Abstract
Background
Trials on the effect of systemic chemotherapy on survival and recurrence in adults with high‐grade glioma have had inconclusive results. We undertook a systematic review and meta‐analysis to assess the effects of such treatment on survival and recurrence.
Objectives
To compare radiotherapy plus chemotherapy with radiotherapy alone in completely resected adults with high‐grade glioma. To investigate whether or not pre‐defined patient subgroups benefit more or less from chemotherapy.
Search methods
MEDLINE and CancerLit searches were supplemented with information from trial registers and by hand searching relevant meeting proceedings and by discussion with relevant trialists and organisations. These searches were carried out in June 1997, June 1999, December 2000 and August 2003.
Selection criteria
Trials comparing radiotherapy versus radiotherapy + chemotherapy were eligible for inclusion provided that they randomized adult patients using a method which precluded prior knowledge of treatment assignment.
Data collection and analysis
A quantitative meta‐analysis using updated information from individual patients from all available randomized trials was carried out. Data from all patients randomized in all eligible trials were sought directly from those responsible. Updated information on survival and date of follow‐up were obtained, as were details of treatment allocation, date of randomization, age, sex, histological cell type, stage and performance status. To avoid potential bias, information was requested for all randomized patients including those who had been excluded from the investigators' original analyses. All analyses were done on an intention to treat basis on the endpoint of survival. For trials using cisplatin‐based regimens, subgroup analyses by age, sex, histological cell type, tumour stage and performance status were also done.
Main results
Data from 12 randomized trials and 3004 patients were included. The results show a significant prolongation of survival associated with chemotherapy, with a hazard ratio of 0.85 (95% CI 0.78‐0.91, p=0.00004) or 15% relative decrease in the risk of death. This is equivalent to an absolute increase in one year survival rate of 6% (95% confidence interval 3% to 9%) from 40% to 46% and a two‐month increase in median survival time (95% confidence interval one month to three months). There was no evidence that the effect of chemotherapy was different in any group of patients defined by age, sex, histology, performance status or extent of resection.
Authors' conclusions
This small but clear improvement in survival from chemotherapy encourages further study of drug treatment of these tumours
Plain language summary
Including chemotherapy in the treatment of high‐grade glioma improves survival
High‐grade glioma is a brain tumour that is difficult to treat successfully. Standard treatment is by surgery to reduce the tumour size, followed by radiotherapy. Adding chemotherapy to the treatment results in a small but significant prolongation of survival. Few of the original studies measured quality of life during and post chemotherapy, so it was impossible to assess this. Further randomized controlled trials, which include quality of life assessment, are encouraged.
Background
Malignant gliomas are amongst the most devastating of cancers, frequently producing profound and progressive disability and usually leading to death. They are difficult to diagnose and challenging to treat. Incidence peaks in children and at 50 to 60 years of age (Souhami 1995). These tumours are therefore a major cause of mortality in a relatively young population and improving survival by even a moderate amount could potentially result in many years of life saved. The infiltrating nature of high‐grade glioma makes complete resection virtually impossible, and even if possible it may be associated with severe neurological damage. Thus standard treatment generally consists of cytoreductive surgery followed by radiotherapy. However, prognosis remains poor with a median survival time of nine months and only five to ten per cent of patients surviving to two years (Bleehen 1991). Consequently, a number of randomized trials have explored the use of adjuvant chemotherapy, with research mostly focusing on the use of nitrosoureas which are used because they are lipid soluble and cross the blood‐brain barrier. The majority of these trials, which have been carried out over a period of almost thirty years, have been relatively small, and many have randomized between multiple treatment arms. It is not surprising then, that most have shown inconclusive results and that there is consequently no consensus on the value of chemotherapy treatment
Combining the results of trials in a meta‐analysis increases statistical power and may provide sufficient information to answer the question of survival benefit more reliably. Two meta‐analyses based on summary data extracted from trial reports have been published (Stenning 1987; Fine 1993). However, these suffer from a number of limitations and potential biases. Each identified only a proportion of currently relevant trials and included trials that used pseudo‐random methods of allocation which are known to be liable to bias (Schulz 1995). They were limited to published trials, thereby susceptible to publication bias (Dickersin 1990), and many of the trials excluded considerable numbers of patients (on average 10‐15%) from their published analyses potentially introducing further bias. Importantly, there is strong evidence from the cancer field that meta‐analyses based on data extracted from published reports can give different results from those based on updated individual patient data (Stewart 1993; Clarke 1995) We therefore initiated a systematic review and individual patient data (IPD) meta‐analysis to collect, validate and re‐analyse trial data on all randomized patients from all relevant trials. There are many advantages of collecting IPD in a meta‐analysis such as this (Stewart 1995). In particular, it permits time‐to‐event analyses which, in a disease such a malignant glioma where prolongation of survival rather than cure is anticipated, is extremely important. It also allows us to conduct analyses to assess whether chemotherapy may be more or less effective in different subgroups of patients. The IPD meta‐analysis was initiated and coordinated by the British Medical Research Council Clinical Trials Unit and carried out by the Glioma Meta‐analysis Trialists (GMT) group.
Objectives
To compare radiotherapy plus chemotherapy with radiotherapy alone in completely resected adults with high‐grade glioma. To investigate whether or not pre‐defined patient subgroups benefit more or less from chemotherapy. .
Methods
Criteria for considering studies for this review
Types of studies
Both published and unpublished trials were eligible for inclusion. Trials should have been properly randomized in a way which precluded prior knowledge of treatment assignment (trials which allocated treatment by pseudo‐random methods such as birthdate were excluded). Trials should have aimed to randomize patients with high‐grade glioma, who had undergone surgery and were then allocated radiotherapy and chemotherapy or radiotherapy alone. Recruitment should have started after January 1st 1965 and completed by June 30th 1997.
Types of participants
Eligible trials included individuals with high‐grade glioma who have not received any prior treatment for any other malignancy likely to interfere with protocol treatments or comparisons. Individual data from all randomized patients were included in the meta‐analysis and where possible data were obtained for individuals who had been excluded from the original trial analyses. These individuals were included in the meta‐analysis.
Types of interventions
Surgery + radiotherapy + chemotherapy versus surgery + radiotherapy
Details of surgery, radiotherapy and chemotherapy are given in Characteristics of Included Studies
Types of outcome measures
Survival Recurrence‐free survival
Search methods for identification of studies
To avoid publication bias, both published and unpublished trials were included. Computerised bibliographic searches of MEDLINE and CancerLit were made (06/1997, 06/1999, 12/2000) using a version of the Cochrane Collaboration optimal search strategy (Dickersin 1994). Searches were not limited to trials reported in English language journals. This strategy was also modified and used to search Embase. These electronic searches were supplemented by hand searching the reference lists of identified trials, and bibliographies of relevant books and review articles. The National Cancer Institute Physicians Data Query (PDQ) Clinical Protocols and the United Kingdom Coordinating Committee for Cancer Research Trials Register were also searched to identify both completed and ongoing trials. All trialists who took part in the meta‐analysis were asked to help to identify trials. All titles identified by search strategies were assessed for relevance independently by two reviewers. Abstracts were downloaded for all titles of potential relevance and full papers obtained for all abstracts judged potentially relevant. Where there was uncertainty about the eligibility of a trial, or particular treatment arms within a trial, this was discussed and resolved by consensus within the project secretariat, and ratified by the GMT group at a meeting held in July 1999.
1 Randomized‐Controlled‐Trial.pt. 2 Randomized Controlled Trials/ 3 Random Allocation/ 4 Double‐Blind Method/ 5 Single‐Blind Method/ 6 1 or 2 or 3 or 4 or 5 7 Animal.DE. 8 Human.DE. 9 7 NOT (7 AND 8) 10 6 NOT 9 11 Clinical‐Trial.pt. 12 Clinical‐Trials/ 13 (Clin$ WITH Trial$).ab,ti. 14 ((Sing$ OR Doub$ OR Trebl$ OR Tripl$) ADJ (Blind$ OR MaskS)).ab,ti. 15 Placebo$.ab,ti. 16 Random.ab,ti. 17 Research Design/ 18 11 OR 12 OR 13 OR 14 OR 15 OR 16 OR 17 19 18 NOT 9 20 19 NOT 10 21 10 NOT 20 22 Brain‐Neoplasms.DE. 23 Drug‐Therapy.DE. 24 22 AND 23 25 21 AND 24 26 Brain ADJ Neoplasm$ 27 Glioma$ 28 26 OR 27 29 Chemotherapy 30 28 AND 29 31 25 OR 30 32 Child#.DE. 33 31 NOT 32
Data collection and analysis
This review is based on individual patient data obtained directly from the responsible trialist or data centre. It does not use information extracted from published papers. All data were collected, checked and analysed centrally.
Data were sought for all patients randomized in all eligible randomized trials (published or unpublished) and updated follow‐up requested. For all comparisons the following data were collected: patient identifier, treatment allocated, date of randomization, survival status, date of last follow‐up or death and whether the individual was excluded from the original analyses. Data on age, sex, stage, histology and performance status were also collected. Collection and validation of data were carried out at the MRC Cancer Trials Office (now the Cancer Division of the MRC Clinical Trials Unit).
All data were checked thoroughly and a common database was agreed. The final database entries for each trial were verified by the responsible trialist or data centre.
For trials with multiple treatment arms the eligibility of each individual arm was assessed and only the relevant arms were included. Characterisitics of Included Studies provides further information on this.
All analyses were carried out on an intention‐to‐treat basis, that is, patients were analysed according to their allocated treatment, irrespective of whether they received that treatment. Survival analyses were stratified by trial, and the log rank expected number of deaths and variance used to calculate individual trials and overall pooled hazard ratios (HR) using the fixed effect model (Yusuf 1985). Thus, the times to event (progression or death) for individual patients were used within trials to calculate the HR, representing the overall risk of an event for those patients allocated to adjuvant chemotherapy compared with those allocated to no chemotherapy. To investigate the effects of chemotherapy within subgroups of patients, similar stratified analyses were done. Analyses were performed for each pre‐specified category, for example, comparing treatment and control for males and for females within each individual trial. These results were then combined to give overall HRs for males and for females. Results are also presented as absolute differences at one and two years, calculated using the overall HRs and event rate on the control (Parmar 1995). Absolute effects for different types of patients defined by categories used in our sub‐group analyses were calculated using the overall HR and event rates on the surgery alone arm for each subgroup. Confidence intervals for absolute differences were calculated from the baseline event rate and the HR at the 95% confidence interval boundary values. Chi‐square heterogeneity tests (EBCTCG 1990) were used to test for gross statistical heterogeneity across trials. Survival curves are presented as simple (non‐stratified) Kaplan‐Meier curves (Kaplan 1958) . All p‐values quoted are two‐sided.
Results
Description of studies
In total, 24 trials were identified as potentially eligible for the meta‐analysis. Five of these were found to be ineligible and therefore excluded. The reasons for exclusion are listed in the table of excluded studies. Of the 19 eligible trials, data were not available from seven trials as they had been lost, destroyed or were untraceable. These trials are also listed in the table of excluded trials. Data from 12 randomized trials and 3004 patients are therefore included in this meta‐analysis. This represents 81% of individuals from all known eligible randomized trials. Data were collected for 210 of the 253 patients who had been excluded from the original published analyses and were reinstated in the meta‐analysis. For one trial (Alberta) we were unable to obtain information from eight patients. As the missing patients were few and distributed evenly across treatment arms the trial was included. In another trial, (Poland) data had to be read from archived computer printouts and we were unable to retrieve information on 19 patients that had become detached from the end of the listing. As these missing patients were not evenly distributed on treatment arms, the main analyses were done both with and without this trial. Design features of all eligible trials are shown in Characteristics of Included Studies. Of the included trials, total radiotherapy doses ranged from 40 Gy to 60 Gy given in between 25 and 35 fractions. Four trials delivered whole brain irradiation whilst eight irradiated the tumour plus margins. The maximum planned delay between surgery and radiotherapy/chemotherapy ranged from two to six weeks and all but one trial (EORTC 26741) randomized prior to radiotherapy. All trials included at least one nitrosourea compound, given either as a single agent or in combination with other drugs. Chemotherapy regimens and planned drug doses are given in Characteristics of Included Studies. Although trials were able to provide most of the baseline patient characteristic data requested, some data were unavailable. Information on age, sex, histology and extent of resection was provided for all trials and data on performance status for nine trials. Grade data were available for only four trials and so were insufficient for subgroup analyses. Cause of death data (coded as glioma, treatment related and other) were provided for eight trials although the trialists themselves questioned the reliability of this information for many of the trials. The average median follow up is two years for surviving patients (eight months to three years six months for individual trials). The patient characteristics which reflect the eligibility criteria of individual trials are given in Table 1. Patients were mostly male, fairly young with glioblastoma multiforme and had received an incomplete resection.
1. Patient Characteristics.
| RT + Chemotherapy | RT alone | Total | ||
| Age | <=40 | 291 (17%) | 218 (17%) | 509 |
| 41‐59 | 914 (54%) | 714 (55%) | 1628 | |
| >=60 | 474 (28%) | 362 (27%) | 836 | |
| Unknown | 19 (1%) | 12 (1%) | 31 | |
| Sex | Male | 971 (57%) | 776 (59%) | 1747 |
| Female | 709 (42%) | 518 (40%) | 1227 | |
| Unknown | 18 (1%) | 12 (1%) | 30 | |
| Histology | AA | 400 (24%) | 306 (24%) | 706 |
| GBM | 1062 (62%) | 838 (64%) | 1900 | |
| Other | 98 (6%) | 80 (6%) | 178 | |
| Unknown | 138 (8%) | 82 (6%) | 220 | |
| Performance Status | Good | 636 (37%) | 591 (45%) | 1225 |
| Poor | 560 (33%) | 438 (34%) | 998 | |
| Unknown | 504 (30%) | 277 (21%) | 781 | |
| Extent of Resection | Complete | 432 (25%) | 317 (24%) | 749 |
| Incomplete | 953 (56%) | 723 (55%) | 1676 | |
| Biopsy | 262 (16%) | 231 (18%) | 493 | |
| Unknown | 51 (3%) | 35 (3%) | 86 | |
Risk of bias in included studies
Only trials with adequate methods of randomization (those which did not allow prior knowledge of treatment assignment) were included. All data received were checked thoroughly to ensure both the accuracy of the meta‐analysis database and the quality of randomization and follow up. Any queries were resolved and the final database entries verified by the responsible trial investigator or statistician.
Effects of interventions
Survival data were available for all 12 trials and included information on 3004 patients and 2659 deaths. Although the confidence intervals for individual trial results are wide and the results of most inconclusive, all but one HR estimate is in favour of adjuvant chemotherapy (Outcome 01: Survival). There is no clear evidence of statistical heterogeneity (p=0.275) between trials. The combined results show a statistically significant increase in survival (p=0.00004) associated with the use of chemotherapy. The hazard ratio of 0.85 (95% CI 0.78 ‐ 0.92), representing a 15% relative reduction in the risk of death, is equivalent to an absolute improvement of 6% at one year (95% CI 3% to 9%) increasing overall survival from 40% to 46%. At two years it is equivalent to a five per cent (95%CI 2% to 8%) increase from 15% to 20%. This advantage of chemotherapy is also illustrated in the survival curves, which are presented in the meta‐analysis publication (GMT Group), which appear to separate at around six months and then remain apart over time. A sensitivity analysis excluding one trial (Poland) for the reasons discussed above had minimal impact on the pooled result (HR=0.84, 95% CI 0.78‐0.92, p=0.00003). There was no difference in the results (interaction p=0.84) between trials using single‐agent chemotherapy (HR=0.84, 95% CI 0.75‐0.93) and those using combination chemotherapy (HR=0.85, 95%CI 0.76‐0.94). In a supplementary analysis, there was no clear evidence that those trials giving higher total doses of radiotherapy (>=60Gy) showed substantially different results to those using lower radiotherapy doses (<60Gy), with HRs of 0.88 and 0.77 (95%CI) respectively, (interaction p=0.11). A further analysis excluding the individual trial (MRC BR05) that had suggested an interaction between radiotherapy dose and effect of chemotherapy also showed no evidence that results of trials using lower radiotherapy doses of >=60Gy were any different to those using doses of <60Gy (interaction p= 0.68) with HRs of 0.83 and 0.79 respectively. A sensitivity analysis based on only those patients with glioblastoma multiforme and anaplastic astrocytoma gave a very similar estimate to the main result (HR=0.83, 95%CI 0.76‐0.90, p=0.000013).
Progression‐Free Survival
Information on disease progression was available from eight trials and a total of 2022 patients. A total of 1859 events were observed. Results show a similar pattern to survival. The overall HR of 0.83 (95% CI = 0.75 ‐ 0.91) (Figure 1), indicates a statistically significant (p=0.00008) 17% reduction in the risk of progression or death, This is equivalent to an absolute benefit of five per cent at two years (95% confidence interval 2 to 8%) increasing progression‐free survival from 10% to 15%. Median progression‐free survival is increased by one and a half months (95% CI 0.5 to 2.5 months) from six months to seven and a half months.
1.
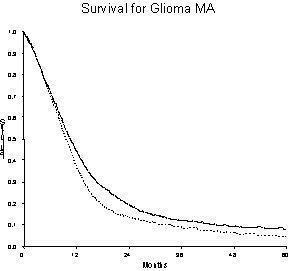
Events Total RT + Chemo ______ 1484 1698 RT alone ‐‐‐‐‐‐‐‐‐‐‐ 1175 1306
Patients at risk RT + Chemo ‐ 1698 ‐ 720 (1 yr), 295 (2 yrs), 149 (3 yrs), 96 (4 yrs), 68 (5yrs) RT alone ‐ 1306 ‐ 456 (1 yr), 155 (2 yrs), 86 (3 yrs), 45 (4 yrs), 28 (5 yrs)
Subsidiary Analyses in Patient Subgroups
Analyses were performed to determine whether there was evidence of a differential effect of chemotherapy in pre‐defined subgroups of patients. For survival there was no evidence to suggest that chemotherapy was differentially effective in any group of patients defined by age (trend p=0.313), sex (interaction p=0.874), histology (interaction, p=0.995), performance status (interaction p=0.872) or extent of resection (trend p=0.291) (Outcomes 03‐07).
Analysis of published data for unavailable trials
Because information was not available from seven trials accounting for 683 patients, an analysis based on data extracted from publications was done for the six trials (EORTC 26741; EORTC 26742; Hatlevoll 1985; Eagan 1979; Reagan 1976; Kristiansen 1981) for which appropriate data could be extracted. This used numbers of patients who had died by two years to calculate an odds ratio (OR). This analysis gave results that were broadly similar to the results of our IPD analysis. (OR = 0.92, 95%CI 0.79 ‐ 1.09).
Discussion
At the outset of this project, despite the enrolment of more than 3500 patients in randomized trials, it remained unclear whether or not chemotherapy was effective in the treatment of high‐grade glioma. Current clinical practice varies both nationally and internationally. The aim of this systematic review and meta‐analysis was to provide a comprehensive, reliable and up‐to‐date summary of the average effect of chemotherapy in adult patients with high‐grade glioma, to provide guidance for clinical practice and future research.
For the primary endpoint of survival, there is clear evidence of a beneficial effect of adjuvant chemotherapy. The 15% relative reduction in the risk of death associated with chemotherapy is equivalent to an overall increase in survival from 40% to 46% at one year, to an increase from 10% to 15% at two years, and to a two month increase in median survival time from 10 to 12 months. Although many trials were completed some years ago and did not undergo central pathology review, there is no indication that these results are being driven by inclusion of chemo‐sensitive tumours e.g. oligodendrogliomas. A sensitivity analysis based on only the anaplastic astrocytoma and glioblastoma multiforme tumours gave results that were very close to the main result. In addition, the results of subgroup analyses illustrate a benefit of chemotherapy irrespective of histology and are applicable to present‐day patients with a confirmed diagnosis of glioblastoma. Further supplementary analyses by age, sex, performance status and extent of tumour resection also gave no indication that the relative effect of chemotherapy varies in the different patient subgroups included in the meta‐analysis. Thus, the best estimate for any individual patient is that they are likely to gain around 15% reduction in the overall risk of death. However, given that the underlying prognoses for different categories of patients vary considerably, these relative effects are likely to translate to different absolute improvements in outcome rates. Baseline survival rates and corresponding absolute increases are shown in Table 2. This shows, for example, that the two‐year survival rate for individuals with glioblastoma multiforme is increased from nine per cent to 13%, whereas for those with anaplastic astrocytoma it is increased from 31% to 37%.
2. Baseline survival and equivalent absolute increases.
| 1 yr survival | 1 yr survival | 2 yr survival | 2 yr survival | ||
| Baseline | Absolute increase | Baseline | Absolute increase | ||
| Age | <=40 | 78% | 3% | 50% | 5% |
| 41‐49 | 45% | 6% | 14% | 5% | |
| >=60 | 22% | 6% | 4% | 2% | |
| Sex | Male | 45% | 6% | 18% | 5% |
| Female | 40% | 6% | 16% | 5% | |
| Histology | AA | 58% | 5% | 31% | 6% |
| GBM | 35% | 6% | 9% | 4% | |
| Other | 72% | 4% | 52% | 5% | |
| Performance Status | Good | 54% | 5% | 22% | 6% |
| Poor | 31% | 6% | 9% | 4% | |
| Extent of Resection | Complete | 50% | 5% | 19% | 5% |
| Incomplete | 40% | 6% | 16% | 5% | |
| Biopsy | 36% | 6% | 19% | 5% | |
As data were not available from around 19% of the total randomized evidence, we conducted a comparative analysis based on data presented in publications for the missing trials. Although there are many potential problems and biases with this approach, it is useful to compare how results from the unavailable trials compare to those included in the IPD analysis. In particular, it allows us to explore whether there is any obvious bias associated with trial availability, for example, did we only have access to the positive trials? The results of this analysis of survival at two years showed broadly similar results to our IPD analysis and apparent efficacy of chemotherapy. Thus we can be reasonably confident that, had we successfully obtained the missing data, it would not have substantially altered the results of our IPD analysis.
Undoubtedly, there are design differences in the trials included in the meta‐analysis, particularly with respect to the radiotherapy regimens and techniques used. It could be suggested that rather than giving an additional advantage, chemotherapy is simply making up for inadequate radiotherapy. However, there was no compelling evidence that the effect of chemotherapy was moderated by radiotherapy total dose. The HR 0.88 for just those trials delivering a total dose of 60Gy or more is not significantly different to that of the remainder of trials and very similar to the overall HR. Thus the effect of chemotherapy is apparent in those trials delivering radiotherapy doses similar to those widely used in current clinical practice and there is no strong evidence that chemotherapy is merely compensating for inadequate radiotherapy techniques.
Although this meta‐analysis has shown a clear benefit of chemotherapy of around six per cent at two years, and an improvement in median survival time of two months (from 10 to 12 months), whether this is of benefit clinically remains open to interpretation. This is, of course, likely to vary depending upon the clinical situation and individual patient and family preference. Clearly, tolerability of treatment and quality of life, including cognitive impairment, are major issues in judging this for patients who will usually survive only a short time after their treatment has finished. Few of the trials included in this meta‐analysis, formally measured quality of life or undertook cognitive function tests in ways that would allow data to be combined in a meta‐analysis. We are therefore unable to assess the quality of the demonstrated prolongation of survival. However, when making decisions about treatment, the interpretation of such information is likely to be influenced by a myriad of personal beliefs and preferences, so that interpretation of these data in isolation may not be particularly helpful. In this respect the nitrosoureas, whilst not a novel method of treatment, are fairly well tolerated, easily administered, may be of practical use in the clinic for those individuals to whom it is important to extend their likely survival time, if only by a modest amount. Importantly, the clear effect observed in this comprehensive review, does demonstrate that high‐grade gliomas can respond to chemotherapy and that further research into newer chemotherapies and methods of delivery is justified. The size of the benefit and remaining uncertainty concerning quality of life, is such that some clinical trialists would consider radiotherapy alone to be a justified standard therapy arm, whereas others may feel that the appropriate standard therapy arm should now include a nitrosourea. The small but clear improvement in survival from chemotherapy encourages further study of drug treatment of these tumours.
Authors' conclusions
Implications for practice.
Although this meta‐analysis has shown a clear benefit of chemotherapy of around six per cent at two years, and an improvement in median survival time of two months (from 10 to 12 months), whether this is of benefit clinically remains open to interpretation
Implications for research.
This small but clear improvement in survival from chemotherapy encourages further study of drug treatment of these tumours
What's new
| Date | Event | Description |
|---|---|---|
| 27 March 2014 | Amended | Contact details updated. |
| 21 July 2009 | Review declared as stable | IPD data |
History
Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 13 October 2008 | Amended | Converted to new review format. |
| 21 May 2002 | New citation required and conclusions have changed | Substantive amendment |
Notes
This review originated as an individual patient data (IPD) review, therefore the text has been agreed upon by the collaborative group who undertook the review.
The manuscript has been scrutinised by the collaborative group and has been through peer review prior to publication in a journal as well as being assessed by the Cochrane Review Group.
Searches will be updated according to usual Cochrane guidelines, however, the analyses will only be updated if substantial new evidence emerges.
Acknowledgements
The UK Medical Research Council funded the coordination of the meta‐analysis and the collaborators' meeting. We thank all those patients who took part in the trials and contributed to this research. The meta‐analysis would not have been possible without their participation or without the collaborating institutions that kindly supplied their trial data. We are also thank Richard Kaplan and the US National Cancer Institute for supporting data retrieval by the Radiation Therapy Oncology Group (RTOG), Jayne Tierney for comments and assistance at all stages of the project, Claire Vale and Janet Darbyshire for helpful comments on the report.
Contributors All aspects of the meta‐analysis were carried out under the auspices of the GMT Group. D Áfra, B Baron, G Bonadonna, WJ Curran Jr, SB Green, J Hildebrand, CB Scott, W Shapiro, D Thomas, T Trojanowski, R Urtasun and MD Walker collated and supplied the individual patient data, contributed to the discussions of the results and commented on the drafts of the reports. The project was organised by the secretariat, S Burdett, MKB Parmar, RL Souhami, SP Stenning and LA Stewart, who were responsible for formulating the question, developing the protocol, receiving, checking and analysing the data. The Project was managed by S Burdett. The report was drafted by LA Stewart and S Burdett with detailed input from RL Souhami and SP Stenning. GMT Group D Áfra (National Institute of Neurosurgery, Budapest, Hungary), B Baron (EORTC Data Center, Brussels, Belgium), G Bonadonna (Istituto Nazionale Tumori, Milan, Italy), S Burdett, MKB Parmar, SP Stenning, LA Stewart (MRC Clinical Trials Unit, London, UK), WJ Curran Jr. (Jefferson Medical College, Philadelphia, PA, USA), SB Green (Case Western Reserve University, Cleveland, OH, USA), J Hildebrand (Hopital Universitaire Erasme, Brussels, Belgium), CB Scott (Radiation Therapy Oncology Group, Philadelphia, PA, USA), W Shapiro (Barrow Neurological Institute, Phoenix, AZ, USA), RL Souhami (Royal Free and University College Medical School, London, UK), D Thomas (The National Hospital, London, UK), T Trojanowski (Medical School, Lublin, Poland), RC Urtasun (University of Alberta, Edmonton, Alberta, Canada), MD Walker (National Institute of Neurological Disorders and Stroke, Bethesda, MD, USA)
Data and analyses
Comparison 1. RT + Chemo vs RT alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Survival | 12 | 3004 | Peto Odds Ratio (99% CI) | 0.85 [0.78, 0.92] |
| 2 Progression‐free Survival | 8 | 2022 | Peto Odds Ratio (99% CI) | 0.83 [0.75, 0.91] |
| 3 Survival subgroup analysis ‐ age | 3 | 2972 | Peto Odds Ratio (99% CI) | 0.83 [0.76, 0.90] |
| 4 Survival subgroup analysis ‐ sex | 2 | 2659 | Peto Odds Ratio (99% CI) | 0.83 [0.76, 0.90] |
| 5 Survival subgroup analysis ‐ histology | 3 | 2501 | Peto Odds Ratio (99% CI) | 0.79 [0.73, 0.87] |
| 6 Survival subgroup analysis ‐ performance status | 2 | 2520 | Peto Odds Ratio (99% CI) | 0.82 [0.75, 0.89] |
| 7 Survival subgroup analysis ‐ extent of resection | 3 | 2631 | Peto Odds Ratio (99% CI) | 0.82 [0.75, 0.89] |
1.1. Analysis.
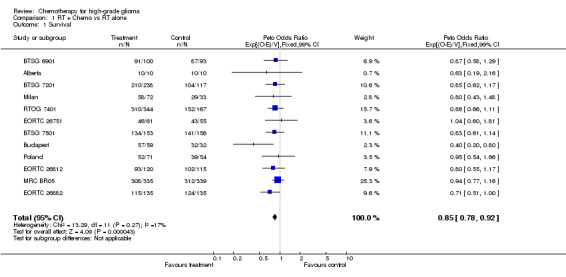
Comparison 1 RT + Chemo vs RT alone, Outcome 1 Survival.
1.2. Analysis.
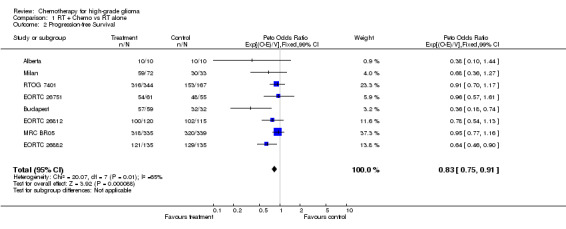
Comparison 1 RT + Chemo vs RT alone, Outcome 2 Progression‐free Survival.
1.3. Analysis.
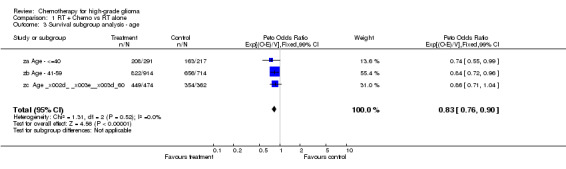
Comparison 1 RT + Chemo vs RT alone, Outcome 3 Survival subgroup analysis ‐ age.
1.4. Analysis.
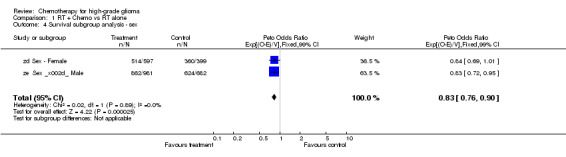
Comparison 1 RT + Chemo vs RT alone, Outcome 4 Survival subgroup analysis ‐ sex.
1.5. Analysis.
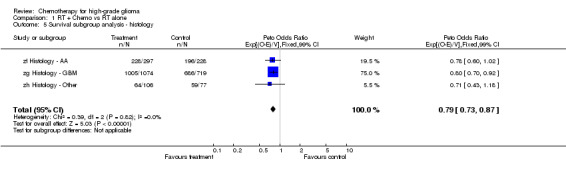
Comparison 1 RT + Chemo vs RT alone, Outcome 5 Survival subgroup analysis ‐ histology.
1.6. Analysis.
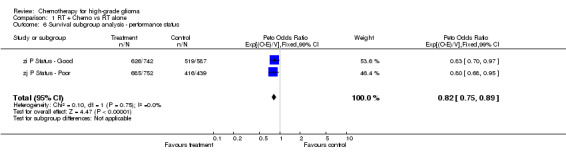
Comparison 1 RT + Chemo vs RT alone, Outcome 6 Survival subgroup analysis ‐ performance status.
1.7. Analysis.
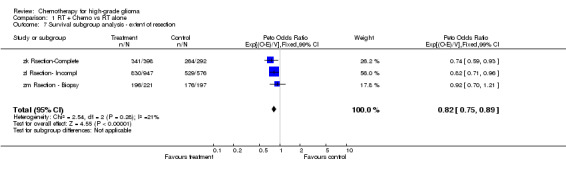
Comparison 1 RT + Chemo vs RT alone, Outcome 7 Survival subgroup analysis ‐ extent of resection.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alberta.
| Methods | RCT | |
| Participants | 28 ‐High grade astrocytoma ‐Resection biopsy | |
| Interventions | CT + RT vs RT ‐CCNU 130mg/m2 oral q 6 wks ‐RT 40‐45 Gy 25f 4‐5 wks | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1971‐1973 2 of 3 arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
BTSG 6901.
| Methods | RCT | |
| Participants | 193 ‐Anaplastic astrocytoma ‐Definitive surgical resection | |
| Interventions | CT + RT vs RT ‐BNCU 80 mg/m2 x3 iv q 6‐8 wks ‐RT 50‐60 Gy 30‐35f 6‐7 wks | |
| Outcomes | Survival | |
| Notes | 1969‐1972 2 of 4 arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
BTSG 7201.
| Methods | RCT | |
| Participants | 356 ‐Malignant glioma ‐Definitive surgery | |
| Interventions | CT + RT vs RT ‐MeCCNU 220mg/m2 oral q 6‐8 wks ‐BCNU 80 mg/m2 x3 iv q 6‐8 wks ‐RT 60 Gy 30‐35f 6‐7 wks | |
| Outcomes | Survival | |
| Notes | 1972‐1975 3 of 4 arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
BTSG 7501.
| Methods | RCT | |
| Participants | 309 ‐Malignant glioma ‐Definitive surgery | |
| Interventions | CT + RT vs RT ‐BCNU 80mg/m2 x3 iv q 8 wks ‐PCZ 150mg/m2 x 28days q 8wks | |
| Outcomes | Survival | |
| Notes | 1974‐1978 2 of 4 arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Budapest.
| Methods | RCT | |
| Participants | 91 ‐Glioblastoma malignant astrocytoma grade III (WHO zulch) ‐At least sub‐total resection | |
| Interventions | CT + RT vs RT ‐DBD 400 mg/m2 q 5 days during RT month rest then repeat ‐DBD 400 mg/m2 q 5 days during RT 5‐6 wks rest then CCNU 200 mg/m2 x5 q 4‐6 wks ‐RT 51 Gy 25‐30f 5‐6 wks | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1978‐1981 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
EORTC 26751.
| Methods | RCT | |
| Participants | 116 ‐Malignant glioma ‐Optimal resection | |
| Interventions | CT + RT vs RT ‐CCNU 130mg/m2 oral % VM‐26 60mg/m2 iv q 6 wks | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1975‐1978 1st random | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
EORTC 26812.
| Methods | RCT | |
| Participants | 335 ‐Malignant: astrocytoma, glioblastoma, ependymoblastoma, oligodendroglioma. | |
| Interventions | CT + RT vs RT ‐CCNU 130 mg/m2 oral + VM‐26 100mg/m2 IV q 6 wks | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1982‐1987 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
EORTC 26882.
| Methods | RCT | |
| Participants | 270 ‐Anaplastic astrocytoma, glioblastoma ‐Resection stereotactic biopsy | |
| Interventions | CT + RT vs RT ‐DBD 700 mg/m2 x7 oral during RT then BCNU 150 mg/m2 iv DBD 1000 mg/m2 oral q 6 wks | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1989‐1991 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Milan.
| Methods | RCT | |
| Participants | 105 ‐Glioblastoma multiforme ‐Total or subtotal resection | |
| Interventions | CT + RT vs RT ‐BCNU 80mg/m2 x3 iv q 6‐8 wks ‐CCNU 130mg/m2 oral q 6‐8 wks | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1972‐1973 2 of 3 arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
MRC BR05.
| Methods | RCT | |
| Participants | 674 ‐Astrocytoma grade III/IV (WHO/Zulch) | |
| Interventions | CT + RT vs RT ‐CCNU 100mg/m2 PCZ 100mg/m2 oral x 10 VCR 1.5mg/m2 q 6 wks | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1986‐1997 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Poland.
| Methods | RCT | |
| Participants | 149 ‐Glioma, high and low grade | |
| Interventions | CT + RT vs RT ‐CCNU 100mg/m2 oral q 6‐8 wks | |
| Outcomes | Survival | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
RTOG 7401.
| Methods | RCT | |
| Participants | 512 ‐Astrocytoma grade III/IV (Kernohan) | |
| Interventions | CT + RT vs RT ‐BCNU 80mg/m2 x3 iv q 6‐8 wks ‐MeCCNU 125mg/m2 oral q 8 wks DTIC 150mg/m2 x5 iv q 4 wks (doses initially 150mg/m2 & 175mg/m2 but reduced owing to severe toxicity) | |
| Outcomes | Survival Recurrence‐free survival | |
| Notes | 1974‐1979 3 of 4 arms (institutions chose to randomise to 2 or 3 of the 4 arms. RTOG indicate that all institutes randomised between RT vs RT + chemo) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
za Age ‐ <=40.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zb Age ‐ 41‐59.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zc Age ‐ >=60.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zd Sex ‐ Female.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
ze Sex ‐ Male.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zf Histology ‐ AA.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zg Histology ‐ GBM.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zh Histology ‐ Other.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zi P Status ‐ Good.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zj P Status ‐ Poor.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zk Rsection‐Complete.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zl Rsection‐ Incompl.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
zm Rsection ‐ Biopsy.
| Methods | ||
| Participants | ||
| Interventions | ||
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
CT=chemotherapy, RT=radiotherapy, f=fractions, q=every Za‐Zm are sub‐group categories not trials
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Brisman 1976 | Confounded by use of hyperbaric oxygen on control arm |
| Cianfriglia 1980 | alternate allocation |
| Eagan 1979 | Eligible but data unavailable |
| EORTC 26741 | Eligible but data lost by data centre |
| EORTC 26742 | Eligible but data lost by data centre |
| Garrett 1978 | 'randomized' by date of birth |
| Hatlevoll 1985 | Eligible but data unavailable |
| Kristiansen 1981 | Eligible but data unavailable |
| Muller 1985 | 'randomized' by date of birth |
| Reagan 1976 | Eligible but data unavailable |
| Takakura 1986 | Eligible but unable to participate |
| Ushio 1981 | Several treatment groups operational at different times |
Contributions of authors
All reviewers participated in the design, execution and analysis of the review.
Declarations of interest
None known
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Alberta {published and unpublished data}
- Weir B, Band P, Urtasun R, Blain G, McLean D, Wilson F, Mielke B, Grace M. Radiotherapy and CCNU in the treatment of high‐grade supratentorial astrocytomas. Journal of Neurosurgery 1976;45:129‐34. [DOI] [PubMed] [Google Scholar]
BTSG 6901 {published and unpublished data}
- Walker MD, Alexander Jr E, Hunt WE, MacCarty CS, Mahaley MS, Mealey Jr J, Norrell HA, Owens G, Ransohoff J, Wilson CB. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. Journal of Neurosurgery 1978;49:333‐43. [DOI] [PubMed] [Google Scholar]
BTSG 7201 {published and unpublished data}
- Walker MD, Green SB, Byar DP, Alexander E, Batzdorf U, Brooks WH, Hunt WE, MacCarty CS, MacCarty CS, Mahaley MS. Randomized comparisons of radiotherapy and nitrosoureas for the treatment of malignant glioma after surgery. The New England journal of medicine 1980;303:1323‐9. [DOI] [PubMed] [Google Scholar]
BTSG 7501 {published and unpublished data}
- Green SB, Byar DP, Walker MD, Pistenmaa DA, Alexander Jr E, Batzdorf U, Brooks WH, Hunt WE, Mealey Jr J, Odom GL. Comparisons of carmustine, procarbazine and high‐dose methylprednisolone as additions to surgery and radiotherapy for the treatment of malignant glioma. Cancer Treatment Reports 1983;67(2):121‐32. [PubMed] [Google Scholar]
Budapest {published and unpublished data}
- Áfra D, Kocsis B, Dobay J, Eckhardt S. Combined radiotherapy and chemotherapy with dibromoducitol and CCNU in the postoperative treatment of malignant gliomas. Journal of Neurosurgery 1983;59:106‐10. [DOI] [PubMed] [Google Scholar]
EORTC 26751 {published and unpublished data}
- E.O.R.T.C. Brain Tumor Group. Evaluation of CCNU, VM‐26 plus CCNU and procarbazine in supratentorial brain gliomas. Journal of Neurosurgery 1981;55:27‐31. [DOI] [PubMed] [Google Scholar]
EORTC 26812 {unpublished data only}
- E.O.R.T.C. Brain Tumor Group. Phase III adjuvant therapy with radiotherapy versus radiotherapy plus VM‐26/CCNU for resected malignant glioma (as supplied 2002). Data on file.
EORTC 26882 {published and unpublished data}
- Hildebrand J, Sahmoud T, Mignolet F, Brucher JM, Áfra D. Adjuvant therapy with dibromoducitol and BCNU increases survival of adults with malignant gliomas. Neurology 1994;44:1479‐83. [DOI] [PubMed] [Google Scholar]
Milan {published and unpublished data}
- Solero CL, Monfardini S, Brambilla C, Vaghi A, Valagussa P, Morello G, Bonadonna G. Controlled study with BCNU versus CCNU as adjuvant chemotherapy following surgery plus radiotherapy for glioblastoma multiforme. Cancer Clinical Trials 1979;2:43‐8. [PubMed] [Google Scholar]
MRC BR05 {unpublished data only}
- Medical Research Council Brain Tumour Working Party. A randomised trial of adjuvant chemotherapy in malignant glioma. Journal of Clinical Oncology 2001;19:509‐18. [Google Scholar]
Poland {published and unpublished data}
- Trojanowski T, Peszynski J, Turowski K, Kaminski S, Goscinski I, Reinfus M, Krzyszkowski T, Pyrich M, Bielawski A, Leszczyk C. Post‐operative radiotherapy and radiotherapy combined with CCNU chemotherapy for treatment of brain gliomas. Journal of Neuro‐Oncology 1988;6:285‐91. [DOI] [PubMed] [Google Scholar]
RTOG 7401 {published and unpublished data}
- Chang CH, Horton J, Schoenfeld D, Salazar OM, Perez‐Tamayo R, Kramer S, Weinstein A, Nelson JS, Tsukada Y. Comparison of postoperative radiotherapy and combined postoperative radiotherapy and chemotherapy in the multidisciplinary management of malignant gliomas. Cancer 1983;52:997‐1007. [DOI] [PubMed] [Google Scholar]
za Age ‐ <=40 {published and unpublished data}
zb Age ‐ 41‐59 {published and unpublished data}
zc Age ‐ >=60 {published and unpublished data}
zd Sex ‐ Female {published and unpublished data}
ze Sex ‐ Male {published and unpublished data}
zf Histology ‐ AA {published and unpublished data}
zg Histology ‐ GBM {published data only}
zh Histology ‐ Other {published and unpublished data}
zi P Status ‐ Good {published and unpublished data}
zj P Status ‐ Poor {published and unpublished data}
zk Rsection‐Complete {published and unpublished data}
zl Rsection‐ Incompl {published and unpublished data}
zm Rsection ‐ Biopsy {published and unpublished data}
References to studies excluded from this review
Brisman 1976 {published and unpublished data}
- Brisman R, Housepian E, Chang C, Duffy P, Balis E. Adjuvant nitrosourea therapy for glioblastoma. Archives of Neurology 1976;33:745‐50. [DOI] [PubMed] [Google Scholar]
Cianfriglia 1980 {published and unpublished data}
- Cianfriglia F, Pompili A, Riccio A, Grassi A. CCNU‐chemotherapy of hemispheric supratentorial glioblastoma multiforme. Cancer 1980;45:1289‐99. [DOI] [PubMed] [Google Scholar]
Eagan 1979 {published and unpublished data}
- Eagan RT, Childs DS, Layton DD, Laws ER, Bisel HF, Holbrook MA, Fleming TR. Dianhydrogalactitol and radiation therapy: treatment of supratentorial glioma. Journal of the American Medical Association 1979;241(19):2046‐50. [PubMed] [Google Scholar]
EORTC 26741 {published and unpublished data}
- EORTC Brain Tumor Group. Effect of CCNU on survival rate of objective remission and duration of free interval in patients with malignant brain glioma ‐ Final evaluation. European Journal of Cancer 1978;14:851‐6. [DOI] [PubMed] [Google Scholar]
EORTC 26742 {published and unpublished data}
- EORTC Brain Tumor Group. Effect of CCNU on survival rate of objective remission and duration of free interval in patients with malignant brain glioma ‐ Final evaluation. European Journal of Cancer 1978;14:851‐6. [DOI] [PubMed] [Google Scholar]
Garrett 1978 {published and unpublished data}
- Garrett MJ, Hughes HJ, Freedman LS. A comparison of radiotherapy alone with radiotherapy and CCNU in cerebral glioma. Clinical Oncology 1978;4:71‐6. [PubMed] [Google Scholar]
Hatlevoll 1985 {published and unpublished data}
- Hatlevoll R, Lindegaard K, Hagen S, Kristiansen K, Nesbakken R, Torvik A, Ganz JC, Mella O, Rosengren B, Ringkjöb R. Combined modality treatment of operated astrocytomas grade 3 and 4; A prospective and randomised study of misonidazole and radiotherapy with two different radiation schedules and subsequent CCNU chemotherapy. Stage II of a prospective multicentre trial of the Scandinavian Glioblastoma Study Group. Cancer 1985;56:41‐7. [DOI] [PubMed] [Google Scholar]
Kristiansen 1981 {published and unpublished data}
- Kristiansen K, Hagen S, Kollevold T, Torvik A, Holme I, Nesbakken R, Hatlevoll R, Lindgren M, Brun A, Lindgren S. Combined modality therapy of operated astrocytomas grade III and IV. Confirmation of the value of postoperative irradiation and lack of potentiation of bleomycin on survival time. Cancer 1981;47:649‐52. [DOI] [PubMed] [Google Scholar]
Muller 1985 {published and unpublished data}
- Müller H, Brock M, Ernst H. Long‐term survival and recurrence free interval in combined surgical, radio‐ and chemotherapy of malignancy brain gliomas. Clinical Neurology and Neurosurgery 1985;87(3):167‐71. [DOI] [PubMed] [Google Scholar]
Reagan 1976 {published and unpublished data}
- Reagan TJ, Bisel HF, Childs DS, Layton DD, Rhoton AL, Taylor WF. Controlled study of CCNU and radiation therapy in malignant astrocytoma. Journal of Neurosurgery 1976;44:186‐90. [DOI] [PubMed] [Google Scholar]
Takakura 1986 {published data only}
- Takakura K, Abe H, Tanaka R, Kitamura K, Miwa T, Takeuchi K, Yamamoto S, Kageyama N, Handa H, Mogami H. Effects of ACNU and radiotherapy on malignant glioma. Journal of Neurosurgery 1986;64:53‐7. [DOI] [PubMed] [Google Scholar]
Ushio 1981 {published and unpublished data}
- Ushio Y, Akagi K, Bitoh S, Hayakawa T, Ikeda H, Kamikawa K, Mogami H, Oku Y, Yamada K. Phase 3 study of methyl‐CCNU and bleomycin combination chemotherapy in the treatment of malignant gliomas. Proceedings of the 7th International Congress of Neurological Surgery, Munich. 1981:362.
Additional references
Bleehen 1991
- Bleehen NM, Stenning SP. A Medical Research Council trial of two radiotherapy doses in the treatment of grades 3 and 4 astrocytoma. British Journal of Cancer 1991;64:769‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Clarke 1995
- Clarke MJ. Ovarian ablation: why the Early Breast Cancer Trialists' Group individual patient data overview was needed. Controlled Clinical Trials. 1995; Vol. 16, issue 3S:67S.
Dickersin 1990
- Dickersin K. The existence of publication bias and risk factors for its occurrence. JAMA : the journal of the American Medical Association 1990;263(10):1385‐9. [PubMed] [Google Scholar]
Dickersin 1994
- Dickersin K, Scherer R, Lefebvre C. Identifying relevant studies for systematic reviews. BMJ 1994;309:1286‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
EBCTCG 1990
- Early Breast Cancer Trialists Collaborative Group. Treatment of Early Breast Cancer Vol 1. Worldwide evidence 1985‐1990. Oxford: Oxford University Press, 1990. [Google Scholar]
Fine 1993
- Fine HA, Dear KBG, Loeffler JS, et al. Meta‐analysis of radiation therapy with and without adjuvant chemotherapy for malignant gliomas in adults.. Cancer 1993;71:2585‐97. [DOI] [PubMed] [Google Scholar]
Kaplan 1958
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observation. Journal of the American Statistical Association 1958;53:457‐81. [Google Scholar]
Parmar 1995
- Parmar MKB, Machin D. Survival analysis: a practical approach. John Wiley & Sons Ltd, 1995. [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DA. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials.. JAMA : the journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Souhami 1995
- Souhami RL, Tobias J. Cancer and its Management. Vol. Second Edition, Oxford: Blackwell Scientific Publications, 1995. [Google Scholar]
Stenning 1987
- Stenning SP, Freedman LS, Bleehen NM. An overview of published results from randomised studies of nitrosoureas in primary high‐grade malignant glioma. British Journal of Cancer 1987;56:89‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 1993
- Stewart LA, Parmar MKB. Meta‐analysis of the literature or of individual patient data: is there a difference?. The Lancet 1993;341:418‐22. [DOI] [PubMed] [Google Scholar]
Stewart 1995
- Stewart LA, Clarke MJ on behalf of the Cochrane Collaboration Working Party on meta‐analyses using individual patient data. Practical methodology on meta‐analyses (overviews) using individual patient data. Statistics in Medicine 1995;14:2057‐79. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta‐blockade during and after myocardial infarction: An overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27(5):335‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
GMT Group
- Glioma Meta‐analysis Trialists Group. Chemotherapy in adult high‐grade glioma: a systematic review and meta‐analysis of individual patient data from 12 randomised trials. The Lancet 2002;359:1011‐8. [DOI] [PubMed] [Google Scholar]


