Abstract
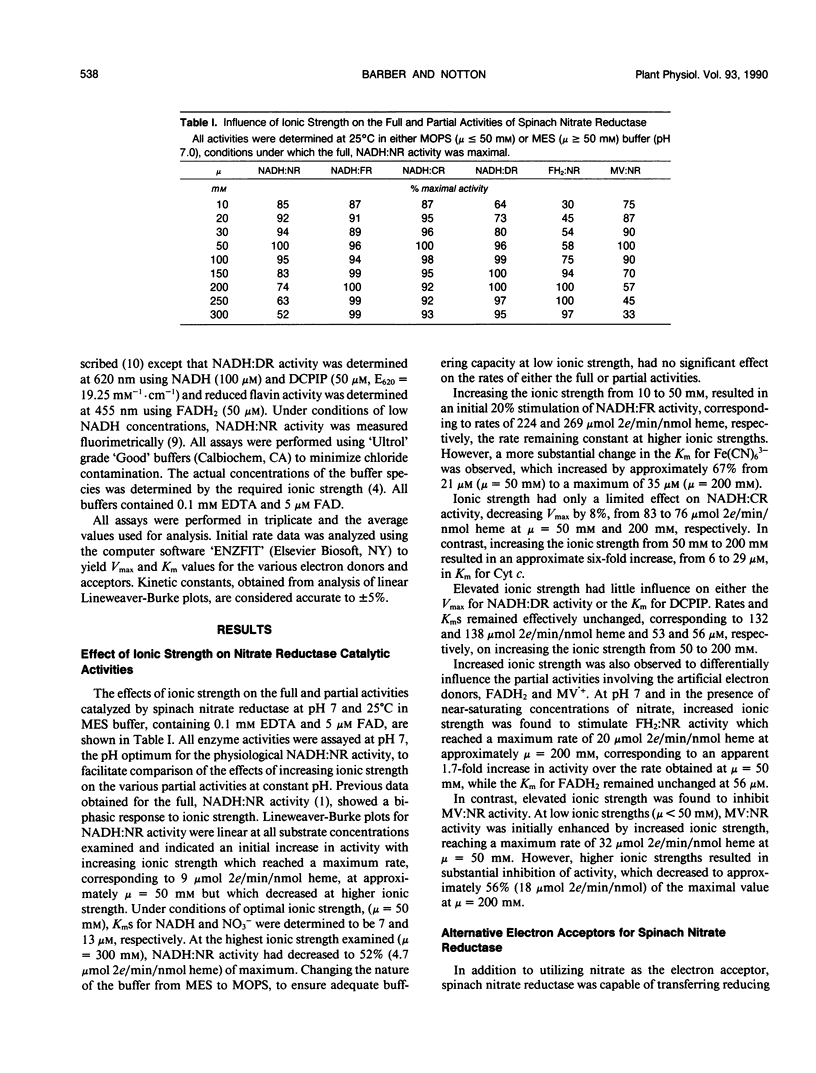
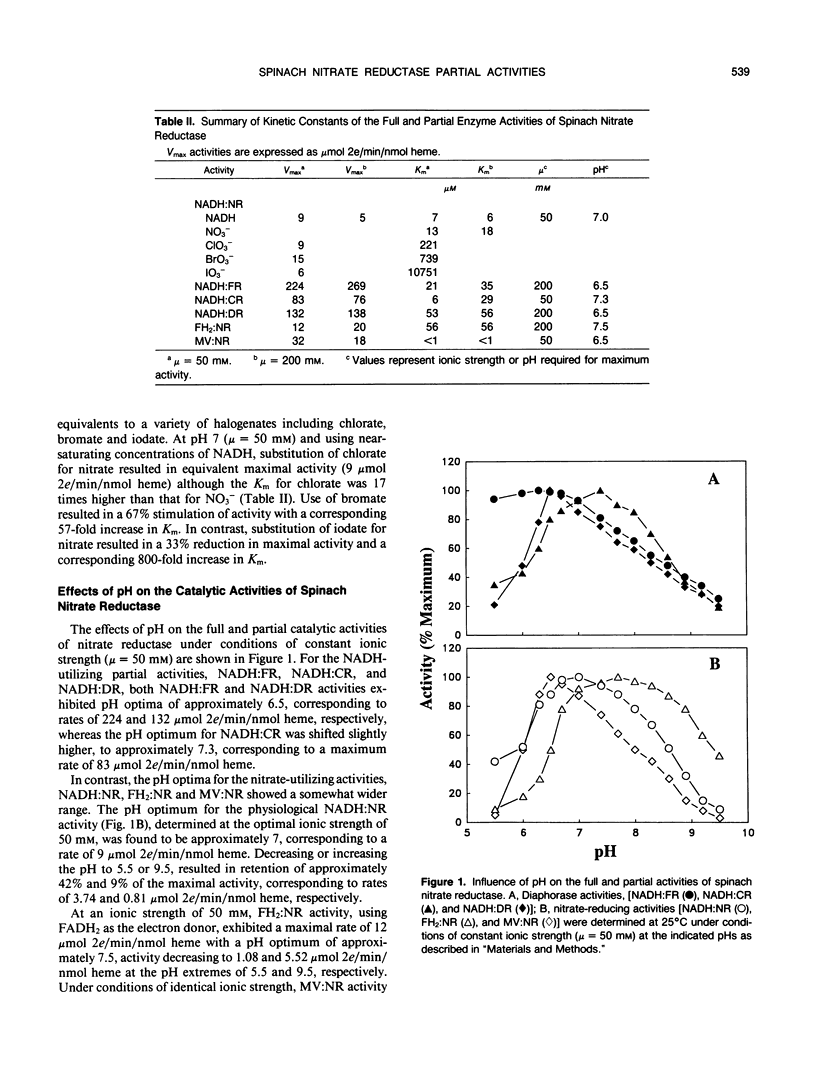
Initial velocity studies of immunopurified spinach nitrate reductase have been performed under conditions of controlled ionic strength and pH and in the absence of chloride ions. Increased ionic strength stimulated NADH:ferricyanide reductase and reduced flavin:nitrate reductase activities and inhibited NADH:nitrate reductase, NADH:cytochrome c reductase and reduced methyl viologen:nitrate reductase activities. NADH:dichlorophenolindophenol reductase activity was unaffected by changes in ionic strength. All of the partial activities, expressed in terms of micromole 2 electron transferred per minute per nanomole heme, were faster than the overall full, NADH:nitrate reductase activity indicating that none of the partial activities included the rate limiting step in electron transfer from NADH to nitrate. The pH optimum for NADH:nitrate reductase activity was determined to be 7 while values for the various partial activities ranged from 6.5 to 7.5. Chlorate, bromate, and iodate were determined to be alternate electron acceptors for the reduced enzyme. These results indicate that unlike the enzyme from Chlorella vulgaris, intramolecular electron transfer between reduced heme and Mo is not rate limiting for spinach nitrate reductase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barber M. J., Notton B. A., Kay C. J., Solomonson L. P. Chloride inhibition of spinach nitrate reductase. Plant Physiol. 1989 May;90(1):70–74. doi: 10.1104/pp.90.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W. H., Smarrelli J. Purification and Kinetics of Higher Plant NADH:Nitrate Reductase. Plant Physiol. 1978 Apr;61(4):611–616. doi: 10.1104/pp.61.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis K. J., Morrison J. F. Buffers of constant ionic strength for studying pH-dependent processes. Methods Enzymol. 1982;87:405–426. doi: 10.1016/s0076-6879(82)87025-0. [DOI] [PubMed] [Google Scholar]
- Gewitz H. S., Piefke J., Vennesland B. Purification and characterization of demolybdo nitrate reductase (NADH-cytochrome c oxidoreductase) of Chlorella vulgaris. J Biol Chem. 1981 Nov 25;256(22):11527–11531. [PubMed] [Google Scholar]
- Howard W. D., Solomonson L. P. Kinetic mechanism of assimilatory NADH:nitrate reductase from Chlorella. J Biol Chem. 1981 Dec 25;256(24):12725–12730. [PubMed] [Google Scholar]
- Kay C. J., Barber M. J. Assimilatory nitrate reductase from Chlorella. Effect of ionic strength and pH on catalytic activity. J Biol Chem. 1986 Oct 25;261(30):14125–14129. [PubMed] [Google Scholar]
- Kay C. J., Barber M. J. EPR and kinetic analysis of the interaction of halides and phosphate with nitrate reductase. Biochemistry. 1989 Jul 11;28(14):5750–5758. doi: 10.1021/bi00440a008. [DOI] [PubMed] [Google Scholar]
- Solomonson L. P., Vennesland B. Nitrate Reductase and Chlorate Toxicity in Chlorella vulgaris Beijerinck. Plant Physiol. 1972 Oct;50(4):421–424. doi: 10.1104/pp.50.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]


