Abstract
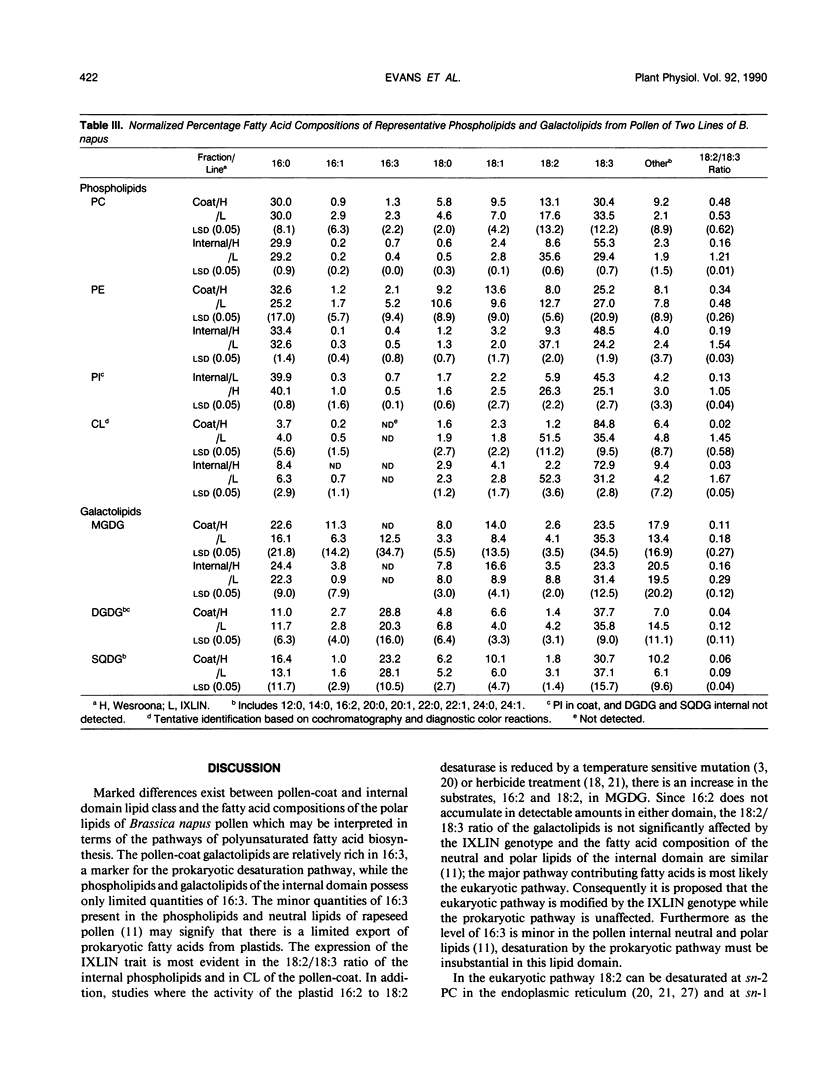
The phospholipids and galactolipids of the pollen-coat and internal domains of two lines of Brassica napus, Wesroona and IXLIN, with different linoleic/linolenic acid ratios (18:2/18:3) have been characterized by normal phase silica high performance liquid chromatography and gas liquid chromatography. The polar lipids of the pollen-coat are similar to leaf lipids in the high proportion of galactolipids (almost 50%) and the fatty acids; 18:3, palmitic (16:0) and hexadecatrienoic (16:3). In contrast, the pollen internal domain, although rich in 18:3, 18:2 and 16:0, is composed primarily of phosphatidyl-choline, -ethanolamine, and -inositol whose 18:2/18:3 ratio is correlated with that of the seed generation. The difference between the two divergent 18:2/18:3 ratio lines is most evident in the internal domain phospholipids. The 18:2/18:3 ratio of the galactolipids of both pollen domains is not significantly effected by the line genotype. The results are interpreted in terms of the previously described `prokaryotic' and `eukaryotic' plant desaturation pathways (PG Roughan, CR Slack [1982] Annu Rev Plant Physiol 33: 97-132). We propose that the eukaryotic pathway is the major desaturation pathway providing polyunsaturated fatty acids to the haploid-specified internal domain in which the IXLIN genotype modifies the activity of the sn-2 linoleoyl phosphatidylcholine desaturase/s of the endoplasmic reticulum. In the diploid-specified pollen-coat, our evidence suggests that a combination of the prokaryotic and eukaryotic pathways contribute polyunsaturated fatty acids.
Full text
PDF
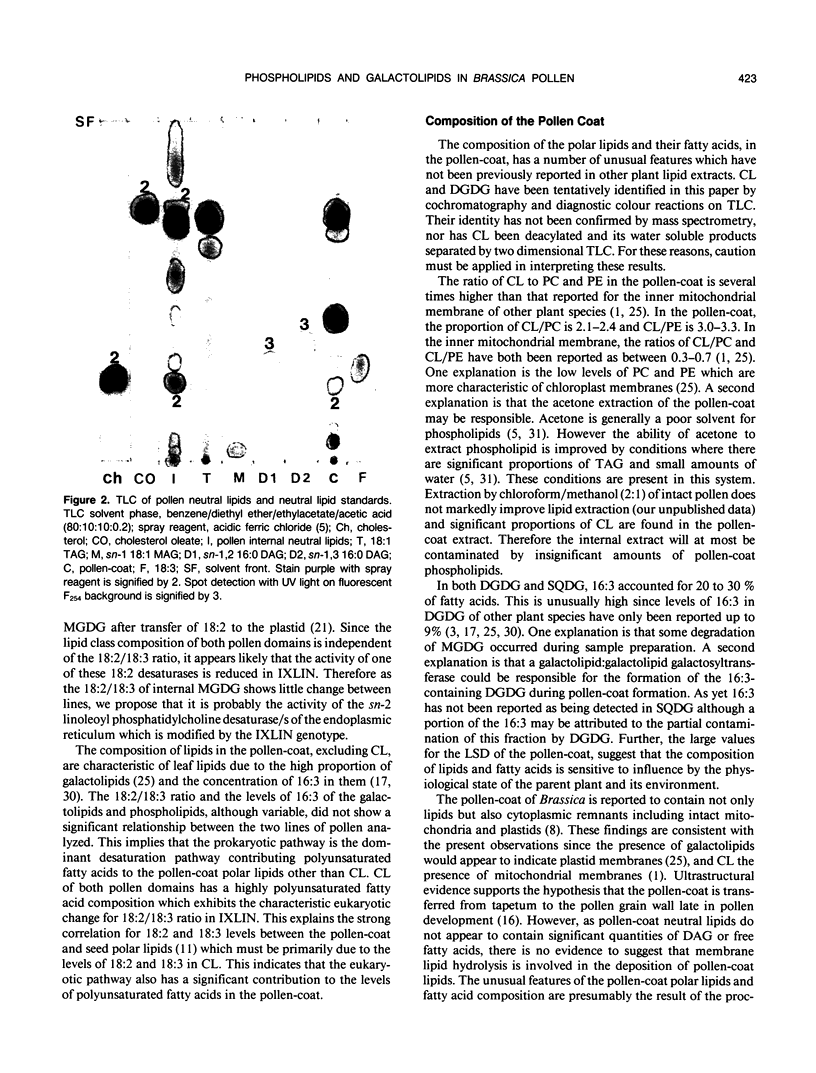

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bligny R., Douce R. A precise localization of cardiolipin in plant cells. Biochim Biophys Acta. 1980 Feb 22;617(2):254–263. doi: 10.1016/0005-2760(80)90168-x. [DOI] [PubMed] [Google Scholar]
- Browse J., McCourt P., Somerville C. A mutant of Arabidopsis deficient in c(18:3) and c(16:3) leaf lipids. Plant Physiol. 1986 Jul;81(3):859–864. doi: 10.1104/pp.81.3.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie W. W. Separation of lipid classes by high-performance liquid chromatography with the "mass detector". J Chromatogr. 1986 Jun 27;361:396–399. doi: 10.1016/s0021-9673(01)86932-6. [DOI] [PubMed] [Google Scholar]
- Dickinson H. G. The role of plastids in the formation of pollen grain coatings. Cytobios. 1973 Sep-Oct;8(29):25–40. [PubMed] [Google Scholar]
- Frentzen M., Heinz E., McKeon T. A., Stumpf P. K. Specificities and selectivities of glycerol-3-phosphate acyltransferase and monoacylglycerol-3-phosphate acyltransferase from pea and spinach chloroplasts. Eur J Biochem. 1983 Jan 1;129(3):629–636. doi: 10.1111/j.1432-1033.1983.tb07096.x. [DOI] [PubMed] [Google Scholar]
- Norman H. A., John J. B. Differential Effects of a Substituted Pyridazinone, BASF 13-338, on Pathways of Monogalactosyldiacylglycerol Synthesis in Arabidopsis. Plant Physiol. 1987 Nov;85(3):684–688. doi: 10.1104/pp.85.3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman H. A., John J. B. Metabolism of Unsaturated Monogalactosyldiacylglycerol Molecular Species in Arabidopsis thaliana Reveals Different Sites and Substrates for Linolenic Acid Synthesis. Plant Physiol. 1986 Jul;81(3):731–736. doi: 10.1104/pp.81.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]