Abstract
Urban vegetation is associated with numerous public health benefits; however, urban tree canopies may be threatened by fugitive methane exposure from leaky natural gas distribution systems. Despite anecdotal evidence of the harmful impacts of natural gas leaks on urban tree decline, the relationship between soil gas exposure and tree health has not been formally quantified in an urban setting. We conducted a case-control study to compare soil natural gas exposure in sidewalk tree pits of healthy and dead or dying trees in Chelsea, Massachusetts, during summer 2019. We measured soil concentrations of methane and oxygen at four points around the trunks of 84 case and 97 control trees. We determined that case trees had 30 times the odds of being exposed to detectable levels of soil methane relative to the control trees sampled (95% CI=3.93, 229). Among tree pits with elevated soil gas, we also found that methane concentrations were highest on the side of the tree pit closest to the street. These results contribute evidence to support the widespread belief that soil methane exposure can negatively impact urban tree health. They also suggest that fugitive methane leakage from urban natural gas distribution systems beneath the street surface may be responsible for elevated soil gas concentrations in sidewalk tree pits and subsequent tree death.
Keywords: Methane, gas leaks, urban tree canopy, tree pit soil
Summary capsule:
Urban street trees exposed to elevated soil methane as a result of leaky natural distribution infrastructure have increased odds of death.
Graphical Abstract
Introduction
Urban vegetation, in particular tree canopy, is known to have multiple public health benefits, including improving air quality and associated respiratory outcomes among residents, moderating ambient temperature through shading and mitigating urban heat island effects, and improving human physical and mental health by promoting physical activity, reducing stress, and facilitating positive social interactions (Ulmer et al. 2016, Nowak et al. 2006, Donovan and Butry 2009, Tyrvanien et al 2005, Kweon et al. 1998). Higher percentages of urban tree canopy cover are also associated with increased perceptions of personal safety (Mouratidis 2019). Despite broad and general acceptance of these benefits and major investments in urban tree planting over the last several decades, urban tree canopy in the United States is declining (Nowak and Greenfield 2012, Roman 2014). Among the many causes of urban tree death (e.g., drought, excess moisture, construction/development), asphyxia from natural gas has received attention from the tree industry, extension agents, and activists (Fraedrich nd, Extension 2019, Taliesen 2019). To date, there have been only individual case studies and anecdotal evidence of tree death near natural gas leaks, particularly in urban environments. In an effort to contribute to understanding of tree health in proximity to gas leaks, we conducted a formal assessment of tree death and fugitive natural gas exposure in a densely populated urban community in Massachusetts, USA.
Despite its reputation as a fuel source that emits relatively low levels of carbon dioxide, natural gas is primarily made up of methane (CH4) which is also a potent greenhouse gas (Brandt et al. 2014, Howarth et al. 2011). Natural gas leaks, which can cause explosive hazards, occur throughout the gas extraction and distribution processes, posing as the largest source of anthropogenic methane emissions in the United States (Jackson et al. 2014).
Fugitive methane emissions, caused by leaks in the distribution infrastructure, are of particular concern in Massachusetts which has one of the oldest natural gas pipeline systems in the United States. Over eleven percent of the pipeline distribution network in Massachusetts is made up of leak-prone materials such as cast iron, wrought iron, and bare steel relative to an average of only 0.9% for the rest of the country where older pipes have been replaced with improved manufactured or lined steel pipes (PHMSA 2018, Gallagher et al. 2015). The material of these aged pipes subject them to corrosion and pose a high risk of rupture and leaks (Gallagher et al. 2015). Previous studies have documented the abundance of natural gas leaks in east coast cities, including Boston and the District of Columbia, where the average density of leaks was found to be 4.2 and 3.9 leaks per mile of road, respectively (Phillips et al. 2013, Jackson et al. 2014). In Boston, researchers found 3356 leaks across the 785 miles of road surveyed, with ambient methane concentrations exceeding up to 15 times background concentrations (Phillips et al. 2013). Ambient methane concentrations were even higher in the District of Columbia, where the maximum concentration measured reached up to 45 times background levels (Jackson et al. 2014). The density and magnitude of natural gas leaks found in these two major east coast cities highlight the severity of this leaky infrastructure in densely populated urban areas.
Beyond explosive risks and global warming impacts associated with fugitive methane emissions, leaky natural gas infrastructure also decreases the efficiency of gas distribution, which can increase utility costs for consumers (Phillips et al. 2013). Methane emissions are also known to negatively impact soil and vegetation health (Davis 1977, Flower 1981). Although methane is not directly toxic to plant matter, methane-rich soil can induce anaerobic soil conditions that are harmful for tree root systems (Adamse et al. 1972, Smith et al. 2005, Steven et al. 2006, Costello et al. 1991, Kozlowski 1985). Previous studies, which experimentally injected natural gas into soil in controlled settings and monitored changes in soil chemistry found an inverse relationship between methane and soil oxygen, which is characteristic of direct oxygen displacement; however, these studies also observed declines in soil oxygen concentrations to levels greater than the amount of methane introduced, which the authors concluded was indicative of further oxygen consumption by methanotrophs (methane-oxidizing bacteria) (Adamse et al. 1972, Smith et al. 2005, Steven et al. 2006). Elevated methane-oxidizing methanotroph counts, along with decreased oxygen levels, have also been documented in studies of soil cover over methane-producing landfills (Whalen et al. 1990, Nozhevnikova et al. 2003). These anaerobic soil conditions can limit respiration and growth in tree roots, which may be particularly damaging during early development (Costello et al. 1991). Root stress resulting from anaerobic soil conditions can also decrease tree root resilience to other factors such as pests and fungi, which contribute to overall tree decline and death (Costello et al. 1991, Kozlowski 1985). These impacts may be of particular concern to urban vegetation which often lies in close proximity to subsurface natural gas distribution infrastructure.
This study quantifies the relationships between subsurface exposure to natural gas and street tree deaths in Chelsea, Massachusetts. Using a case-control study design, we compared measured soil methane concentrations in sidewalk tree pits of healthy and dying or dead trees throughout the city to determine the odds of soil gas exposure among dead or dying trees. Our hypothesis was that soil gas concentrations would be higher among the tree pits of dead or dying trees compared with soil gas concentrations in the tree pits of healthy trees. We also sought to determine if elevated soil gas concentrations in tree pits could be attributed to urban natural gas distribution systems.
Materials and Methods
Study Area
We conducted our study in the City of Chelsea, MA. Chelsea is one of the most densely populated and diverse cities in the nation, with more than 35 languages spoken among approximately ~40,000 residents, 78.9% of whom identify as ethnic/racial minorities (RWJF, U.S. Census Bureau). Twenty-four percent of residents live below the federal poverty level (compared to the state’s 10.5%) (Healthy Chelsea). Despite its characterization as an environmental justice community (Mass.gov), for over 15 years the City has been awarded Tree City USA status by the Arbor Day Foundation (Arbor Day Foundation). Tree City USA status is accomplished by meeting the four core standards of urban forestry: an active tree board, a tree care ordinance, an annual urban forest budget of at least $2 per capita, and observance of Arbor Day (Arbor Day Foundation). In 2016 the City contracted arborists to conduct a thorough inventory of Chelsea’s tree inventory using risk assessment methods defined by the International Society of Arboriculture, which considers multiple factors including trunk condition, root health, foliage condition, canopy, branch structure, and presence of pests (Davey Resource Group 2016, Smiley et al. 2011). Each tree is rated as Dead, Critical, Poor, Fair, Good, Very Good, or Excellent for each factor and general health is determined by the most commonly assigned rating across all factors (Smiley et al. 2001). This inventory resulted in records, maps, and unique identification numbers for approximately 4000 trees, including both street trees (i.e. trees planted in sidewalk tree pits that line the streets) and park trees. This inventory is publicly available via the OpenTreeMap app (OpenTreeMap). We divided the City into seven geographic regions that could each conveniently be printed on single-page, zoomed-in maps for the field team, and identified cases and controls from six of the seven regions. We focused primarily on residential and commercial land use zones in order to minimize the impacts of other potentially harmful ambient or soil exposures thought to be associated with industrial zones. The majority of Chelsea street trees are also planted in the residential and commercial zones. However, the maps we used were not perfectly aligned with zoning usages, nor did they include zoning uses when the field team ascertained cases and controls.
Case definition and ascertainment
We defined cases as dead trees (or very close to dead, if not technically absent of all life). Cases were selected by one of our team members upon visual inspection of every tree in each region during July 2019 and noting if the tree appeared to be dead. OpenTreeMap was used to identify the unique tree ID and examine its history (i.e., the 2016 inventory). Dead trees with any record of improper pruning, human-caused physical damage, interference with overhead utility lines, or any other condition that may indicate a non-gas related cause of death were removed from the sample. Cases were then confirmed separately by an arborist certified by the Massachusetts Arborist Association in 2011 (Massachusetts Certified Arborist ID Number: 2408).
Control definition and ascertainment
Control trees were selected from the 2016 tree inventory from the same six regions. Trees were deemed controls if their 2016 health assessment indicated that they were in ‘Good’ or ‘Very good’ condition, if tree failure potential was ‘Improbable’, and if there were no additional assessor notes indicating poor health or damage. Control trees were selected to accomplish a comparable distribution of tree diameter breast height (DBH) and genus diversity relative to the cases. We essentially sought to match controls to cases, but not on an individual basis. We also mapped all sample trees using ArcGIS Pro 2.1.0 to ensure cases and controls were sufficiently spatially distributed throughout the study area. A certified arborist confirmed the health status of all control trees identified from the 2016 tree inventory.
Exposure: Soil Methane and Oxygen Sampling
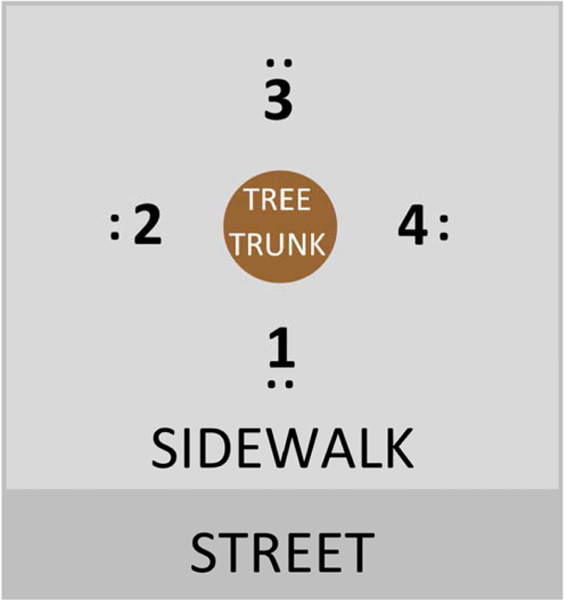
Methane and oxygen concentrations were measured separately from the ascertainment of cases and control by different members of our research team. We chose to measure subsurface methane concentrations as opposed to ambient methane because we had previously conducted an exploratory study in Chelsea, MA of ambient methane measurements collected using a mobile Picarro G2301 Cavity Ring-Down Spectrometer and did not find an association between ambient methane and tree health. We hypothesized soil gas may be a more reliable measure of a tree’s exposure to methane. Previous studies which examined at the impact of methane on vegetation health have also used soil gas concentrations as their exposure metric (Smith et al. 2005, Steven et al. 2006). At each tree pit, gas was measured approximately 15–25 centimeters below the soil surface at four points (sampling holes) around the tree trunk. Each sampling hole was 20–25 centimeters from the tree trunk (Jim 1998, Kargar et al. 2015). Figure 1 diagrams the sampling schema in each tree pit. We used a plunger bar to puncture holes into the root zone of the tree pit then immediately inserted a Bascom-Turner Gas Sentry multi-gas detector into the hole to obtain a methane gas measurement. The limit of detection of the multi-gas detector was 0.01%. Methane measurements were taken on all four sides of the tree using this method. Because elevated soil methane is known to be associated with anaerobic soil conditions, we created four new holes directly next to the original holes to collect measured oxygen concentrations from each side of the tree (Adamse et al. 1972, Smith et al. 2005, Steven et al. 2006). Leaks with subsurface methane concentrations greater than 4% and within 1.5 meters from a building foundation were immediately reported to the utility company. Such leaks are characterized as a “probable hazard to person or property… and require repair as immediately as possible and continuous action until the conditions are no longer hazardous” according to Massachusetts state law, which is informed by guidance from the Gas Piping Technology Committee (Mass. Gen. Laws ch. 164, § 144).
Figure 1.
Diagram of the protocol for sampling methane and oxygen concentrations in sidewalk tree pits. The black dots represent the two adjacent holes, one for the methane sample and one of oxygen sample, that were created at each of the four locations around the tree trunk.
Statistical analysis
In all analyses, oxygen and methane measurements below the limit of detection were converted to the limit of detection and divided by the square root of two. We averaged the four methane and oxygen measurements from each pit and used within-pit means to calculate the mean methane and oxygen concentrations in the case and control groups. We calculated the minimum methane and oxygen concentrations by taking the lowest concentration measured at any point within the tree pits (i.e. not based on the average of the four measurements across the pit). Similarly, we calculated the maximum methane and oxygen concentrations across the case and control groups by taking the highest concentrations measured at any of the four points in each pit. Concentrations are reported as percent gas content.
We calculated an odds ratio to predict odds of tree death based on exposure to soil methane in tree pits. We defined exposed trees as those in tree pits where at least one of the four methane measurements was greater than the limit of detection of the instrument. Unexposed trees were defined as those trees in pits where all four soil methane measurements were below the limit of detection. We generated histograms showing the distributions of methane concentrations in each hole position across the exposed trees. We conducted a one-way ANOVA with Tukey HSD post hoc comparisons to test for differences in mean methane concentrations between tree pits in different zoning regions. A two sample t-test was used to assess the difference in mean oxygen levels between case and control trees. Finally, we conducted a multiple linear regression adjusting for case status and zoning region to evaluate the relationship between soil methane and oxygen concentrations measured in each tree pit. All statistical analyses were carried out in R 3.6.1.
Results
Our final sample consisted of 84 dead or dying case trees and 97 healthy control trees, distributed across residential and commercial zones throughout the city (Figure 2). The case group consisted of 17 unique tree genera with Acer, Prunus, and Amelanchier (Maple, Cherry, and Serviceberry trees) being the most common genera represented. The control group was made up of 16 unique genera with Quercus (Oak), Acer (Maple), and Zelkova being the most common genera. The average trunk diameter was 16.0 cm in case trees was and 10.7 cm in control trees (Table 1).
Figure 2.
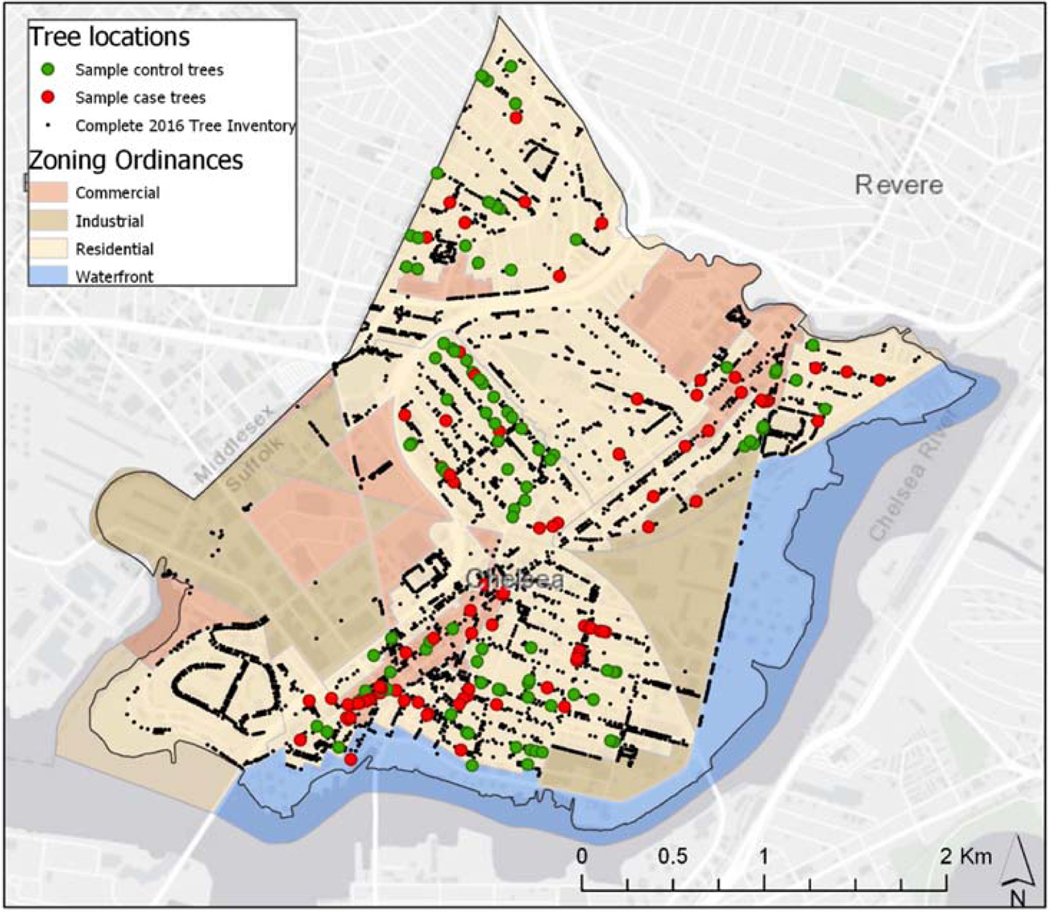
Map of spatial distribution of case and control trees overlaid on top of Chelsea zoning ordinances.
Table 1:
Characteristics of case and control trees and exposure means in each group.
| Case(n=84) | Controls (n=97) | |
|---|---|---|
| Unique Genus Count | 17 | 16 |
| Mean 2016 DBH (cm) (min, max) | 16.0 (5.0, 64.5) | 10.7 (5.0, 30.5) |
| Land Use Type | ||
| Residential | 31.2% | 49.1% |
| Commercial | 11.5% | 5.8% |
| Industrial | 0.6% | 0.6% |
| Waterfront | 0.6% | 0.6% |
| Mean CH4 soil content (%) (min, max) | 1.55% (0, 58) | 0.08% (0, 31) |
| Mean O2 soil content (%) (min, max) | 15.8% (0.5, 21.1) | 16.9% (4.3, 21.2) |
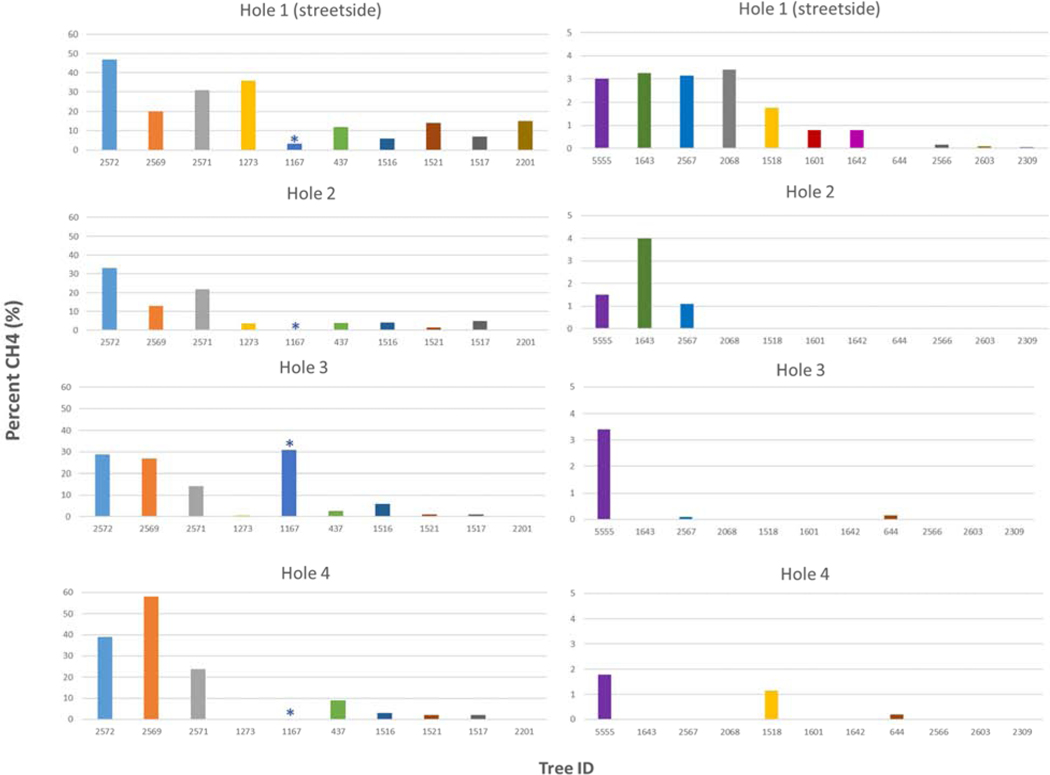
Of the 84 case trees, 20 were exposed to CH4 above the limit of detection of the multi-gas sensor. Of the 97 control trees sampled, only one was exposed to CH4 via tree pit soil. Based on these exposure frequencies, we determined that case trees had 30 times the odds of being exposed to CH4 relative to the control trees sampled, with a minimum odds of nearly four times and a max of over 200 times more likely (Table 2). In the tree pits where methane was detected, the average methane concentration was highest in the sample location closest to the street (Table 3). Figure 3 shows the distribution of methane concentrations across all four sampling holes in the tree pits of exposed trees. There was a significant difference in methane concentrations across the four zoning regions [F(180)= 4.171, p= 0.0427], with a Tukey HSD post hoc comparison indicating that tree pits in commercial zones (M=3.14, SD=9.24) had significantly higher soil methane levels than in residential zones (M= 0.244, SD=1.21), on average.
Table 2.
Odds of tree death among trees in pits with measured natural gas.
| Cases | Controls | Odds Ratio (p-value, 95% CI) | |
|---|---|---|---|
| Exposed | 20 | 91 | OR=30.0 (p=0.001, CI=[3.93, 229]) |
| Unexposed | 64 | 1 |
Table 3:
Descriptive statistics of soil CH4 content by sample hole in exposed trees.
| Hole 1 (street side) | Hole 2 | Hole 3 | Hole 4 | |
|---|---|---|---|---|
| Mean % CH4 (Min, Max) | 2.45% (0.00, 47.0) | 1.09% (0.00, 31.0) | 1.37% (0.00, 33.0) | 1.65% (0.00, 58.0) |
Figure 3.
Distribution of CH4 content by sampling hole in all exposed trees (n=21). The * indicates the exposed control tree.
Mean oxygen concentration measured in case tree pits was significantly lower than the mean oxygen concentration measured in control tree pits [t(141)= 2.10, p= 0.037]. Our multiple linear regression result, adjusting for case status and zoning region, indicated a significant negative association between soil methane concentrations and corresponding soil oxygen concentrations [β=−0.52, R2 =0.24, p>0.0001].
Discussion
In this study, we found that trees exposed to detectable levels of soil methane had higher odds of being dead or dying relative to unexposed trees. We also found the greatest soil methane concentrations on the side of the tree pit closest to the street, nearest to where natural gas distribution pipelines are located. These results suggest that elevated soil methane may contribute to urban street tree decline and that the fugitive methane may be the result of leaking pipeline infrastructure beneath the street surface. We found that case tree pits had significantly lower mean oxygen concentrations than controls as well as a significant negative association between soil methane and soil oxygen levels. Despite the fact that these results are based on a limited sample size, they may point to a potential mechanism for vegetation decline that has been cited in previous studies related to direct oxygen displacement by methane and the proliferation of methane-oxidizing methanotrophs that thrive in methane-rich soil, both which contribute to anaerobic soil conditions (Adamse et al. 1972, Smith et al. 2005, Steven et al. 2006, Whalen et al. 1990, Nozhevnikova et al. 2003).
While measuring soil oxygen and methane concentrations we called the utility company to report 3/21 tree pits that we identified as most hazardous based on the Massachusetts Department of Public Utilities-mandated uniform leak classification system. Leaks are graded on a scale from 1 to 3 based on explosive potential, where a Grade 1 leak is considered a hazard to humans or property and requires immediate repair and surveillance (PHMSA 2015). Grade 2 and 3 leaks are considered non-hazardous at the time of evaluation and are flagged for reevaluation after 6 and 12 months, respectively (PHMSA 2015). These grades do not represent leak severity in terms of any other factors besides explosive potential; thus, they do not account for severity of hazards to climate and vegetation (Hendrick et al. 2016). Under this classification system, 85% of the leaks we detected would not be classified as Grade 1, despite associated tree decline. The current reporting and repair processes may not be sufficiently protective of urban vegetation.
Beyond the loss of the human health benefits of urban vegetation cited above, this street tree damage has financial implications for municipalities. The City of Chelsea spends approximately $50,000/year on tree removals throughout the city and approximately $500 to replant a single tree (Maltez, Personal Communication, 2019). While not all trees removed each year are due to methane exposure-related decline, our results indicate that fugitive natural gas could be an important contributor to urban tree death and that these deaths could have notable ecological, human health, and economic impacts.
One limitation of this study was that we were unable to look retrospectively at leaks and repairs due to imprecise geolocated leak and repair locations by utility companies (i.e. companies often report the street address nearest to the leak instead of the exact coordinates of the leak beneath the street surface). This lack of knowledge of previous leaks and repairs may have contributed to exposure misclassification if we were unaware of a recently repaired leak near a case tree and did not detect soil methane in the tree pit as a result. Additional limitations stem from our small sample size and limited information on individual tree histories; however, we attempted to address this by creating case and control groups with similar distributions of trunk diameter, number of unique tree genera, frequencies of those genera, and land-use categories. We also tried to control for other factors which are often attributed to urban tree decline, such as age, vandalism, over or under pruning, and poor species selection by excluding trees in the inventory with record of these stressors or stressors that were obvious upon visual inspection; however, it is still possible that some of these factors were not recorded in the inventory and that other factors, such as lack of water, pests, or too deep or too shallow planting, could have contributed to tree decline in the case group. Despite these limitations, our study demonstrated a significant association between soil methane exposure in tree pits and street tree decline.
Conclusion
Our study quantifies the association between subsurface methane exposure and likelihood of street tree death. We found that exposure to elevated soil methane concentrations was associated with significant increased odds of tree death, supporting the hypothesis that fugitive emissions from natural gas distribution infrastructure negatively impact urban vegetation health. Additional research on urban tree pit soil conditions, including studies of methanotroph populations in these environments, could contribute to a better understanding of the mechanisms leading to tree decline in close proximity to leaky natural gas infrastructure.
Highlights.
First study to quantify the effects of natural gas leaks on urban tree health
Urban street trees exposed to soil methane have increased odds of death
Leaky natural gas pipes may be responsible for elevated methane in tree pits
Acknowledgements
The authors would like to acknowledge GreenRoots, Inc in Chelsea for use of their meeting space and staff support, especially the Environmental Chelsea Organizers (the ECO Crew), for their assistance with data collection efforts. We also want to thank Nathan Phillips, PhD from Boston University and Fidel Maltez at Chelsea Department of Public Works for sharing data.
Funding
This work is part of the Center for Research on Environmental and Social Stressors in Housing across the Life Course (CRESSH), funded by the National Institute on Minority Health and Health Disparities (Award No. P50MD010428), the U.S. Environmental Protection Agency (Award No. RD-836156). MKS, RA and AD were supported by a JPB Environmental Health Fellowship to MKS by the JPB Foundation and managed by the Harvard T.H. Chan School of Public Health. The content of this manuscript is solely the responsibility of the grantee and does not represent official views of any funding entity.
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamse AD, Hoeks J, Bont J. A. M. de, & Kessel J. F. van. (1972). Microbial activities in soil near natural gas leaks. Archiv Für Mikrobiologie, 83(1), 32–51. 10.1007/BF00425043 [DOI] [PubMed] [Google Scholar]
- Arbor Day Foundation. Tree City USA. Accessed: November 17, 2019. https://www.arborday.org/programs/treecityusa/ [Google Scholar]
- Brandt AR, Heath GA, Kort EA, O’Sullivan F, Petron G, Jordaan SM,Harriss R. (2014). Methane Leaks from North American Natural Gas Systems. Science, 343(6172), 733–735. 10.1126/science.1247045 [DOI] [PubMed] [Google Scholar]
- Costello LR. MacDonald JD Jacobs KA (1991). Soil Aeration and Tree Health: Correlating Soil Oxygen Measurements With the Decline of Established Oaks. In: Standiford, Richard B., tech. coord. Proceedings of the symposium on oak woodlands and hardwood rangeland management; October 31 - November 2, 1990; Davis, California. Gen. Tech. Rep. PSW-GTR-126. Berkeley, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture; p. 295–299 [Google Scholar]
- Davey Resource Group. (2016). Tree Management Plan: City of Chelsea, MA. The Davey Tree Expert Company. [Google Scholar]
- Davis S. (1977). The Effect of Natural Gas on Trees and Other Vegetation. Journal of Aboriculture. 3(8): 153–154. [Google Scholar]
- Donovan G. Butry D. (2009). The value of shade: Estimating the effect of urban trees on summertime electricity use. Energy and Buildings. 41: 662–668. 10.1016/j.enbuild.2009.01.002 [DOI] [Google Scholar]
- Extension (2019). Natural Gas Injury. University of Maryland Extension: Home & Garden Information Center. Accessed: November 17, 2019. https://extension.umd.edu/hgic/topics/natural-gas-injury [Google Scholar]
- Flower F. Gilman E. Leone I. 1981. Landfill gas, what it does to trees and how its injurious effects may be prevented. Journal of Aboricultue. 7(2): 43–52. [Google Scholar]
- Fraedrich BR. Nd. Gas injury to trees and shrubs. Research Laboratory Technical Report. Bartlett Tree Experts. https://www.bartlett.com/resources/gas-injury-to-trees.pdf [Google Scholar]
- Gallagher ME, Down A, Ackley RC, Zhao K, Phillips N, & Jackson RB (2015). Natural Gas Pipeline Replacement Programs Reduce Methane Leaks and Improve Consumer Safety. Environmental Science & Technology Letters, 2(10), 286–291. 10.1021/acs.estlett.5b00213 [DOI] [Google Scholar]
- Healthy Chelsea. (2019). Who we are. Accessed: November 17, 2019. http://healthychelsea.org/who-we-are/ [Google Scholar]
- Hendrick M. Ackley R. Sanaie-Movahed B. Tang X. Phillips N. (2016). Fugitive methane emissions from leak-prone natural gas distribution infrastructure in urban environments. Environmental Pollution. 213: 710–716. 10.1016/j.envpol.2016.01.094 [DOI] [PubMed] [Google Scholar]
- Howarth RW, Santoro R, & Ingraffea A. (2011). Methane and the greenhouse-gas footprint of natural gas from shale formations: A letter. Climatic Change, 106(4), 679–690. 10.1007/s10584-011-0061-5 [DOI] [Google Scholar]
- Jackson RB, Down A, Phillips NG, Ackley RC, Cook CW, Plata DL, & Zhao K. (2014). Natural Gas Pipeline Leaks Across Washington, DC. Environmental Science & Technology, 48(3), 2051–2058. 10.1021/es404474x [DOI] [PubMed] [Google Scholar]
- Jim CY (1998). Physical and chemical properties of a Hong Kong roadside soil in relation to urban tree growth. Urban Ecosystems, 2(2), 171–181. 10.1023/A:1009585700191 [DOI] [Google Scholar]
- Kargar M., Jutras P., Clark OG., Hendershot WH., & Prasher SO. (2015). Macro-nutrient availability in surface soil of urban tree pits influenced by land use, soil age, and soil organi matter content. Urban Ecosystems, 18(3), 921–936. 10.1007/s11252-015-0439-7 [DOI] [Google Scholar]
- Kozlowski TT (1985). Soil aeration, flooding and tree growth. Journal of Arboriculture 11:85–96. [Google Scholar]
- Kweon B. Sullivan W. Wiley A. (1998). Green Common Spaces and the Social Integration of Inner-City Older Adults. Environment and Behavior. 30(6): 832–858. 10.1177/001391659803000605 [DOI] [Google Scholar]
- Malzez F. (2019). Personal Communication. City of Chelsea Department of Public Works. [Google Scholar]
- Mass. Gen. Laws ch. 164, § 144. Uniform natural gas leaks classification system; grading of reported natural gas leaks; projects on public ways; school zones; gas company response and reporting.
- Mass.gov. (2010). Environmental Justice Populations. MA Executive Office of Energy And Environmental Affairs. https://www.mass.gov/environmental-justice [Google Scholar]
- Mouratidis K. (2019). The impact of urban tree cover on perceived safety. Urban Forestry: 44. 10.1016/j.ufug.2019.126434 [DOI] [Google Scholar]
- Nowak D. Crane D. Stevens J. (2006). Air pollution removal by urban trees and shrubs in the United States. Urban Forestry & Urban Greening. 3(3):115–123. 10.1016/j.ufug.2006.01.007 [DOI] [Google Scholar]
- Nowak DJ and Greenfield EJ. (2012). Tree and impervious cover change in U.S. cities. Urban Forestry &Urban Greening. 11:21–30. 10.1016/j.ufug.2011.11.005 [DOI] [Google Scholar]
- Nozhevnikova A. Glagolev M. Nekrasova V. Einola J. Sormunen K. Rintala J. (2003) The analysis of methods for measurement of methane oxidation in landfills. Water Science and Technology/ 48, 45–52. [PubMed] [Google Scholar]
- OpenTreeMap Cloud. (2019). Accessed: November 17, 2019. https://www.opentreemap.org/chelseama/map/ [Google Scholar]
- Phillips NG, Ackley R, Crosson ER, Down A, Hutyra LR, Brondfield M, Jackson RB (2013). 536 Mapping urban pipeline leaks: Methane leaks across Boston. Environmental Pollution, 173, 1–4. 10.1016/j.envpol.2012.11.003 [DOI] [PubMed] [Google Scholar]
- PHMSA. (2018). Pipeline Replacement Updates: Cast and Wrought Iron Inventories. U.S. Department of 540 Transportation Pipeline and Hazardous Materials Safety Administration. Accessed: November 17, 2019. https://www.phmsa.dot.gov/data-and-statistics/pipeline-replacement/cast-and-wrought-iron-inventory [Google Scholar]
- Robert Wood Johnson Foundation (RWJF). (2017). Chelsea, Massachusetts. Accessed: November 17, 2019. https://www.rwjf.org/en/library/features/culture-of-health-prize/2017-winner-chelsea-mass.html [Google Scholar]
- Roman LA. (2014). How many trees are enough? Tree death and the urban canopy. Scenario 04: Building the Urban Forest, Scenario Journal, Inc. https://www.fs.fed.us/nrs/pubs/jrnl/2014/nrs_2014_roman_001.pdf [Google Scholar]
- Smiley ET, Matheny N, and Lilly S. (2011). Best Management Practices: Tree Risk Assessment. Champaign: International Society of Arboriculture. [Google Scholar]
- Smith K. Colls J. Steven M. (2005). A Facility to Investigate Effects on Elevated Soil and Gas Concentration on Vegetation. Water, Air, and Soil Pollution. 161:75–96. 10.1007/s11270-005-2833-x [DOI] [Google Scholar]
- Steven MD, Smith KL, Beardsley MD, & Colls JJ (2006). Oxygen and methane depletion in soil 558 affected by leakage of natural gas. European Journal of Soil Science, 57(6), 800–807. 10.1111/j.1365-2389.2005.00770.x [DOI] [Google Scholar]
- Taliesen J. (2019). Somerville mothers demand National Grid fix gas leaks. Wicked Local: Somerville. Accessed: November 17, 2019. https://somerville.wickedlocal.com/news/20190724/somerville-mothers-demand-national-grid-fix-gas-leaks [Google Scholar]
- Tyrväinen L. Pauleit S. Seeland K. De Vries S. (2005). Benefits and uses of urban Forests and Trees. In Konijnendijk et al. (Eds.) Urban Forests and Trees. (pp. 81–114) Springer. [Google Scholar]
- Ulmer JM, Wolf KL, Backan DR, Tretheway RL, Blain CJA, O’Neil-Dunne JPM, Frank LD. (2016). Multiple health benefits of urban tree canopy: The mounting evidence for a green prescription. Health & Place. 42:54–62. 10.1016/j.healthplace.2016.08.011 [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2018). QuickFacts: Chelsea city, Massachusetts. Accessed: November 17, 2019. https://www.census.gov/quickfacts/chelseacitymassachusetts [Google Scholar]
- Whalen S. Reeburgh W. Sandbeck K. (1990). Rapid methane oxidation in a landfill cover soil. Appl. Environ. Microbiol 56: 3405–3411. [DOI] [PMC free article] [PubMed] [Google Scholar]