Abstract
Background
Primary studies have demonstrated the effectiveness of noninvasive respiratory supports, including noninvasive positive pressure ventilation (NIPPV) and high flow nasal cannula (HFNC), for improving oxygenation and ventilation in patients with interstitial lung diseases (ILDs) and acute respiratory failure (ARF). These studies have not been synthesized and are not included in current practice guidelines. This systematic review with meta-analysis synthesizes studies that compared the effectiveness of NIPPV, HFNC and conventional oxygen therapy (COT) for improving oxygenation and ventilation in ILD patients with ARF.
Methods
MEDLINE, EMBASE and the Cochrane Library searches were conducted from inception to August 2023. An additional search of relevant primary literature and review articles was also performed. A random effects model was used to estimate the PF ratio (ratio of arterial oxygen partial pressure to fractional inspired oxygen), PaCO2 (partial pressure of carbon dioxide), mortality, intubation rate and hospital length of stay.
Results
Ten studies were included in the systematic review and meta-analysis. Noninvasive respiratory supports demonstrated a significant improvement in PF ratio compared to conventional oxygen therapy (COT); the mean difference was 55.92 (95% CI [18.85-92.99]; p=0.003). Compared to HFNC, there was a significant increase in PF ratio in NIPPV (mean difference 0.45; 95% CI [0.12–0.79]; p=0.008). There were no mortality and intubation rate benefits when comparing NIPPV and HFNC; the mean difference was 1.1; 95% CI [0.83-1.44]; p=0.51 and 1.86; 95% CI [0.42-8.33]; p=0.42, respectively. In addition, there was a significant decrease in hospital length of stay in HFNC compared to NIPPV (mean difference 9.27; 95% Cl [1.45 – 17.1]; p=0.02).
Conclusions
Noninvasive respiratory supports might be an alternative modality in ILDs with ARF. NIPPV demonstrated a potential to improve the PF ratio compared to HFNC. There was no evidence to support the benefit of NIPPV or HFNC in terms of mortality and intubation rate.
Keywords: interstitial lung disease, noninvasive positive pressure ventilation, noninvasive ventilation, high-flow nasal cannula, acute respiratory failure, acute exacerbation, noninvasive respiratory support
Introduction
Interstitial lung diseases (ILDs) are groups of diffuse parenchymal lung conditions with overlapping clinical presentation and radiological features. The 2013 Global Burden of Disease Study reported that ILDs were ranked 40th in relation to global years of mortality.1 The global incidence of ILDs ranged from 1 to 31.5 per 100,000 patients per year, and the prevalence ranged from 6.3 to 7.1 per 100,000.2 The diagnosis and treatment of ILDs require a multidisciplinary approach. Professions involved in the management of ILDs include but are not limited to, respirologists, radiologists and pathologists.3 The trajectory of ILDs is unpredictable; thus, prognostication may be challenging. Interstitial lung disease classification and terminology have been modified and updated over the past decade.4 The American Thoracic Society (ATS)/European Respiratory Society (ERS)/Japanese Respiratory Society (JRS)/Latin American Thoracic Association have collaborated to endorse practice guidelines for ILD management.3–6
Hypoxemia in ILDs consists of multiple physiologic derangements, including diffusion impairment, ventilation–perfusion mismatch, and abnormalities of the pulmonary vasculature leading to pulmonary hypertension.7,8 Chronic repetitive inflammatory processes and aberrant wound healing can lead to progressive destruction in alveolar units (i.e., fibrosis) and consequently limitations to oxygen diffusion from alveoli to capillaries.9,10 Consequently, oxygen supplementation is a main treatment approach in both acute and chronic respiratory failure patients. However, achieving targeted oxygen levels can be challenging. Acute exacerbations of ILDs have been reported to be the most common causes of respiratory deterioration and are associated with poor outcomes. Specifically, acute exacerbation of ILDs accounts for 29-55% of respiratory hospitalizations and 20-33% of lower respiratory tract infections.11,12 ILD patients who require mechanical ventilation generally have poor outcomes due to the irreversible nature of their disease.13–17 Although extracorporeal membrane oxygenation (ECMO) is stated as a rescue modality in ILDs with refractory hypoxemia, it is unable to alter the mortality, particularly when patients do not qualify for lung transplantation.18
Current practice guidelines reflect certain favourable outcomes associated with noninvasive positive pressure ventilation (NIPPV) or high flow nasal cannula (HFNC) in selected acute respiratory failure (ARF) patients.19–21 Although ILDs were not included in those recommendations, primary studies suggest that NIPPV or HFNC might be potential approaches in this subgroup.22–24 The common ventilatory settings of NIPPV used in acute and/or chronic respiratory failure are known as Continuous Positive Airway Pressure (CPAP) and Bilevel Positive Airway Pressure (BiPAP).21 This review synthesizes current studies that compared the effectiveness of noninvasive respiratory supports (NIPPV and/or HFNC) and conventional oxygen therapy (COT) for improving oxygenation and ventilation in ILD patients with ARF.
Method
Eligibility criteria
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines.25 We applied the following inclusion criteria: experimental studies (randomized, quasi-randomized, prospective and retrospective trials) that examined the benefit of NIPPV or HFNC on patients with ILDs and ARF and/or distress; adults (aged ≥ 18 years. The exclusion criteria were case reports and case series. The outcome measures included improvements in oxygenation and ventilation at 24 h or as defined by the study, using PaO2/FiO2 (PF ratio) and PaCO2, respectively; mortality; intubation rate; hospital length of stay and complications.
Information sources and search strategy
Systematic literature searches were conducted for studies published from inception to August 6, 2023, in MEDLINE, EMBASE and the Cochrane Library. Search terms included the medical subject headings (MeSH) “noninvasive positive pressure ventilation” OR “noninvasive ventilation” OR “high flow nasal cannula” AND “interstitial lung disease” (Supplemental Table 1). There were no language restrictions.
Study selection and data collection
Two authors (VP and NO) independently performed article selection by title and abstract screening based on predetermined eligibility criteria. The references of the included studies were manually reviewed for additional eligible studies. Disagreements relating to any aspect of the data extraction process were discussed and resolved by a third reviewer (JN), with the final decision made by consensus. The full-text articles of the selected studies were reviewed independently for the final study selection. The data were extracted and analyzed from the included studies (NS, VP, and NJ).
Quality Assessment
Two investigators (VP and NO) assessed the quality of included studies using the Cochrane risk-of-bias tool for randomized trials (RoB 2) and the Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) for non-randomized studies tool25,26 (Table 1, Supplemental Tables 2 and 3).
Table 1. Characteristics of included studies.
| Study and Country | Age (years; mean ± SD) and Sex (%) | Study Design | Number of patients (N) | Patient Characteristics | Duration of NIPPV/HFNC (days) | Device setting (mmHg) /Initial PF ratio |
Location of interventions | Overall risk of bias (ROBINS-I or RoB 2) |
| Yokoyama T et al. 2010, Japan | 72.3 ± 7.7 Male 63.6% |
Retrospective cohort study | 11 - NIPPV | IPF with ARF | 5.4 ± 3.8 | CPAP - PEEP 10.1 ± 2.5 BiPAP - IPAP 15 ±3.3, EPAP 10.2 ± 2.9 /138.9 ± 55.7 |
University hospital, N/A | Serious |
| Gungor G et al. 2013, Turkey | 66 ± 3.2 Male 59.2% |
Retrospective cohort study | 75 - NIPPV | ILDs with ARF 56 - IPF (no biopsy proven) 7 - CVD 8 - Silicosis 2 - Drug induced ILD 2 – EP |
5 ± 3.7 | BiPAP - Target PS to keep TV 6-8 ml/Kg, PEEP 5-7 /143 ± 60.7 |
Pulmonary disease and Thoracic surgery hospital, ICU | Moderate |
| Aliberti S et al. 2014, Italy | 71 ± 3 Male 63.3% |
Retrospective cohort study | 60 - NIPPV | ILDs with ARF 28 - IPF 8 - CTD 3 - COP 1 – HP 16 - idiopathic NSIP 4 - others |
N/A | CPAP - PEEP 8 ± 1.48 BiPAP - PS 15 ±7.4, PEEP 5 ± 2.2 /125 ± 57.8 |
General hospital, RICU | Moderate |
| Shebl E et al. 2018, Egypt | 61.1 ± 12.3 Male 35.7% |
Prospective randomized control trial | 36 - NIPPV 34 - HFNC |
ILDs with ARF 19 - IPF 9 - HP 9 - CTD 2 - Drug induced ILD 4 - LCH 3 - Pneumoconiosis 7 - Sarcoidosis 17 - non-IPF IIP |
N/A | NIPPV - CPAP with PEEP up to 12 HFNC - 60 L/min /172 ± 48.5 |
General hospital, ICU | Some concerns |
| Koyauchi T et al. 2018, Japan | 78.4 ± 2.8 Male 72.6% |
Retrospective cohort study | 30 - NIPPV 54 - HFNC |
ILDs with ARF in DNI patients 44 - IPF 27 - Non-IPF IIP 10 - CTD 2 - HP 1 - Sarcoidosis |
6 ± 8.9 | NIPPV - N/A HFNC - 40 ± 14.8 L/min /N/A |
General hospital, N/A | Serious |
| Vianello A et al. 2019, Italy | 68.6 ± 9.5 Male 82.4% |
Retrospective cohort study | 17 - HFNC | IPF with ARF | N/A | NIPPV - BiPAP target TV 6-8 ml/Kg, PEEP 5 HFNC - up to 70 L/min /145 ± 180 |
University Hospital, RICU | Moderate |
| Imai R et al. 2019, Japan | 78.2 ± 4.1 Male 48.6% |
Retrospective cohort study | 14 - NIPPV 21 - HFNC |
ILDs with ARF in DNI patients 13 - IPF 11 - CTD 11 - others |
8 ± 17.8 | NIPPV - BiPAP PS as tolerate, PEEP 5 HFNC - up to 60 L/min /N/A |
General hospital, N/A | Moderate |
| Omote N et al. 2020, Japan | 70.9 ± 3.9 Male 81.3% |
Retrospective cohort study | 19 - NIPPV 13 - HFNC |
ILDs with ARF 20 - IPF 8 - CTD 4 - others |
N/A | NIPPV - BiPAP PS 2 ± 2.9, PEEP 6 ±2.9 HFNC - up to 50 L/min /138.5 ± 57 |
University hospital, ED and ICU | Serious |
| Koyauchi T et al. 2020, Japan | 78 ± 2.3 Male 77.3% |
Retrospective cohort study | 66 - HFNC | ILDs with ARF 31 - IPF 35 - non-IPF |
6.5 ± 7.4 | HFNC - 40 ± 3.7 L/min /199.18 ± 53.9 |
University Hospital, ICU | Serious |
| Ahmed N et al. 2023, Egypt | 45.67 ± 14.48 Male 30% |
Prospective cohort study | 30 - NIPPV | ILDs with ARF 16 - HP 14 - others (non-IPF) |
2.93 ± 1.26 | BiPAP - PS 4, PEEP 4 /160.67 ± 41.26 |
University Hospital, RICU | Moderate |
Abbreviation: PF ratio – the ratio of arterial oxygen partial pressure to fractional inspired oxygen, NIPPV – noninvasive positive pressure ventilation, HFNC – high flow nasal cannula, IPF – idiopathic pulmonary fibrosis, ARF – acute respiratory failure, CPAP – continuous positive airway pressure, BiPAP – bi-level positive airway pressure, PEEP – positive end-expiratory pressure, PS – pressure support, IPAP – inspiratory positive airway pressure, EPAP – expiratory positive airway pressure, ILDs – interstitial lung diseases, CVD – collagen vascular disease, EP – eosinophilic pneumonia, CTD – connective tissue disease, COP – cryptogenic organizing pneumonia, HP – hypersensitivity pneumonitis, NSIP – non-specific interstitial pneumonia, LCH – Langerhans cell histiocytosis, IIP – idiopathic interstitial pneumonia, DNI – do not intubation, ED – emergency department, ICU – intensive care unit, RICU – respiratory intensive care unit.
Statistical Analysis
All statistical analyses were performed using Review Manager (RevMan) version 5.4.1 (The Cochrane Collaboration, 2020). We extracted the proportions and 95% confidence intervals (CIs) from each study and pooled them using the random effect model. Cochrane’s Q test was performed and quantified using the I2 statistic to determine the statistical heterogeneity among the included studies. An I2 value of 0% to 25% represents insignificant heterogeneity, greater than 25% but less than or equal to 50% represents low heterogeneity, greater than 50% but less than or equal to 75% represents moderate heterogeneity, and greater than 75% represents high heterogeneity. P-values less than 0.05 were considered statistically significant. A funnel plot visualized the presence of a publication bias (Supplemental Figure 1). The protocol for this study was registered at www.inplasy.com (No. 202260104). Ethical approval was not required.
Results
Search Results
Systematic literature searches identified 1,000 unique citations. A review of titles and abstracts resulted in the elimination of 981 studies. The full text of these 19 studies was reviewed to determine eligibility (Figure 1). This systematic review included ten studies (including a total of 480 patients). One randomized control trial, one prospective cohort study and eight retrospective cohort studies were included.
Figure 1. Flow diagram of the article selection procedure based on the PRISMA guideline.
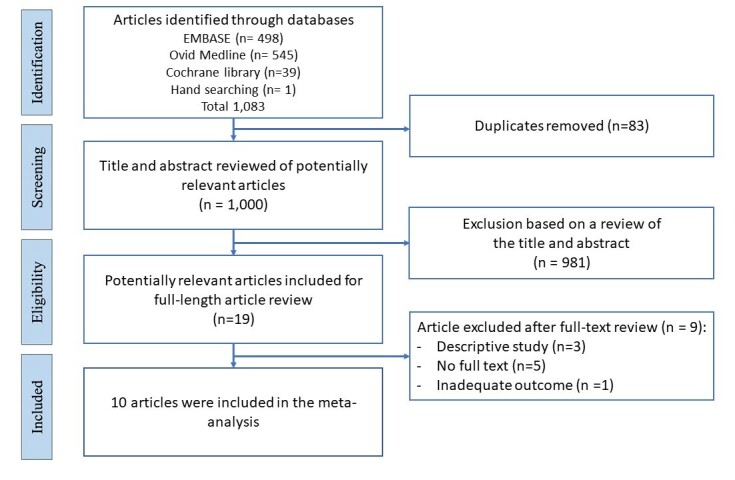
Study characteristics are described in Table 1. The included studies consisted of NIPPV application (four studies),27–30 HFNC application (two studies)31,32 and NIPPV and HFNC application (four studies).33–36 Idiopathic pulmonary fibrosis (IPF) was a major type of ILDs in this study (239 patients, 49.7% of total ILDs). Three of ten studies included cardiac failure in the definition of acute exacerbation.28,34,36 The ICU was the main location of the interventions (seven of ten studies). The mean duration of noninvasive respiratory supports was 5.76 ± 8.55 days. The mean initial PF ratio before noninvasive respiratory supports was 156.94 ± 64.74.
Publication bias assessment
The funnel plot of the PF ratio outcome of the conventional oxygen therapy and noninvasive respiratory supports was symmetric and showed no publication bias (Supplemental Figure 1).
Effect of intervention
Primary outcome
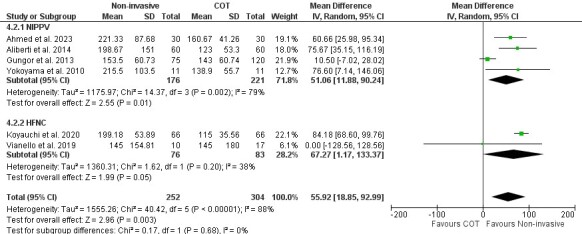
The PF ratio (a clinical indicator of hypoxemia) is the primary outcome measure of the effect of noninvasive respiratory supports (NIPPV or HFNC) compared to COT on ILD patients with ARF. Of ten studies, pooled analysis was performed on six (NIPPV-four studies, HFNC-two studies)27–32,37 using a random-effect model. This analysis showed that noninvasive respiratory support significantly improved the PF ratio compared to COT. The mean difference was 55.92 (95% CI [18.85–92.99]; I2=88%; p=0.003. In subgroup analysis, both NIPPV and HFNC demonstrated a significant improvement in oxygenation compared to COT (mean difference 51.06, 95%Cl [11.88-90.24]; I2=79%; p=0.01 and (mean difference 67.27, 95% CI [1.17–133.37]; I2=38%; p=0.05), respectively (Figure 2).
Figure 2. Effect of noninvasive respiratory supports on PF ratio.
Secondary outcomes
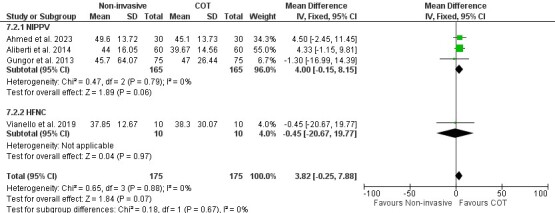
Secondary outcomes measures included i.) PaCO2 (a clinical indicator of alveolar ventilation) to examine the effect of noninvasive respiratory supports (NIPPV or HFNC) compared to COT on ILD patients with ARF, ii.) PF ratio to compare the effects of NIPPV with HFNC, iii.) mortality, iv.) intubation rate and v.) hospital length of stay. Four studies (three studies–NIPPV and one study–HFNC)27,28,30,32 reported the effects of noninvasive respiratory supports on PaCO2 outcomes. There was no significant difference in PaCO2 reduction between COT and noninvasive respiratory supports. The mean difference was 3.82 (95% CI [-0.25–7.88]; I2=0%; p=0.07). (Figure 3).
Figure 3. Effect of noninvasive respiratory supports on PaCO2.
Four studies compared NIPPV directly to HFNC in ILD patients with ARF. Three of four studies33–35 demonstrated a significant increase in PF ratio for NIPPV when compared to HFNC (mean difference 0.45; 95% CI [0.12–0.79]; I2=0%; p=0.008) (Figure 4). The comparison between NIPPV and HFNC revealed that neither method demonstrated a significant impact on mortality (four studies)33–36 (Figure 5) or intubation rates (two studies)35,36 (Figure 6). Risk Ratio (RR) was 1.1; 95% CI [0.83–1.44]; I2=67%; p=0.51 and 1.86; 95% CI [0.42–8.33]; I2=54%; p=0.42, respectively. Lastly, patient groups receiving HFNC experienced significantly shorter hospital lengths of stay when compared to those receiving NIPPV (two studies33,34; the mean difference was 9.27 (95% Cl [1.45–17.1]; I2=17; p=0.02) (Figure 7).
Figure 4. Effect of NIPPV or HFNC on PF ratio.
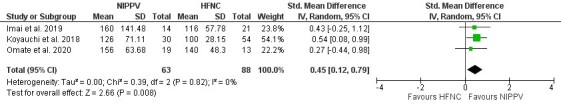
Figure 5. Effect of NIPPV or HFNC on mortality.
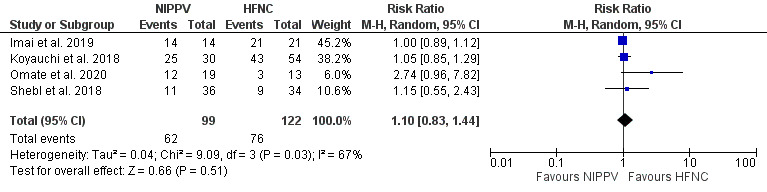
Figure 6. Effect of NIPPV or HFNC on intubation rate.
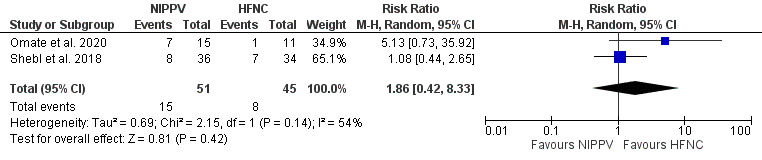
Figure 7. Effect of NIPPV or HFNC on hospital length of stay.
Other effects of NIPPV and HFNC
Two studies conducted on do-not-intubate patients (DNI) examined the effect of oral alimentation and the time of loss of cognitive function before death as outcomes33,34 The HFNC showed a significant oral intake ability before death compared to NIPPV in Imai et al. and Koyauchi et al.; p=0.002 and p=0.037, respectively. In addition, there was significantly less cognitive dysfunction in HFNC compared to NIPPV in Imai et al. and Koyauchi et al.; p=0.03 and p=0.037, respectively.
Koyauchi et al. reported eight adverse events, including seven in patients receiving NIPPV and one in a patient receiving HFNC.34 Specifically, seven of 30 patients (23.3%) receiving NIPPV reported injuries (5 reports of skin damage, one gingival ulcer and one pneumo-mediastinum), while one of 54 patients (1.85%) receiving HFNC reported nasal bleeding. In addition, patients’ requests for interface discontinuation were significantly higher for NIPPV (3 of 30; 10%) compared to HFNC (0 of 54), p=0.043.
In Yogoyama et al.,37 the time to initiate NIPPV determined survival outcomes significantly (p=0.006). The survivor group showed 2.3 ± 2.9 days to initiate NIPPV, whereas the non-survivor group showed 4.4 ± 3.1 days.
Gungor et al.28 demonstrated the APACHE II score greater than 20 and continuous NIPPV demand indicated a significant risk for NIPPV failure: hazard ratio (HR) 2.77 (95% CI 1.19–6.45); p<0.02, and HR 5.12 (95% Cl 1.44–18.19); p<0.01, respectively. On the contrary, the result from Vianello et al.32 conducted in HFNC demonstrated a significantly higher APACHE II score in a success group than a failure group (p=0.043).
Discussion
To our knowledge, this is the first systematic review with meta-analysis to compare the effectiveness of noninvasive respiratory supports (NIPPV and/or HFNC) and conventional oxygen therapy (COT) for improving oxygenation and ventilation in ILD patients with ARF.
Compared with COT, noninvasive respiratory supports significantly increased PF ratio. The subgroup analysis suggested a significant benefit of both NIPPV and HFNC on PF ratio. When comparing NIPPV and HFNC, PF ratio was significantly increased in NIPPV. There was no difference in mortality and intubation rate between the two groups. However, the hospital length of stay showed a significantly shorter duration with HFNC compared to NIPPV. The number of reported serious complications (i.e., pneumothorax, nasal bleeding) was low.
According to ERS recommendation, NIPPV is recommended for ARF with chronic obstructive pulmonary disease exacerbation and/or weaning, cardiogenic pulmonary edema and immunocompromised patients.21 In contrast, HFNC is strongly recommended in acute hypoxemic respiratory failure and conditionally recommended in any high-risk features following extubation or in high-risk and/or obese patients following cardiac or thoracic surgery.20 Notwithstanding, none of the studies explicitly evaluated patients with ILDs.
In our study, noninvasive respiratory supports significantly improved PF ratio compared to COT, and both NIPPV and HFNC subgroups demonstrated this improvement. An earlier systematic review determined that NIPPV improves ventilation-perfusion mismatch and decreases the work of respiratory muscles38 Furthermore, NIPPV has been described as decreasing venous return against pulmonary edema, improving oxygenation, particularly in concomitant cardiac dysfunction patients.39 Similarly, HFNC subgroup analysis demonstrated a significant improvement on PF ratio compared to COT. Prior studies have demonstrated that HFNC improves mucociliary clearance, reducing upper-airway dead space, generating a low level of positive airway pressure with consistent FiO2 regardless of inspiratory flow rate and ultimately providing comfort for the patient.40,41 In our study, PF ratio was significantly increased in NIPPV. This result may be potentially explained by lung recruitment after receiving adequate positive pressure.42,43
Noninvasive respiratory supports did not show a significant reduction in carbon dioxide (CO2) levels. This may reflect many variables (patient’s conditions, various settings of HFNC and NIPPV (CPAP and/or BiPAP), different types of interfaces) across studies. Notably, the risk of carbon dioxide rebreathing was higher on helmet interface.44,45 In a recent meta-analysis of trials of acute hypoxemic respiratory failure, treatment with noninvasive respiratory support strategies was associated with a lower risk of death and intubation46; however, our study demonstrated none of those benefits. Of note, acute pneumonia was a frequent cause of acute hypoxemic respiratory failure in the aforementioned meta-analysis, and the high reversible potential of this acute pneumonia may have contributed to the favourable outcomes described. In our study, HFNC showed a significant decrease in hospital length of stay compared to NIPPV. This finding was consistent with recent studies demonstrating decreased length of stay in patients with hypercapnic respiratory failure and hypoxemia related to COVID pneumonia when using HFNC.47,48 Recent studies have proposed that diaphragm atrophy, a result of positive pressure ventilation, may cause prolonged hospital length of stay in NIPPV patients; however, these positive pressure effects were only related to mechanical ventilation.49,50 Therefore, this issue may require further scientific study.
A strength of this meta-analysis is that it includes a large number of subjects with ILDs with ARF. Our meta-analysis was guided by a registered protocol and strengthened by an extensive search, duplicate citation screening, data abstraction and conducting of prespecified subgroup analyses. However, there are limitations. First, data was obtained primarily from retrospective studies (eight of ten). Thus, the overall level of evidence is low to moderate. Second, summary estimates were limited by heterogeneous types of ILDs, which may interfere with a treatment response and an overall prognosis.
Conclusion
In summary, NIPPV or HFNC might be an alternative modality in ILD patients with ARF, mainly to avoid the negative consequences of intubation. NIPPV showed a significant improvement in PF ratio compared to HFNC. However, there were no mortality and intubation rate benefits when comparing NIPPV and HFNC.
Contributors
NS, VP, and NO conducted the literature searches and resolved discrepancies between citation reviewers, selected studies meeting inclusion criteria, assessed study quality, conducted risk of bias assessment, double-checked data entry, and prepared initial and subsequent drafts of the manuscript and revised versions. NS, NJ and JN conducted literature searches, assisted with screening articles’ study quality, provided methodologic guidance, and integrated comments into the manuscript. All authors revised and approved the final version of the manuscript.
Prior conference presentation
This study was presented as a poster presentation at the 42nd International Symposium on Intensive Care & Emergency Medicine (ISICEM), March 21, 2023.
Competing interests
The authors declare no conflict of interest.
Ethics approval
Not required.
Data availability statement
Data are available on request.
Supplementary Material
Funding Statement
This study did not receive any funding in any form.
References
- Global, regional, and national age–sex specific all-cause and cause-specific mortality for 240 causes of death, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. GBD 2013 Mortality and Causes of Death Collaborators Jan;2015 The Lancet. 385(9963):117–171. doi: 10.1016/s0140-6736(14)61682-2. doi: 10.1016/s0140-6736(14)61682-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Variability in Global Prevalence of Interstitial Lung Disease. Kaul Bhavika, Cottin Vincent, Collard Harold R., Valenzuela Claudia. Nov 4;2021 Frontiers in Medicine. 8:751181. doi: 10.3389/fmed.2021.751181. doi: 10.3389/fmed.2021.751181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interstitial Lung Disease in 2020: A History of Progress. Guler Sabina A., Corte Tamera J. Jun;2021 Clinics in Chest Medicine. 42(2):229–239. doi: 10.1016/j.ccm.2021.03.001. doi: 10.1016/j.ccm.2021.03.001. [DOI] [PubMed] [Google Scholar]
- An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Travis William D., Costabel Ulrich, Hansell David M., King Talmadge E. Jr., Lynch David A., Nicholson Andrew G., Ryerson Christopher J., Ryu Jay H., Selman Moisés, Wells Athol U., Behr Jurgen, Bouros Demosthenes, Brown Kevin K., Colby Thomas V., Collard Harold R., Cordeiro Carlos Robalo, Cottin Vincent, Crestani Bruno, Drent Marjolein, Dudden Rosalind F., Egan Jim, Flaherty Kevin, Hogaboam Cory, Inoue Yoshikazu, Johkoh Takeshi, Kim Dong Soon, Kitaichi Masanori, Loyd James, Martinez Fernando J., Myers Jeffrey, Protzko Shandra, Raghu Ganesh, Richeldi Luca, Sverzellati Nicola, Swigris Jeffrey, Valeyre Dominique. Sep 15;2013 American Journal of Respiratory and Critical Care Medicine. 188(6):733–748. doi: 10.1164/rccm.201308-1483st. doi: 10.1164/rccm.201308-1483st. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Raghu Ganesh, Remy-Jardin Martine, Myers Jeffrey L., Richeldi Luca, Ryerson Christopher J., Lederer David J., Behr Juergen, Cottin Vincent, Danoff Sonye K., Morell Ferran, Flaherty Kevin R., Wells Athol, Martinez Fernando J., Azuma Arata, Bice Thomas J., Bouros Demosthenes, Brown Kevin K., Collard Harold R., Duggal Abhijit, Galvin Liam, Inoue Yoshikazu, Jenkins R. Gisli, Johkoh Takeshi, Kazerooni Ella A., Kitaichi Masanori, Knight Shandra L., Mansour George, Nicholson Andrew G., Pipavath Sudhakar N. J., Buendía-Roldán Ivette, Selman Moisés, Travis William D., Walsh Simon L. F., Wilson Kevin C. Sep 1;2018 American Journal of Respiratory and Critical Care Medicine. 198(5):e44–e68. doi: 10.1164/rccm.201807-1255st. doi: 10.1164/rccm.201807-1255st. [DOI] [PubMed] [Google Scholar]
- Diagnosis of Hypersensitivity Pneumonitis in Adults. An Official ATS/JRS/ALAT Clinical Practice Guideline [published correction appears in Am J Respir Crit Care Med. 2021 Jan 1;203(1):150-151] [published correction appears in Am J Respir Crit Care Med. 2022 Aug 15;206(4):518] Raghu Ganesh, Remy-Jardin Martine, Ryerson Christopher J., Myers Jeffrey L., Kreuter Michael, Vasakova Martina, Bargagli Elena, Chung Jonathan H., Collins Bridget F., Bendstrup Elisabeth, Chami Hassan A., Chua Abigail T., Corte Tamera J., Dalphin Jean-Charles, Danoff Sonye K., Diaz-Mendoza Javier, Duggal Abhijit, Egashira Ryoko, Ewing Thomas, Gulati Mridu, Inoue Yoshikazu, Jenkins Alex R., Johannson Kerri A., Johkoh Takeshi, Tamae-Kakazu Maximiliano, Kitaichi Masanori, Knight Shandra L., Koschel Dirk, Lederer David J., Mageto Yolanda, Maier Lisa A., Matiz Carlos, Morell Ferran, Nicholson Andrew G., Patolia Setu, Pereira Carlos A., Renzoni Elisabetta A., Salisbury Margaret L., Selman Moises, Walsh Simon L. F., Wuyts Wim A., Wilson Kevin C. Aug 1;2020 American Journal of Respiratory and Critical Care Medicine. 202(3):e36–e69. doi: 10.1164/rccm.202005-2032st. doi: 10.1164/rccm.202005-2032st. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechanisms of gas-exchange impairment in idiopathic pulmonary fibrosis. Agustí Alvar G. N., Roca Josep, Gea Joaquim, Wagner Peter D., Xaubet Antoni, Rodriguez-Roisin Robert. Feb;1991 American Review of Respiratory Disease. 143(2):219–225. doi: 10.1164/ajrccm/143.2.219. doi: 10.1164/ajrccm/143.2.219. [DOI] [PubMed] [Google Scholar]
- Pulmonary hypertension in interstitial lung disease. Strange Charlie, Highland Kristin B. Sep;2005 Current Opinion in Pulmonary Medicine. 11(5):452–455. doi: 10.1097/01.mcp.0000174250.38188.6d. doi: 10.1097/01.mcp.0000174250.38188.6d. [DOI] [PubMed] [Google Scholar]
- Pathogenesis of Interstitial Lung Disease in Children and Adults. Glasser Stephan W., Hardie William D., Hagood James S. Mar 5;2010 Pediatric Allergy, Immunology, and Pulmonology. 23(1):9–14. doi: 10.1089/ped.2010.0004. doi: 10.1089/ped.2010.0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physiology of the lung in idiopathic pulmonary fibrosis. Plantier Laurent, Cazes Aurélie, Dinh-Xuan Anh-Tuan, Bancal Catherine, Marchand-Adam Sylvain, Crestani Bruno. Jan 24;2018 European Respiratory Review. 27(147):170062. doi: 10.1183/16000617.0062-2017. doi: 10.1183/16000617.0062-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outcomes with newly proposed classification of acute respiratory deterioration in idiopathic pulmonary fibrosis. Teramachi Ryo, Kondoh Yasuhiro, Kataoka Kensuke, Taniguchi Hiroyuki, Matsuda Toshiaki, Kimura Tomoki, Yokoyama Toshiki, Yamano Yasuhiko, Furukawa Taiki, Sakamoto Koji, Hashimoto Naozumi, Hasegawa Yoshinori. Oct;2018 Respiratory Medicine. 143:147–152. doi: 10.1016/j.rmed.2018.09.011. doi: 10.1016/j.rmed.2018.09.011. [DOI] [PubMed] [Google Scholar]
- Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Song J. W., Hong S.-B., Lim C.-M., Koh Y., Kim D. S. 2011European Respiratory Journal. 37(2):356–363. doi: 10.1183/09031936.00159709. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- Outcome of patients with idiopathic pulmonary fibrosis admitted to the ICU for respiratory failure. Blivet Sandra, Philit François, Sab Jean Michel, Langevin Bruno, Paret Micheline, Guérin Claude, Robert Dominique. Jul;2001 Chest. 120(1):209–212. doi: 10.1378/chest.120.1.209. doi: 10.1378/chest.120.1.209. [DOI] [PubMed] [Google Scholar]
- Outcomes of patients admitted to the intensive care unit with idiopathic pulmonary fibrosis. Rangappa Pradeep, Moran John L. Jun;2009 Critical Care and Resuscitation. 11(2):102–109. doi: 10.1016/s1441-2772(23)01533-8. doi: 10.1016/s1441-2772(23)01533-8. [DOI] [PubMed] [Google Scholar]
- Outcome of patients admitted to the intensive care unit for acute exacerbation of idiopathic pulmonary fibrosis. Al-Hameed Fahad M, Sharma Sat. 2004Canadian Respiratory Journal. 11(2):117–122. doi: 10.1155/2004/379723. doi: 10.1155/2004/379723. [DOI] [PubMed] [Google Scholar]
- Ventilator settings and outcome of respiratory failure in chronic interstitial lung disease. Fernández-Pérez Evans R., Yilmaz Murat, Jenad Hussam, Daniels Craig E., Ryu Jay H., Hubmayr Rolf D., Gajic Ognjen. May;2008 Chest. 133(5):1113–1119. doi: 10.1378/chest.07-1481. doi: 10.1378/chest.07-1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Management of acute respiratory failure in interstitial lung diseases: overview and clinical insights. Faverio Paola, De Giacomi Federica, Sardella Luca, Fiorentino Giuseppe, Carone Mauro, Salerno Francesco, Ora Jousel, Rogliani Paola, Pellegrino Giulia, Sferrazza Papa Giuseppe Francesco, Bini Francesco, Bodini Bruno Dino, Messinesi Grazia, Pesci Alberto, Esquinas Antonio. May 15;2018 BMC Pulmonary Medicine. 18(1):70. doi: 10.1186/s12890-018-0643-3. doi: 10.1186/s12890-018-0643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Extrakorporale Membranoxygenierung bei akutem respiratorischem Versagen: Outcome von Patienten mit interstitieller Lungenerkrankung [Extracorporeal membrane oxygenation for treatment of acute respiratory failure: Outcome of patients with interstitial lung disease] Trudzinski F. C., Lepper P. M. Aug 15;2017 Medizinische Klinik - Intensivmedizin und Notfallmedizin. 112(6):552–556. doi: 10.1007/s00063-017-0326-5. doi: 10.1007/s00063-017-0326-5. [DOI] [PubMed] [Google Scholar]
- Failure of high-flow nasal cannula therapy may delay intubation and increase mortality. Kang Byung Ju, Koh Younsuck, Lim Chae-Man, Huh Jin Won, Baek Seunghee, Han Myongja, Seo Hyun-Suk, Suh Hee Jung, Seo Ga Jin, Kim Eun Young, Hong Sang-Bum. Feb 18;2015 Intensive Care Medicine. 41(4):623–632. doi: 10.1007/s00134-015-3693-5. doi: 10.1007/s00134-015-3693-5. [DOI] [PubMed] [Google Scholar]
- The role for high flow nasal cannula as a respiratory support strategy in adults: a clinical practice guideline. Rochwerg Bram, Einav Sharon, Chaudhuri Dipayan, Mancebo Jordi, Mauri Tommaso, Helviz Yigal, Goligher Ewan C., Jaber Samir, Ricard Jean-Damien, Rittayamai Nuttapol, Roca Oriol, Antonelli Massimo, Maggiore Salvatore Maurizio, Demoule Alexandre, Hodgson Carol L., Mercat Alain, Wilcox M. Elizabeth, Granton David, Wang Dominic, Azoulay Elie, Ouanes-Besbes Lamia, Cinnella Gilda, Rauseo Michela, Carvalho Carlos, Dessap-Mekontso Armand, Fraser John, Frat Jean-Pierre, Gomersall Charles, Grasselli Giacomo, Hernandez Gonzalo, Jog Sameer, Pesenti Antonio, Riviello Elisabeth D., Slutsky Arthur S., Stapleton Renee D., Talmor Daniel, Thille Arnaud W., Brochard Laurent, Burns Karen E. A. Nov 17;2020 Intensive Care Medicine. 46(12):2226–2237. doi: 10.1007/s00134-020-06312-y. doi: 10.1007/s00134-020-06312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Rochwerg Bram, Brochard Laurent, Elliott Mark W., Hess Dean, Hill Nicholas S., Nava Stefano, Navalesi Paolo (members o, Antonelli Massimo, Brozek Jan, Conti Giorgio, Ferrer Miquel, Guntupalli Kalpalatha, Jaber Samir, Keenan Sean, Mancebo Jordi, Mehta Sangeeta, Raoof Suhail (members o. Aug;2017 European Respiratory Journal. 50(2):1602426. doi: 10.1183/13993003.02426-2016. doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- Mechanical ventilation in patients with end-stage idiopathic pulmonary fibrosis. Mollica Corrado, Paone Gregorino, Conti Vittoria, Ceccarelli Daniela, Schmid Giovanni, Mattia Paolo, Perrone Nicola, Petroianni Angelo, Sebastiani Alfredo, Cecchini Luca, Orsetti Remo, Terzano Claudio. 2010Respiration. 79(3):209–215. doi: 10.1159/000225932. doi: 10.1159/000225932. [DOI] [PubMed] [Google Scholar]
- Acute and subacute idiopathic interstitial pneumonias. Taniguchi Hiroyuki, Kondoh Yasuhiro. Apr 28;2016 Respirology. 21(5):810–820. doi: 10.1111/resp.12786. doi: 10.1111/resp.12786. [DOI] [PubMed] [Google Scholar]
- Benefits of high-flow nasal cannula oxygen therapy on exercise capacity following acute exacerbation in ILD patients. Arizono Shinichi, Oomagari Masaki, Yanagita Yorihide, Machiguchi Hikaru, Tawara Yuichi, Yokomura Koshi. Sep 7;2020 European Respiratory Journal. 56(suppl 64):4662. doi: 10.1183/13993003.congress-2020.4662. doi: 10.1183/13993003.congress-2020.4662. [DOI] [Google Scholar]
- Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Moher David, Liberati Alessandro, Tetzlaff Jennifer, Altman Douglas G., PRISMA Group Jul 21;2009 PLoS Medicine. 6(7):e1000097. doi: 10.1371/journal.pmed.1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Sterne Jonathan AC, Hernán Miguel A, Reeves Barnaby C, Savović Jelena, Berkman Nancy D, Viswanathan Meera, Henry David, Altman Douglas G, Ansari Mohammed T, Boutron Isabelle, Carpenter James R, Chan An-Wen, Churchill Rachel, Deeks Jonathan J, Hróbjartsson Asbjørn, Kirkham Jamie, Jüni Peter, Loke Yoon K, Pigott Theresa D, Ramsay Craig R, Regidor Deborah, Rothstein Hannah R, Sandhu Lakhbir, Santaguida Pasqualina L, Schünemann Holger J, Shea Beverly, Shrier Ian, Tugwell Peter, Turner Lucy, Valentine Jeffrey C, Waddington Hugh, Waters Elizabeth, Wells George A, Whiting Penny F, Higgins Julian PT. Oct 12;2016 BMJ. 355:i4919. doi: 10.1136/bmj.i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Non-invasive mechanical ventilation in patients with diffuse interstitial lung diseases. Aliberti Stefano, Messinesi Grazia, Gamberini Silvia, Maggiolini Sveva, Visca Dina, Galavotti Vanni, Giuliani Fabio, Cosentini Roberto, Brambilla Anna Maria, Blasi Francesco, Scala Raffaele, Carone Mauro, Luisi Francesca, Harari Sergio, Voza Antonio, Esquinas Antonio, Pesci Alberto. Dec;2014 BMC Pulmonary Medicine. 14:194. doi: 10.1186/1471-2466-14-194. doi: 10.1186/1471-2466-14-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Why do patients with interstitial lung diseases fail in the ICU? a 2-center cohort study. Güngör Gökay, Tatar Dursun, Saltürk Cüneyt, Çimen Pinar, Karakurt Zuhal, Kirakli Cenk, Adıgüzel Nalan, Ediboğlu Özlem, Yılmaz Huri, Moçin Özlem Yazıcıoglu, Balcı Merih, Yılmaz Adnan. Feb 25;2013 Respiratory Care. 58(3):525–531. doi: 10.4187/respcare.01734. doi: 10.4187/respcare.01734. [DOI] [PubMed] [Google Scholar]
- Noninvasive ventilation in acute exacerbation of idiopathic pulmonary fibrosis. Yokoyama Toshiki, Kondoh Yasuhiro, Taniguchi Hiroyuki, Kataoka Kensuke, Kato Keisuke, Nishiyama Osamu, Kimura Tomoki, Hasegawa Ryuichi, Kubo Keishi. 2010Internal Medicine. 49(15):1509–1514. doi: 10.2169/internalmedicine.49.3222. doi: 10.2169/internalmedicine.49.3222. [DOI] [PubMed] [Google Scholar]
- Noninvasive ventilation in acute exacerbation of interstitial lung diseases. Abo Elwafa Gihan S, Ahmed Naglaa B, Abou Zeid Amany A, Abo Elhasab Mai A. 2023The Egyptian Journal of Chest Diseases and Tuberculosis. 72(1):99. doi: 10.4103/ecdt.ecdt_40_22. doi: 10.4103/ecdt.ecdt_40_22. [DOI] [Google Scholar]
- Pulse oximetric saturation to fraction of inspired oxygen (SpO2/FIO2) ratio 24 hours after high-flow nasal cannula (HFNC) initiation is a good predictor of HFNC therapy in patients with acute exacerbation of interstitial lung disease. Koyauchi Takafumi, Yasui Hideki, Enomoto Noriyuki, Hasegawa Hirotsugu, Hozumi Hironao, Suzuki Yuzo, Karayama Masato, Furuhashi Kazuki, Fujisawa Tomoyuki, Nakamura Yutaro, Inui Naoki, Yokomura Koshi, Suda Takafumi. Jan;2020 Therapeutic Advances in Respiratory Disease. 14:1753466620906327. doi: 10.1177/1753466620906327. doi: 10.1177/1753466620906327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High-flow nasal cannula oxygen therapy to treat acute respiratory failure in patients with acute exacerbation of idiopathic pulmonary fibrosis. Vianello Andrea, Arcaro Giovanna, Molena Beatrice, Turato Cristian, Braccioni Fausto, Paladini Luciana, Vio Stefania, Ferrarese Silvia, Peditto Piera, Gallan Federico, Saetta Marina. Jan;2019 Therapeutic Advances in Respiratory Disease. 13:1753466619847130. doi: 10.1177/1753466619847130. doi: 10.1177/1753466619847130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noninvasive Oxygenation Strategies for Acute Exacerbation of Interstitial Lung Disease: A Retrospective Single-center Study and a Review of the Literature. Imai Ryosuke, Tsugitomi Ryosuke, Nakaoka Hiroshi, Jinta Torahiko, Tamura Tomohide. May;2019 Clinical Pulmonary Medicine. 26(3):87–91. doi: 10.1097/cpm.0000000000000312. doi: 10.1097/cpm.0000000000000312. [DOI] [Google Scholar]
- Efficacy and Tolerability of High-Flow Nasal Cannula Oxygen Therapy for Hypoxemic Respiratory Failure in Patients with Interstitial Lung Disease with Do-Not-Intubate Orders: A Retrospective Single-Center Study. Koyauchi Takafumi, Hasegawa Hirotsugu, Kanata Kei, Kakutani Takuya, Amano Yusuke, Ozawa Yuichi, Matsui Takashi, Yokomura Koshi, Suda Takafumi. 2018Respiration. 96(4):323–329. doi: 10.1159/000489890. doi: 10.1159/000489890. [DOI] [PubMed] [Google Scholar]
- High-flow nasal cannula therapy for acute respiratory failure in patients with interstitial pneumonia: a retrospective observational study. Omote Norihito, Matsuda Naoyuki, Hashimoto Naozumi, Nishida Kazuki, Sakamoto Koji, Ando Akira, Nakahara Yoshio, Nishikimi Mitsuaki, Higashi Michiko, Matsui Shigeyuki, Hasegawa Yoshinori. May;2020 Nagoya Journal of Medical Science. 82(2):301–313. doi: 10.18999/nagjms.82.2.301. doi: 10.18999/nagjms.82.2.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High-flow nasal oxygen therapy versus noninvasive ventilation in chronic interstitial lung disease patients with acute respiratory failure. Shebl Eman, Embarak Sameh. 2018The Egyptian Journal of Chest Diseases and Tuberculosis. 67(3):270–5. doi: 10.4103/ejcdt.ejcdt_33_18. doi: 10.4103/ejcdt.ejcdt_33_18. [DOI] [Google Scholar]
- Potential benefits of early continuous positive pressure ventilation in patients with rapidly progressive interstitial pneumonia. Yokoyama TOSHIKI, Tsushima KENJI, Yamamoto HIROSHI, Koizumi TOMONOBU, Kubo KEISHI. Jan 24;2012 Respirology. 17(2):315–321. doi: 10.1111/j.1440-1843.2011.02051.x. doi: 10.1111/j.1440-1843.2011.02051.x. [DOI] [PubMed] [Google Scholar]
- Non-invasive positive pressure ventilation to treat respiratory failure resulting from exacerbations of chronic obstructive pulmonary disease: Cochrane systematic review and meta-analysis. Lightowler J. V, Wedzicha J A, Elliott M W, Ram F S. Jan 25;2003 BMJ. 326(7382):185. doi: 10.1136/bmj.326.7382.185. doi: 10.1136/bmj.326.7382.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indications and practical approach to non-invasive ventilation in acute heart failure. Masip Josep, Peacock W Frank, Price Susanna, Cullen Louise, Martin-Sanchez F Javier, Seferovic Petar, Maisel Alan S, Miro Oscar, Filippatos Gerasimos, Vrints Christiaan, Christ Michael, Cowie Martin, Platz Elke, McMurray John, DiSomma Salvatore, Zeymer Uwe, Bueno Hector, Gale Chris P, Lettino Maddalena, Tavares Mucio, Ruschitzka Frank, Mebazaa Alexandre, Harjola Veli-Pekka, Mueller Christian, Acute Heart Failure Study Group of the Acute Cardiovascular Care Association and the Committee on Acute Heart Failure of the Heart Failure Association of the European Society of Cardiology 2018Eur Heart J. 39(1):17–25. doi: 10.1093/eurheartj/ehx580. doi: 10.1093/eurheartj/ehx580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasal high flow therapy: a novel treatment rather than a more expensive oxygen device. Ischaki Eleni, Pantazopoulos Ioannis, Zakynthinos Spyros. Aug 9;2017 European Respiratory Review. 26(145):170028. doi: 10.1183/16000617.0028-2017. doi: 10.1183/16000617.0028-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heated Humidified High-Flow Nasal Oxygen in Adults: Mechanisms of Action and Clinical Implications. Spoletini Giulia, Alotaibi Mona, Blasi Francesco, Hill Nicholas S. Jul;2015 Chest. 148(1):253–261. doi: 10.1378/chest.14-2871. doi: 10.1378/chest.14-2871. [DOI] [PubMed] [Google Scholar]
- Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Cosentini Roberto, Brambilla Anna Maria, Aliberti Stefano, Bignamini Angelo, Nava Stefano, Maffei Antonino, Martinotti Renato, Tarsia Paolo, Monzani Valter, Pelosi Paolo. Jul;2010 Chest. 138(1):114–120. doi: 10.1378/chest.09-2290. doi: 10.1378/chest.09-2290. [DOI] [PubMed] [Google Scholar]
- Physiologic effects of noninvasive ventilation during acute lung injury. L'Her Erwan, Deye Nicolas, Lellouche François, Taille Solenne, Demoule Alexandre, Fraticelli Amanda, Mancebo Jordi, Brochard Laurent. Nov 1;2005 American Journal of Respiratory and Critical Care Medicine. 172(9):1112–1118. doi: 10.1164/rccm.200402-226oc. doi: 10.1164/rccm.200402-226oc. [DOI] [PubMed] [Google Scholar]
- Noninvasive positive pressure ventilation delivered by helmet vs. standard face mask. Chiumello Davide, Pelosi Paolo, Carlesso Eleonora, Severgnini Paolo, Aspesi Michele, Gamberoni Chiara, Antonelli Massimo, Conti Giorgio, Chiaranda Maurizio, Gattinoni Luciano. Jun 12;2003 Intensive Care Medicine. 29(10):1671–1679. doi: 10.1007/s00134-003-1825-9. doi: 10.1007/s00134-003-1825-9. [DOI] [PubMed] [Google Scholar]
- Non-invasive ventilation in chronic obstructive pulmonary disease patients: helmet versus facial mask. Navalesi Paolo, Costa Roberta, Ceriana Piero, Carlucci Annalisa, Prinianakis George, Antonelli Massimo, Conti Giorgio, Nava Stefano. 2007Intensive Care Medicine. 33(1):74–81. doi: 10.1007/s00134-006-0391-3. doi: 10.1007/s00134-006-0391-3. [DOI] [PubMed] [Google Scholar]
- Association of Noninvasive Oxygenation Strategies With All-Cause Mortality in Adults With Acute Hypoxemic Respiratory Failure: A Systematic Review and Meta-analysis. Ferreyro Bruno L., Angriman Federico, Munshi Laveena, Del Sorbo Lorenzo, Ferguson Niall D., Rochwerg Bram, Ryu Michelle J., Saskin Refik, Wunsch Hannah, da Costa Bruno R., Scales Damon C. Jul 7;2020 JAMA. 324(1):57–67. doi: 10.1001/jama.2020.9524. doi: 10.1001/jama.2020.9524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High-flow nasal cannula versus noninvasive ventilation in patients with COVID-19: a systematic review and meta-analysis. He Yuewen, Liu Na, Zhuang Xuhui, Wang Xia, Ma Wuhua. Jan;2022 Therapeutic Advances in Respiratory Disease. 16:17534666221087847. doi: 10.1177/17534666221087847. doi: 10.1177/17534666221087847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- High-flow nasal cannula versus non-invasive ventilation for acute hypercapnic respiratory failure in adults: a systematic review and meta-analysis of randomized trials. Ovtcharenko N., Ho E., Alhazzani W., Cortegiani A., Ergan B., Scala R., Sotgiu G., Chaudhuri D., Oczkowski S., Lewis K. Nov 9;2022 Critical Care. 26(1):348. doi: 10.1186/s13054-022-04218-3. doi: 10.1186/s13054-022-04218-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. Levine Sanford, Nguyen Taitan, Taylor Nyali, Friscia Michael E., Budak Murat T., Rothenberg Pamela, Zhu Jianliang, Sachdeva Rajeev, Sonnad Seema, Kaiser Larry R., Rubinstein Neal A., Powers Scott K., Shrager Joseph B. Mar 27;2008 New England Journal of Medicine. 358(13):1327–1335. doi: 10.1056/nejmoa070447. doi: 10.1056/nejmoa070447. [DOI] [PubMed] [Google Scholar]
- Positive End-Expiratory Pressure Ventilation Induces Longitudinal Atrophy in Diaphragm Fibers. Lindqvist Johan, van den Berg Marloes, van der Pijl Robbert, Hooijman Pleuni E., Beishuizen Albertus, Elshof Judith, de Waard Monique, Girbes Armand, Spoelstra-de Man Angelique, Shi Zhong-Hua, van den Brom Charissa, Bogaards Sylvia, Shen Shengyi, Strom Joshua, Granzier Henk, Kole Jeroen, Musters René J. P., Paul Marinus A., Heunks Leo M. A., Ottenheijm Coen A. C. Aug 15;2018 American Journal of Respiratory and Critical Care Medicine. 198(4):472–485. doi: 10.1164/rccm.201709-1917oc. doi: 10.1164/rccm.201709-1917oc. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on request.


