMed] [Google Scholar]
- 5.Vrijheid M The exposome: a new paradigm to study the impact of environment on health. Thorax. Sep 2014;69(9):876–8. doi: 10.1136/thoraxjnl-2013-204949 [DOI] [PubMed] [Google Scholar]
- 6.Wild CP, Scalbert A, Herceg Z. Measuring the exposome: A powerful basis for evaluating environmental exposures and cancer risk. Environmental and Molecular Mutagenesis. 2013;54(7):480–499. doi: 10.1002/em.21777 [DOI] [PubMed] [Google Scholar]
- 7.Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. Feb 15 2007;175(4):367–416. doi: 10.1164/rccm.200604-571ST [DOI] [PubMed] [Google Scholar]
- 8.Daley CL, Iaccarino JM, Lange C, et al. Treatment of Nontuberculous Mycobacterial Pulmonary Disease: An Official ATS/ERS/ESCMID/IDSA Clinical Practice Guideline. Clin Infect Dis. Aug 14 2020;71(4):e1–e36. doi: 10.1093/cid/ciaa241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dahl VN, Mølhave M, Fløe A, et al. Global trends of pulmonary infections with nontuberculous mycobacteria: a systematic review. Int J Infect Dis. Oct 13 2022;125:120–131. doi: 10.1016/j.ijid.2022.10.013 [DOI] [PubMed] [Google Scholar]
- 10.Henkle E, Hedberg K, Schafer S, Novosad S, Winthrop KL. Population-based Incidence of Pulmonary Nontuberculous Mycobacterial Disease in Oregon 2007 to 2012. Ann Am Thorac Soc. May 2015;12(5):642–7. doi: 10.1513/AnnalsATS.201412-559OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lipner EM, Crooks JL, French J, Strong M, Nick JA, Prevots DR. Nontuberculous mycobacterial infection and environmental molybdenum in persons with cystic fibrosis: a case-control study in Colorado. J Expo Sci Environ Epidemiol. Mar 2022;32(2):289–294. doi: 10.1038/s41370-021-00360-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lipner EM, French JP, Falkinham JO, 3rd, et al. Nontuberculous Mycobacteria Infection Risk and Trace Metals in Surface Water: A Population-based Ecologic Epidemiologic Study in Oregon. Ann Am Thorac Soc. Apr 2022;19(4):543–550. doi: 10.1513/AnnalsATS.202101-053OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andréjak C, Thomsen V, Johansen IS, et al. Nontuberculous pulmonary mycobacteriosis in Denmark: incidence and prognostic factors. Am J Respir Crit Care Med. Mar 1 2010;181(5):514–21. doi: 10.1164/rccm.200905-0778OC [DOI] [PubMed] [Google Scholar]
- 14.Marras TK, Mehta M, Chedore P, May K, Al Houqani M, Jamieson F. Nontuberculous mycobacterial lung infections in Ontario, Canada: clinical and microbiological characteristics. Lung. Aug 2010;188(4):289–99. doi: 10.1007/s00408-010-9241-8 [DOI] [PubMed] [Google Scholar]
- 15.Ghio AJ, Smith GS, DeFlorio-Barker S, et al. Application of diagnostic criteria for non-tuberculous mycobacterial disease to a case series of mycobacterial-positive isolates. J Clin Tuberc Other Mycobact Dis. Dec 2019;17:100133. doi: 10.1016/j.jctube.2019.100133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prevots DR, Shaw PA, Strickland D, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. Oct 1 2010;182(7):970–6. doi: 10.1164/rccm.201002-0310OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winthrop KL, Baxter R, Liu L, et al. The reliability of diagnostic coding and laboratory data to identify tuberculosis and nontuberculous mycobacterial disease among rheumatoid arthritis patients using anti-tumor necrosis factor therapy. Pharmacoepidemiol Drug Saf. Mar 2011;20(3):229–35. doi: 10.1002/pds.2049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ku JH, Henkle EM, Carlson KF, Marino M, Winthrop KL. Validity of Diagnosis Code-Based Claims to Identify Pulmonary NTM Disease in Bronchiectasis Patients. Emerg Infect Dis. Mar 2021;27(3):982–985. doi: 10.3201/eid2703.203124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Winthrop KL, McNelley E, Kendall B, et al. Pulmonary nontuberculous mycobacterial disease prevalence and clinical features: an emerging public health disease. Am J Respir Crit Care Med. Oct 1 2010;182(7):977–82. doi: 10.1164/rccm.201003-0503OC [DOI] [PubMed] [Google Scholar]
- 20.Mercaldo RA, Marshall JE, Cangelosi GA, et al. Environmental risk of nontuberculous mycobacterial infection: Strategies for advancing methodology. Tuberculosis. 2023/01/10/ 2023:102305. doi: 10.1016/j.tube.2023.102305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grigg C, Jackson KA, Barter D, et al. Epidemiology of Pulmonary and Extrapulmonary Nontuberculous Mycobacteria Infections in Four U.S. Emerging Infections Program Sites: A Six-Month Pilot. Clin Infect Dis. Apr 21 2023;doi: 10.1093/cid/ciad214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinnard C, Longworth S, Mezochow A, Patrawalla A, Kreiswirth BN, Hamilton K. Deaths Related to Nontuberculous Mycobacterial Infections in the United States, 1999–2014. Ann Am Thorac Soc. Nov 2016;13(11):1951–1955. doi: 10.1513/AnnalsATS.201606-474BC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morimoto K, Iwai K, Uchimura K, et al. A steady increase in nontuberculous mycobacteriosis mortality and estimated prevalence in Japan. Ann Am Thorac Soc. Jan 2014;11(1):1–8. doi: 10.1513/AnnalsATS.201303-067OC [DOI] [PubMed] [Google Scholar]
- 24.Marras TK, Vinnard C, Zhang Q, et al. Relative risk of all-cause mortality in patients with nontuberculous mycobacterial lung disease in a US managed care population. Respir Med. Dec 2018;145:80–88. doi: 10.1016/j.rmed.2018.10.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mourad A, Baker AW, Stout JE. Reduction in Expected Survival Associated With Nontuberculous Mycobacterial Pulmonary Disease. Clin Infect Dis. May 18 2021;72(10):e552–e557. doi: 10.1093/cid/ciaa1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diel R, Jacob J, Lampenius N, et al. Burden of non-tuberculous mycobacterial pulmonary disease in Germany. Eur Respir J. Apr 2017;49(4)doi: 10.1183/13993003.02109-2016 [DOI] [PubMed] [Google Scholar]
- 27.Tzou CL, Dirac MA, Becker AL, et al. Association between Mycobacterium avium Complex Pulmonary Disease and Mycobacteria in Home Water and Soil. Annals of the American Thoracic Society. 2020;17(1):57–62. doi: 10.1513/AnnalsATS.201812-915OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomson R, Tolson C, Carter R, Coulter C, Huygens F, Hargreaves M. Isolation of Nontuberculous Mycobacteria (NTM) from Household Water and Shower Aerosols in Patients with Pulmonary Disease Caused by NTM. Journal of Clinical Microbiology. 2013/09/01 2013;51(9):3006–3011. doi: 10.1128/JCM.00899-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dirac MA, Horan KL, Doody DR, et al. Environment or host?: A case-control study of risk factors for Mycobacterium avium complex lung disease. Am J Respir Crit Care Med. Oct 1 2012;186(7):684–91. doi: 10.1164/rccm.201205-0825OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park Y, Kwak SH, Yong SH, et al. The Association between Behavioral Risk Factors and Nontuberculous Mycobacterial Pulmonary Disease. Yonsei Med J. Aug 2021;62(8):702–707. doi: 10.3349/ymj.2021.62.8.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prevots DR, Adjemian J, Fernandez AG, Knowles MR, Olivier KN. Environmental Risks for Nontuberculous Mycobacteria. Individual Exposures and Climatic Factors in the Cystic Fibrosis Population. Annals of the American Thoracic Society. 2014;11(7):1032–1038. doi: 10.1513/AnnalsATS.201404-184OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bouso JM, Burns JJ, Amin R, Livingston FR, Elidemir O. Household proximity to water and nontuberculous mycobacteria in children with cystic fibrosis. Pediatr Pulmonol. Mar 2017;52(3):324–330. doi: 10.1002/ppul.23646 [DOI] [PubMed] [Google Scholar]
- 33.Maekawa K, Ito Y, Hirai T, et al. Environmental risk factors for pulmonary Mycobacterium avium-intracellulare complex disease. Chest. Sep 2011;140(3):723–729. doi: 10.1378/chest.10-2315 [DOI] [PubMed] [Google Scholar]
- 34.Reed C, von Reyn CF, Chamblee S, et al. Environmental risk factors for infection with Mycobacterium avium complex. Am J Epidemiol. Jul 1 2006;164(1):32–40. doi: 10.1093/aje/kwj159 [DOI] [PubMed] [Google Scholar]
- 35.Gebert MJ, Delgado-Baquerizo M, Oliverio AM, et al. Ecological Analyses of Mycobacteria in Showerhead Biofilms and Their Relevance to Human Health. mBio. 2018;9(5):e01614–18. doi:doi: 10.1128/mBio.01614-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lipner EM, French JP, Nelson S, et al. Vanadium in groundwater aquifers increases the risk of MAC pulmonary infection in O’ahu, Hawai’i. Environ Epidemiol. Oct 2022;6(5):e220. doi: 10.1097/ee9.0000000000000220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh J, Shin SH, Choi R, et al. Assessment of 7 trace elements in serum of patients with nontuberculous mycobacterial lung disease. J Trace Elem Med Biol. May 2019;53:84–90. doi: 10.1016/j.jtemb.2019.02.004 [DOI] [PubMed] [Google Scholar]
- 38.DeFlorio-Barker S, Egorov A, Smith GS, et al. Environmental risk factors associated with pulmonary isolation of nontuberculous mycobacteria, a population-based study in the southeastern United States. Sci Total Environ. Apr 1 2021;763:144552. doi: 10.1016/j.scitotenv.2020.144552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adjemian J, Olivier KN, Seitz AE, Falkinham JO, 3rd, Holland SM, Prevots DR. Spatial clusters of nontuberculous mycobacterial lung disease in the United States. Am J Respir Crit Care Med. Sep 15 2012;186(6):553–8. doi: 10.1164/rccm.201205-0913OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Adjemian J, Olivier KN, Prevots DR. Nontuberculous mycobacteria among patients with cystic fibrosis in the United States: screening practices and environmental risk. Am J Respir Crit Care Med. 2014;190(5):581–586. doi: 10.1164/rccm.201405-0884OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomson RM, Furuya-Kanamori L, Coffey C, Bell SC, Knibbs LD, Lau CL. Influence of climate variables on the rising incidence of nontuberculous mycobacterial (NTM) infections in Queensland, Australia 2001–2016. Sci Total Environ. Oct 20 2020;740:139796. doi: 10.1016/j.scitotenv.2020.139796 [DOI] [PubMed] [Google Scholar]
- 42.Chou MP, Clements ACA, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infectious Diseases. 2014/05/21 2014;14(1):279. doi: 10.1186/1471-2334-14-279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maki T, Noda J, Morimoto K, et al. Long-range transport of airborne bacteria over East Asia: Asian dust events carry potentially nontuberculous Mycobacterium populations. Environ Int. Oct 2022;168:107471. doi: 10.1016/j.envint.2022.107471 [DOI] [PubMed] [Google Scholar]
- 44.Schildkraut JA, Gallagher J, Morimoto K, et al. Epidemiology of nontuberculous mycobacterial pulmonary disease in Europe and Japan by Delphi estimation. Respir Med. Nov 2020;173:106164. doi: 10.1016/j.rmed.2020.106164 [DOI] [PubMed] [Google Scholar]
- 45.Winthrop KL, Marras TK, Adjemian J, Zhang H, Wang P, Zhang Q. Incidence and Prevalence of Nontuberculous Mycobacterial Lung Disease in a Large U.S. Managed Care Health Plan, 2008–2015. Ann Am Thorac Soc. Feb 2020;17(2):178–185. doi: 10.1513/AnnalsATS.201804-236OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strollo SE, Adjemian J, Adjemian MK, Prevots DR. The Burden of Pulmonary Nontuberculous Mycobacterial Disease in the United States. Ann Am Thorac Soc. Oct 2015;12(10):1458–64. doi: 10.1513/AnnalsATS.201503-173OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pyarali FF, Schweitzer M, Bagley V, et al. Increasing Non-tuberculous Mycobacteria Infections in Veterans With COPD and Association With Increased Risk of Mortality. Front Med (Lausanne). 2018;5:311. doi: 10.3389/fmed.2018.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spaulding AB, Lai YL, Zelazny AM, et al. Geographic Distribution of Nontuberculous Mycobacterial Species Identified among Clinical Isolates in the United States, 2009–2013. Ann Am Thorac Soc. Nov 2017;14(11):1655–1661. doi: 10.1513/AnnalsATS.201611-860OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dean SG, Ricotta EE, Fintzi J, et al. Mycobacterial Testing Trends, United States, 2009–2015(1). Emerg Infect Dis. Sep 2020;26(9):2243–2246. doi: 10.3201/eid2609.200749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Donohue MJ. Increasing nontuberculous mycobacteria reporting rates and species diversity identified in clinical laboratory reports. BMC Infect Dis. Apr 10 2018;18(1):163. doi: 10.1186/s12879-018-3043-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donohue MJ, Wymer L. Increasing Prevalence Rate of Nontuberculous Mycobacteria Infections in Five States, 2008–2013. Annals of the American Thoracic Society. 2016;13(12):2143–2150. doi: 10.1513/AnnalsATS.201605-353OC [DOI] [PubMed] [Google Scholar]
- 52.Adjemian J, Frankland TB, Daida YG, et al. Epidemiology of Nontuberculous Mycobacterial Lung Disease and Tuberculosis, Hawaii, USA. Emerg Infect Dis. Mar 2017;23(3):439–447. doi: 10.3201/eid2303.161827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Blakney RA, Ricotta EE, Frankland TB, et al. Incidence of Nontuberculous Mycobacterial Pulmonary Infection, by Ethnic Group, Hawaii, USA, 2005–2019. Emerg Infect Dis. Aug 2022;28(8):1543–1550. doi: 10.3201/eid2808.212375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin C, Russell C, Soll B, et al. Increasing Prevalence of Nontuberculous Mycobacteria in Respiratory Specimens from US-Affiliated Pacific Island Jurisdictions(1). Emerg Infect Dis. Mar 2018;24(3):485–491. doi: 10.3201/eid2403.171301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kambali S, Quinonez E, Sharifi A, et al. Pulmonary nontuberculous mycobacterial disease in Florida and association with large-scale natural disasters. BMC Public Health. Nov 10 2021;21(1):2058. doi: 10.1186/s12889-021-12115-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Garcia CV, Teo GE, Zeitler K, et al. The epidemiology, demographics, and comorbidities of pulmonary and extra-pulmonary non-tuberculous mycobacterial infections at a large central Florida Academic Hospital. J Clin Tuberc Other Mycobact Dis. Dec 2021;25:100289. doi: 10.1016/j.jctube.2021.100289. eCollection 2021 Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adjemian J, Olivier KN, Prevots DR. Epidemiology of Pulmonary Nontuberculous Mycobacterial Sputum Positivity in Patients with Cystic Fibrosis in the United States, 2010–2014. Annals of the American Thoracic Society. 2018/07/01 2018;15(7):817–826. doi: 10.1513/AnnalsATS.201709-727OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith GS, Ghio AJ, Stout JE, et al. Epidemiology of nontuberculous mycobacteria isolations among central North Carolina residents, 2006–2010. J Infect. Jun 2016;72(6):678–686. doi: 10.1016/j.jinf.2016.03.008 [DOI] [PubMed] [Google Scholar]
- 59.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis. Nov 2013;19(11):1889–91. doi: 10.3201/eid1911.130737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raats D, Brode SK, Mehrabi M, Marras TK. Increasing and More Commonly Refractory Mycobacterium avium Pulmonary Disease, Toronto, Ontario, Canada. Emerg Infect Dis. Aug 2022;28(8):1589–1596. doi: 10.3201/eid2808.220464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chaptal M, Andrejak C, Bonifay T, et al. Epidemiology of infection by pulmonary non-tuberculous mycobacteria in French Guiana 2008–2018. PLoS Negl Trop Dis. Sep 2022;16(9):e0010693. doi: 10.1371/journal.pntd.0010693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lopez-Luis BA, Sifuentes-Osornio J, Pérez-Gutiérrez MT, Chávez-Mazari B, Bobadilla-Del-Valle M, Ponce-de-León A. Nontuberculous mycobacterial infection in a tertiary care center in Mexico, 2001–2017. Braz J Infect Dis. May-Jun 2020;24(3):213–220. doi: 10.1016/j.bjid.2020.04.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Carneiro MDS, Nunes LS, David SMM, Dias CF, Barth AL, Unis G. Nontuberculous mycobacterial lung disease in a high tuberculosis incidence setting in Brazil. J Bras Pneumol. Apr 2018;44(2):106–111. doi: 10.1590/s1806-37562017000000213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Marques LRM, Ferrazoli L, Chimara É. Pulmonary nontuberculous mycobacterial infections: presumptive diagnosis based on the international microbiological criteria adopted in the state of São Paulo, Brazil, 2011–2014. J Bras Pneumol. Apr 25 2019;45(2):e20180278. doi: 10.1590/1806-3713/e20180278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.De Lima Mota MA, De Melo DM, Christyan Nunes Beserra FL, et al. Clinical-epidemiological profile and factors related to the mortality of patients with nontuberculous mycobacteria isolated at a reference hospital in Ceará, Northeastern Brazil. Int J Mycobacteriol. Jan-Mar 2020;9(1):83–90. doi: 10.4103/ijmy.ijmy_12_20 [DOI] [PubMed] [Google Scholar]
- 66.López A, Acosta F, Sambrano D, et al. Direct Molecular Characterization of Acid-Fast Bacilli Smear of Nontuberculosis Mycobacterium Species Causing Pulmonary Tuberculosis in Guna Yala Region, Panama. Am J Trop Med Hyg. Jul 8 2021;105(3):633–637. doi: 10.4269/ajtmh.21-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Greif G, Coitinho C, van Ingen J, Robello C. Species Distribution and Isolation Frequency of Nontuberculous Mycobacteria, Uruguay. Emerg Infect Dis. May 2020;26(5):1014–1018. doi: 10.3201/eid2605.191631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermansen TS, Ravn P, Svensson E, Lillebaek T. Nontuberculous mycobacteria in Denmark, incidence and clinical importance during the last quarter-century. Scientific Reports. 2017/07/27 2017;7(1):6696. doi: 10.1038/s41598-017-06931-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Veziris N, Andréjak C, Bouée S, Emery C, Obradovic M, Chiron R. Non-tuberculous mycobacterial pulmonary diseases in France: an 8 years nationwide study. BMC Infect Dis. Nov 17 2021;21(1):1165. doi: 10.1186/s12879-021-06825-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schildkraut JA, Zweijpfenning SMH, Nap M, et al. The epidemiology of nontuberculous mycobacterial pulmonary disease in the Netherlands. ERJ Open Res. Jul 2021;7(3)doi: 10.1183/23120541.00207-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ringshausen FC, Ewen R, Multmeier J, et al. Predictive modeling of nontuberculous mycobacterial pulmonary disease epidemiology using German health claims data. Int J Infect Dis. Mar 2021;104:398–406. doi: 10.1016/j.ijid.2021.01.003 [DOI] [PubMed] [Google Scholar]
- 72.Ringshausen FC, Wagner D, de Roux A, et al. Prevalence of Nontuberculous Mycobacterial Pulmonary Disease, Germany, 2009–2014. Emerg Infect Dis. Jun 2016;22(6):1102–5. doi: 10.3201/eid2206.151642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Oliveira MJ, Gaio AR, Gomes M, Gonçalves A, Duarte R. Mycobacterium avium infection in Portugal. Int J Tuberc Lung Dis. Feb 1 2017;21(2):218–222. doi: 10.5588/ijtld.16.0002 [DOI] [PubMed] [Google Scholar]
- 74.Dakić I, Arandjelović I, Savić B, et al. Pulmonary isolation and clinical relevance of nontuberculous mycobacteria during nationwide survey in Serbia, 2010–2015. PLoS One. 2018;13(11):e0207751. doi: 10.1371/journal.pone.0207751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Santin M, Barrabeig I, Malchair P, et al. Pulmonary Infections with Nontuberculous Mycobacteria, Catalonia, Spain, 1994–2014. Emerg Infect Dis. Jun 2018;24(6):1091–1094. doi: 10.3201/eid2406.172095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alcaide F, Peña MJ, Pérez-Risco D, et al. Increasing isolation of rapidly growing mycobacteria in a low-incidence setting of environmental mycobacteria, 1994–2015. Eur J Clin Microbiol Infect Dis. Aug 2017;36(8):1425–1432. doi: 10.1007/s10096-017-2949-0 [DOI] [PubMed] [Google Scholar]
- 77.Shah NM, Davidson JA, Anderson LF, et al. Pulmonary Mycobacterium avium-intracellulare is the main driver of the rise in non-tuberculous mycobacteria incidence in England, Wales and Northern Ireland, 2007–2012. BMC Infect Dis. May 6 2016;16:195. doi: 10.1186/s12879-016-1521-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pedrero S, Tabernero E, Arana-Arri E, Urra E, Larrea M, Zalacain R. Changing epidemiology of nontuberculous mycobacterial lung disease over the last two decades in a region of the Basque country. ERJ Open Res. Oct 2019;5(4)doi: 10.1183/23120541.00110-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Axson EL, Bloom CI, Quint JK. Nontuberculous mycobacterial disease managed within UK primary care, 2006–2016. Eur J Clin Microbiol Infect Dis. Sep 2018;37(9):1795–1803. doi: 10.1007/s10096-018-3315-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hoefsloot W, van Ingen J, Andrejak C, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. European Respiratory Journal. 2013;42(6):1604. doi: 10.1183/09031936.00149212 [DOI] [PubMed] [Google Scholar]
- 81.Modrá H, Ulmann V, Caha J, et al. Socio-Economic and Environmental Factors Related to Spatial Differences in Human Non-Tuberculous Mycobacterial Diseases in the Czech Republic. Int J Environ Res Public Health. Oct 17 2019;16(20)doi: 10.3390/ijerph16203969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dahl VN, Fløe A, Wejse C. Nontuberculous mycobacterial infections in a Danish region between 2011 and 2021: evaluation of trends in diagnostic codes. Infect Dis (Lond). Mar 30 2023:1–5. doi: 10.1080/23744235.2023.2194411 [DOI] [PubMed] [Google Scholar]
- 83.Mejia-Chew C, Yaeger L, Montes K, Bailey TC, Olsen MA. Diagnostic Accuracy of Health Care Administrative Diagnosis Codes to Identify Nontuberculous Mycobacteria Disease: A Systematic Review. Open forum infectious diseases. 2021;8(5):ofab035. doi: 10.1093/ofid/ofab035 Accessed 2021/05//. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Panagiotou M, Papaioannou AI, Kostikas K, et al. The epidemiology of pulmonary nontuberculous mycobacteria: data from a general hospital in Athens, Greece, 2007–2013. Pulm Med. 2014;2014:894976. doi: 10.1155/2014/894976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lange C, Böttger EC, Cambau E, et al. Consensus management recommendations for less common non-tuberculous mycobacterial pulmonary diseases. Lancet Infect Dis. Jul 2022;22(7):e178–e190. doi: 10.1016/s1473-3099(21)00586-7 [DOI] [PubMed] [Google Scholar]
- 86.Russell CD, Claxton P, Doig C, Seagar AL, Rayner A, Laurenson IF. Non-tuberculous mycobacteria: a retrospective review of Scottish isolates from 2000 to 2010. Thorax. Jun 2014;69(6):593–5. doi: 10.1136/thoraxjnl-2013-204260 [DOI] [PubMed] [Google Scholar]
- 87.Okoi C, Anderson STB, Antonio M, Mulwa SN, Gehre F, Adetifa IMO. Non-tuberculous Mycobacteria isolated from Pulmonary samples in sub-Saharan Africa - A Systematic Review and Meta Analyses. Sci Rep. Sep 20 2017;7(1):12002. doi: 10.1038/s41598-017-12175-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gharbi R, Mhenni B, Ben Fraj S, Mardassi H. Nontuberculous mycobacteria isolated from specimens of pulmonary tuberculosis suspects, Northern Tunisia: 2002–2016. BMC Infect Dis. Sep 18 2019;19(1):819. doi: 10.1186/s12879-019-4441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Agizew T, Basotli J, Alexander H, et al. Higher-than-expected prevalence of non-tuberculous mycobacteria in HIV setting in Botswana: Implications for diagnostic algorithms using Xpert MTB/RIF assay. PLoS One. 2017;12(12):e0189981. doi: 10.1371/journal.pone.0189981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Otchere ID, Asante-Poku A, Osei-Wusu S, Aboagye SY, Yeboah-Manu D. Isolation and characterization of nontuberculous mycobacteria from patients with pulmonary tuberculosis in Ghana. Int J Mycobacteriol. Jan-Mar 2017;6(1):70–75. doi: 10.4103/2212-5531.201895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bjerrum S, Oliver-Commey J, Kenu E, et al. Tuberculosis and non-tuberculous mycobacteria among HIV-infected individuals in Ghana. Trop Med Int Health. Sep 2016;21(9):1181–90. doi: 10.1111/tmi.12749 [DOI] [PubMed] [Google Scholar]
- 92.Kone B, Sarro YS, Maiga M, et al. Clinical characteristics of non-tuberculous mycobacterial pulmonary infections in Bamako, Mali. Epidemiol Infect. Feb 2018;146(3):354–358. doi: 10.1017/s0950268817003090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Alemayehu A, Kebede A, Neway S, et al. A glimpse into the genotype and clinical importance of non tuberculous mycobacteria among pulmonary tuberculosis patients: The case of Ethiopia. PLoS One. 2022;17(9):e0275159. doi: 10.1371/journal.pone.0275159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mwangi ZM, Mukiri NN, Onyambu FG, Wallace BD. Genetic diversity of nontuberculous mycobacteria among symptomatic tuberculosis negative patients in Kenya. Int J Mycobacteriol. Jan-Mar 2022;11(1):60–69. doi: 10.4103/ijmy.ijmy_224_21 [DOI] [PubMed] [Google Scholar]
- 95.Hoza AS, Mfinanga SG, Rodloff AC, Moser I, König B. Increased isolation of nontuberculous mycobacteria among TB suspects in Northeastern, Tanzania: public health and diagnostic implications for control programmes. BMC Res Notes. Feb 17 2016;9:109. doi: 10.1186/s13104-016-1928-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chanda-Kapata P, Kapata N, Klinkenberg E, et al. Non-tuberculous mycobacteria (NTM) in Zambia: prevalence, clinical, radiological and microbiological characteristics. BMC Infect Dis. Nov 6 2015;15:500. doi: 10.1186/s12879-015-1264-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Epola Dibamba Ndanga M, Achimi Agbo Abdul JBP, Edoa JR, et al. Non-tuberculous mycobacteria isolation from presumptive tuberculosis patients in Lambaréné, Gabon. Trop Med Int Health. Apr 2022;27(4):438–444. doi: 10.1111/tmi.13736 [DOI] [PubMed] [Google Scholar]
- 98.Park SC, Kang MJ, Han CH, et al. Prevalence, incidence, and mortality of nontuberculous mycobacterial infection in Korea: a nationwide population-based study. BMC Pulm Med. Aug 1 2019;19(1):140. doi: 10.1186/s12890-019-0901-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park JH, Shin S, Kim TS, Park H. Clinically refined epidemiology of nontuberculous mycobacterial pulmonary disease in South Korea: overestimation when relying only on diagnostic codes. BMC Pulm Med. May 13 2022;22(1):195. doi: 10.1186/s12890-022-01993-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park Y, Kim CY, Park MS, Kim YS, Chang J, Kang YA. Age- and sex-related characteristics of the increasing trend of nontuberculous mycobacteria pulmonary disease in a tertiary hospital in South Korea from 2006 to 2016. Korean J Intern Med. Nov 2020;35(6):1424–1431. doi: 10.3904/kjim.2019.395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lee YM, Kim MJ, Kim YJ. Increasing Trend of Nontuberculous Mycobacteria Isolation in a Referral Clinical Laboratory in South Korea. Medicina (Kaunas). Jul 16 2021;57(7)doi: 10.3390/medicina57070720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ahn K, Kim YK, Hwang GY, Cho H, Uh Y. Continued Upward Trend in Non-Tuberculous Mycobacteria Isolation over 13 Years in a Tertiary Care Hospital in Korea. Yonsei Med J. Oct 2021;62(10):903–910. doi: 10.3349/ymj.2021.62.10.903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kim N, Yi J, Chang CL. Recovery Rates of Non-Tuberculous Mycobacteria from Clinical Specimens Are Increasing in Korean Tertiary-Care Hospitals. J Korean Med Sci. Aug 2017;32(8):1263–1267. doi: 10.3346/jkms.2017.32.8.1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ko RE, Moon SM, Ahn S, et al. Changing Epidemiology of Nontuberculous Mycobacterial Lung Diseases in a Tertiary Referral Hospital in Korea between 2001 and 2015. J Korean Med Sci. Feb 19 2018;33(8):e65. doi: 10.3346/jkms.2018.33.e65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Morimoto K, Hasegawa N, Izumi K, et al. A Laboratory-based Analysis of Nontuberculous Mycobacterial Lung Disease in Japan from 2012 to 2013. Ann Am Thorac Soc. Jan 2017;14(1):49–56. doi: 10.1513/AnnalsATS.201607-573OC [DOI] [PubMed] [Google Scholar]
- 106.Morimoto K, Ato M, Hasegawa N, Mitarai S. Population-Based Distribution of Mycobacterium avium and Mycobacterium intracellulare in Japan. Microbiology Research. 2021;12(3):739–743. [Google Scholar]
- 107.Izumi K, Morimoto K, Hasegawa N, et al. Epidemiology of Adults and Children Treated for Nontuberculous Mycobacterial Pulmonary Disease in Japan. Ann Am Thorac Soc. Mar 2019;16(3):341–347. doi: 10.1513/AnnalsATS.201806-366OC [DOI] [PubMed] [Google Scholar]
- 108.Ito Y, Hirai T, Fujita K, et al. Increasing patients with pulmonary Mycobacterium avium complex disease and associated underlying diseases in Japan. J Infect Chemother. May 2015;21(5):352–6. doi: 10.1016/j.jiac.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 109.Furuuchi K, Morimoto K, Yoshiyama T, et al. Interrelational changes in the epidemiology and clinical features of nontuberculous mycobacterial pulmonary disease and tuberculosis in a referral hospital in Japan. Respir Med. Jun 2019;152:74–80. doi: 10.1016/j.rmed.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 110.Harada K, Hagiya H, Funahashi T, Koyama T, Kano MR, Otsuka F. Trends in the Nontuberculous Mycobacterial Disease Mortality Rate in Japan: A Nationwide Observational Study, 1997–2016. Clin Infect Dis. Jul 15 2021;73(2):e321–e326. doi: 10.1093/cid/ciaa810 [DOI] [PubMed] [Google Scholar]
- 111.Hagiwara E, Katano T, Isomoto K, et al. Clinical characteristics and early outcomes of patients newly diagnosed with pulmonary Mycobacterium avium complex disease. Respir Investig. Jan 2019;57(1):54–59. doi: 10.1016/j.resinv.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 112.Lin CK, Yang YH, Lu ML, et al. Incidence of nontuberculous mycobacterial disease and coinfection with tuberculosis in a tuberculosis-endemic region: A population-based retrospective cohort study. Medicine (Baltimore). Dec 24 2020;99(52):e23775. doi: 10.1097/md.0000000000023775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen HH, Lin CH, Chao WC. Mortality association of nontuberculous mycobacterial infection requiring treatment in Taiwan: a population-based study. Ther Adv Respir Dis. Jan-Dec 2022;16:17534666221103213. doi: 10.1177/17534666221103213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chiang CY, Yu MC, Yang SL, Yen MY, Bai KJ. Surveillance of Tuberculosis in Taipei: The Influence of Nontuberculous Mycobacteria. PLoS One. 2015;10(11):e0142324. doi: 10.1371/journal.pone.0142324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chien JY, Lai CC, Sheng WH, Yu CJ, Hsueh PR. Pulmonary infection and colonization with nontuberculous mycobacteria, Taiwan, 2000–2012. Emerg Infect Dis. Aug 2014;20(8):1382–5. doi: 10.3201/eid2008.131673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang HL, Cheng MH, Lu PL, et al. Epidemiology and Predictors of NTM Pulmonary Infection in Taiwan - a Retrospective, Five-Year Multicenter Study. Sci Rep. Nov 24 2017;7(1):16300. doi: 10.1038/s41598-017-16559-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan Y, Deng Y, Yan X, et al. Nontuberculous mycobacterial pulmonary disease and associated risk factors in China: A prospective surveillance study. J Infect. Jul 2021;83(1):46–53. doi: 10.1016/j.jinf.2021.05.019 [DOI] [PubMed] [Google Scholar]
- 118.Ji LC, Chen S, Piao W, Hong CY, Li JL, Jiang Q. Increasing Trends and Species Diversity of Nontuberculous Mycobacteria in A Coastal Migrant City-Shenzhen, China. Biomed Environ Sci. Feb 20 2022;35(2):146–150. doi: 10.3967/bes2022.020 [DOI] [PubMed] [Google Scholar]
- 119.Sun Q, Yan J, Liao X, et al. Trends and Species Diversity of Non-tuberculous Mycobacteria Isolated From Respiratory Samples in Northern China, 2014–2021. Front Public Health. 2022;10:923968. doi: 10.3389/fpubh.2022.923968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Duan H, Han X, Wang Q, et al. Clinical Significance of Nontuberculous Mycobacteria Isolated From Respiratory Specimens in a Chinese Tuberculosis Tertiary Care Center. Sci Rep. Nov 3 2016;6:36299. doi: 10.1038/srep36299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sumbul B, Doymaz MZ. A Current Microbiological Picture of Mycobacterium Isolates from Istanbul, Turkey. Pol J Microbiol. 2020;69(2):1–7. doi: 10.33073/pjm-2020-021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karamat A, Ambreen A, Ishtiaq A, Tahseen S, Rahman MA, Mustafa T. Isolation of non-tuberculous mycobacteria among tuberculosis patients, a study from a tertiary care hospital in Lahore, Pakistan. BMC Infect Dis. Apr 24 2021;21(1):381. doi: 10.1186/s12879-021-06086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Khosravi AD, Mirsaeidi M, Farahani A, et al. Prevalence of nontuberculous mycobacteria and high efficacy of d-cycloserine and its synergistic effect with clarithromycin against Mycobacterium fortuitum and Mycobacterium abscessus. Infect Drug Resist. 2018;11:2521–2532. doi: 10.2147/idr.S187554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ayoubi S, Aghajani J, Farnia P, Farnia P, Ghanavi J, Velayati AA. Prevalence of Mycobacterium abscessus among the Patients with Nontuberculous Mycobacteria. Arch Iran Med. Mar 1 2020;23(3):163–168. [PubMed] [Google Scholar]
- 125.Suresh P, Kumar A, Biswas R, et al. Epidemiology of Nontuberculous Mycobacterial Infection in Tuberculosis Suspects. Am J Trop Med Hyg. Aug 23 2021;105(5):1335–1338. doi: 10.4269/ajtmh.21-0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Umrao J, Singh D, Zia A, et al. Prevalence and species spectrum of both pulmonary and extrapulmonary nontuberculous mycobacteria isolates at a tertiary care center. Int J Mycobacteriol. Sep 2016;5(3):288–293. doi: 10.1016/j.ijmyco.2016.06.008 [DOI] [PubMed] [Google Scholar]
- 127.Sebastian G, Nagaraja SB, Vishwanatha T, Voderhobli M, Vijayalakshmi N, Kumar P. Non-Tuberculosis mycobacterium speciation using HPLC under Revised National TB Control Programme (RNTCP) in India. J Appl Microbiol. Jan 2018;124(1):267–273. doi: 10.1111/jam.13604 [DOI] [PubMed] [Google Scholar]
- 128.Phelippeau M, Osman DA, Musso D, Drancourt M. Epidemiology of Nontuberculous Mycobacteria in French Polynesia. J Clin Microbiol. Dec 2015;53(12):3798–804. doi: 10.1128/jcm.01560-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ley S, Carter R, Millan K, et al.Non-tuberculous mycobacteria: baseline data from three sites in Papua New Guinea, 2010–2012. Western Pac Surveill Response J. Oct-Dec 2015;6(4):24–9. doi: 10.5365/wpsar.2015.6.2.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Foote SL, Lipner EM, Prevots DR, Ricotta EE. Environmental predictors of pulmonary nontuberculous mycobacteria (NTM) sputum positivity among persons with cystic fibrosis in the state of Florida. PLoS One. 2021;16(12):e0259964. doi: 10.1371/journal.pone.0259964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brode SK, Marchand-Austin A, Jamieson FB, Marras TK. Pulmonary versus Nonpulmonary Nontuberculous Mycobacteria, Ontario, Canada. Emerg Infect Dis. Nov 2017;23(11):1898–1901. doi: 10.3201/eid2311.170959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ide S, Nakamura S, Yamamoto Y, et al. Epidemiology and clinical features of pulmonary nontuberculous mycobacteriosis in Nagasaki, Japan. PLoS One. 2015;10(5):e0128304. doi: 10.1371/journal.pone.0128304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kim HO, Lee K, Choi HK, Ha S, Lee SM, Seo GH. Incidence, comorbidities, and treatment patterns of nontuberculous mycobacterial infection in South Korea. Medicine (Baltimore). Nov 2019;98(45):e17869. doi: 10.1097/md.0000000000017869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nasiri MJ, Dabiri H, Darban-Sarokhalil D, Hashemi Shahraki A. Prevalence of Non-Tuberculosis Mycobacterial Infections among Tuberculosis Suspects in Iran: Systematic Review and Meta-Analysis. PLoS One. 2015;10(6):e0129073. doi: 10.1371/journal.pone.0129073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boyle DP, Zembower TR, Reddy S, Qi C. Comparison of Clinical Features, Virulence, and Relapse among Mycobacterium avium Complex Species. Am J Respir Crit Care Med. 2015/06/01 2015;191(11):1310–1317. doi: 10.1164/rccm.201501-0067OC [DOI] [PubMed] [Google Scholar]
- 136.Hannah CE, Ford BA, Chung J, Ince D, Wanat KA. Characteristics of Nontuberculous Mycobacterial Infections at a Midwestern Tertiary Hospital: A Retrospective Study of 365 Patients. Open Forum Infect Dis. Jun 2020;7(6):ofaa173. doi: 10.1093/ofid/ofaa173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Martin A, Colmant A, Verroken A, Rodriguez-Villalobos H. Laboratory diagnosis of nontuberculous mycobacteria in a Belgium Hospital. Int J Mycobacteriol. Apr-Jun 2019;8(2):157–161. doi: 10.4103/ijmy.ijmy_40_19 [DOI] [PubMed] [Google Scholar]
- 138.Vande Weygaerde Y, Cardinaels N, Bomans P, et al. Clinical relevance of pulmonary non-tuberculous mycobacterial isolates in three reference centres in Belgium: a multicentre retrospective analysis. BMC Infect Dis. Dec 17 2019;19(1):1061. doi: 10.1186/s12879-019-4683-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Glodić G, Samaržija M, Sabol I, et al. Risk factors for nontuberculous mycobacterial pulmonary disease (NTM-PD) in Croatia. Wien Klin Wochenschr. Nov 2021;133(21–22):1195–1200. doi: 10.1007/s00508-021-01923-x [DOI] [PubMed] [Google Scholar]
- 140.Blanc P, Dutronc H, Peuchant O, et al. Nontuberculous Mycobacterial Infections in a French Hospital: A 12-Year Retrospective Study. PLoS One. 2016;11(12):e0168290. doi: 10.1371/journal.pone.0168290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bemer P, Peuchant O, Guet-Revillet H, et al. Management of patients with pulmonary mycobacteriosis in France: a multicenter retrospective cohort study. BMC Pulm Med. Oct 26 2021;21(1):333. doi: 10.1186/s12890-021-01701-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wetzstein N, Hügel C, Wichelhaus TA, et al. Species distribution and clinical features of infection and colonisation with non-tuberculous mycobacteria in a tertiary care centre, central Germany, 2006–2016. Infection. Oct 2019;47(5):817–825. doi: 10.1007/s15010-019-01317-2 [DOI] [PubMed] [Google Scholar]
- 143.Chong SG, Kent BD, Fitzgerald S, McDonnell TJ. Pulmonary non-tuberculous mycobacteria in a general respiratory population. Ir Med J. Jul-Aug 2014;107(7):207–9. [PubMed] [Google Scholar]
- 144.Zweijpfenning S, Kops S, Magis-Escurra C, Boeree MJ, van Ingen J, Hoefsloot W. Treatment and outcome of non-tuberculous mycobacterial pulmonary disease in a predominantly fibro-cavitary disease cohort. Respir Med. Oct 2017;131:220–224. doi: 10.1016/j.rmed.2017.08.031 [DOI] [PubMed] [Google Scholar]
- 145.Szturmowicz M, Siemion-Szcześniak I, Wyrostkiewicz D, et al. Factors predisposing to non-tuberculous mycobacterial lung disease in the patients with respiratory isolates of non-tuberculous mycobacteria. Adv Respir Med. Dec 30 2018;doi: 10.5603/ARM.a2018.0043 [DOI] [PubMed] [Google Scholar]
- 146.Kwiatkowska S, Augustynowicz-Kopeć E, Korzeniewska-Koseła M, et al. Nontuberculous mycobacteria strains isolated from patients between 2013 and 2017 in Poland. Our data with respect to the global trends. Adv Respir Med. Dec 30 2018;doi: 10.5603/ARM.a2018.0047 [DOI] [PubMed] [Google Scholar]
- 147.Ramos AL, Carvalho T, Guimarães JT. The importance of multiple samples in mycobacterial recovery: A 10-year retrospective study. Int J Mycobacteriol. Apr-Jun 2019;8(2):175–179. doi: 10.4103/ijmy.ijmy_68_19 [DOI] [PubMed] [Google Scholar]
- 148.Adzic-Vukicevic T, Barac A, Blanka-Protic A, et al. Clinical features of infection caused by non-tuberculous mycobacteria: 7 years’ experience. Infection. Jun 2018;46(3):357–363. doi: 10.1007/s15010-018-1128-2 [DOI] [PubMed] [Google Scholar]
- 149.Blanco Pérez JJ, Pérez González A, Morano Amado LE, et al. Clinical Significance of Environmental Mycobacteria Isolated From Respiratory Specimens of Patients With and Without Silicosis. Arch Bronconeumol. Mar 2016;52(3):145–50. doi: 10.1016/j.arbres.2015.07.007 [DOI] [PubMed] [Google Scholar]
- 150.Matesanz López C, Loras Gallego C, Cacho Calvo J, Thuissard Vasallo IJ, Río Ramírez MT. Patients with non-tuberculous mycobacteria in respiratory samples: a 5-year epidemiological study. Rev Esp Quimioter. Apr 2021;34(2):120–125. doi: 10.37201/req/121.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vongthilath-Moeung R, Plojoux J, Poncet A, et al. Nontuberculous Mycobacteria under Scrutiny in the Geneva Area (2015–2020). Respiration. 2022;101(4):367–375. doi: 10.1159/000520033 [DOI] [PubMed] [Google Scholar]
- 152.Schiff HF, Jones S, Achaiah A, Pereira A, Stait G, Green B. Clinical relevance of non-tuberculous mycobacteria isolated from respiratory specimens: seven year experience in a UK hospital. Sci Rep. Feb 11 2019;9(1):1730. doi: 10.1038/s41598-018-37350-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Pokam BDT, Yeboah-Manu D, Ofori S, et al. Prevalence of non-tuberculous mycobacteria among previously treated TB patients in the Gulf of Guinea, Africa. IJID Reg. Jun 2022;3:287–292. doi: 10.1016/j.ijregi.2022.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maya TG, Komba EV, Mensah GI, et al. Drug susceptibility profiles and factors associated with non-tuberculous mycobacteria species circulating among patients diagnosed with pulmonary tuberculosis in Tanzania. PLoS One. 2022;17(3):e0265358. doi: 10.1371/journal.pone.0265358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Wu J, Zhang Y, Li J, et al. Increase in nontuberculous mycobacteria isolated in Shanghai, China: results from a population-based study. PLoS One. 2014;9(10):e109736. doi: 10.1371/journal.pone.0109736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Zhu Y, Hua W, Liu Z, et al. Identification and characterization of nontuberculous mycobacteria isolated from suspected pulmonary tuberculosis patients in eastern china from 2009 to 2019 using an identification array system. Braz J Infect Dis. Mar-Apr 2022;26(2):102346. doi: 10.1016/j.bjid.2022.102346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Tan Y, Su B, Shu W, et al. Epidemiology of pulmonary disease due to nontuberculous mycobacteria in Southern China, 2013–2016. BMC Pulm Med. Nov 9 2018;18(1):168. doi: 10.1186/s12890-018-0728-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hu C, Huang L, Cai M, Wang W, Shi X, Chen W. Characterization of non-tuberculous mycobacterial pulmonary disease in Nanjing district of China. BMC Infect Dis. Sep 2 2019;19(1):764. doi: 10.1186/s12879-019-4412-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu J, Li P, Zheng S, Shu W, Pang Y. Prevalence and risk factors of pulmonary nontuberculous mycobacterial infections in the Zhejiang Province of China. Epidemiol Infect. Sep 11 2019;147:e269. doi: 10.1017/s0950268819001626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nagano H, Kinjo T, Nei Y, Yamashiro S, Fujita J, Kishaba T. Causative species of nontuberculous mycobacterial lung disease and comparative investigation on clinical features of Mycobacterium abscessus complex disease: A retrospective analysis for two major hospitals in a subtropical region of Japan. PLoS One. 2017;12(10):e0186826. doi: 10.1371/journal.pone.0186826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sharma SK, Upadhyay V. Non-tuberculous mycobacteria: a disease beyond TB and preparedness in India. Expert Rev Respir Med. Jul 2021;15(7):949–958. doi: 10.1080/17476348.2021.1925545 [DOI] [PubMed] [Google Scholar]
- 162.Lim AYH, Chotirmall SH, Fok ETK, et al. Profiling non-tuberculous mycobacteria in an Asian setting: characteristics and clinical outcomes of hospitalized patients in Singapore. BMC Pulm Med. May 22 2018;18(1):85. doi: 10.1186/s12890-018-0637-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhang ZX, Cherng BPZ, Sng LH, Tan YE. Clinical and microbiological characteristics of non-tuberculous mycobacteria diseases in Singapore with a focus on pulmonary disease, 2012–2016. BMC Infect Dis. May 17 2019;19(1):436. doi: 10.1186/s12879-019-3909-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Al-Harbi A, Al-Jahdali H, Al-Johani S, Baharoon S, Bin Salih S, Khan M. Frequency and clinical significance of respiratory isolates of non-tuberculous mycobacteria in Riyadh, Saudi Arabia. Clin Respir J. Mar 2016;10(2):198–203. doi: 10.1111/crj.12202 [DOI] [PubMed] [Google Scholar]
- 165.Gochi M, Takayanagi N, Kanauchi T, Ishiguro T, Yanagisawa T, Sugita Y. Retrospective study of the predictors of mortality and radiographic deterioration in 782 patients with nodular/bronchiectatic Mycobacterium avium complex lung disease. BMJ Open. Aug 5 2015;5(8):e008058. doi: 10.1136/bmjopen-2015-008058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hagiwara E, Katano T, Isomoto K, et al. Clinical characteristics and early outcomes of patients newly diagnosed with pulmonary Mycobacterium avium complex disease. Respir Investig. Jan 2019;57(1):54–59. doi: 10.1016/j.resinv.2018.08.006 [DOI] [PubMed] [Google Scholar]
- 167.Uno S, Asakura T, Morimoto K, et al. Comorbidities associated with nontuberculous mycobacterial disease in Japanese adults: a claims-data analysis. BMC Pulm Med. Oct 9 2020;20(1):262. doi: 10.1186/s12890-020-01304-6 [DOI] [PMC free article] [PubMed] [Google Scholar]


