Abstract
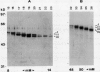
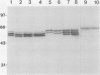
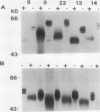
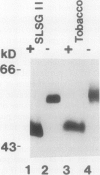
Glycoprotein products of two highly homologous Brassica S gene family members were studied: SLSG (S locus-specific glycoprotein), product of an SLG gene at the S locus, and SLR1 (S locus-related) protein, product of the SLR1 gene, a gene unlinked to the S locus. A polyclonal antibody directed against a trpE-SLR1 fusion protein facilitated study of the SLR1 protein. SLR1 protein was detected in a number of crucifer species. No variation in the level of this protein was found between self-compatible and self-incompatible plants. Both SLSG and SLR1 protein occurred as glycoforms on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Each glycoform had several charge forms, indicated by elution patterns from a high performance liquid chromatography cation exchange column and behavior on two-dimensional gels. Deglycosylation of both SLSG and SLR1 protein caused loss of the glycoforms, which apparently arose from differences in glycosylation. Consistent with their apparent similar post-translational processing, immunolocalization showed that SLR1 protein, like SLSG, accumulated in the stigma papillae cell walls. Thus, both SLSG and SLR1 protein are present at the site of pollen-stigma interaction.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dieckmann C. L., Tzagoloff A. Assembly of the mitochondrial membrane system. CBP6, a yeast nuclear gene necessary for synthesis of cytochrome b. J Biol Chem. 1985 Feb 10;260(3):1513–1520. [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Kandasamy M. K., Paolillo D. J., Faraday C. D., Nasrallah J. B., Nasrallah M. E. The S-locus specific glycoproteins of Brassica accumulate in the cell wall of developing stigma papillae. Dev Biol. 1989 Aug;134(2):462–472. doi: 10.1016/0012-1606(89)90119-x. [DOI] [PubMed] [Google Scholar]
- Karp D. R., Atkinson J. P., Shreffler D. C. Genetic variation in glycosylation of the fourth component of murine complement. Association with hemolytic activity. J Biol Chem. 1982 Jul 10;257(13):7330–7335. [PubMed] [Google Scholar]
- Kleid D. G., Yansura D., Small B., Dowbenko D., Moore D. M., Grubman M. J., McKercher P. D., Morgan D. O., Robertson B. H., Bachrach H. L. Cloned viral protein vaccine for foot-and-mouth disease: responses in cattle and swine. Science. 1981 Dec 4;214(4525):1125–1129. doi: 10.1126/science.6272395. [DOI] [PubMed] [Google Scholar]
- Kresze G. B., Ronft H. Pyruvate dehydrogenase complex from baker's yeast. 1. Purification and some kinetic and regulatory properties. Eur J Biochem. 1981 Oct;119(3):573–579. doi: 10.1111/j.1432-1033.1981.tb05646.x. [DOI] [PubMed] [Google Scholar]
- Lalonde B. A., Nasrallah M. E., Dwyer K. G., Chen C. H., Barlow B., Nasrallah J. B. A highly conserved Brassica gene with homology to the S-locus-specific glycoprotein structural gene. Plant Cell. 1989 Feb;1(2):249–258. doi: 10.1105/tpc.1.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson E., Howlett B., Jagendorf A. Artificial reductant enhancement of the Lowry method for protein determination. Anal Biochem. 1986 Jun;155(2):243–248. doi: 10.1016/0003-2697(86)90432-x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Moore H. M., Nasrallah J. B. A Brassica Self-Incompatibility Gene Is Expressed in the Stylar Transmitting Tissue of Transgenic Tobacco. Plant Cell. 1990 Jan;2(1):29–38. doi: 10.1105/tpc.2.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah J. B., Yu S. M., Nasrallah M. E. Self-incompatibility genes of Brassica oleracea: Expression, isolation, and structure. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5551–5555. doi: 10.1073/pnas.85.15.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrallah M. E. Genetic control of quantitative variation in self-incompatibility proteins detected by immunodiffusion. Genetics. 1974 Jan;76(1):45–50. doi: 10.1093/genetics/76.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio T., Hinata K. Comparative Studies on S-Glycoproteins Purified from Different S-Genotypes in Self-Incompatible BRASSICA Species I. Purification and Chemical Properties. Genetics. 1982 Apr;100(4):641–647. doi: 10.1093/genetics/100.4.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarker R. H., Elleman C. J., Dickinson H. G. Control of pollen hydration in Brassica requires continued protein synthesis, and glycosylation in necessary for intraspecific incompatibility. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4340–4344. doi: 10.1073/pnas.85.12.4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spindler K. R., Rosser D. S., Berk A. J. Analysis of adenovirus transforming proteins from early regions 1A and 1B with antisera to inducible fusion antigens produced in Escherichia coli. J Virol. 1984 Jan;49(1):132–141. doi: 10.1128/jvi.49.1.132-141.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trick M., Flavell R. B. A homozygous S genotype of Brassica oleracea expresses two S-like genes. Mol Gen Genet. 1989 Jul;218(1):112–117. doi: 10.1007/BF00330573. [DOI] [PubMed] [Google Scholar]
- Voelker T. A., Herman E. M., Chrispeels M. J. In vitro mutated phytohemagglutinin genes expressed in tobacco seeds: role of glycans in protein targeting and stability. Plant Cell. 1989 Jan;1(1):95–104. doi: 10.1105/tpc.1.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wassarman P. M. Zona pellucida glycoproteins. Annu Rev Biochem. 1988;57:415–442. doi: 10.1146/annurev.bi.57.070188.002215. [DOI] [PubMed] [Google Scholar]