Abstract
Dyslipidemia can increase the risk of heart attack and stroke due to the restriction of blood flow through the blood vessels. Dietary modification is an appropriate approach to reducing this phenomenon. This cross-sectional study aimed to evaluate major dietary patterns and the dietary inflammatory index (DII) in relation to dyslipidemia. 5954 participants in the Ravansar non-communicable diseases (RaNCD) cohort study were eligible for this study. Dyslipidemia was diagnosed based on the lipid profile under consideration of the RaNCD physician. Dietary patterns were assessed by principal component analysis. The three identified dietary patterns included (1) plant-based pattern; (2) high protein and sugar pattern; and (3) energy-dense dense pattern. DII was also calculated based on the dietary information from a validated semi-quantitative food frequency questionnaire (FFQ). We found that higher adherence to DII was significantly associated with increased odds of dyslipidemia after adjusting for age, sex, and physical activity (OR: 1.24; CI 95% 1.09–1.42). Additionally, higher adherence to the high protein and sugar diet and an energy-dense diet was significantly associated with higher odds for dyslipidemia (OR: 1.31; CI 95% 1.16–1.49) and (OR: 1.28; CI 95% 1.12–1.46). Nevertheless, according to our results, following plant-based diet had no association with dyslipidemia in both crude and adjusted models. Our findings revealed that greater adherence to DII, a high-protein, high-sugar diet, and an energy-dense diet can have undesirable effects on dyslipidemia.
Subject terms: Dyslipidaemias, Nutrition disorders
Introduction
Dyslipidemia is an important predisposing factor in increasing the risk of cardiovascular diseases (CVDs)1,2. Studies have shown that dyslipidemia is associated with a lipid profile imbalance, including higher levels of triglycerides (TG), low-density lipoprotein cholesterol (LDL-C), and total cholesterol (TC) while the amount of high-density lipoprotein cholesterol (HDL-C) is low3. In the last 30 years on the Asian continent, the amount of mortality from CVDs has increased from 5.6 million to 10.8 million4. Dyslipidemia causes about 4 million deaths from CVDs worldwide5.
The main contributors to dyslipidemia are an inactive lifestyle, genetic factors, hormonal abnormalities, obesity, and diet6,7. In recent years, the dietary pattern approach has been the focus of many researches and has been widely used in examining the international effects of nutrients together8. In fact, the dietary pattern approach is related to the amounts, proportions, variety, or combination of different foods, drinks, and nutrients in the usual diet and their consumption frequency9. Examining dietary patterns conceptually provides a more realistic picture of the overall diet8. Therefore, due to the interaction of nutrients in the consumed meal, it is not possible to separate the components of the food, and for this reason, the use of these indicators and food patterns has been expanded as a way to evaluate the effects of the overall dietary intake10.
The dietary inflammatory index (DII) was developed to estimate the inflammatory potential.
of foodandwas first introduced by Shivappa et al.11. The DII has been used in many epidemiological studies involving different ethnicities and different health outcomes12,13 where higher adherence to DII was related to an increase pro-inflammatory cytokines such as c- reactive protein (CRP), interleukin 6 (IL-6), and tumor necrosis factor- a (TNF-a)14. DII can be used to evaluate the inflammatory status of diets, including anti-inflammatory or pro-inflammatory properties11.
According to the high prevalence of dyslipidemia in the Ravansar non-communicable diseases (RaNCD) cohort study15, the present study was designed with the aim of investigating dietary patterns and DII in relation to dyslipidemia in people.
Material and methods
Study design
We designed a cross-sectional study using data from the RaNCD cohort study, a population-based study on the Kurdish population (4764 men and 5283 women) aged 35–65 years, Ravansar, Kermanshah province, Western Iran. The RaNCD cohort study, subset of the PERSIAN (Prospective Epidemiological Research Studies in Iran) mega cohort study, was approved by the Ethics Committees in the Ministry of Health and Medical Education, the Digestive Diseases Research Institute, Tehran University of Medical Sciences, Iran16,17. All procedures for the RaNCD cohort study were approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics approval number: IR.KUMS.REC.1401.507). Furthermore, all participants provided written informed consent.
Participants
In this study, we did not include pregnant women (n = 138). We further did not include participants who had been diagnosed with CVDs (n = 1684), diabetes (n = 490), hypertension (n = 372), thyroid disease (n = 507), or cancer (n = 57). After that, we excluded participants with unusual energy intake (< 800 kcal/day or ≥ 4200 kcal/day for men and (< 600 kcal/day or ≥ 3500 kcal/day for women) (n = 788) and insufficient data (n = 57). A total of 5954 participants were finally selected for the analysis.
Physical activity assessment
Physical activity was assessed by the standard questionnaire designed by the PERSIAN mega cohort study, with twenty two questions about the amount of daily activities. The report of this questionnaire was evaluated based on the metabolic equivalent of tasks per hour per day (MET/h/day). Details of this questionnaire was published in the previous study17.
Socioeconomic status (SES)
Participants' SES was measured based on the asset-based approach. Data on household income, housing conditions (e.g. type of home ownership and number of rooms), infrastructure facilities (sanitary facilities, drinking water supply), ownership of a range of durable assets (e.g. car, dishwasher, television, etc.), and level of education (illiterate, under-diploma, diploma, and university) were used to measure individuals' SES. SES was constructed using the principal component analysis (PCA) technique. PCA generates weights for each selected asset and then estimates a continuous index based on the sum of the weights of the variables included in the PCA for each individual. This index was used to categorize individuals into three SES tertiles (from poorest to richest).
Anthropometric measurement/biochemical assessment
Height and body weight were assessed in the recruitment phase of the RaNCD cohort study. Body mass index (BMI) was calculated by dividing weight (kg) by the square of height (m2). Furthermore, waist circumference (WC) was measured by non-stretched and flexible tape in a standing position at the level of the iliac crest by a trained nutritionist. In the RaNCD cohort study, fasting blood samples were obtained from all RaNCD participants and all serum samples were analyzed at the RaNCD laboratory. To assess lipid profile, Concentrations of total cholesterol (TC), high-density lipoproteins (HDL), triglyceride (TG) and low-density lipoproteins (LDL) were analyzed using enzymatic kits (Pars Azmun, Iran)18.
Dyslipidemia
Dyslipidemia was considered to have an LDL cholesterol of ≥ 160 mg/dl and/or total cholesterol of ≥ 240 mg/dl and/or HDL cholesterol of < 40 mg/dl and/or triglycerides of ≥ 200 mg/dl and/or a history of taking medications for dyslipidemia15.
Dietary intake assessment
The dietary intake of RaNCD participants was analyzed using a validated semi-quantitative food frequency questionnaire (FFQ)19,20.
Dietary inflammatory index
Dietary intake information was also used to calculate DII using the Shivapa method11. Shivapa et al.11 reported that 45 nutrients were associated with one or more inflammatory events, including interleukin-1b (IL-1b), interleukin-6 (IL-6), tumor necrosis factor-a (TNF-a), reactive protein C (CRP), or anti-inflammatory markers, including interleukin-4 (IL-4) and interleukin-10 (IL-10). Therefore, each food parameter that had inflammatory potential was scored + 1. A score of -1 was given to foods that caused a decrease in inflammation or an increase in anti-inflammatory markers, and zero meant no effect on reducing or increasing inflammation.
In this study, based on the RaNCD FFQ questionnaire, 31 food parameters from 45 food items were available to calculate DII, including: vitamin A, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, folic acid, niacin, iron, zinc , selenium, magnesium, beta-carotene, caffeine, thiamin, riboflavin, onion, garlic, tea, omega 3, omega 6, trans fat, saturated fats (SFAs), cholesterol, monounsaturated fatty acids (MUFA), polyunsaturated fatty acids (PUFA), fiber, protein, total fat, carbohydrate, and energy. First, to calculate DII, the intake of each of the mentioned food parameters is subtracted from the global average intake of that food parameter and divided by its global standard deviation, the Z score is obtained, which is standardized for the parameter related to the food itself, and to minimize the effect of right skewness, this value is converted into a percentile score, and the percentile score is multiplied by two and subtracted from the number one. The number obtained in the inflammatory score corresponding to that food parameter is multiplied and the numbers obtained from each food parameter for each person are added together to obtain the total inflammatory score for each person. Finally, all three known food patterns and DII were divided into three groups to check the relationship between different variables. The first tertile and the third tertile represent the least and the most adherence to the respective patterns, respectively.
Statistical analysis
Quantitative variables were described by mean ± standard deviation (SD), and qualitative variables were reported using frequency (%). The comparison of participants’ baseline characteristics was evaluated using the Chi-square and ANOVA tests based on the tertiles of DII. This FFQ covered 118 food items commonly found in the Kurdish eating pattern. The data from these food items were categorized into 31 food groups (principal components) based on the nutrient content similarity21 (Table 1). These 31 food groups were included in PCA as items (input variables). We extracted the major dietary patterns by principal component analysis (PCA) and for factor rotation, we used the varimax rotation method to reduce the number of variables. We identified three main dietary patterns with eigenvalues greater than 1.5 based on the Scree plot and the interpretability of the factors. These dietary patterns were named on the basis of characteristics of the food groups they included (with factor loadings > 0.2). The KMO and Bartlett’s test was 0.798. The radar graph was drawn to better show the factor loadings of food groups in three major dietary patterns. Binary logistic regression in crude and adjusted odds ratios (OR) and 95% confidence intervals (CI) was used to determine the association between dyslipidemia and tertiles of DII and three major dietary patterns. In the adjusted model, we controlled age (continuous), sex (categorical), SES (categorical), and physical activity (continuous) as potential confounders. In all analyses, the first tertile of DII and three major dietary patterns were considered the reference categories. In addition, to better illustrate this association, we considered the figure of linear regression OR across increased DII and three major dietary patterns with adjustment for the mentioned variables in logistic regression. All statistical analyses were conducted using SPSS 20 (IBM Corp, Chicago, IL, USA) and Stata, version 14 (Stata Corp, College Station, TX). P-values were considered significant at the level of < 0.05.
Table 1.
Food groupings used in the dietary pattern analyses.
| Food groups | Dietary components |
|---|---|
| Leafy vegetables | Cauliflower, lettuce, cucumber, onion, green bean, mushroom, pepper, garlic, turnip, others |
| Fresh fruits | Melon, watermelon, honeydew melon, plums, prunes, apples, cherries, sour cherries, peaches, nectarine, pear, fig, date, grapes, kiwi, pomegranate, strawberry, banana, persimmon, berry, pineapple, oranges, others |
| Dried fruits | Dried apricots, Dried berries, raisins, and other type dried fruits |
| Dairy | Milk, yogurt, yogurt drink (doogh), cheese, chocolate milk, crud |
| Tomato | Tomato |
| Carotene-rich vegetables | Yellow squash, carrot |
| Condiments | Spices, pepper powder, tomato paste, mayonnaise |
| Pickles | Pickles |
| Legumes | All type beans, peas, lentils, mung bean, soy |
| Whole grain | Dark breads (Iranian), wheat, barley |
| Starchy vegetables | Corn, eggplant, green peas, green squash |
| Vegetable oil | Vegetable oil |
| Natural juices | All fruit juices |
| Butter | Butter, margarine |
| Olive | Olive and olive oil |
| Organ meat | Heart, kidney, liver, tongue, brain, offal |
| Read meat | Beef, lamb, minced meat |
| Fish | All fish types |
| Processed meat | Hamburger, sausage, delicatessen meat, pizza |
| Soft drink | Soft drink |
| Nuts | Almond, peanut, walnut, pistachio, hazelnut, seeds |
| Egg | Egg |
| Poultry | Chicken |
| Snack | Corn puffs, potato chips, French fries |
| Sweets and desserts | Cookies, cakes, biscuit, muffins, pies, chocolates, ice, honey, jam, sugar cubes, sugar, candies, others |
| Tea and coffee | Tea and coffee |
| Hydrogenated fat | Hydrogenated fats, animal fats including ghee and tallow |
| Salt | Salt |
| Potato | Potato |
| Refined grain | White breads (lavash, baguettes), noodles, pasta, rice |
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the Ethics Committee of Kermanshah University of Medical Sciences (ethics approval number: IR.KUMS.REC.1401.507).
Informed consent
Written informed consent was obtained from each studied subject after explaining the purpose of the study. The right of the subjects to withdraw from the study at any time and the subject’s information are reserved and will not be published.
Results
This current study was conducted on data from 5954 RaNCD participants (51.9% men). 40.2% of these participants had dyslipidemia. The mean of weight and BMI significantly increased with higher adherence to DII (P < 0.001). The prevalence of dyslipidemia in the third was significantly higher than in other tertiles of DII (P < 0.001). Table 2 describes the baseline characteristics of the studied participants in total and based on tertiles of dietary inflammatory index.
Table 2.
Baseline characteristics of studied participants based on tertiles of dietary inflammatory index.
| Variables | Total (n = 5954) | Tertiles of dietary inflammatory index | P | ||
|---|---|---|---|---|---|
| T1 (n = 1984) | T2 (n = 1985) | T3 (n = 1985) | |||
| Age (year) | 45.80 ± 7.82* | 46.74 ± 8.09 | 45.58 ± 7.78 | 45.07 ± 7.50 | < 0.001 |
| Sex, male% | 51.9 | 45.4 | 50.5 | 59.7 | < 0.001 |
| SES | |||||
| Low | 32.4 | 45 | 29 | 23.4 | < 0.001 |
| Moderate | 32.5 | 28.3 | 34.5 | 34.9 | |
| High | 35 | 26.7 | 36.6 | 41.7 | |
| Weight (kg) | 71.87 ± 13.40 | 69.50 ± 13.11 | 72.00 ± 13.27 | 74.12 ± 13.41 | < 0.001 |
| BMI (kg/m2) | 23.78 ± 9.26 | 23.12 ± 9.01 | 24.01 ± 9.19 | 24.22 ± 9.53 | < 0.001 |
| WC (cm) | 96.05 ± 10.27 | 95.93 ± 10.41 | 96.19 ± 10.16 | 96.02 ± 10.25 | 0.716 |
| PA (MET/day) | 41.28 ± 8.39 | 40.96 ± 7.32 | 41.00 ± 8.19 | 41.87 ± 9.47 | 0.001 |
| TG (mg/dl) | 130.85 ± 74.72 | 129.30 ± 76.06 | 128.55 ± 72.34 | 134.71 ± 75.58 | 0.018 |
| TC (mg/dl) | 183.86 ± 36.54 | 185.06 ± 37.44 | 183.43 ± 35.71 | 183.08 ± 36.43 | 0.189 |
| HDL (mg/dl) | 46.61 ± 11.37 | 47.93 ± 11.48 | 46.82 ± 11.53 | 45.09 ± 10.90 | < 0.001 |
| Dyslipidemia,% | 40.2 | 37.1 | 39.1 | 44.3 | < 0.001 |
| Taking medications, % | 1.2 | 0.6 | 1.3 | 1.8 | 0.278 |
| high LDL levels, % | 24.1 | 24 | 23.4 | 23.9 | 0.511 |
| high TC levels, % | 29.3 | 28.9 | 29 | 30.7 | 0.233 |
| low HDL levels, % | 47.4 | 45.1 | 47.6 | 49.7 | 0.014 |
| Smoking, % | 12.8 | 12.5 | 13.2 | 12.6 | 0.756 |
BMI body mass index, WC waist circumference, PA physical activity, TG triglyceride, TC total cholesterol, HDL high density lipoprotein, SES socioeconomic status.
P-values were obtained ANOVA and Chi square test.
*Mean ± SD.
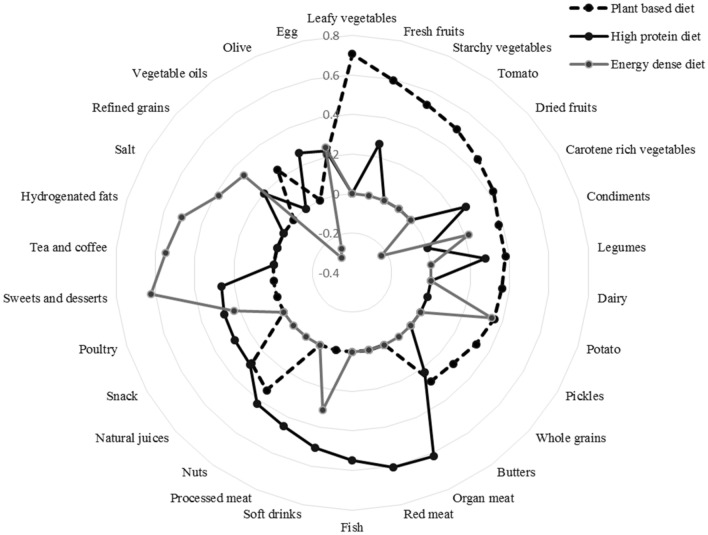
In this present study, three dietary patterns were identified including (1) plant-based diet which was associated with major intake to leafy vegetables, carotene rich vegetables, fresh and dried fruits, starchy vegetables, tomato, potato, condiments, legumes, dairy, pickles, nuts, natural juice, whole grain, vegetable oil, and egg; (2) high protein and sugar diet tend to organ meat, red meat, processed meat, fish, nuts, poultry, sweet and dessert, soft drink, snack, butter, legumes, carotene rich vegetables, refined grain, olive, and, egg; and (3) energy dense diet had the most factor loadings for sweet and dessert, soft drink, tea and coffee, hydrogenated fats, salt, refined grain, potato, egg, condiments, poultry, and negative factor loadings for carotene rich vegetables, vegetable oil, and olive. Factor loadings for all food groups are presented in Table 3 and Fig. 1.
Table 3.
Factor loading of food groups in all dietary patterns.
| Food groups | Major identified dietary pattern | ||
|---|---|---|---|
| Plant based diet | High protein and sugar diet | Energy dense diet | |
| Leafy vegetables | 0.707 | – | – |
| Fresh fruits | 0.595 | 0.266 | – |
| Starchy vegetables | 0.531 | – | – |
| Tomato | 0.498 | – | – |
| Dried fruits | 0.455 | – | – |
| Carotene rich vegetables | 0.425 | 0.264 | − 0.231 |
| Condiments | 0.380 | – | 0.220 |
| Legumes | 0.379 | 0.278 | – |
| Dairy | 0.362 | – | – |
| Potato | 0.356 | – | 0.341 |
| Pickles | 0.324 | – | – |
| Whole grains | 0.288 | – | – |
| Butters | 0.278 | 0.222 | – |
| Organ meat | – | 0.616 | – |
| Red meat | – | 0.606 | – |
| Fish | – | 0.548 | – |
| Soft drinks | – | 0.505 | 0.309 |
| Processed meat | – | 0.451 | – |
| Nuts | 0.335 | 0.420 | – |
| Natural juices | 0.285 | 0.293 | – |
| Snack | – | 0.285 | – |
| Poultry | – | 0.280 | 0.230 |
| Sweets and desserts | – | 0.265 | 0.623 |
| Tea and coffee | – | – | 0.549 |
| Hydrogenated fats | – | – | 0.507 |
| Salt | – | – | 0.378 |
| Refined grains | – | 0.201 | 0.337 |
| Vegetable oils | 0.243 | – | − 0.306 |
| Olive | – | 0.261 | − 0.269 |
| Egg | 0.212 | 0.229 | 0.246 |
| Variance, % | 13.8 | 6.52 | 5.03 |
Values < 0.2 have been removed for clarity.
Figure 1.
Radar graph for factor loading of food groups in all dietary patterns.
In this study, binary logistic regression for dyslipidemia across tertiles of DII revealed that greater adherence to DII was associated with significantly increased odds of dyslipidemia (OR: 1.35; CI 95% 1.19–1.53). This association remained after adjusting for age, sex, and physical activity (OR: 1.24; CI 95% 1.09–1.42). (Table 4, Fig. 2a).
Table 4.
Multivariable-adjusted odds ratios and 95% confidence intervals for dyslipidemia across categories of dietary inflammatory index and major identified dietary patterns.
| Dietary patterns | Tertiles of dietary inflammatory index | P- trend | ||
|---|---|---|---|---|
| T1 | T2 | T3 | ||
| DII | ||||
| Crude | 1 | 1.09 (0.96–1.24)a | 1.35 (1.19–1.53) | < 0.001 |
| Model 1* | 1 | 1.06 (0.93–1.21) | 1.24 (1.09–1.42) | 0.001 |
| Plant based diet | ||||
| Crude | 1 | 0.98 (0.86–1.11) | 1.12 (0.99–1.27) | 0.072 |
| Model 1** | 1 | 0.99 (0.86–1.13) | 1.12 (0.97–1.30) | 0.124 |
| High protein and sugar diet | ||||
| Crude | 1 | 1.07 (0.94–1.22) | 1.31 (1.16–1.49) | < 0.001 |
| Model 1** | 1 | 1.02 (0.9–1.16) | 1.18 (1.04–1.37) | 0.013 |
| Energy dense diet | ||||
| Crude | 1 | 1.04 (0.91–1.18) | 1.19 (1.05–1.35) | 0.008 |
| Model 1** | 1 | 1.01 (0.97–1.25) | 1.28 (1.12–1.46) | < 0.001 |
*Model 1 adjusted for age, sex, SES, and physical activity.
**Model 1 adjusted for age, sex, SES, physical activity, and energy intake.
aOR (CI 95%).
Figure 2.
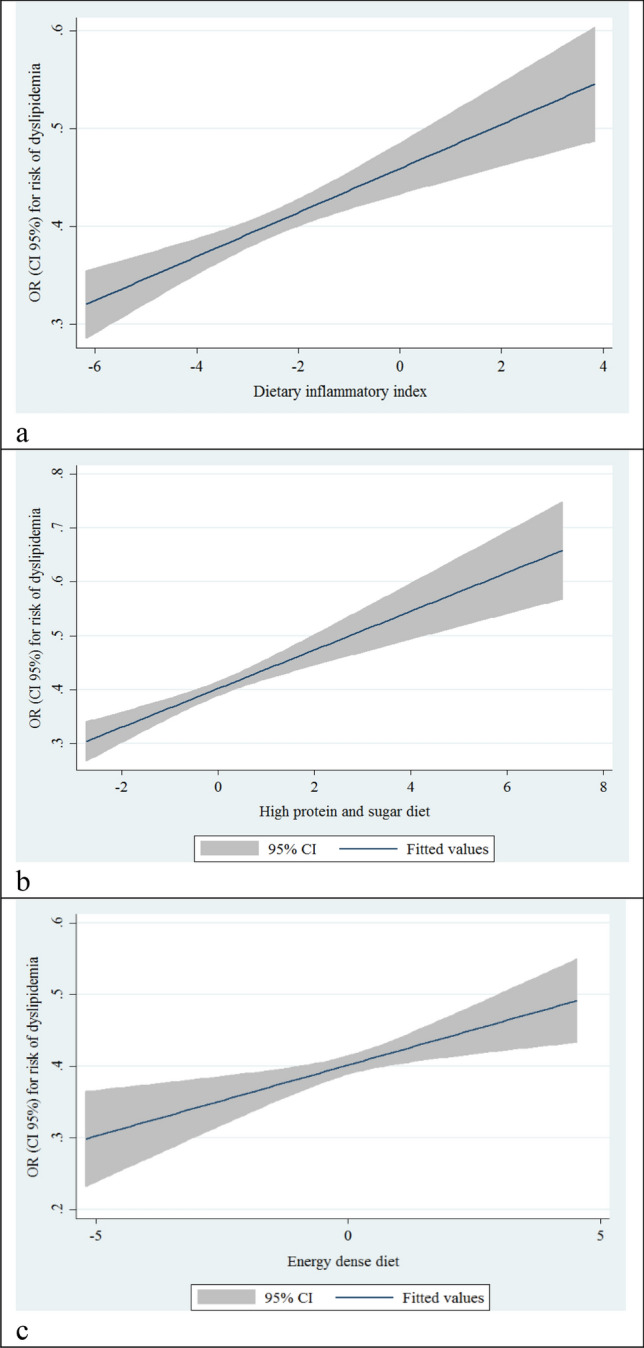
Liner regression odds ratios and 95% confidence intervals for dyslipidemia across categories of (a) dietary inflammatory index; (b) high protein and sugar diet; and (c) energy dense diet.
On the other hand, our findings on the relationship between major identified dietary patterns and dyslipidemia showed that higher adherence to the high protein and sugar diet and energy dense were significantly associated with higher odds for dyslipidemia in both crude and adjusted models (OR: 1.31; CI 95% 1.16–1.49) and (OR: 1.28; CI 95% 1.12–1.46). (Table 4, Fig. 2b,c) Nevertheless, according to our results, plant-based diet had no association with dyslipidemia. We did not find any associations adjusted for the mentioned potential confounders. (Table 4).
Discussion
Findings from our cross-sectional study support the hypothesis of a relationship between a pro-inflammatory diet (as estimated by the DII) and increased dyslipidemia in a Kurdish population.
We observed that greater adherence to DII, high protein and sugar diet or a diet of increased energy intake was associated with higher odds for dyslipidemia.
In our study, it was observed that increased DII was associated with higher serum levels of TG and lower levels of HDL. These findings are in line with Neufcourt et al.,22 study and another study in an Iranian population23. In addition, it was observed that adherence to an anti-inflammatory dietary pattern that is rich in antioxidants ameliorates the lipid profile24,25. Evidence supports an inverse relationship between DII and serum HDL26. However, in several studies, there was no association between DII and TG, which may be due to the smaller number of dietary factors used for the calculation of DII27–29. Also, similar results are found in relation to HDL27,28. The relationship between the anti-inflammatory diet, which contains rich resources of β-Carotene, vitamin C, omega-3, and fiber, and pro-inflammatory diet, such as the western food pattern (rich in energy, hydrogenated fat, and processed foods), has been shown to reduce and increase inflammatory factors such as CRP and IL-6, respectively30. According to the evidence, to explain how diets with a higher DII lead to dyslipidemia, it should be said that these foods activate the NF-kB pathway31 and can stimulate TG production in the liver and then release it into the blood32,33, as well as alters the balance of the lipid profile34.
In our study, adherence to high protein and sugar diet was related to increasing odds of dyslipidemia. In terms of effect on cardiovascular risk factors and metabolic syndrome, it is similar to the effect of a Westernized or energy dense dietary pattern35,36. A western dietary pattern often has more fast food, fats, and meat and less vegetables and grains37. In Zhang et al.'s study, more adherence to this food pattern was associated with an increase in LDL cholesterol38. Red and processed meat in the high protein and sugar diet have higher amounts of heme iron and saturated fatty acids, which decrease LDL receptor-mediated clearance39. Also, heme iron and saturated fatty acids lead to increased oxidative stress, which is a risk factor for dyslipidemia39,40. In other studies, it was observed that if SFAs are replaced with unsaturated fatty acids (UFAs), the number of LDL receptors increases41,42.
In our study, the energy dense diet was related to increasing odds of dyslipidemia. This pattern is characterized by high consumption of sweets and refined grains, as well as salt, and low intake of fruits and vegetables. Similar observations were seen in previous studies, including one study that found a significant relationship between the consumption of carbonated drinks and an increased risk of dyslipidemia43. In the study of Al-Lahou et al., it was observed that a major intake to refined grains led to a higher risk of dyslipidemia44.
According to our findings, the plant based dietary pattern had no association with dyslipidemia, unlike the two patterns mentioned above. Despite this, a recent meta-analysis published in including 30 clinical trials concluded that compared to people eating an omnivorous diet, those who were following a plant-based diet experienced an average reduction in total cholesterol levels of 7% from levels measured at the start of the studies, a 10% reduction in LDL cholesterol levels and a 14% reduction in Apo B levels45. It was also stated that plant-based diets have the potential to reduce the atherosclerotic burden caused by atherogenic lipoproteins and thus reduce the risk of cardiovascular diseases45. Foods of this pattern are rich sources of fiber, vitamins, and polyphenols similar to the Mediterranean diet and the dietary approaches to stop hypertension (DASH) diet46,47, which may have antioxidant and anti-inflammatory properties and may affect the lipid profile6. In past studies, it was observed that greater adherence a plant-based dietary pattern was associated with a reduction in TC and LDL48 as well as an incidence of dyslipidemia44. In a randomized controlled trial, adherence to the DASH diet, compared with the control diet, resulted in a significant decrease in serum triglycerides, and very-low-density lipoprotein cholesterol levels49. In a recent study by Antoniazzi et al., it was shown that higher adherence to a Mediterranean diet was associated with better dyslipidemia and low-grade inflammation profiles in familial hypercholesterolemia50. The neutral effect of the plant-based diet seems to be because this pattern contains a high factor loading for fructose sources (fruit, juice, and dried fruit), as well as butter especially margarine. The most important metabolic side effects of high fructose consumption are postprandial hypertriglyceridemia, which leads to the activation of protein kinase C, hepatic triglyceride accumulation, and hepatic insulin resistance by increasing visceral fat deposition51. Additionally, butter rich in cholesterol, saturated fatty acids, especially trans fatty acids, which increases plasma cholesterol and HDL cholesterol concentration. Hence, the ratio of total cholesterol to HDL remains largely unchanged52. Studies have also observed weak or neutral associations between butter consumption and mortality, CVD, diabetes and cancer53,54. On the other hand, margarine is a type of butter that is produced from vegetable oils in the hydrogenation process, but it has a higher trans fatty acid content than butter55. Excess intake of trans fatty acids can effectively increase LDL levels and subsequently increase the risk of CVDs56. As a result, the reason for not observing the relationship between plant-based dietary patterns and dyslipidemia may be that the benefits of fiber, vitamins, and polyphenols have been neutralized by the higher amount of margarine and fructose consumption.
Among the important limitations of this study is its cross-sectional nature, which cannot show a causal relationship between DII and dyslipidemia. Other limitations of this study are the use of an FFQ and the lack of all the food factors that are used in the calculation of the DII. Further well design follow- up studies in this field seem necessary.
In summary, our findings highlighted that higher adherence to energy dense, and high protein and sugary dietary patterns and also a higher DII score can have undesirable effects on dyslipidemia prevalence. Therefore, the adverse side effects of these aforementioned diets should be noted by dietitians.
Acknowledgements
RaNCD is part of the PERSIAN national cohort and we would like to thank Professor Reza Malekzadeh, Deputy of Research and Technology at the Ministry of Health and Medical Education of Iran and Director of the PERSIAN cohort, and also Dr. Hossein Poustchi, Executive Director of PERSIAN cohort, for all their support during the design and running of RaNCD.
Author contributions
S.M. and Y.P. equally contributed to the conception and design of the research; F.N., M.B., and Y.P. contributed to data collection; S.M., F.M., and M.S. contributed to the acquisition and analysis of the data; S.M. and F.M. contributed to the interpretation of the data; and S.M., F.M., A.S., M.S., and Y.P. contributed to the draft of the manuscript. S.M., S.C., A.S., and F.M. contributed to critical review of the manuscript. All authors are in agreement with the manuscript and declare that the content has not been published elsewhere.
Funding
This study was supported by the Ministry of Health and Medical Education of Iran and Kermanshah University of Medical Science (Grant No: 4020412).
Data availability
Data will be available upon request from the corresponding author.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xiao C, Dash S, Morgantini C, Hegele RA, Lewis GF. Pharmacological targeting of the atherogenic dyslipidemia complex: The next frontier in CVD prevention beyond lowering LDL cholesterol. Diabetes. 2016;65:1767–1778. doi: 10.2337/db16-0046. [DOI] [PubMed] [Google Scholar]
- 2.Tada, H., Kawashiri, M.-a. & Yamagishi, M. Clinical perspectives of genetic analyses on dyslipidemia and coronary artery disease. J. Atheroscler. Thrombosis, RV17002 (2017). [DOI] [PMC free article] [PubMed]
- 3.Peters SA, Singhateh Y, Mackay D, Huxley RR, Woodward M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: A systematic review and meta-analysis. Atherosclerosis. 2016;248:123–131. doi: 10.1016/j.atherosclerosis.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Zhao, D. Epidemiological features of cardiovascular disease in Asia. JACC: Asia1, 1–13 (2021). [DOI] [PMC free article] [PubMed]
- 5.Zhao D, Liu J, Wang M, Zhang X, Zhou M. Epidemiology of cardiovascular disease in China: Current features and implications. Nat. Rev. Cardiol. 2019;16:203–212. doi: 10.1038/s41569-018-0119-4. [DOI] [PubMed] [Google Scholar]
- 6.Kim S-A, Joung H, Shin S. Dietary pattern, dietary total antioxidant capacity, and dyslipidemia in Korean adults. Nutr. J. 2019;18:1–12. doi: 10.1186/s12937-019-0459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trautwein EA, McKay S. The role of specific components of a plant-based diet in management of dyslipidemia and the impact on cardiovascular risk. Nutrients. 2020;12:2671. doi: 10.3390/nu12092671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S-A, Joung H, Shin S. Dietary pattern, dietary total antioxidant capacity, and dyslipidemia in Korean adults. Nutr. J. 2019;18:37. doi: 10.1186/s12937-019-0459-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheung BH, Ho IC, Chan RS, Sea MM, Woo J. Current evidence on dietary pattern and cognitive function. Adv. Food Nutr. Res. 2014;71:137–163. doi: 10.1016/B978-0-12-800270-4.00004-3. [DOI] [PubMed] [Google Scholar]
- 10.Pasdar Y, et al. Healthy eating index 2015 and major dietary patterns in relation to incident hypertension; a prospective cohort study. BMC Public Health. 2022;22:1–11. doi: 10.1186/s12889-022-13166-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hébert JR. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–1696. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowler ME, Akinyemiju TF. Meta-analysis of the association between dietary inflammatory index (DII) and cancer outcomes. Int. J. Cancer. 2017;141:2215–2227. doi: 10.1002/ijc.30922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niclis C, et al. Proinflammatory dietary intake is associated with increased risk of colorectal cancer: Results of a case-control study in Argentina using a multilevel modeling approach. Nutr. Cancer. 2018;70:61–68. doi: 10.1080/01635581.2018.1397710. [DOI] [PubMed] [Google Scholar]
- 14.Matta J, et al. Diet and physical activity in the association between depression and metabolic syndrome: Constances study. J. Affect. Disord. 2019;244:25–32. doi: 10.1016/j.jad.2018.09.072. [DOI] [PubMed] [Google Scholar]
- 15.Rezaei M, Fakhri N, Pasdar Y, Moradinazar M, Najafi F. Modeling the risk factors for dyslipidemia and blood lipid indices: Ravansar cohort study. Lipids Health Dis. 2020;19:1–8. doi: 10.1186/s12944-020-01354-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasdar Y, et al. Cohort profile: Ravansar Non-Communicable Disease cohort study: the first cohort study in a Kurdish population. Inter J Epidemiol. 2019;48:682–683f. doi: 10.1093/ije/dyy296. [DOI] [PubMed] [Google Scholar]
- 17.Poustchi H, et al. Prospective epidemiological research studies in Iran (the PERSIAN Cohort Study): Rationale, objectives, and design. Am. J. Epidemiol. 2018;187:647–655. doi: 10.1093/aje/kwx314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasdar, Y. et al. Cohort profile: Ravansar non-communicable disease cohort study: The first cohort study in a Kurdish population. Int. J. Epidemiol. (2019). [DOI] [PubMed]
- 19.Moradi S, et al. Comparison of 3 nutritional questionnaires to determine energy intake accuracy in Iranian adults. Clin. Nutr. Res. 2018;7:213–222. doi: 10.7762/cnr.2018.7.3.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13:654–662. doi: 10.1017/S1368980009991698. [DOI] [PubMed] [Google Scholar]
- 21.Pasdar, Y. et al. Major dietary patterns in relation to chronic low back pain; A Cross-sectional Study from RaNCD Cohort. (2021). [DOI] [PMC free article] [PubMed]
- 22.Neufcourt, L. et al. Prospective association between the dietary inflammatory index and metabolic syndrome: Findings from the SU. VI. MAX study. Nutr. Metab. Cardiovasc. Dis.25, 988–996 (2015). [DOI] [PubMed]
- 23.Vahid F, et al. Association between Dietary Inflammatory Index (DII) and risk of prediabetes: A case-control study. Appl. Physiol. Nutr. Metab. 2017;42:399–404. doi: 10.1139/apnm-2016-0395. [DOI] [PubMed] [Google Scholar]
- 24.Giannini C, et al. Influence of the Mediterranean diet on carotid intima–media thickness in hypercholesterolaemic children: A 12-month intervention study. Nutr. Metab. Cardiovasc. Dis. 2014;24:75–82. doi: 10.1016/j.numecd.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 25.Vitale M, et al. Impact of a Mediterranean dietary pattern and its components on cardiovascular risk factors, glucose control, and body weight in people with type 2 diabetes: A real-life study. Nutrients. 2018;10:1067. doi: 10.3390/nu10081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neufcourt, L. et al. Prospective association between the dietary inflammatory index and metabolic syndrome: findings from the SU.VI.MAX study. Nutr. Metab. Cardiovasc. Dis. NMCD25, 988–996. 10.1016/j.numecd.2015.09.002 (2015). [DOI] [PubMed]
- 27.Ren Z, et al. Association between dietary inflammatory index, C-reactive protein and metabolic syndrome: A cross-sectional study. Nutrients. 2018;10:831. doi: 10.3390/nu10070831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Naja F, et al. Role of inflammation in the association between the western dietary pattern and metabolic syndrome among Lebanese adults. Int. J. Food Sci. Nutr. 2017;68:997–1004. doi: 10.1080/09637486.2017.1312297. [DOI] [PubMed] [Google Scholar]
- 29.Sokol A, et al. Association between the dietary inflammatory index, waist-to-hip ratio and metabolic syndrome. Nutr. Res. 2016;36:1298–1303. doi: 10.1016/j.nutres.2016.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahluwalia N, Andreeva VA, Kesse-Guyot E, Hercberg S. Dietary patterns, inflammation and the metabolic syndrome. Diabetes Metab. 2013;39:99–110. doi: 10.1016/j.diabet.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Calder PC, et al. Inflammatory disease processes and interactions with nutrition. Br. J. Nutr. 2009;101:1–45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- 32.Rupérez AI, Mesana MI, Moreno LA. Dietary sugars, metabolic effects and child health. Curr. Opin. Clin. Nutr. Metab. Care. 2019;22:206–216. doi: 10.1097/MCO.0000000000000553. [DOI] [PubMed] [Google Scholar]
- 33.Siri-Tarino PW, Krauss RM. Diet, lipids, and cardiovascular disease. Curr. Opin. Lipidol. 2016;27:323–328. doi: 10.1097/MOL.0000000000000310. [DOI] [PubMed] [Google Scholar]
- 34.Rauber F, Campagnolo PDB, Hoffman DJ, Vitolo MR. Consumption of ultra-processed food products and its effects on children's lipid profiles: A longitudinal study. Nutr. Metab. Cardiovasc. Dis. 2015;25:116–122. doi: 10.1016/j.numecd.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Muga MA, Owili PO, Hsu C-Y, Rau H-H, Chao JC. Association between dietary patterns and cardiovascular risk factors among middle-aged and elderly adults in Taiwan: A population-based study from 2003 to 2012. PloS One. 2016;11:e0157745. doi: 10.1371/journal.pone.0157745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amini M, Esmaillzadeh A, Shafaeizadeh S, Behrooz J, Zare M. Relationship between major dietary patterns and metabolic syndrome among individuals with impaired glucose tolerance. Nutrition. 2010;26:986–992. doi: 10.1016/j.nut.2010.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Strate, L. L. et al. Western dietary pattern increases, and prudent dietary pattern decreases, risk of incident diverticulitis in a prospective cohort study. Gastroenterology152, 1023–1030 (2017). [DOI] [PMC free article] [PubMed]
- 38.Zhang J, et al. Association between dietary patterns and blood lipid profiles among Chinese women. Public Health Nutr. 2016;19:3361–3368. doi: 10.1017/S136898001600197X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim S-A, Shin S. Red meat and processed meat consumption and the risk of dyslipidemia in Korean adults: A prospective cohort study based on the Health Examinees (HEXA) study. Nutr. Metab. Cardiovasc. Dis. 2021;31:1714–1727. doi: 10.1016/j.numecd.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 40.Klisic A, et al. Relationship between oxidative stress, inflammation and dyslipidemia with fatty liver index in patients with type 2 diabetes mellitus. Exp. Clin. Endocrinol. Diabetes. 2018;126:371–378. doi: 10.1055/s-0043-118667. [DOI] [PubMed] [Google Scholar]
- 41.Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutr. 2005;135:2075–2078. doi: 10.1093/jn/135.9.2075. [DOI] [PubMed] [Google Scholar]
- 42.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fatty acids and risk of coronary heart disease: Modulation by replacement nutrients. Curr. Atheroscler. Rep. 2010;12:384–390. doi: 10.1007/s11883-010-0131-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enani S, et al. The association between dyslipidemia, dietary habits and other lifestyle indicators among non-diabetic attendees of primary health care centers in Jeddah, Saudi Arabia. Nutrients. 2020;12:2441. doi: 10.3390/nu12082441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Al-Lahou B, et al. Dietary patterns associated with the prevalence of cardiovascular disease risk factors in Kuwaiti adults. J. Acad. Nutr. Diet. 2020;120:424–436. doi: 10.1016/j.jand.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Koch, C. A., Kjeldsen, E. W. & Frikke-Schmidt, R. Vegetarian or vegan diets and blood lipids: A meta-analysis of randomized trials. Eur. Heart J. ehad211 (2023). [DOI] [PMC free article] [PubMed]
- 46.Visioli F, Galli C. The role of antioxidants in the Mediterranean diet. Lipids. 2001;36:S49–S52. doi: 10.1007/s11745-001-0682-z. [DOI] [PubMed] [Google Scholar]
- 47.Pirouzeh R, et al. Effect of DASH diet on oxidative stress parameters: A systematic review and meta-analysis of randomized clinical trials. Diabetes Metab. Syndrome Clin. Res. Rev. 2020;14:2131–2138. doi: 10.1016/j.dsx.2020.10.031. [DOI] [PubMed] [Google Scholar]
- 48.Melina V, Craig W, Levin S. Position of the Academy of Nutrition and Dietetics: vegetarian diets. J. Acad. Nutr. Dietetics. 2016;116:1970–1980. doi: 10.1016/j.jand.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 49.Asemi Z, et al. Effects of DASH diet on lipid profiles and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: A randomized clinical trial. Nutrition. 2014;30:1287–1293. doi: 10.1016/j.nut.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 50.Antoniazzi L, et al. Adherence to a Mediterranean diet, dyslipidemia and inflammation in familial hypercholesterolemia. Nutr. Metab. Cardiovasc. Dis. 2021;31:2014–2022. doi: 10.1016/j.numecd.2021.04.006. [DOI] [PubMed] [Google Scholar]
- 51.Stanhope KL, Havel PJ. Fructose consumption: Potential mechanisms for its effects to increase visceral adiposity and induce dyslipidemia and insulin resistance. Curr. Opin. Lipidol. 2008;19:16. doi: 10.1097/MOL.0b013e3282f2b24a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pfeuffer M, Watzl B. Nutrition and health aspects of milk and dairy products and their ingredients. Ernahrungs Umschau. 2018;65:22–33. [Google Scholar]
- 53.Pimpin L, Wu JH, Haskelberg H, Del Gobbo L, Mozaffarian D. Is butter back? A systematic review and meta-analysis of butter consumption and risk of cardiovascular disease, diabetes, and total mortality. PloS One. 2016;11:e0158118. doi: 10.1371/journal.pone.0158118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lu W, et al. Dairy products intake and cancer mortality risk: A meta-analysis of 11 population-based cohort studies. Nutr. J. 2016;15:1–11. doi: 10.1186/s12937-016-0210-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber C, et al. Nutrient comparisons of margarine/margarine-like products, butter blend products and butter in the US marketplace in 2020 post-FDA ban on partially hydrogenated oils. Public Health Nutr. 2021;25:1123–1130. doi: 10.1017/S1368980021004511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Islam MA, et al. Trans fatty acids and lipid profile: A serious risk factor to cardiovascular disease, cancer and diabetes. Diab. Metab. Syndrome Clin. Res. Rev. 2019;13:1643–1647. doi: 10.1016/j.dsx.2019.03.033. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be available upon request from the corresponding author.