Abstract
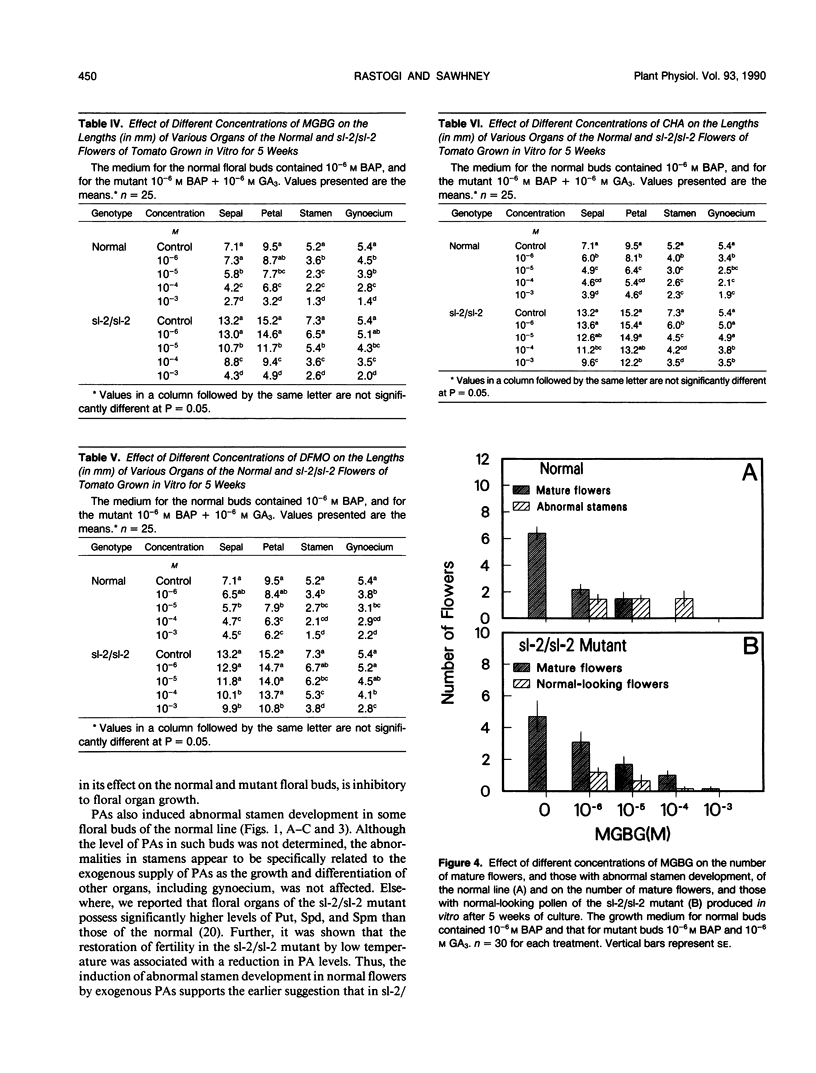
The floral organs of the male sterile stamenless-2 (sl-2/sl-2) mutant of tomato (Lycopersicon esculentum Mill.) contain significantly higher level of polyamines than those of the normal (R Rastogi, VK Sawhney [1990] Plant Physiol 93: 439-445). The effects of putrescine, spermidine and spermine, and three different inhibitors of polyamine biosynthesis on the in vitro development of floral buds of the normal and sl-2/sl-2 mutant were studied. The polyamines were inhibitory to the in vitro growth and development of both the normal and mutant floral buds and they induced abnormal stamen development in normal flowers. The inhibitors of polyamine biosynthesis also inhibited the growth and development of floral organs of the two genotypes, but the normal flowers showed greater sensitivity than the mutant. The inhibitors also promoted the formation of normal-looking pollen in stamens of some mutant flowers. The effect of the inhibitors on polyamine levels was not determined. The polyamine-induced abnormal stamen development in the normal, and the inhibitor-induced production of normal-looking pollen in mutant flowers support the suggestion that the elevated polyamine levels contribute to abnormal stamen development in the sl-2/sl-2 mutant of tomato.
Full text
PDF
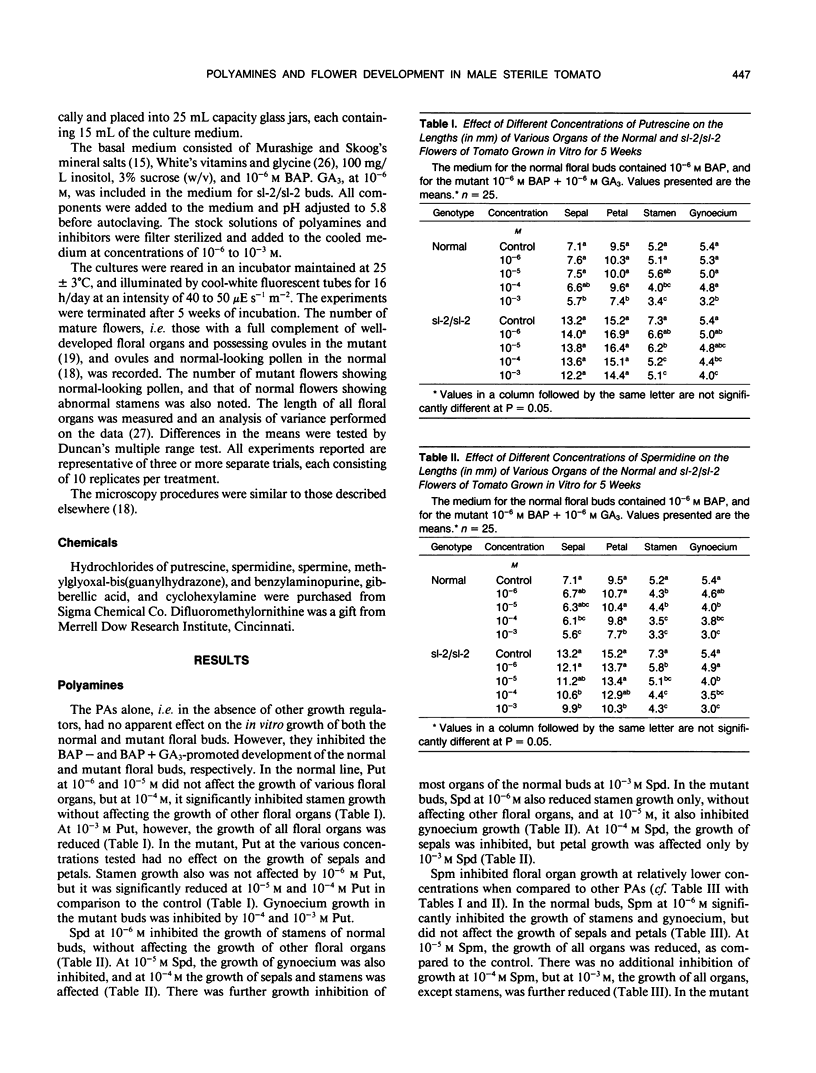
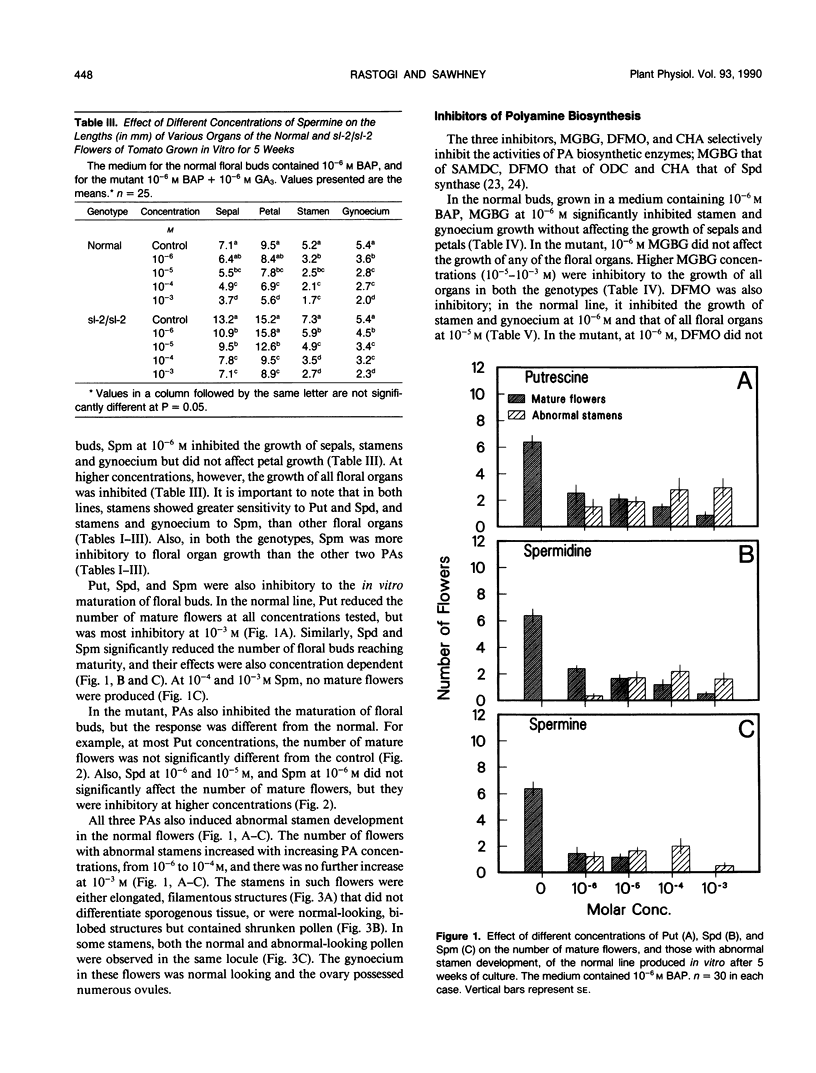
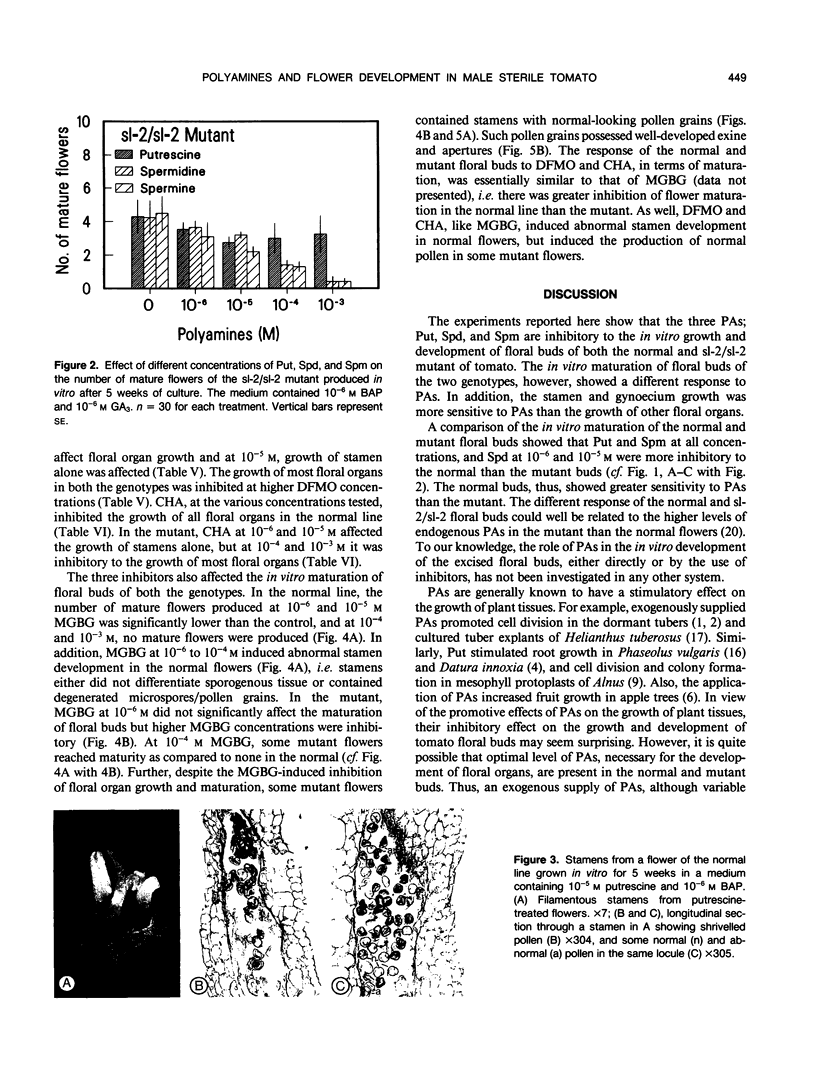


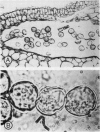
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen E., Arad S. M., Heimer Y. M., Mizrahi Y. Participation of ornithine decarboxylase in early stages of tomato fruit development. Plant Physiol. 1982 Aug;70(2):540–543. doi: 10.1104/pp.70.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg R. L., McIndoo J., Hiatt A. C., Lowe B. A. Genetics of polyamine synthesis in tobacco: developmental switches in the flower. Cold Spring Harb Symp Quant Biol. 1985;50:475–482. doi: 10.1101/sqb.1985.050.01.059. [DOI] [PubMed] [Google Scholar]
- Montague M. J., Koppenbrink J. W., Jaworski E. G. Polyamine Metabolism in Embryogenic Cells of Daucus carota: I. Changes in Intracellular Content and Rates of Synthesis. Plant Physiol. 1978 Sep;62(3):430–433. doi: 10.1104/pp.62.3.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi R., Sawhney V. K. Polyamines and Flower Development in the Male Sterile Stamenless-2 Mutant of Tomato (Lycopersicon esculentum Mill.) : I. Level of Polyamines and Their Biosynthesis in Normal and Mutant Flowers. Plant Physiol. 1990 Jun;93(2):439–445. doi: 10.1104/pp.93.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocum R. D., Galston A. W. In vivo inhibition of polyamine biosynthesis and growth in tobacco ovary tissues. Plant Cell Physiol. 1985;26(8):1519–1526. [PubMed] [Google Scholar]
- Slocum R. D., Kaur-Sawhney R., Galston A. W. The physiology and biochemistry of polyamines in plants. Arch Biochem Biophys. 1984 Dec;235(2):283–303. doi: 10.1016/0003-9861(84)90201-7. [DOI] [PubMed] [Google Scholar]