Abstract
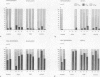
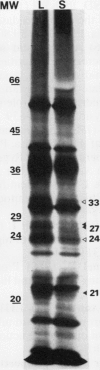
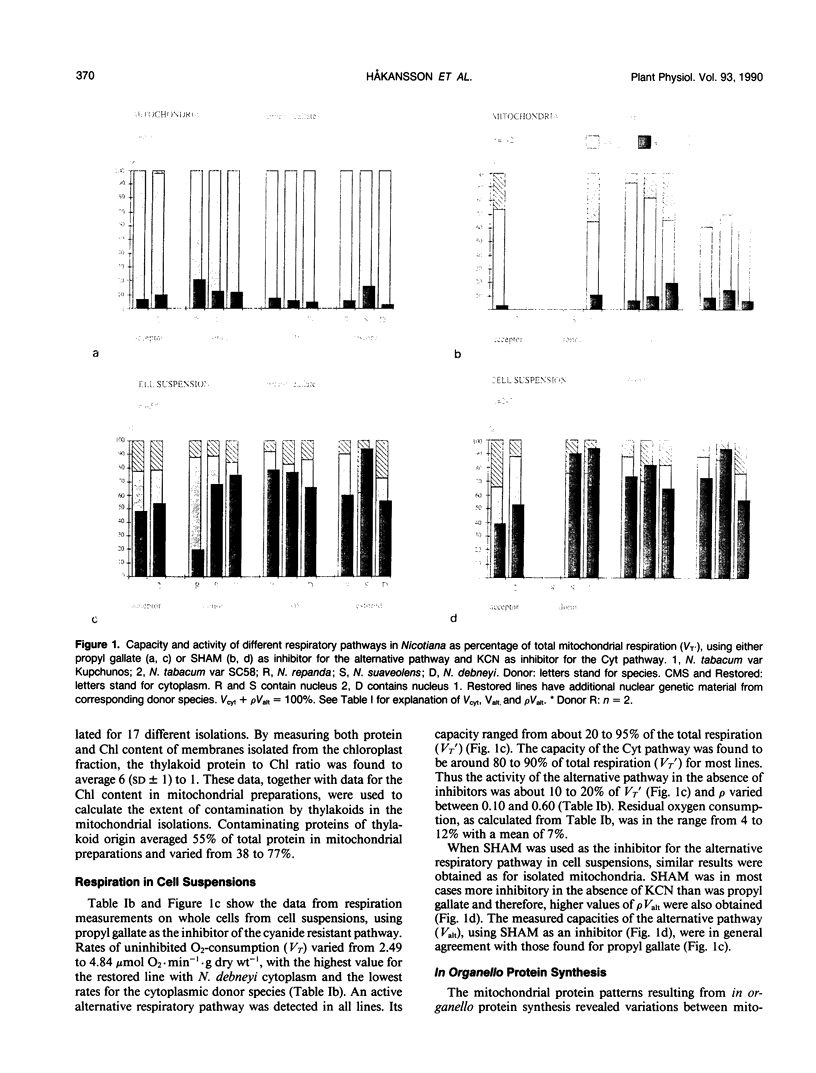
Three cytoplasmic male-sterile Nicotiana cultivars together with corresponding male-fertile progenitors and restored lines were investigated in order to find possible correlations between respiratory characteristics and male sterility. Oxygen consumption measurements were performed on cells from suspension cultures as well as on mitochondria isolated from green leaves. Inhibitors, which have been reported to specifically block either the cytochrome (KCN) or the alternative (propyl gallate and sali-cylhydroxamic acid [SHAM] respiratory pathways, were used in order to measure the capacity and activity of the two pathways. One of the inhibitors, SHAM, was found unsuitable to measure the activity of the alternative pathway due to the lack of specificity of SHAM for this pathway. A great difference in the capacity of the alternative pathway was detected between the two types of cell materials tested. Mitochondria isolated from green leaves showed a capacity of the alternative pathway of 5 to 20% of total mitochondrial repiration, while the capacity of cells from suspension cultures generally ranged from 50 to 80%. In addition to this, in organello synthesis of mitochondrial proteins revealed differences between mitochondria isolated from green leaves and from cell suspensions. No correlation, however, could be found between respiratory characteristics and male sterility.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. D., Clarke S. D., Rich P. R. Partial Purification and Characterization of the Quinol Oxidase Activity of Arum maculatum Mitochondria. Plant Physiol. 1986 Apr;80(4):838–842. doi: 10.1104/pp.80.4.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Day D. A. Malate Decarboxylation by Kalanchoë daigremontiana Mitochondria and Its Role in Crassulacean Acid Metabolism. Plant Physiol. 1980 Apr;65(4):675–679. doi: 10.1104/pp.65.4.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Glycine metabolism and oxalacetate transport by pea leaf mitochondria. Plant Physiol. 1981 Aug;68(2):425–429. doi: 10.1104/pp.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D. L., Rosenberg E. Regulation of cyanide-insensitive respiration in Neurospora. Eur J Biochem. 1976 Feb 16;62(2):217–221. doi: 10.1111/j.1432-1033.1976.tb10150.x. [DOI] [PubMed] [Google Scholar]
- Elthon T. E., McIntosh L. Identification of the alternative terminal oxidase of higher plant mitochondria. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8399–8403. doi: 10.1073/pnas.84.23.8399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elthon T. E., Nickels R. L., McIntosh L. Monoclonal antibodies to the alternative oxidase of higher plant mitochondria. Plant Physiol. 1989 Apr;89(4):1311–1317. doi: 10.1104/pp.89.4.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn M. E., Mertz D. Cyanide-Resistant Respiration in Suspension Cultured Cells of Nicotiana glutinosa L. Plant Physiol. 1982 Jun;69(6):1439–1443. doi: 10.1104/pp.69.6.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly B. M., Wiskich J. T. Respiration of Mitochondria Isolated from Leaves and Protoplasts of Avena sativa. Plant Physiol. 1988 Jul;87(3):705–710. doi: 10.1104/pp.87.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obenland D., Hiser C., McIntosh L., Shibles R., Stewart C. R. Occurrence of alternative respiratory capacity in soybean and pea. Plant Physiol. 1988 Nov;88(3):528–531. doi: 10.1104/pp.88.3.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinzen F. W., van der Vusse G. J., Reneman R. S. Blood flow distribution in the left ventricular free wall in open-chest dogs. Basic Res Cardiol. 1981 Jul-Aug;76(4):431–437. doi: 10.1007/BF01908337. [DOI] [PubMed] [Google Scholar]
- Schwitzguebel J. P., Siegenthaler P. A. Purification of peroxisomes and mitochondria from spinach leaf by percoll gradient centrifugation. Plant Physiol. 1984 Jul;75(3):670–674. doi: 10.1104/pp.75.3.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesay A., Stewart C. R., Shibles R. Cyanide-resistant respiration in light- and dark-grown soybean cotyledons. Plant Physiol. 1988 Jul;87(3):655–659. doi: 10.1104/pp.87.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siedow J. N., Bickett D. M. Structural features required for inhibition of cyanide-insensitive electron transfer by propyl gallate. Arch Biochem Biophys. 1981 Mar;207(1):32–39. doi: 10.1016/0003-9861(81)90004-7. [DOI] [PubMed] [Google Scholar]
- Theologis A., Laties G. G. Relative Contribution of Cytochrome-mediated and Cyanide-resistant Electron Transport in Fresh and Aged Potato Slices. Plant Physiol. 1978 Aug;62(2):232–237. doi: 10.1104/pp.62.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke H. E., Lee S. L. Pollen Abortion in T Cytoplasmic Male-Sterile Corn (Zea mays): A Suggested Mechanism. Science. 1978 May 5;200(4341):561–563. doi: 10.1126/science.200.4341.561. [DOI] [PubMed] [Google Scholar]