Abstract
In methanogenic sediments from a petroleum-contaminated aquifer, [14C]benzene was converted to 14CH4 and 14CO2 without an apparent lag. Phenol, acetate, and propionate were intermediates in benzene mineralization. These results suggest that alternative electron acceptors need not be available for there to be significant natural attenuation of benzene in some petroleum-contaminated aquifers.
The development of rational strategies for the remediation of petroleum-contaminated aquifers requires an understanding of the ability of microorganisms to degrade the aromatic hydrocarbon contaminants. Of greatest concern is benzene, due to its toxicity and water solubility. It is well known that aerobic microorganisms can degrade benzene and other aromatic hydrocarbons and limit the spread of benzene plumes in the subsurface (16). However, many petroleum-contaminated aquifers contain extensive anaerobic zones, especially near the source of contamination (3, 8). Previous studies have indicated that microbial populations in aquifer sediments may be adapted for anaerobic benzene degradation, but benzene degradation took place only after long adaptation periods and/or after various amendments were made to stimulate microbial activity (7, 12, 14, 15, 19, 20). Thus, it did not appear that benzene was being degraded under anaerobic conditions in situ.
Sediment sampling and incubation.
In order to further evaluate the potential for anaerobic degradation of benzene in petroleum-contaminated aquifers, benzene degradation was investigated in an aquifer located in Ponca City, Okla., that has been contaminated with aromatic hydrocarbons for over 50 years. At the sampling site, benzene, which is typically at concentrations of 130 to 640 μM, was the principal aromatic hydrocarbon in the groundwater. Sediments were collected at a depth of 2 to 3 m with a hand auger (ca. 7.6 cm in diameter) and placed in canning jars. In order to exclude oxygen, the bottles were completely filled with anaerobic groundwater delivered to the surface with a peristaltic pump. The samples were shipped by overnight carrier to the laboratory. Sediments (ca. 30 g) were loaded into serum bottles (50 ml) under N2 in a glove bag. The bottles were sealed with thick butyl rubber stoppers, removed from the glove bag, and then flushed with N2-CO2 (93:7) that had been passed over heated copper filings to remove any traces of O2. Additional sediment aliquots (ca. 750 ml) were transferred in a similar manner to 1-liter bottles and refed benzene when they became benzene depleted. These provided a source of sediments for subsequent studies. Rates of benzene degradation were measured by adding 0.3 to 0.5 μCi of [14C]benzene (58.2 mCi/mmol) from an anaerobic aqueous stock (3.3 μCi/ml) and monitoring the production of 14CO2 and 14CH4 with gas-proportional counting as previously described (12). Incubations were conducted in the dark at 20°C.
Conversion of [14C]benzene to 14CH4 and 14CO2.
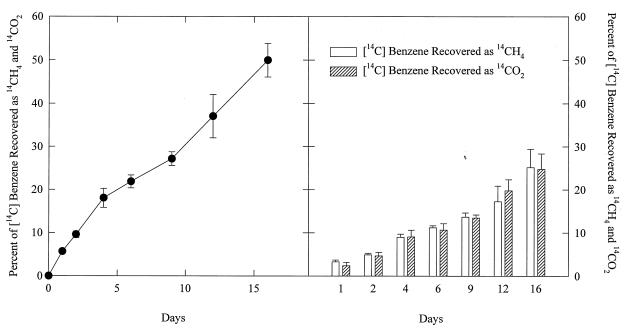
Based on previous studies (7, 12, 13) it was assumed that benzene degradation would be slow and that there would be little if any benzene degradation unless soluble Fe(III) was added to the sediments. However, at the first sampling time, which was 13 days after the addition of [14C]benzene, 53% of the benzene had been mineralized in sediments which had received no amendments. To evaluate this metabolism further, a detailed time course was initiated (Fig. 1). The added [14C]benzene was steadily converted to 14CH4 and 14CO2 over time without an apparent lag.
FIG. 1.
Production of 14CH4 and 14CO2 over time in methanogenic aquifer sediments amended with [14C]benzene. The results are the mean of triplicate incubations. Error bars designate one standard deviation from the mean.
Parallel studies in which [2-14C]acetate was added to the sediments further confirmed that methane production was the predominant terminal electron-accepting process (TEAP), as 14CH4 accounted for 83% of the radiolabel recovered as 14CH4 and 14CO2. Geochemical evidence obtained with previously described methods (9) also suggested that methanogenesis was the predominant TEAP, as the groundwater contained high concentrations of dissolved methane (675 μM), Fe(II) (339 μM), and Mn(II) (140 μM). No sulfate or nitrate was detectable by ion chromatography. Furthermore, over 90% of the total iron (21.1 μmol/g) that could be extracted from the sediments with 0.5 N HCl (11) was in the Fe(II) state.
14CH4 and 14CO2 were produced in equal proportions from [14C]benzene (Fig. 1). If benzene was converted solely to methane and carbon dioxide according to the following equation, 4C6H6 + 27H2O→15CH4 + 9HCO3− + 9H+, then it would be expected that, of the total radiolabel recovered in methane and carbon dioxide, 62.5% would be recovered as methane. The fact that the recovery of 14CH4 from [14C]benzene was 80% of that expected compares favorably with the finding, noted above, that the recovery of 14CH4 from [2-14C]acetate was 83% of that expected in an environment in which methanogenesis is the only TEAP. These results suggest that, because of isotope exchange or other factors, the fate of 14C-labeled compounds might not be exactly the same as that predicted from balanced redox reactions. Alternatively, there might have been some other, as yet unidentified, minor TEAP taking place simultaneously with methanogenesis. However, this latter alternative is not supported by the finding that similar percentages of 14CH4 were recovered even after long-term incubations of the sediments with repeated feedings of benzene that would be expected to deplete alternative electron acceptors from the sediments.
In methanogenic environments, many aromatic compounds are partially metabolized to extracellular fatty acids and H2, which are then metabolized to methane (6, 17). To determine whether benzene was metabolized to extracellular intermediates prior to complete conversion to methane and carbon dioxide, isotope trapping studies were conducted. In this approach (10), potential intermediates are added to the sediments to artificially increase the size of the pool of potential extracellular intermediates. If the added compounds are extracellular intermediates, then increasing their pool size will slow the turnover of the intermediate pool. Thus, any radiolabel that enters the artificially elevated intermediate pool from the metabolism of [14C]benzene will be released as 14CO2 and 14CH4 more slowly than it will in sediments in which the size of the pool of extracellular intermediate has not been increased.
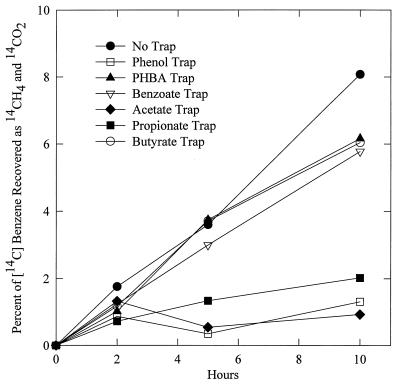
Sediments (5 ml) were added to anaerobic pressure tubes, and some of the tubes were amended with potential extracellular intermediates from anaerobic stock solutions at concentrations that would increase the estimated size of the pool of the potential intermediates by 10- to 100-fold. The addition of benzoate (200 μM added), p-hydroxybenzoate (200 μM), or butyrate (100 μM) had no effect on mineralization of added [14C]benzene (Fig. 2). However, added phenol (20 μM), acetate (1 mM), and propionate (100 μM) greatly inhibited benzene mineralization, suggesting that these compounds could be intermediates in benzene degradation. To further evaluate this, the sediments with no added isotope traps from the study summarized in Fig. 2 were centrifuged after 8 h of incubation in order to collect the pore water. Aromatic compounds and fatty acids in the pore water were separated by high-performance liquid chromatography, and fractions representing the potential intermediates were collected as described previously (10). Radiolabel was recovered in the phenol, acetate, and propionate pools at levels equivalent to 51%, 50%, and 21% of the [14C]benzene that had been mineralized to 14CH4 and 14CO2 at the time of pore water collection. These results demonstrate that phenol, propionate, and acetate are important extracellular intermediates in benzene degradation in these methanogenic sediments.
FIG. 2.
Effect of potential extracellular intermediates in benzene degradation on the mineralization of [14C]benzene in methanogenic aquifer sediments. The initial benzene concentration was 5 μM. The results are the means of duplicate incubations for each treatment.
The metabolism of benzene through the extracellular intermediates phenol, acetate, and propionate contrasts with the finding that phenol and acetate were not detected as extracellular intermediates in benzene degradation coupled to sulfate reduction in either marine or freshwater sediments (10, 19). Propionate was not evaluated as a potential extracellular intermediate in those studies. A previous study demonstrated that phenol was formed from benzene in anaerobic enrichment cultures in which methanogenesis was considered to be the TEAP (5, 18). However, in those studies benzene mineralization was minor, with less than 6% of the added [14C]benzene being converted to 14CO2.
Conclusions.
This is the first report of rapid conversion of benzene to methane and carbon dioxide in methanogenic aquifer sediments. The fact that benzene was degraded without a lag suggests that the microbial population was adapted for benzene degradation and that benzene was being degraded in situ. In a previous study, slurries constructed with aquifer sediments were found to convert benzene to methane and carbon dioxide, but only after a lag period of over 400 days (7). The long lag period in that study suggested that the microbial population had to be adapted for benzene degradation and raised doubts about whether benzene was actually being metabolized in situ. In a related study, benzene disappearance was observed in aquifer material which was considered to be methanogenic (20). However, data demonstrating that methanogenesis was the predominant TEAP were not provided and the fate of the benzene that disappeared was not determined. Furthermore, there was a lag period of over 140 days prior to benzene loss, which suggested that the microorganisms in the sediment were not adapted for benzene degradation at the time of collection. Other studies have found that benzene persists under methanogenic conditions in aquifer sediments or enrichment cultures established with aquifer sediments (1, 4, 12).
Several factors may be contributing to the active methane production from benzene in the aquifer sediments investigated here. One factor is that the sediments at this site have a long history of exposure to relatively high concentrations of benzene. The finding that benzene degradation under methanogenic conditions is likely to require the cooperation of a microbial consortium may mean that a significant period of time is required for the appropriate consortium to develop. Furthermore, these studies were conducted with minimal disturbance of the sediments, whereas previous studies have significantly diluted the aquifer sediments with groundwater, enrichment media, or water. Such dilution may disturb the potentially delicate consortia required for benzene mineralization.
Now that it is recognized that benzene can be degraded in the methanogenic zone of petroleum-contaminated aquifers, it will eventually be important to determine if this is a common phenomenon. The depletion of alternative electron acceptors, such as nitrate, Fe(III), and sulfate, as the result of microbial metabolism may leave methanogenesis as the predominant TEAP in large zones of heavily contaminated aquifers (3, 8). Estimates of the rates at which naturally occurring microbial activity can remove aromatic hydrocarbons from petroleum-contaminated aquifers are generally based on the assumption that benzene will only be degraded under aerobic conditions. However, in aquifers in which benzene is degraded under methanogenic conditions, benzene mineralization may be faster than would be calculated for aerobic degradation alone. Recent studies (2) have demonstrated that benzene may also be degraded under Fe(III)-reducing conditions in some petroleum-contaminated aquifers, further enhancing the removal of benzene under anaerobic conditions. Thus, the possibility of anaerobic benzene degradation should be considered when assessing the time necessary for natural attenuation to remediate petroleum-contaminated aquifers.
Acknowledgments
This work was supported by the National Science Foundation (grant DEB9523932), the American Petroleum Institute, and Conoco, Inc.
We thank Terry Lauck and Jeff Meyers of Conoco, Inc., for suggesting the sampling site and for support in obtaining sediment samples.
REFERENCES
- 1.Acton D W, Barker J F. In situ biodegradation potential of aromatic hydrocarbons in anaerobic groundwaters. J Contam Hydrol. 1992;9:325–352. [Google Scholar]
- 2.Anderson, R. T., K. Gaw, J. Rooney-Varga, and D. R. Lovley. Anaerobic benzene oxidation in the Fe(III)-reduction zone of petroleum-contaminated aquifers. Environ. Sci. Technol., in press.
- 3.Anderson R T, Lovley D R. Ecology and biogeochemistry of in situ groundwater bioremediation. Adv Microb Ecol. 1997;15:289–350. [Google Scholar]
- 4.Edwards E A, Grbic-Galic D. Anaerobic degradation of toluene and o-xylene by a methanogenic consortium. Appl Environ Microbiol. 1994;60:313–322. doi: 10.1128/aem.60.1.313-322.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grbic-Galic D, Vogel T. Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol. 1987;53:254–260. doi: 10.1128/aem.53.2.254-260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heider J, Fuchs G. Microbial anaerobic aromatic metabolism. Anaerobe. 1997;3:1–22. doi: 10.1006/anae.1997.0073. [DOI] [PubMed] [Google Scholar]
- 7.Kazumi J, Caldwell M E, Suflita J M, Lovley D R, Young L Y. Anaerobic degradation of benzene in diverse environments. Environ Sci Technol. 1997;31:813–818. [Google Scholar]
- 8.Lovley D R. Potential for anaerobic bioremediation of BTEX in petroleum-contaminated aquifers. J Ind Microbiol. 1997;18:75–81. [Google Scholar]
- 9.Lovley D R, Chapelle F H, Woodward J C. Use of dissolved H2 concentrations to determine the distribution of microbially catalyzed redox reactions in anoxic ground water. Environ Sci Technol. 1994;28:1205–1210. doi: 10.1021/es00056a005. [DOI] [PubMed] [Google Scholar]
- 10.Lovley D R, Coates J D, Woodward J C, Phillips E J P. Benzene oxidation coupled to sulfate reduction. Appl Environ Microbiol. 1995;61:953–958. doi: 10.1128/aem.61.3.953-958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovley D R, Phillips E J P. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal Potomac River. Appl Environ Microbiol. 1986;52:751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lovley D R, Woodward J C, Chapelle F H. Stimulated anoxic biodegradation of aromatic hydrocarbons using Fe(III) ligands. Nature. 1994;370:128–131. doi: 10.1038/370128a0. [DOI] [PubMed] [Google Scholar]
- 13.Lovley D R, Woodward J C, Chapelle F H. Rapid anaerobic benzene oxidation with a variety of chelated Fe(III) forms. Appl Environ Microbiol. 1996;62:288–291. doi: 10.1128/aem.62.1.288-291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Major D W, Mayfield C I, Barker J F. Biotransformation of benzene by denitrification in aquifer sand. Ground Water. 1988;26:8–14. [Google Scholar]
- 15.Morgan P, Lewis S T, Watkinson R J. Biodegradation of benzene, toluene, ethylbenzene, and xylenes in gas-condensate-contaminated ground-water. Environ Pollut. 1993;82:181–190. doi: 10.1016/0269-7491(93)90115-5. [DOI] [PubMed] [Google Scholar]
- 16.Salanitro J P. The role of bioattenuation in the management of aromatic hydrocarbon plumes in aquifers. Ground Water Monit Remediation. 1993;13:150–161. [Google Scholar]
- 17.Schink B. Energetics of syntrophic cooperation in methanogenic degradation. Microbiol Mol Biol Rev. 1997;61:262–280. doi: 10.1128/mmbr.61.2.262-280.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vogel T M, Grbic-Galic D. Incorporation of oxygen from water into toluene and benzene during anaerobic fermentative transformation. Appl Environ Microbiol. 1986;52:200–202. doi: 10.1128/aem.52.1.200-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weiner J, Lovley D R. Stimulation of anaerobic benzene oxidation in petroleum-contaminated sediments with a freshwater, benzene-oxidizing, sulfate-reducing inoculum. Appl Environ Microbiol. 1997;64:775–778. doi: 10.1128/aem.64.2.775-778.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson B H, Smith G B, Rees J F. Biotransformations of selected alkylbenzenes and halogenated aliphatic hydrocarbons in methanogenic aquifer material: a microcosm study. Environ Sci Technol. 1986;20:997–1002. doi: 10.1021/es00152a005. [DOI] [PubMed] [Google Scholar]