Abstract
The members of the International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support Task Force have written a comprehensive summary of trials of the effectiveness of induced hypothermia (IH) or targeted temperature management (TTM) in comatose patients after cardiac arrest (CA). However, in-depth analysis of these studies is incomplete, especially since there was no significant difference in primary outcome between hypothermia versus normothermia in the recently reported TTM2 trial. We critically appraise trials of IH/TTM versus normothermia to characterize reasons for the lack of treatment effect, based on a previously published framework for what to consider when the primary outcome fails. We found a strong biologic rationale and external clinical evidence that IH treatment is beneficial. Recent TTM trials mainly included unselected patients with a high rate of bystander cardiopulmonary resuscitation. The treatment was not applied as intended, which led to a large delay in achievement of target temperature. While receiving intensive care, sedative drugs were likely used that might have led to increased neurologic damage as were antiplatelet drugs that could be associated with increased acute stent thrombosis in hypothermic patients. It is reasonable to still use or evaluate IH treatment in patients who are comatose after CA as there are multiple plausible reasons why IH compared to normothermia did not significantly improve neurologic outcome in the TTM trials.
Keywords: temperature management, therapeutic hypothermia, review
Introduction
The members of the International Liaison Committee on Resuscitation (ILCOR) Advanced Life Support Task Force recently published a summary of trials which evaluated the effectiveness of induced hypothermia (IH) or targeted temperature management (TTM) in comatose patients after resuscitation from cardiac arrest (CA) (Granfeldt et al, 2021). However, in-depth analysis of these studies is incomplete, especially as the pooled estimate of the effect of IH on the primary outcome in these studies showed no significant difference between hypothermia versus normothermia.
In 2016, Pocock and Stone published a framework for considering trial results when a significant difference in the primary outcome between treatment groups was not observed (Pocock and Stone, 2016). Below, we use a modification of this method to comment on the ILCOR systematic review. Although initial trials (Bernard et al, 2002; Hypothermia after Cardiac Arrest Study Group, 2002) and a recent large French trial reported that TTM improves outcomes in patients resuscitated from CA, the clinical community seems to have preferentially weighted the lack of benefit observed in the TTM (Nielsen et al, 2013) and TTM2 (Dankiewicz et al, 2021) trials. Therefore, we focus our observations on the latter two trials.
Is There A Strong Biologic Rationale That Favors Treatment?
Restoration of flow (called reperfusion) causes the release of circulating inflammatory molecules that lead to cellular injury (Madathil et al, 2016; Yellon and Hausenloy, 2007). In the brain after CA, cell injury is associated with activation of N-methyl-D-aspartate receptors; opening of mitochondrial permeability transition pores (MPTPs); impaired oxygen and glucose metabolism; release of free radicals, reactive oxygen species (ROS) and cytokines; and seizures (Allan and Rothwell, 2001; Barone and Parsons, 2000; Chan, 2001; Green and Shuaib, 2006; Love, 1999; Matsuo et al, 1995). In the heart after CA, cell injury is associated with activation of glutamate receptors; opening of MPTPs; impaired oxygen and glucose metabolism; microvascular obstruction; myocardial dysfunction; and arrhythmia (Yellon and Hausenloy, 2007).
This cell injury includes rapid release of ROS, cytokines, adhesion molecules, and leukocytes, (Adrie et al, 2004; Bisschops 2004; Adrie et al, 2002; Bottiger et al, 2002; Gando et al, 1999; Niemann et al, 2009; Shyu et al, 1997) upregulation of DNA, activation of protein kinases, and endothelial dysfunction (Adrie et al, 2002; Bottiger et al, 1995; Gando et al, 1997; Laurent et al, 2005). The latter plays a key role in myocardial necrosis (Miura and Tanno, 2012; Peart and Headrick, 2009). During ischemia and reperfusion, ROS lead to oxidative injury to fatty acids and cell membranes, lipid peroxidation, and damage to proteins (Becker, 2004). Cell injury begins within seconds and peaks within 15 minutes of reperfusion (Ambrosio and Tritto, 1999; Khalid and Ashraf, 1993; Zweier et al, 1987).
IH reduces global reperfusion injury in the brain and heart via a variety of protective mechanisms (Sun et al, 2019). The neuroprotective effect is attributed to reduced metabolic rate, reduced formation of free radicals, reduced inflammation, inhibition of excitotoxicity and reduction in apoptosis. Multiple studies in small and large animal models of CA demonstrate that hypothermia to a target temperature of 34°C or less improves outcome compared to normothermia (Arrich et al, 2021; Che et al, 2011; Chen et al, 2013; Gong et al, 2015a; Gong et al, 2015b; Hu et al, 2011; Kuboyama et al, 1993; Leonov et al, 1990; Li et al, 2012; Safar et al, 1996; Sterz et al, 1991; Tadler et al, 1998; Weinrauch et al, 1992; Zhao et al, 2015).
Also, multiple studies in small and large animal models of CA demonstrate that rapid hypothermia improved outcome compared to delayed hypothermia (Arrich et al, 2021; Che et al, 2011; Kim et al, 2016; Kuboyama et al, 1993; Yuan et al, 2017). In humans resuscitated from CA, briefer time from restoration of spontaneous circulation (ROSC) to target temperature is associated with significantly reduced likelihood of death or neurologic impairment (Lee et al, 2017; Nagao et al, 2010; Schock et al, 2016; Uribarri et al, 2015). Collectively, these data demonstrate that there is a strong biologic rationale that favors treatment with rapid IH to 34°C or less to reduce morbidity and mortality after resuscitation from CA.
Is There Some Indication of Potential Benefit?
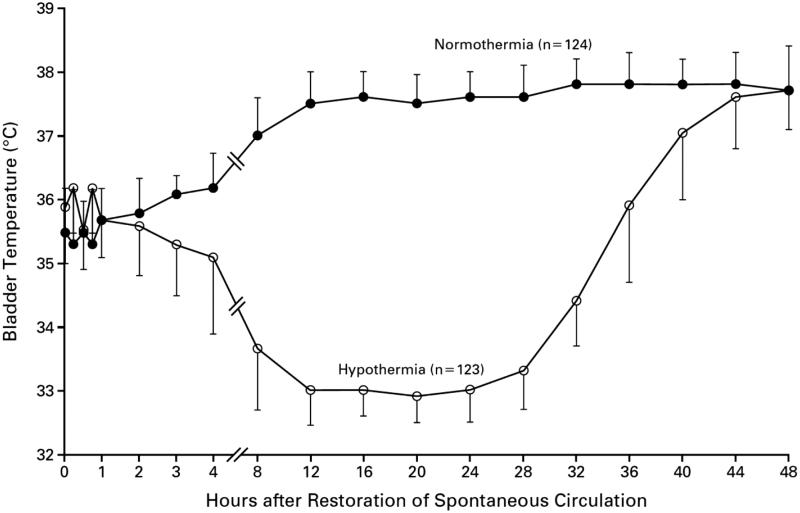
A key difference among trials of IH or TTM in patients resuscitated from CA is time to each trial's intended target temperature. Holzer and Sterz reported time from ROSC versus core temperature (Fig. 1): (Hypothermia after Cardiac Arrest Study Group, 2002) in the intervention group, this was ∼8 hours to 34°C. Generally, subsequent trials have reported time from randomization to target temperature rather than time from ROSC. This difference is important, as there is a large variation in the estimated time from ROSC to target temperature in each trial (Table 1). Note that the TTM2 investigators stated in their methods article that “rapid cooling in the hypothermia group will be achieved by means of cold fluids and cooling devices” (Dankiewicz et al, 2019).
FIG. 1.
Temperature versus Time from Restoration of Spontaneous Circulation in HACA Trial (Hypothermia after Cardiac Arrest Study Group, 2002).
Table 1.
Characteristics of Randomized Trials of Hypothermia Versus Normothermia in Patients Resuscitated from Cardiac Arrest
| Population | Treatment group | Treatment method | Temperature target,°C | Estimated time from onset of arrest to target temperature, mins. | Favorable neurologic outcome, % | p-Value | |
|---|---|---|---|---|---|---|---|
| Hachimi-Idrissi (Hachimi-Idrissi et al, 2001) | Unconscious adults Resuscitated from Witnessed Out of Hospital Asystole or PEA | Normothermia (n = 14) | n/a | 0a | 0.49 | ||
| Hypothermia (n = 16) | Cold Cap | 34°C | 282 | 13a | |||
| Bernard (Bernard et al, 2002) | Unconscious adults Resuscitated from Out of Hospital VF | Normothermia (n = 34) | n/a | n/a | 26b | 0.046 | |
| Hypothermia (n = 43) | Cold Packs | 33°C | ∼270 | 49b | |||
| HACA (Hypothermia after Cardiac Arrest Study, 2002) | Unconscious adults Resuscitated from Witnessed Out of Hospital VF or Pulseless VT | Normothermia (n = 138) | n/a | n/a | 39a | 0.009 | |
| Hypothermia (n = 137) | Cooling tent | 32–34°C | ∼420 | 55a | |||
| Laurent (Laurent et al, 2005) | Unconscious adults Resuscitated from Witnessed Out of Hospital VF or Asystole | Normothermia (n = 19) | n/a | 21a | 0.50 | ||
| Hemofiltration + Hypothermia (n = 22) | Hemofiltration | 32°C | ∼270 | 32a | |||
| Lopez-de-Sa (Lopez-de-Sa et al, 2012) | Unconscious adults Resuscitated from Witnessed Out of Hospital VF or Asystole |
Mild Hypothermia (n = 18) | IVTM | 34°C | 302 | 22a | 0.08 |
| Moderate Hypothermia (n = 18) | IVTM | 32°C | 512 | 50a | |||
| TTM (Nielsen et al, 2013) | Unconscious adults Resuscitated from Out of Hospital VF, Pulseless Electrical Activity (PEA) or Witnessed Asystole | Mild Hypothermia (n = 466) | 76% Surface; 24% IVTM | 36°C | n/a | 48c | 0.51 |
| Moderate Hypothermia (n = 473) | 76% Surface; 24% IVTM | 33°C | >660 | 47c | |||
| Lopez-de-Sa (Lopez-de-Sa et al, 2018) | Unconscious adults Resuscitated from Witnessed Out of Hospital VF or Asystole |
Milder Hypothermia (n = 49) | IVTM | 34°C | ∼230 | 66a | 0.14 |
| Mild Hypothermia (n = 49) | IVTM | 33°C | ∼260 | 66a | |||
| Moderate Hypothermia (n = 52) | IVTM | 32°C | ∼280 | 65a | |||
| Hyperion (Lascarrou et al, 2019) | Unconscious adults Resuscitated from PEA or Asystole of Any Cause | Normothermia (n = 297) | 81% Surface; 15% IVTM | 37°C | n/a | 5d | 0.04 |
| Moderate Hypothermia (n = 284) | 89% Surface; 15% IVTM | 33°C | ∼710 | 10d | |||
| TTM2 (Dankiewicz et al, 2021) | Unconscious adults Resuscitated from Out of Hospital CA of Presumed Cardiac or Unknown Cause | Hypothermia (n = 931) | 69% Surface; 31% IVTM | 36°C | n/a | 45e | Not Stated |
| Normothermia (n = 930) | 70% Surface; 30% IVTM | 33°C | >550 | 45e | |||
| Le May (Le May et al, 2021) | Unconscious adults Resuscitated from Out of Hospital CA All rhythms |
Mild Hypothermia (n = 196) | Ice packs + IVTM | 34°C | 324 | 55a | 0.56 |
| Moderate Hypothermia (n = 193) | Ice packs + IVTM | 31°C | 436 | 52a | |||
| Kwon (Kwon et al, 2021) | Unconscious adults Resuscitated from Out of Hospital CA of Presumed Cardiac Cause | Mild Hypothermia (n = 28) | Controlled surface | 36°C | n/a | 14a | 0.21 |
| Moderate Hypothermia (n = 29) | Controlled surface | 33°C | ∼390 | 31a |
Primary outcome was favorable neurologic outcome within 6 months, defined as a Pittsburgh cerebral-performance category of 1 (good recovery) or 2 (moderate disability) on a five-category scale.
Primary outcome was good neurologic outcome, defined as discharge home or to a rehabilitation facility.
Primary outcome was mortality at end of study follow-up. Patients followed for mean 256 days.
Primary outcome was survival with a favorable neurologic outcome 90 days after randomization.
Primary outcome was death at 6 months.
n/a, not applicable.
Excluded from the TTM2 trial were patients with greater than 180 minutes from ROSC to eligibility screening. In their primary report of the results, (Dankiewicz et al, 2021) the TTM2 investigators emphasized the time from randomization to target temperature, rather than from ROSC. Despite their stated goal of applying rapid cooling, they reported a similar time to their intended target temperature in TTM2 as they achieved in the original TTM trial (Nielsen et al, 2013).
A lead investigator of the TTM2 trial stated that patients enrolled in the trial were cooled as fast as is feasible using contemporary medical devices (https://web.archive.org/web/*/https://twitter.com/DogICUma/status/1405348094594621444). However, the recent TTM24vs48 trial (Kirkegaard et al, 2017) achieved a faster time to target temperature than that in TTM2: (281 [IQR, 217–360] minutes in the 48 hours group versus 320 [IQR 241–410] minutes in the 24 hours group [p = 0.01]). Importantly, the mean core temperature did not achieve the intended target in the intervention group in either TTM or TTM2 (i.e., mean temperature did not cross 33°C). Collectively, these data suggest that the relative benefit of IH was attenuated versus the control group in the TTM and TTM2 trials because the intervention was neither delivered as intended nor as quickly as feasible.
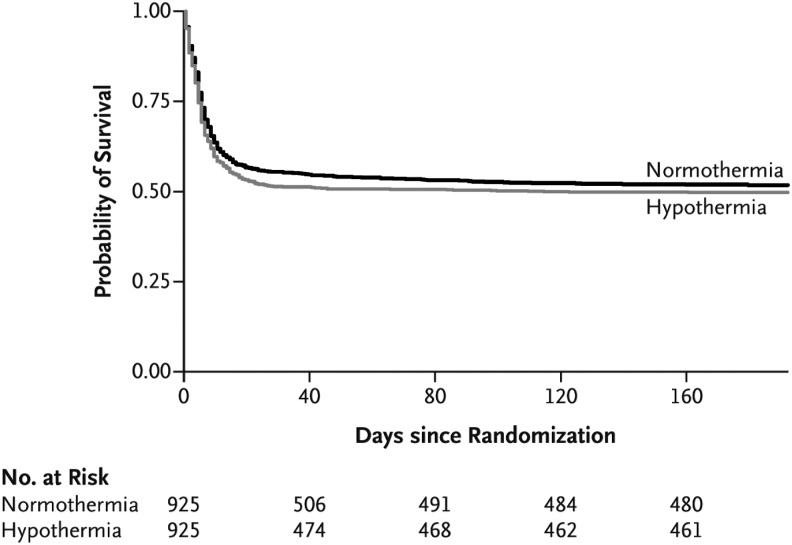
The planned primary outcome of TTM2 was all-cause mortality at 6 months (Dankiewicz et al, 2021). Other outcomes were assessed at 30 days, 6 months, and 24 months after randomization. Although there was no significant difference in mortality between the intervention and control group at 6 months, it appears that the intervention was associated with increased early mortality as the survival curves have wider separation around 30 days but come together later (Fig. 2). A plausible interpretation of this is that hypothermia in the TTM2 trial was associated with increased early mortality.
FIG. 2.
Survival with Hypothermia versus Normothermia Over Time in TTM2 Trial (Dankiewicz et al, 2021).
In the TTM2 trial, hypothermia was initiated with chilled intravenous (IV) saline (Dankiewicz et al, 2019; Jakobsen et al, 2020). In humans resuscitated from CA, chilled IV saline to initiate hypothermia was associated with no survival benefit and possibly increased adverse events (Bernard et al, 2012; Kim et al, 2014). Collectively, these data suggest that the use of chilled IV saline to initiate TTM could have contributed to the apparent increased early mortality in the intervention group versus the control group in the TTM2 trial.
Was The Treatment Regimen Appropriate?
Limited information is available about the quality of postresuscitation care that patients enrolled in the TTM2 trial received. A prior retrospective analysis of observational data demonstrated that the quality of postresuscitation care, including how TTM is initiated and maintained, is associated with outcome after resuscitation from CA (Stub et al, 2015). Note that the TTM2 investigators stated in their methods article that “General intensive care management [was] according to standard practice at participating hospitals (Dankiewicz et al, 2019).” But limited information is available about the quality of postresuscitation care that patients enrolled in the TTM2 trial received. Below, we describe why the quality of concurrent care is relevant to interpretation of the TTM2 trial.
Cooling method
The majority of patients enrolled in the TTM2 trial had hypothermia induced and maintained with surface cooling methods (SCM) as opposed to intravascular temperature management (IVTM). But SCM cools at slower rates than IVTM (Sonder et al, 2018). Multiple systematic reviews show that use of SCM is associated with worse outcomes than IVTM (Bartlett et al, 2020; Calabro et al, 2019). Collectively, these data suggest that it is plausible that preferential use of SCM rather than IVTM attenuated the benefit of moderate cooling in the TTM2 trial.
Propofol
The intervention group received significantly more propofol than the control group in the TTM2 trial: median (interquartile range) 8,768 (3,683, 13,365) mg versus 7,744 (3,183, 12,595) mg (p value not stated). (Dankiewicz et al, 2021) Similarly, a substudy from a hospital that enrolled a large number of patients in the TTM trial reported that the moderate hypothermia group received significantly more propofol than the mild hypothermia group (Bro-Jeppesen et al, 2014). Propofol was not available for clinical use in early trials of IH in patients with OHCA (Bernard et al, 2002; Hypothermia after Cardiac Arrest Study Group, 2002). Note that propofol has dose-dependent effects on mitochondria. At low doses, it reduces ROS (Branca et al, 1995). At higher doses, it reduces adenosine triphosphate synthesis (Branca et al, 1995; Branca et al, 1991a; Branca et al, 1991b; Rigoulet et al, 1996; Sztark et al, 1995).
Small observational and randomized studies of the use of propofol for sedation in patients with CA have not demonstrated consistent effect (Bjelland et al, 2012; Paul et al, 2018; Staudacher et al, 2018). But in a systematic review of volatile anesthetics versus propofol in patients undergoing cardiac surgery with cardiopulmonary bypass (N = 42 trials, n = 8,197 patients), (Bonanni et al, 2020) volatile anesthetics had lower 1-year mortality (5.5% vs. 6.8%; odds ratio, 0.76 [95% confidence interval [CI]: 0.60–0.96]; p = 0.023) and less myocardial infarction (odds ratio, 0.60 [95% CI: 0.39–0.92]; p = 0.023).
Propofol has also been compared to other methods of sedation in patients requiring intensive care. In a systematic review of use of volatile agents versus IV sedation, including propofol in patients receiving critical care (N = 13 trials, n = 1,027 patients), (Kim et al, 2017) volatile agents had less myocardial injury as measured by serum troponin levels 6 hours after ICU admission than patients who received IV sedation (p < 0.05). Collectively, these clinical data suggest that it is plausible that the frequent use and higher dose of propofol in the hypothermia group could have attenuated the relative benefit of hypothermia compared to normothermia in the TTM2 trial.
Antiplatelet therapy during revascularization
Information about which TTM2 patients received which of P2Y12 inhibitor or experienced stent thrombosis or reinfarction is not available. Overall, about 40% of patients had ST-segment myocardial infarction (STEMI); 38% of patients underwent percutaneous coronary intervention (PCI) (Dankiewicz et al, 2021). Evidence-based practice guidelines strongly recommend administration of P2Y12 inhibitors as early as possible to reduce the risk of stent thrombosis (O'Gara et al, 2013). Currently available oral (clopidogrel, prasugrel, ticagrelor) or parenteral (cangrelor) P2Y12 inhibitors are acceptable in normothermic patients.
But in a trial enrolling patients with STEMI undergoing PCI (Nichol et al, 2015), clopidogrel significantly increased acute stent thrombosis in the setting of intraperitoneal hypothermia. The ability of oral P2Y12 inhibitors to achieve timely and adequate platelet inhibition in hypothermic patients is limited (Bednar et al, 2016; Bjelland et al, 2010; Flierl et al, 2016; Steblovnik et al, 2016). Patients resuscitated from CA who received IH and oral P2Y12 inhibitors did not achieve satisfactory platelet inhibition (Bjelland et al, 2010; Buchtele et al, 2020; Ibrahim et al, 2014). In contrast, cangrelor inhibit platelets when given concurrent with hypothermia (Pruller et al, 2018).
It seems plausible that most patients who underwent PCI in the TTM2 trial did not receive cangrelor, as 99% of patients were enrolled outside the United States, where health care costs are more constrained. If a significant proportion of hypothermic patients received oral P2Y12 inhibitors, as seems likely, they would be at risk of stent thrombosis and worse outcomes. This could have contributed to the apparent short-term increase in mortality observed in the moderate hypothermia group in the TTM2 trial.
Implantable defibrillator
Overall, 16% of patients enrolled in the TTM2 trial received an implantable cardioverter defibrillator (ICD) during follow-up. Evidence-based practice guidelines strongly recommend implantation of ICDs in survivors of ventricular fibrillation without a completely reversible cause, as it is associated with a significant and important mortality benefit (Al-Khatib et al, 2018). In contrast to the low rate of ICD use in the TTM2 trial, 42% of a national US sample of patients admitted to hospital after resuscitation from CA in 2002/2003 underwent ICD insertion (Birnie et al, 2007). The large divergence in ICD implantation rates between the TTM2 trial and usual practice could have been associated with reduced survival, attenuating differences in outcome between the control and intervention group.
Neuroprognostication
Modern neuroprognostication guidelines such as those used in the TTM2 trial attempt to predict poor functional outcomes. But these neuroprognostication guidelines lack high diagnostic accuracy (Pouplet et al, 2022). Despite efforts to standardize neuroprognostication in the TTM2 trial (Dankiewicz et al, 2021), a high proportion of patients had withdrawal of life-sustaining therapies (WLST) at 96 hours. Moreover, a high proportion of patients had WLST for reasons other than poor neurologic prognosis (Supplementary Fig. S3 in in Dankiewicz NEJM 2021 Supplement).
Possible accumulation of sedative drugs in hypothermic patients (Bjelland et al, 2013) may have contributed to premature decisions to withdraw therapy in patients with ongoing sedation as opposed to severe brain injury. In a prospective cohort of patients who did not have early WLST, 49% of those who survived to sedation withdrawal after 72 hours were still unconscious (Nakstad et al, 2020). In this study, the time frame arrest to awakening was mean (SD) 6.1 (4.0) days among those discharged with favourable neurologic outcome. Of these, 32% eventually obtained good neurologic outcome. It seems plausible that the high early rate of WLST could have attenuated the effect of IH/TTM.
Do Secondary Outcomes Elicit Reveal Positive Findings?
The TTM2 trial reported a significant increase in the rate of bradycardia requiring pacing in the intervention group compared to the control group (Dankiewicz et al, 2021). It is unclear whether the site investigators were given specific guidance on when pacing was required in the TTM2 trial protocol (Dankiewicz et al, 2019). Bradycardia is commonly observed during application of hypothermia. But such bradycardia is associated with increased cardiac output due to increased stroke volume (Forkmann et al, 2015).
The TTM investigators and others previously reported that early bradycardia is associated with improved survival and neurologic outcome after CA (Staer-Jensen et al, 2014; Thomsen et al, 2016). The TTM2 trial reported that although total arrhythmias did not differ between the two randomized groups, arrhythmias with hemodynamic compromise were more common in the hypothermia group (HR, 1.45 [95% CI: 1.21–1.75] p < 0.00). Possible reasons posited for an apparent higher arrhythmia risk in the hypothermia group are electrolyte disturbances (e.g., hypokalemia) or a direct effect of hypothermia.
In the TTM2 trial, the authors reported that hypokalemia was more frequent in the 33°C compared to the 36°C group (19% vs. 13%). The incidence of VT/VF was similar between randomized groups.
In contrast to the observations of TTM2, significant differences in rates of arrhythmia were not reported in other recent trials of IH in patients with CA (Lascarrou et al, 2019; Le May et al, 2021). In a prospective observational study (Adler et al, 2020), hypokalemia and malignant arrhythmias (VT/VF) were associated with a more rapid time to target temperature. VT/VF occurred in 20% of patients. Additional predictors of VT/VF were a higher number of shocks during resuscitation and higher intensive care use of epinephrine.
Collectively, these data suggest that the clinical significance of the arrhythmia reported in the TTM2 is incompletely defined.
Can Alternative Analyses Help?
The systematic review of the effectiveness of IH or TTM in patients resuscitated from CA reports significant or near significant statistical heterogeneity among the study results. Statistical heterogeneity in a systematic review implies that the observed intervention effects are more different from each other than one would expect due to random error (i.e., chance) alone. The Cochrane Collaboration Handbook on Systematic Reviews states (Granfeldt et al, 2021) as follows:
“Meta-analysis should only be considered when a group of studies is sufficiently homogeneous in terms of participants, interventions, and outcomes to provide a meaningful summary.”
Following from this, (Salcido et al, 2021) clinicians should be cautious about concluding whether TTM does or does not improve outcomes in patients resuscitated from CA based upon this meta-analysis.
Post hoc analyses of the TTM2 trial data could provide further insights. Such analyses might include examining the interaction of mode of cooling, time to cooling from ROSC, enrollment site, and concurrent care (e.g., type of P2Y12 inhibitor; sedation without propofol; insertion of an ICD) (Dankiewicz et al, 2019; Jakobsen et al, 2020). These analyses would generate hypotheses about how to optimize postresuscitation care and inform the design of further trials.
Does More Positive External Evidence Exist?
Evidence external to that of the TTM2 trial suggests that hypothermia improves outcomes compared to normothermia. The HYPERION trial demonstrated that in patients with a first-recorded rhythm that is nonshockable, 10.2% of patients in the hypothermia group were alive with a CPC score of 1 or 2 at 90 days, compared to 5.7% in the normothermia group (difference, 4.5 percentage points; 95% CI: 0.1–8.9; p = 0.04) (Lascarrou et al, 2019). Also, there was no significant difference in adverse events between the hypothermia and normothermia group.
One of the main mechanisms of benefit of IH/TTM is posited to be attenuation of hypoxic brain injuries (HIBI) (Sun et al, 2019). Such HIBI can lead to brain death or permanent alteration of brain function. To be effective, IH needs to be applied to those at risk of moderate to high level of HIBI. A high level of HIBI would contribute to a high level of dependency, that is, higher proportion of patient with poor functional outcome (Taccone et al, 2021). Regarding recent trials, HYPERION is the only one with a proportion of poor functional outcome higher than 10% in the control group (Lascarrou et al, 2019).
When the original TTM trial was published, postresuscitation practices changed at many hospitals to favor use of a target temperature of 36°C as opposed to 33°C. Several large multicenter before and after studies conducted outside the United States have demonstrated that this change in practice was associated with increased mortality (Nolan et al, 2021; Salter et al, 2018).
Multiple retrospective analyses of data from the United States demonstrate that there is a significant relationship between the duration of ischemia and the effect of IH in patients with CA (Reynolds et al, 2016; Sawyer et al, 2020). It seems unlikely that if there is truly no benefit to IH, that such a dose-response relationship would have been observed.
Collectively, these data suggest that it is plausible that, notwithstanding the results of the ILCOR systematic review and TTM2 trial, hypothermia may improve outcomes compared to normothermia in patients resuscitated from CA.
Conclusion
It is reasonable to still use or evaluate IH treatment in patients who are comatose after CA. There are multiple plausible reasons why IH compared to normothermia did not significantly improve neurologic outcome in the TTM trials.
Author Disclosure Statement
Graham Nichol is a consultant to ZOLL Circulation (San Jose, CA), which manufactures and markets devices for IVTM. He is also a consultant to Heartbeam, Inc. (Santa Clara, CA), Invero Health LLC. (Montvale, NJ), and Orixha, Inc. (Saint Cyr Au Mont d'Or, France). He has research funding from ZOLL Medical (Chelmsford, MA), Vapotherm, Inc. (Exeter, NH), and Abiomed, Inc. (Danvers, MA) and is a member of the steering committee of the PRINCESS 2 Trial of Ultrafast Hypothermia after cardiac arrest. Michael Holzer received honoraria for lectures and consulting from C.R. Bard, Inc. (Murray Hill, NJ), which manufactures and markets devices for surface temperature management, and Zoll Medical (Chelmsford, MA) and is a member of the steering committee of the PRINCESS 2 Trial of Ultrafast Hypothermia after cardiac arrest.
Jeanne E. Poole and Ken Fujise have no relevant conflicts related to this article. Jean-Baptiste Lascarrou received lecture fees from C.R. Bard, Inc. (Murray Hill, NJ), and Zoll Medical (Chelmsford, MA). None of these companies or their representatives subsidized preparation of this work, or reviewed it before publication.
Funding Information
No funding was received in support of this article.
References
- Adler C, Schregel F, Heller T, et al. Malignant arrhythmias during induction of target temperature management after cardiac arrest. Ther Hypothermia Temp Manag 2020;10(4):229–236; doi: 10.1089/ther.2019.0025 [DOI] [PubMed] [Google Scholar]
- Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 2002;106(5):562–568; doi: 10.1161/01.cir.0000023891.80661.ad [DOI] [PubMed] [Google Scholar]
- Adrie C, Laurent I, Monchi M, et al. Postresuscitation disease after cardiac arrest: A sepsis-like syndrome? Curr Opin Crit Care 2004;10(3):208–212; doi: 10.1097/01.ccx.0000126090.06275.fe [DOI] [PubMed] [Google Scholar]
- Al-Khatib SM, Stevenson WG, Ackerman MJ, et al. 2017 AHA/ACC/HRS Guideline for Management of Patients With Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation 2018;138(13):e210-e271; doi: 10.1161/CIR.0000000000000548 [DOI] [PubMed] [Google Scholar]
- Allan SM, Rothwell NJ. Cytokines and acute neurodegeneration. Nat Rev Neurosci 2001;2(10):734–744; doi: 10.1038/35094583 [DOI] [PubMed] [Google Scholar]
- Ambrosio G, Tritto I. Reperfusion injury: Experimental evidence and clinical implications. Am Heart J 1999;138(2 Pt 2):S69–S75; doi: 10.1016/s0002-8703(99)70323-6 [DOI] [PubMed] [Google Scholar]
- Arrich J, Herkner H, Mullner D, et al. Targeted temperature management after cardiac arrest. A systematic review and meta-analysis of animal studies. Resuscitation 2021;162:47–55; doi: 10.1016/j.resuscitation.2021.02.002 [DOI] [PubMed] [Google Scholar]
- Barone FC, Parsons AA. Therapeutic potential of anti-inflammatory drugs in focal stroke. Expert Opin Invest Drugs 2000;9(10):2281–2306; doi: 10.1517/13543784.9.10.2281 [DOI] [PubMed] [Google Scholar]
- Bartlett ES, Valenzuela T, Idris A, et al. Systematic review and meta-analysis of intravascular temperature management vs. surface cooling in comatose patients resuscitated from cardiac arrest. Resuscitation 2020;146:82–95; doi: 10.1016/j.resuscitation.2019.10.035 [DOI] [PubMed] [Google Scholar]
- Becker LB. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc Res 2004;61(3):461–470; doi: 10.1016/j.cardiores.2003.10.025 [DOI] [PubMed] [Google Scholar]
- Bednar F, Kroupa J, Ondrakova M, et al. Antiplatelet efficacy of P2Y12 inhibitors (prasugrel, ticagrelor, clopidogrel) in patients treated with mild therapeutic hypothermia after cardiac arrest due to acute myocardial infarction. J Thromb Thrombolysis 2016;41(4):549–555; doi: 10.1007/s11239-015-1274-7 [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346(8):557–563; doi: 10.1056/NEJMoa003289 [DOI] [PubMed] [Google Scholar]
- Bernard SA, Smith K, Cameron P, et al. Induction of prehospital therapeutic hypothermia after resuscitation from nonventricular fibrillation cardiac arrest*. Crit Care Med 2012;40(3):747–753; doi: 10.1097/CCM.0b013e3182377038 [DOI] [PubMed] [Google Scholar]
- Birnie DH, Sambell C, Johansen H, et al. Use of implantable cardioverter defibrillators in Canadian and US survivors of out-of-hospital cardiac arrest. CMAJ 2007;177(1):41–46; doi: 10.1503/cmaj.060730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisschops L. van der Hoeven JG, Mollnes TE, et al. Seventy-two hours of mild hypothermia after cardiac arrest is associated with a lowered inflammatory response during rewarming in a prospective observational study. Crit Care 2004;18(5):546; doi: 10.1186/s13054-014-0546-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjelland TW, Dale O, Kaisen K, et al. Propofol and remifentanil versus midazolam and fentanyl for sedation during therapeutic hypothermia after cardiac arrest: A randomised trial. Intensive Care Med 2012;38(6):959–967; doi: 10.1007/s00134-012-2540-1 [DOI] [PubMed] [Google Scholar]
- Bjelland TW, Hjertner O, Klepstad P, et al. Antiplatelet effect of clopidogrel is reduced in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation 2010;81(12):1627–1631; doi: 10.1016/j.resuscitation.2010.07.002 [DOI] [PubMed] [Google Scholar]
- Bjelland, TW, Klepstad P, Haugen BO, et al. Effects of hypothermia on the disposition of morphine, midazolam, fentanyl, and propofol in intensive care unit patients. Drug Metab Dispos 2013;41(1):214–223; doi: 10.1124/dmd.112.045567 [DOI] [PubMed] [Google Scholar]
- Bonanni A, Signori A, Alicino C, et al. Volatile anesthetics versus propofol for cardiac surgery with cardiopulmonary bypass: Meta-analysis of randomized trials. Anesthesiology 2020;132(6):1429–1446; doi: 10.1097/ALN.0000000000003236 [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Motsch J, Bohrer H, et al. Activation of blood coagulation after cardiac arrest is not balanced adequately by activation of endogenous fibrinolysis. Circulation 1995;92(9):2572–2578; doi: 10.1161/01.cir.92.9.2572 [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Motsch J, Braun V, et al. Marked activation of complement and leukocytes and an increase in the concentrations of soluble endothelial adhesion molecules during cardiopulmonary resuscitation and early reperfusion after cardiac arrest in humans. Crit Care Med 2002;30(11):2473–2480; doi: 10.1097/00003246-200211000-00012 [DOI] [PubMed] [Google Scholar]
- Branca D, Roberti MS, Lorenzin P, et al. Influence of the anesthetic 2,6-diisopropylphenol on the oxidative phosphorylation of isolated rat liver mitochondria. Biochem Pharmacol 1991a;42(1):87–90; doi: 10.1016/0006-2952(91)90684-w [DOI] [PubMed] [Google Scholar]
- Branca D, Roberti MS, Vincenti E, et al. Uncoupling effect of the general anesthetic 2,6-diisopropylphenol in isolated rat liver mitochondria. Arch Biochem Biophys 1991b;290(2):517–521; doi: 10.1016/0003-9861(91)90575-4 [DOI] [PubMed] [Google Scholar]
- Branca D, Vincenti E, Scutari. Influence of the anesthetic 2,6-diisopropylphenol (propofol) on isolated rat heart mitochondria. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 1995;110(1):41–45; doi: 10.1016/0742-8413(94)00078-o [DOI] [PubMed] [Google Scholar]
- Bro-Jeppesen J, Hassager C, Wanscher M, et al. Targeted temperature management at 33 degrees C versus 36 degrees C and impact on systemic vascular resistance and myocardial function after out-of-hospital cardiac arrest: A sub-study of the Target Temperature Management Trial. Circ Cardiovasc Interv 2014;7(5):663–672; doi: 10.1161/CIRCINTERVENTIONS.114.001556 [DOI] [PubMed] [Google Scholar]
- Buchtele N, Herkner H, Schorgenhofer C, et al. High platelet reactivity after transition from cangrelor to ticagrelor in hypothermic cardiac arrest survivors with ST-segment elevation myocardial infarction. J Clin Med 2020;9(2):583; doi: 10.3390/jcm9020583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro L, Bougouin W, Cariou A, et al. Effect of different methods of cooling for targeted temperature management on outcome after cardiac arrest: A systematic review and meta-analysis. Crit Care 2019;23(1):285; doi: 10.1186/s13054-019-2567-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab 2001;21(1):2–14; doi: 10.1097/00004647-200101000-00002 [DOI] [PubMed] [Google Scholar]
- Che D, Li L, Kopil CM, et al. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med 2011;39(6):1423–1430; doi: 10.1097/CCM.0b013e318212020a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Song FQ, Sun LL, et al. Improved early postresuscitation EEG activity for animals treated with hypothermia predicted 96hr neurological outcome and survival in a rat model of cardiac arrest. Biomed Res Int 2013:312137; doi: 10.1155/2013/312137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankiewicz J, Cronberg T, Lilja G, et al. Targeted hypothermia versus targeted Normothermia after out-of-hospital cardiac arrest (TTM2): A randomized clinical trial-Rationale and design. Am Heart J 2019;217:23–31; doi: 10.1016/j.ahj.2019.06.012 [DOI] [PubMed] [Google Scholar]
- Dankiewicz J, Cronberg T, Lilja G, et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N Engl J Med 2021;384(24):2283–2294; doi: 10.1056/NEJMoa2100591 [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, Altman DG, on behalf of the Cochrane Statistical Methods Group. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions. (Higgins J, Thomas J. eds.) https://training.cochrane.org/handbook/current, Cochrane Collaboration: London, England, 2021. [Google Scholar]
- Flierl U, Rontgen P, Zauner F, et al. Platelet inhibition with prasugrel in patients with acute myocardial infarction undergoing therapeutic hypothermia after cardiopulmonary resuscitation. Thromb Haemost 2016;115(5):960–968; doi: 10.1160/TH15-07-0599 [DOI] [PubMed] [Google Scholar]
- Forkmann M, Kolschmann S, Holzhauser L, et al. Target temperature management of 33 degrees C exerts beneficial haemodynamic effects after out-of-hospital cardiac arrest. Acta Cardiol 2015;70(4):451–459; doi: 10.1080/ac.70.4.3096893 [DOI] [PubMed] [Google Scholar]
- Gando S, Kameue T, Nanzaki et al. Massive fibrin formation with consecutive impairment of fibrinolysis in patients with out-of-hospital cardiac arrest. Thromb Haemost 1997;77(2):278–282; doi: 10.1055/s-0038-1655953 [DOI] [PubMed] [Google Scholar]
- Gando S, Nanzak Si, Morimoto Y, et al. Alterations of soluble L- and P-selectins during cardiac arrest and CPR. Intensive Care Med 1999;25(6):588–593; doi: 10.1007/s001340050907 [DOI] [PubMed] [Google Scholar]
- Gong P, Zhao H, Hua R, et al. Mild hypothermia inhibits systemic and cerebral complement activation in a swine model of cardiac arrest. J Cereb Blood Flow Metab 2015a;35(8):1289–1295; doi: 10.1038/jcbfm.2015.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Zhao S, Wang J, et al. Mild hypothermia preserves cerebral cortex microcirculation after resuscitation in a rat model of cardiac arrest. Resuscitation 2015b;97:109–114; doi: 10.1016/j.resuscitation.2015.10.003 [DOI] [PubMed] [Google Scholar]
- Granfeldt A, Holmberg MJ, Nolan JP, et al. Targeted temperature management in adult cardiac arrest: Systematic review and meta-analysis.” Resuscitation 2021;167:160–172; doi: 10.1016/j.resuscitation.2021.08.040 [DOI] [PubMed] [Google Scholar]
- Green AR, Shuaib A. Therapeutic strategies for the treatment of stroke. Drug Discov Today 2006;11(15–16):681–693; doi: 10.1016/j.drudis.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Hachimi-Idrissi S, Corne L, Ebinger G, et al. Mild hypothermia induced by a helmet device: A clinical feasibility study. Resuscitation 2001;51(3):275–281; doi: 10.1016/s0300-9572(01)00412-9 [DOI] [PubMed] [Google Scholar]
- Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346(8):549–556; doi: 10.1056/NEJMoa012689 [DOI] [PubMed] [Google Scholar]
- Hu CL, Wen J, Liao XX, et al. Effects of therapeutic hypothermia on coagulopathy and microcirculation after cardiopulmonary resuscitation in rabbits. Am J Emerg Med 2011;29(9):1103–1110; doi: 10.1016/j.ajem.2010.07.016 [DOI] [PubMed] [Google Scholar]
- Ibrahim K, Christoph M, Schmeinck S, et al. High rates of prasugrel and ticagrelor non-responder in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation 2014; 85(5):649–656; doi: 10.1016/j.resuscitation.2014.02.004 [DOI] [PubMed] [Google Scholar]
- Jakobsen JC, Dankiewicz J, Lange T, et al. Targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest: A statistical analysis plan. Trials 2020;21(1):831; doi: 10.1186/s13063-020-04654-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalid MA, Ashraf M. Direct detection of endogenous hydroxyl radical production in cultured adult cardiomyocytes during anoxia and reoxygenation. Is the hydroxyl radical really the most damaging radical species? Circ Res 1993;72(4):725–736; doi: 10.1161/01.res.72.4.725 [DOI] [PubMed] [Google Scholar]
- Kim F, Nichol G, Maynard C, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: A randomized clinical trial. JAMA 2014;311(1):45–52; doi: 10.1001/jama.2013.282173 [DOI] [PubMed] [Google Scholar]
- Kim HY, Lee JE, Kim HY, et al. Volatile sedation in the intensive care unit: A systematic review and meta-analysis. Medicine (Baltimore) 2017;96(49):e8976; doi: 10.1097/MD.0000000000008976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Paine MG, Meng H, et al. Combined intra- and post-cardiac arrest hypothermic-targeted temperature management in a rat model of asphyxial cardiac arrest improves survival and neurologic outcome compared to either strategy alone. Resuscitation 2016;107:94–101; doi: 10.1016/j.resuscitation.2016.07.232 [DOI] [PubMed] [Google Scholar]
- Kirkegaard H, Soreide E, de Haas I, et al. Targeted Temperature Management for 48 vs 24 Hours and Neurologic Outcome After Out-of-Hospital Cardiac Arrest: A Randomized Clinical Trial. JAMA 2017;318(4):341–350; doi: 10.1001/jama.2017.8978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboyama K, Safar P, Radovsky A, et al. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: A prospective, randomized study. Crit Care Med 1993;21(9):1348–1358; doi: 10.1097/00003246-199309000-00019 [DOI] [PubMed] [Google Scholar]
- Kwon WY, Jung YS, Suh GJ, et al. Regional cerebral oxygen saturation in cardiac arrest survivors undergoing targeted temperature management 36 degrees C versus 33 degrees C: A randomized clinical trial. Resuscitation 2021;167:362–371; doi: 10.1016/j.resuscitation.2021.07.026 [DOI] [PubMed] [Google Scholar]
- Lascarrou JB, Merdji H, Le Gouge A, et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N Engl J Med 2019;381(24):2327–2337; doi: 10.1056/NEJMoa1906661 [DOI] [PubMed] [Google Scholar]
- Laurent I, Adrie C, Vinsonneau C, et al. High-volume hemofiltration after out-of-hospital cardiac arrest: A randomized study. J Am Coll Cardiol 2005;46(3):432–437; doi: 10.1016/j.jacc.2005.04.039 [DOI] [PubMed] [Google Scholar]
- Le May M, Osborne C, Russo J, et al. Effect of Moderate vs Mild Therapeutic Hypothermia on Mortality and Neurologic Outcomes in Comatose Survivors of Out-of-Hospital Cardiac Arrest: The CAPITAL CHILL Randomized Clinical Trial. JAMA 2021;326(15):1494–1503; doi: 10.1001/jama.2021.15703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee BK, Jeung KW, Jung YH, et al. Relationship between timing of cooling and outcomes in adult comatose cardiac arrest patients treated with targeted temperature management. Resuscitation 2017;113:135–141; doi: 10.1016/j.resuscitation.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Leonov Y, Sterz F, Safar P, et al. Moderate hypothermia after cardiac arrest of 17minutes in dogs. Effect on cerebral and cardiac outcome. Stroke 1990;21(11):1600–1606; doi: 10.1161/01.str.21.11.1600 [DOI] [PubMed] [Google Scholar]
- Li Y, Ristagno G, Guan J, et al. Preserved heart rate variability during therapeutic hypothermia correlated to 96hrs neurological outcomes and survival in a pig model of cardiac arrest. Crit Care Med 2012;40(2):580–586; doi: 10.1097/CCM.0b013e31822ef9e4 [DOI] [PubMed] [Google Scholar]
- Lopez-de-Sa E, Juarez M, Armada E, et al. A multicentre randomized pilot trial on the effectiveness of different levels of cooling in comatose survivors of out-of-hospital cardiac arrest: the FROST-I trial. Intensive Care Med 2018;44(11):1807–1815; doi: 10.1007/s00134-018-5256-z [DOI] [PubMed] [Google Scholar]
- Lopez-de-Sa E, Rey JR, Armada E, et al. Hypothermia in comatose survivors from out-of-hospital cardiac arrest: pilot trial comparing 2 levels of target temperature. Circulation 2012;126(24):2826–2833; doi: 10.1161/CIRCULATIONAHA.112.136408 [DOI] [PubMed] [Google Scholar]
- Love S. Oxidative stress in brain ischemia. Brain Pathol 1999;9(1):119–131; doi: 10.1111/j.1750-3639.1999.tb00214.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madathil RJ, Hira RS, Stoeckl M, et al. Ischemia reperfusion injury as a modifiable therapeutic target for cardioprotection or neuroprotection in patients undergoing cardiopulmonary resuscitation. Resuscitation 2016;105:85–91; doi: 10.1016/j.resuscitation.2016.04.009 [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Kihara T, Ikeda M, et al. Role of neutrophils in radical production during ischemia and reperfusion of the rat brain: effect of neutrophil depletion on extracellular ascorbyl radical formation. J Cereb Blood Flow Metab 1995;15(6):941–947; doi: 10.1038/jcbfm.1995.119 [DOI] [PubMed] [Google Scholar]
- Miura T, Tanno M. The mPTP and its regulatory proteins: final common targets of signalling pathways for protection against necrosis. Cardiovasc Res 2012;94(2):181–189; doi: 10.1093/cvr/cvr302 [DOI] [PubMed] [Google Scholar]
- Nagao K, Kikushima K, Watanabe K, et al. Early induction of hypothermia during cardiac arrest improves neurological outcomes in patients with out-of-hospital cardiac arrest who undergo emergency cardiopulmonary bypass and percutaneous coronary intervention. Circ J 2010;74(1):77–85; doi: 10.1253/circj.cj-09-0502 [DOI] [PubMed] [Google Scholar]
- Nakstad ER, Staer-Jensen H, Wimmer H, et al. Late awakening, prognostic factors and long-term outcome in out-of-hospital cardiac arrest - results of the prospective Norwegian Cardio-Respiratory Arrest Study (NORCAST). Resuscitation 2020;149:170–179; doi: 10.1016/j.resuscitation.2019.12.031 [DOI] [PubMed] [Google Scholar]
- Nichol G, Strickland W, Shavelle D, et al. Prospective, multicenter, randomized, controlled pilot trial of peritoneal hypothermia in patients with ST-segment- elevation myocardial infarction. Circ Cardiovasc Interv 2015;8(3):e001965; doi: 10.1161/CIRCINTERVENTIONS.114.001965 [DOI] [PubMed] [Google Scholar]
- Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med 2013;369(23):2197–2206; doi: 10.1056/NEJMoa1310519 [DOI] [PubMed] [Google Scholar]
- Niemann JT, Rosborough JP, Youngquist S, et al. Cardiac function and the proinflammatory cytokine response after recovery from cardiac arrest in swine. J Interferon Cytokine Res 2009;29(11):749–758; doi: 10.1089/jir.2009.0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan JP, Orzechowska I, Harrison DA, et al. Changes in temperature management and outcome after out-of-hospital cardiac arrest in United Kingdom intensive care units following publication of the targeted temperature management trial. Resuscitation 2021;162:304–311; doi: 10.1016/j.resuscitation.2021.03.027 [DOI] [PubMed] [Google Scholar]
- O'Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013;127(4):e362–e425; doi: 10.1161/CIR.0b013e3182742cf6 [DOI] [PubMed] [Google Scholar]
- Paul M, Bougouin W, Dumas F, et al. Comparison of two sedation regimens during targeted temperature management after cardiac arrest. Resuscitation 2018;128:204–210; doi: 10.1016/j.resuscitation.2018.03.025 [DOI] [PubMed] [Google Scholar]
- Peart JN, Headrick JP. Clinical cardioprotection and the value of conditioning responses. Am J Physiol Heart Circ Physiol 2009;296(6):H1705–H1720; doi: 10.1152/ajpheart.00162.2009 [DOI] [PubMed] [Google Scholar]
- Pocock SJ, Stone GW. The Primary Outcome Fails - What Next? N Engl J Med 2016;375(9):861–870; doi: 10.1056/NEJMra1510064 [DOI] [PubMed] [Google Scholar]
- Pouplet C, Colin G, Guichard E, et al. The accuracy of various neuro-prognostication algorithms and the added value of neurofilament light chain dosage for patients resuscitated from shockable cardiac arrest: An ancillary analysis of the ISOCRATE study. Resuscitation 2022;171:1–7; doi: 10.1016/j.resuscitation.2021.12.009 [DOI] [PubMed] [Google Scholar]
- Pruller F, Bis L, Milke OL, et al. Cangrelor induces more potent platelet inhibition without increasing bleeding in resuscitated patients. J Clin Med 2018;7(11); doi: 10.3390/jcm7110442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JC, Grunau BE, Rittenberger JC, et al. Association Between Duration of Resuscitation and Favorable Outcome After Out-of-Hospital Cardiac Arrest: Implications for Prolonging or Terminating Resuscitation. Circulation 2016;134(25):2084–2094; doi: 10.1161/CIRCULATIONAHA.116.023309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoulet M, Devin A, Averet N, et al. Mechanisms of inhibition and uncoupling of respiration in isolated rat liver mitochondria by the general anesthetic 2,6-diisopropylphenol. Eur J Biochem 1996;241(1):280–285; doi: 10.1111/j.1432-1033.1996.0280t.x [DOI] [PubMed] [Google Scholar]
- Safar P, Xiao F, Radovsky A, et al. Improved cerebral resuscitation from cardiac arrest in dogs with mild hypothermia plus blood flow promotion. Stroke 1996;27(1):105–113; doi: 10.1161/01.str.27.1.105 [DOI] [PubMed] [Google Scholar]
- Salcido DD, Fujise K, Nichol G. Re: Targeted temperature management in adult cardiac arrest: Systematic review and meta-analysis. Resuscitation 2021;170:70; doi: 10.1016/j.resuscitation.2021.11.020 [DOI] [PubMed] [Google Scholar]
- Salter R, Bailey M, Bellomo R, et al. Changes in temperature management of cardiac arrest patients following publication of the target temperature management trial. Crit Care Med 2018;46(11):1722–1730; doi: 10.1097/CCM.0000000000003339 [DOI] [PubMed] [Google Scholar]
- Sawyer KN, Humbert A, Leroux BG, et al. Relationship Between Duration of Targeted Temperature Management, Ischemic Interval, and Good Functional Outcome From Out-of-Hospital Cardiac Arrest. Crit Care Med 2020;48(3):370–377; doi: 10.1097/CCM.0000000000004160 [DOI] [PubMed] [Google Scholar]
- Schock RB, Janata A, Peacock WF, et al. Time to cooling is associated with resuscitation outcomes. Ther Hypothermia Temp Manag 2016;6(4):208–217; doi: 10.1089/ther.2016.0026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu KG, Chang H, Lin CC, et al. Concentrations of serum interleukin-8 after successful cardiopulmonary resuscitation in patients with cardiopulmonary arrest. Am Heart J 1997;134(3):551–556; doi: 10.1016/s0002-8703(97)70094-2 [DOI] [PubMed] [Google Scholar]
- Sonder P, Janssens GN, Beishuizen A, et al. Efficacy of different cooling technologies for therapeutic temperature management: A prospective intervention study. Resuscitation 2018;124:14–20; doi: 10.1016/j.resuscitation.2017.12.026 [DOI] [PubMed] [Google Scholar]
- Staer-Jensen H, Sunde K, Olasveengen TM, et al. Bradycardia during therapeutic hypothermia is associated with good neurologic outcome in comatose survivors of out-of-hospital cardiac arrest. Crit Care Med 2014;42(11):2401–2408; doi: 10.1097/CCM.0000000000000515 [DOI] [PubMed] [Google Scholar]
- Staudacher DL, Hamilton SK, Duerschmied D, et al. Isoflurane or propofol sedation in patients with targeted temperature management after cardiopulmonary resuscitation: A single center study. J Crit Care 2018;45:40–44; doi: 10.1016/j.jcrc.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Steblovnik K, Blinc A, Mijovski MB, et al. Ticagrelor Versus Clopidogrel in Comatose Survivors of Out-of-Hospital Cardiac Arrest Undergoing Percutaneous Coronary Intervention and Hypothermia: A Randomized Study. Circulation 2016;134(25):2128–2130; doi: 10.1161/CIRCULATIONAHA.116.024872 [DOI] [PubMed] [Google Scholar]
- Sterz F, Safar P, Tisherman S, et al. Mild hypothermic cardiopulmonary resuscitation improves outcome after prolonged cardiac arrest in dogs. Crit Care Med 1991;19(3):379–389; doi: 10.1097/00003246-199103000-00017 [DOI] [PubMed] [Google Scholar]
- Stub D, Schmicker RH, Anderson ML, et al. Association between hospital post-resuscitative performance and clinical outcomes after out-of-hospital cardiac arrest. Resuscitation 2015;92:45–52; doi: 10.1016/j.resuscitation.2015.04.015 [DOI] [PubMed] [Google Scholar]
- Sun YJ, Zhang ZY, Fan B, et al. Neuroprotection by Therapeutic Hypothermia. Front Neurosci 2019;13:586; doi: 10.3389/fnins.2019.00586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sztark F, Ichas F, Ouhabi R, et al. Effects of the anaesthetic propofol on the calcium-induced permeability transition of rat heart mitochondria: direct pore inhibition and shift of the gating potential. FEBS Lett 1995;368(1):101–104; doi: 10.1016/0014-5793(95)00610-l [DOI] [PubMed] [Google Scholar]
- Taccone FS, Lascarrou JB, Skrifvars MB. Targeted temperature management and cardiac arrest after the TTM-2 study. Crit Care 2021;25(1):275; doi: 10.1186/s13054-021-03718-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadler SC, Callaway CW, Menegazzi JJ. Noninvasive cerebral cooling in a swine model of cardiac arrest. Acad Emerg Med 1998;5(1):25–30; doi: 10.1111/j.1553-2712.1998.tb02570.x [DOI] [PubMed] [Google Scholar]
- Thomsen JH, Nielsen N, Hassager C, et al. Bradycardia during targeted temperature management: An early marker of lower mortality and favorable neurologic outcome in comatose out-of-hospital cardiac arrest patients. Crit Care Med 2016;44(2):308–318; doi: 10.1097/CCM.0000000000001390 [DOI] [PubMed] [Google Scholar]
- Uribarri A, Bueno H, Perez-Castellanos A, et al. Impact of time to cooling initiation and time to target temperature in patients treated with hypothermia after cardiac arrest. Eur Heart J Acute Cardiovasc Care 2015;4(4):365–372; doi: 10.1177/2048872614557241 [DOI] [PubMed] [Google Scholar]
- Weinrauch V, Safar P, Tisherman S, et al. Beneficial effect of mild hypothermia and detrimental effect of deep hypothermia after cardiac arrest in dogs. Stroke 1992;23(10):1454–1462; doi: 10.1161/01.str.23.10.1454 [DOI] [PubMed] [Google Scholar]
- Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007;357(11):1121–1135; doi: 10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- Yuan W, Wu JY, Zhao YZ, et al. Comparison of early sequential hypothermia and delayed hypothermia on neurological function after resuscitation in a swine model. Am J Emerg Med 2017;35(11):1645–1652; doi: 10.1016/j.ajem.2017.05.013 [DOI] [PubMed] [Google Scholar]
- Zhao H, Chen Y, Jin Y. The effect of therapeutic hypothermia after cardiopulmonary resuscitation on ICAM-1 and NSE levels in sudden cardiac arrest rabbits. Int J Neurosci 2015;125(7):540–546; doi: 10.3109/00207454.2014.951887 [DOI] [PubMed] [Google Scholar]
- Zweier JL, Flaherty JT, Weisfeldt ML. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A 1987;84(5):1404–1407; doi: 10.1073/pnas.84.5.1404 [DOI] [PMC free article] [PubMed] [Google Scholar]