Abstract
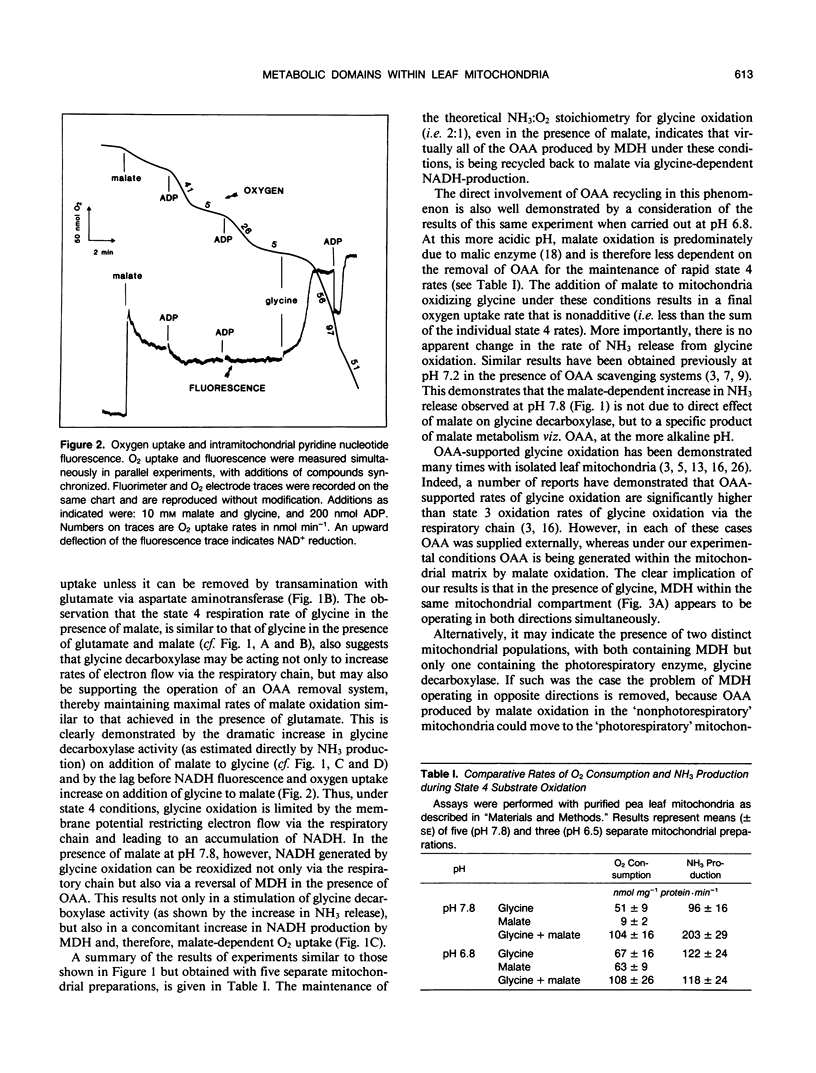
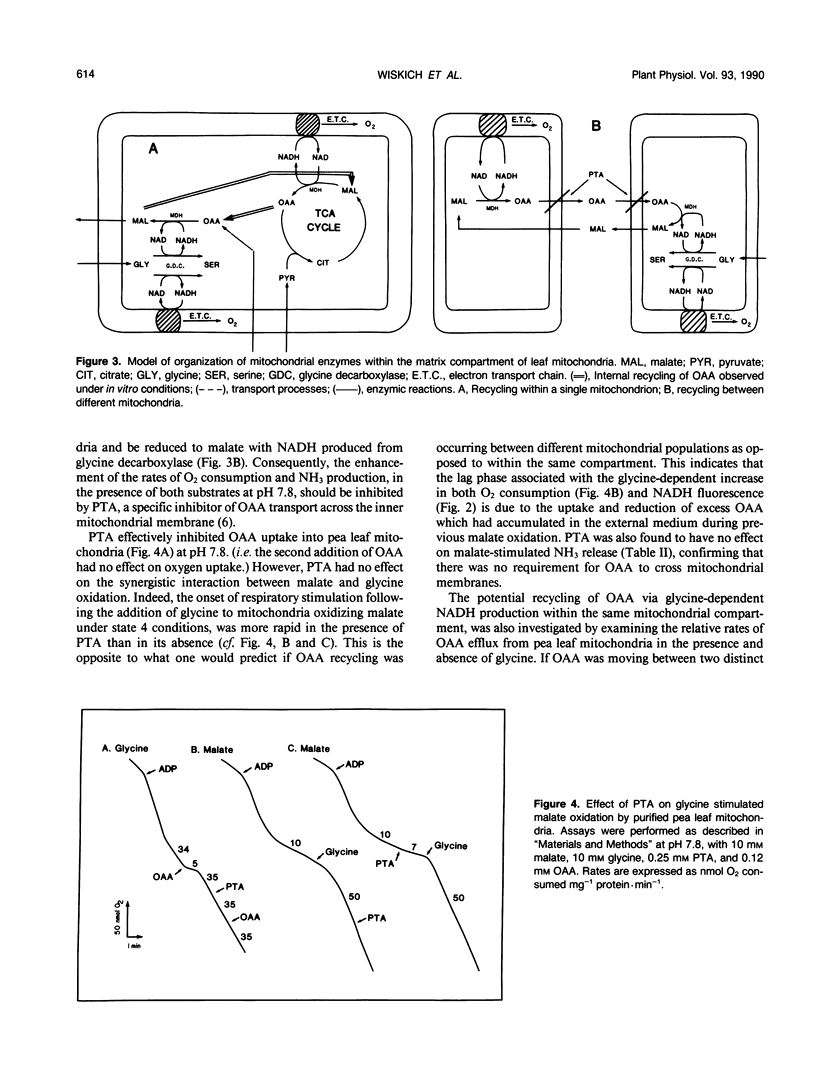
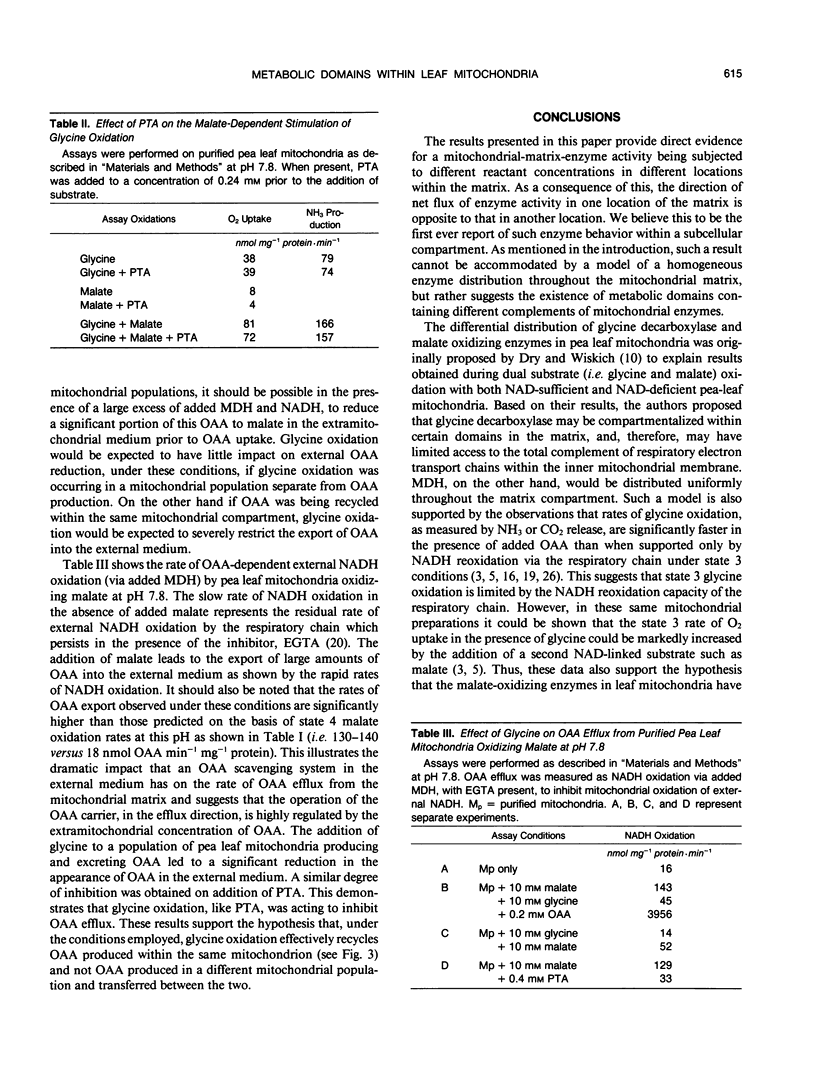
The simultaneous oxidation of malate and of glycine was investigated in pea (Pisum sativum) leaf mitochondria. Adding malate to state 4 glycine oxidation did not inhibit, and under some conditions stimulated, glycine oxidation. State 4 oxygen uptake with glycine is restricted because of the control exerted by the membrane potential but reoxidation of NADH by oxaloacetate reduction can still occur. Thus, malate addition stimulates glycine metabolism by producing oxaloacetate. The malate dehydrogenase (EC 1.1.1.37) enzyme fraction remote from glycine decarboxylase (EC 2.1.2.10) oxidizes malate whereas that closely associated with it produces malate, i.e. they function in opposite directions. It is shown that these opposing directions of malate dehydrogenase activity occur within the same mitochondrial matrix compartment and not in different mitochondrial populations. It is concluded that metabolic domains containing different complements of mitochondrial enzymes exist within the one mitochondrial matrix without physical barriers separating them. The differential spatial organization within the matrix may account for the previously reported limited access of some enzymes to the respiratory electron transport chain. The implications for leaf mitochondrial metabolism are discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeckmans S., Kanarek L. Demonstration of physical interactions between consecutive enzymes of the citric acid cycle and of the aspartate-malate shuttle. A study involving fumarase, malate dehydrogenase, citrate synthesis and aspartate aminotransferase. Eur J Biochem. 1981 Jul;117(3):527–535. doi: 10.1111/j.1432-1033.1981.tb06369.x. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Effect of phthalonic acid on respiration and metabolite transport in higher plant mitochondria. Arch Biochem Biophys. 1981 Oct 1;211(1):100–107. doi: 10.1016/0003-9861(81)90434-3. [DOI] [PubMed] [Google Scholar]
- Day D. A., Wiskich J. T. Glycine metabolism and oxalacetate transport by pea leaf mitochondria. Plant Physiol. 1981 Aug;68(2):425–429. doi: 10.1104/pp.68.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dry I. B., Dimitriadis E., Ward A. D., Wiskich J. T. The photorespiratory hydrogen shuttle. Synthesis of phthalonic acid and its use in the characterization of the malate/aspartate shuttle in pea (Pisum sativum) leaf mitochondria. Biochem J. 1987 Aug 1;245(3):669–675. doi: 10.1042/bj2450669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearl W. G., Churchich J. E. Interactions between 4-aminobutyrate aminotransferase and succinic semialdehyde dehydrogenase, two mitochondrial enzymes. J Biol Chem. 1984 Sep 25;259(18):11459–11463. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lilley R. M., Ebbighausen H., Heldt H. W. The simultaneous determination of carbon dioxide release and oxygen uptake in suspensions of plant leaf mitochondria oxidizing glycine. Plant Physiol. 1987 Feb;83(2):349–353. doi: 10.1104/pp.83.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. L., Jackson C., Halliwell B., Dench J. E., Hall D. O. Intramitochondrial localisation of glycine decarboxylase in spinach leaves. Biochem Biophys Res Commun. 1977 Sep 23;78(2):483–491. doi: 10.1016/0006-291x(77)90204-2. [DOI] [PubMed] [Google Scholar]
- Nash D., Wiskich J. T. Properties of substantially chlorophyll-free pea leaf mitochondria prepared by sucrose density gradient separation. Plant Physiol. 1983 Mar;71(3):627–634. doi: 10.1104/pp.71.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson J. B., Jr, Srere P. A. Organization of Krebs tricarboxylic acid cycle enzymes in mitochondria. J Biol Chem. 1985 Sep 5;260(19):10800–10805. [PubMed] [Google Scholar]
- Sumegi B., Srere P. A. Complex I binds several mitochondrial NAD-coupled dehydrogenases. J Biol Chem. 1984 Dec 25;259(24):15040–15045. [PubMed] [Google Scholar]
- Tobin A., Djerdjour B., Journet E., Neuburger M., Douce R. Effect of NAD on Malate Oxidation in Intact Plant Mitochondria. Plant Physiol. 1980 Aug;66(2):225–229. doi: 10.1104/pp.66.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. H., Oliver D. J., Sarojini G. Simultaneous oxidation of glycine and malate by pea leaf mitochondria. Plant Physiol. 1982 Nov;70(5):1465–1469. doi: 10.1104/pp.70.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]


