Abstract
Purpose
To provide a comprehensive guide of all implantable collamer lens (ICL) sizing nomograms and the respective preoperative diagnostic devices that are required. This guide would help clinicians in choosing the appropriate ICL size for myopic patients to optimize postoperative vault height.
Methods
A literature search of peer-reviewed journals describing methods and postoperative outcomes of ICL sizing was conducted. Research articles containing ICL nomograms or formulas were identified from this search. Preoperative variables necessary for these nomograms and the required diagnostic devices to measure these parameters such as topography, biometry, or ultrasound biomicroscopy (UBM) were noted. An additional search was conducted to identify artificial intelligence (AI) or machine learning (ML)-derived nomograms.
Results
Eighteen ICL sizing nomograms were identified through literature search. Five of these nomograms are available for use and require topography or biometry devices. Of these, four include the manufacturer’s, optimized white-to-white (WTW), Kang, Kim, and Rocamora Nomograms. Eight of the 18 nomograms available for use require UBM. Eight of these include the Kojima, Nakamura, KS, ZZ, Dougherty, Parkhurst, Russo, and Reinstein Nomograms. Four of the 18 nomograms are ML-derived including Shen, Rocamora, Russo, and Kang Nomograms.
Conclusion
ICL nomograms are a vital tool in helping clinicians select the right ICL size for myopic patients to optimize postoperative vault reducing risk of postoperative complications. Based on available diagnostic devices such as topography, biometry, or UBM clinicians can integrate specific nomograms into practice.
Keywords: phakic intraocular lens, pIOL, myopia, implantable contact lens, EVO, Visian ICL, artificial intelligence, machine learning model, ICL sizing, ICL formula
Introduction
The development of the phakic intraocular lens (pIOL) has provided an alternative therapy for patients with severe refractive error that have contraindication to corneal refractive surgery.1 A type of posterior chamber pIOL known as the EVO implantable collamer lens (EVO+-ICL, STAAR surgical, Monrovia, CA, USA) is a US FDA-approved treatment for refractive pathology such as myopia and myopic astigmatism.2 One area of focus regarding pIOL is proper sizing and placement of the lens which is dependent upon conformity of the lens with surrounding structures. Optimizing these factors contributes to better physiological outcomes such as improved aqueous humoral flow, stable anterior chamber angle, and better refraction.3,4 Thus, a reduction in postoperative complications associated with pIOL implantation can be expected with appropriate sizing. Low vault complications include cataract formation while high vault complications include angle-closure glaucoma, pupil block, and pigment dispersion.
Several nomograms aid in the pursuit of accurate pIOL sizing.3,5–18 These tools utilize various measurements to predict optimal sizing such as sulcus-to-sulcus (STS) length, white-to-white (WTW) distance, anterior chamber depth (ACD), etc.3 Adding further complexity to implantable collamer lens (ICL) sizing are the instruments used to visualize the various structures of the anterior segment pertaining to these measurements. These diagnostic tools have evolved in resolution and precision over several years. Historically, ICL was based on simple manual caliper measurement at the slit lamp for determination of the WTW limbus diameter.3 In the modern era topography and biometry devices have largely replaced this device for WTW and other preoperative measurements.3 In addition, with the advent of artificial intelligence, a much larger dataset of patients can be analyzed regarding ICL sizing. Current nomograms provide options in calculating a predicted ICL size and postoperative vault. This review will act as a guide for ophthalmologists to explore the existing nomograms so that they are provided tools in determining an ICL size for myopic patients. However, it is beyond the scope of this paper to evaluate or compare the existing nomograms for accuracy and safety of vault prediction.
Methods
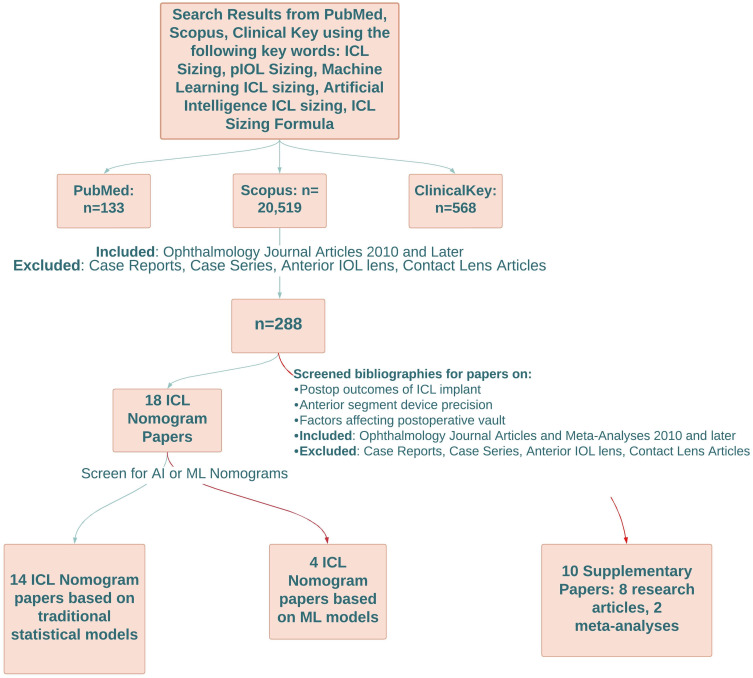
Literature review was conducted by two independent examiners through PubMed, ClinicalKey, and Scopus utilizing search terms such as “ICL sizing”, “pIOL lens sizing”, “machine learning ICL sizing”, and “artificial intelligence ICL sizing”, and “ICL sizing formula”. Studies were limited to ophthalmology journal articles and meta-analyses published in 2010 and later. Case reports and case series were excluded in addition to articles concerning anterior intraocular lenses or contact lenses. This search yielded 288 results and duplicate research articles were noted between databases with removal. The next phase involved scanning research articles and meta-analyses written in English that described methods and limitations of ICL sizing and ICL sizing or predictive vault formulas with preoperative variables that could be measured by topography, biometry, ultrasound biomicroscopy (UBM). Furthermore, research papers were surveilled for any machine learning (ML) models that predict ICL sizing or postoperative vault. Ultimately, this search method distilled 18 papers of distinct ICL nomograms, four of which were based upon ML models. The bibliography of each research paper was scanned for references of additional topics that supplement this literature review including postoperative outcomes of ICL implantation, anterior segment device precision, meta-analysis of ICL, and factors affecting postoperative vault such as light or ICL orientation. Specific criteria for this search involved selecting papers from ophthalmology journals published in 2010 and later. In addition, papers were filtered to only include journal articles or meta-analyses that focused on phakic intraocular lenses (pIOL) of the posterior chamber and various, commonly used devices that measure anterior segment structures including optical coherence tomography (OCT) and UBM. Case reports and case series were excluded as well as articles regarding anterior intraocular lenses or contact lenses. In total 10 references were used including eight research articles and two meta-analyses that were utilized to aid in creating and writing the results and discussion sections. Refer to the flowchart in Figure 1 for a visual representation of these methods.
Figure 1.
Method of Literature Search. Red arrows indicate research articles based on machine learning models and supplementary papers. Green arrows indicate a particular research database with the number of research articles, number of ophthalmology research articles dated 2010 or later with exclusion criteria, total number of implantable collamer lens nomogram papers, or number of implantable collamer lens papers based on traditional statistical models.
Abbreviations: AI, artificial intelligence; ICL, implantable collamer lens; IOL, intraocular lens; ML, machine learning; pIOL, phakic intraocular lens.
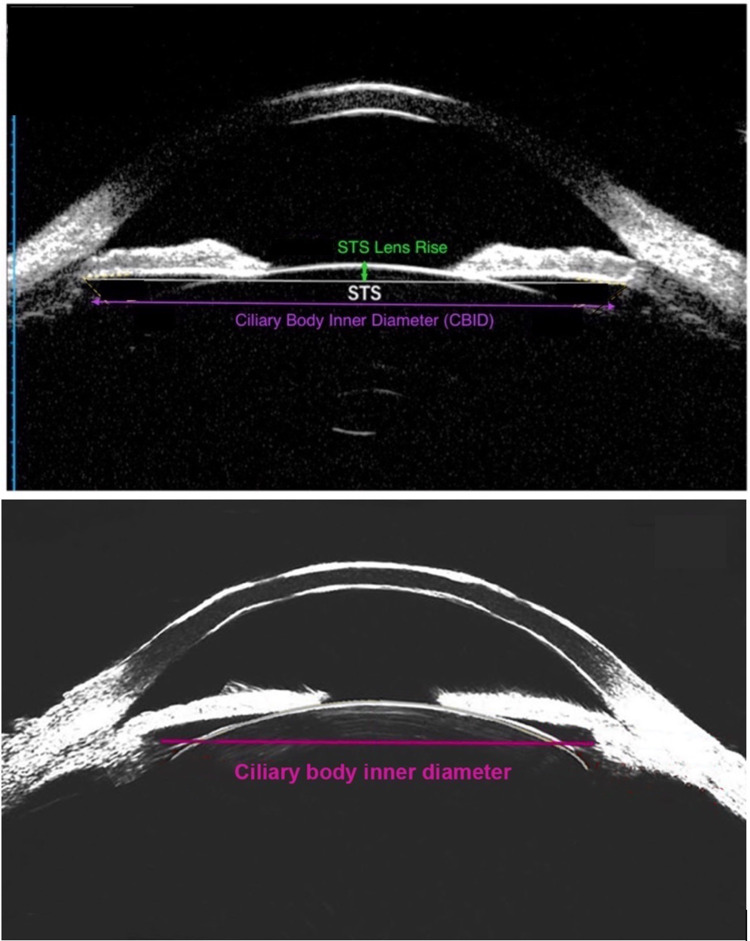
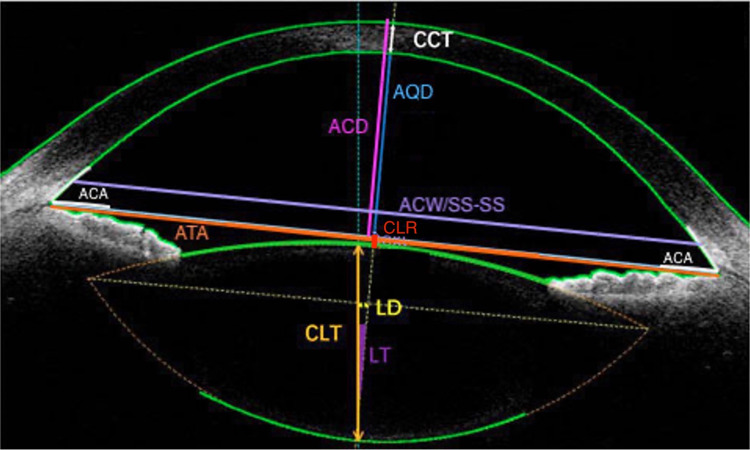
Other database sources from open-access scientific journals were searched for OCT and UBM images by utilizing Google image search with search terms such as “OCT image”, “OCT imaging”, “UBM image” or “ArcScan imaging”. Three clear images from OCT and UBM including one image from a very high frequency UBM called ArcScan Insight® 100 (ArcScan Inc., Golden, CO, USA) were selected that depict the anterior and posterior chamber structures (Figures 2 and 3).19,20 Images with excessive labelling or zoom were excluded. Images selected were from open-access articles that allowed modification of images. Labels and measurement lines indicating the origin of preoperative measurements were drafted via Photoshop.
Figure 3.
Ultrasound Biomicroscopy Images of Posterior Chamber Structures with Specific Measurements Used in Nomograms. The bottom image depicts ArcScan Insight® Ultrasound Biomicroscopy. Reprinted from Ruan et al. Creative Commons.20
Abbreviations: CBID, ciliary body inner diameter; STS, sulcus-to-sulcus.
Figure 2.
Optical Coherence Tomography Image of Anterior and Posterior Chamber Structures with Specific Measurements Used in Nomograms. Reprinted from Lin et al. Creative Commons.19
Abbreviations: ACA, anterior chamber angle; ACD, anterior chamber depth; ACW, anterior chamber width; AQD, aqueous depth; ATA, angle to angle distance; CCT, central corneal thickness; CLR, crystalline lens rise; CLT, crystalline lens thickness; LD, lens decentration; LT, lens tilt; SS-SS, scleral spur to scleral spur distance.
Results
Based upon the literature search method 18 ICL sizing nomograms were identified (Table 1). 14 nomograms were derived from traditional statistical models and four were derived from ML models (Table 1). Five of the 18 nomograms available for use require either optical topography or biometry and eight of the 18 nomograms available for use require UBM which uses very high frequency ultrasound waves (35–100 MHz) to detect structures of the posterior chamber. All nomograms except the Kamiya and Shen are freely available (Table 2). Nomograms will be discussed in the following sections: those requiring topography or biometry and those requiring UBM.
Table 1.
Comprehensive Summary of Compiled Nomograms
| Nomogram | Patients | Country | Imaging | Method | Preoperative Variables | Advantages | Disadvantages |
|---|---|---|---|---|---|---|---|
| Manufacturer (STAAR)3 | WTW, ACD | Fast | Larger size inaccuracy (13.7 mm) | ||||
| Optimized WTW3 | WTW | Fast | |||||
| Parkhurst3 | UBM | STS, STSL | Fast. Considers lens rise | Operator error in UBM | |||
| Dougherty3 | 73 eyes, 48 patients | USA | UBM (VuMax-II) | Linear regression | STS, ICL power | Fast | Operator error in UBM. No CLR. |
| Igarashi/KS Formula V111 | 44 eyes, 23 patients | Japan | AS-OCT (CASIA2) | Spearman rank correlation | ICL size, ATA distance | Small population, low external validity | |
| Igarashi/KS Formula V212 | 121 eyes, 65 patients | Japan | AS-OCT (CASIA2) | Corrected KS formula | ICL size, ATA distance | Improved predicted vault accuracy for 12.1, 13.2, and 13.7 mm sizes. | |
| NK Version 113 | 46 eyes, 23 patients | Japan | AS-OCT (CASIA2) | Linear regression | ACW (distance between scleral spurs), CLR | Considers lens rise | Small population, low external validity. Overestimates small vaults. Underestimates large vaults. |
| NK Version 214 | 81 eyes, 35 patients | Japan | AS-OCT (CASIA2) | Linear regression with stepwise variable selection | ACW (distance between scleral spurs), CLR | Considers lens rise | Same as NK V1 |
| NK Version 315 | 85 eyes, 45 patients | Japan | AS-OCT (CASIA2) | Stepwise multiple regression analysis w /partial regression coefficient to update formula | ACW (distance between scleral spurs), CLR | Considers lens rise | Vault measurements made under mesopic conditions only. |
| Zheng and Zhang (ZZ formula)8 | 164 eyes, 84 patients | China | UBM (AVISO), Biometer (IOLMaster700), OCT (Cirrus) | Horizontal/vertical STS, ACD, AL, CLT, effective lens position | UBM operator error. May be unreliable in patients with predicted low postoperative vault | ||
| Reinstein9 | 147 eyes | UK | UBM (ArcScan Insight® 100) | Linear regression | CBID, STSL, scotopic pupil size | Calculator compares to other nomograms | |
| Kojima10 | 47 eyes, 25 patients | Japan | UBM (VuMax-II) | Linear regression | ACD, STS, STSL | Small population, low external validity | |
| Shen (Machine Learning)16 | 6,297 eyes, 3,536 patients | China | Pentacam, biometer IOLMaster | RF, GB, XGBoost | Pupil size, ACA, CT, AL. Time after surgery, K2, K2 axis, K1, K1 axis, WTW | Large population | Low external validity. High importance on WTW. |
| Kang (Machine Learning)5 | 3,506 eyes, 1753 patients | South Korea | AS-OCT (CASIA2) | XGBoost, light GBM | Age, sex, preoperative spherical equivalent, ICL refractive power, type of ICL (toric lens or not), WTW, ATA, ACD, ACW, CLR, CCT, pupil size, and lens size. | Large population | Low use of 13.7 mm ICL size. Low external validity. Scotopic conditions only. |
| Kamiya (Machine Learning)17 | 1,745 eyes, 1,745 patients | Japan, South Korea | AS-OCT (CASIA2) | RF, SV, LR, GB | Age, sex, refractive power (sphere/cylinder), SE, BCVA, lens type, WTW, ACD, ATA, CLR, ACW, LV, CCT, AOD500, TIA500, logMAR | Large population | Low external validity |
| Russo (Machine Learning)18 | 561 eyes, 300 patients | Italy | AS-OCT (MS39) | Random forest, XG boost, extra tree | AQD, ICL size, refractive power (sphere/cylinder), SE, K1, K2, SP-SP distance, WTW, CLR, age, sex, CCT, target sphere and cylinder, ICL model | Large population | Low external validity. Not available for Mac operating system |
| Kim6 | 894 eyes, 471 patients | South Korea | AS-OCT (ANTERION) | LASSO | AQD, ACA distance, LT | Large population. High resolution CLT | Low external validity |
| Rocamora7 | 115 eyes, 59 patients | Belgium | AS-OCT (MS39) | LASSO | Mean total keratometry, CV, CLR, SS-SS, ICL diameter, Km 3 mm, Km 5 mm, ACD, CCT, age | Three formulas based on equipment |
Abbreviations: ACA, anterior chamber angle; ACD, anterior chamber depth; ACW, anterior chamber width (equal to SS-SS or SP-SP); AL, axial length; AQD, aqueous depth; ATA, angle to angle distance (equal to ACA distance); BCVA, best corrected visual acuity; CBID, ciliary body inner diameter; CCT, central corneal thickness; CLR, crystalline lens rise; CLT, crystalline lens thickness; CV, corneal volume; GB, gradient boost; K1, flat keratometry; K2, steep keratometry; Km 3 mm, mean posterior keratometry 3 mm zone; Km 5 mm, mean anterior keratometry 5 mm zone; logMAR, logarithm of minimal angle of resolution; LR, linear regressor; LV, lens vault; ICL, implantable collamer lens; RF, random forest; SE, spherical equivalent; SP-SP, scleral spur to scleral spur (equals ACW and SS-SS); STS, sulcus-to-sulcus; STSL, sulcus-to-sulcus lens rise; SV, support vector; UBM, ultrasound biomicroscopy; WTW, white-to-white.
Table 2.
Summary of Links to Nomogram Calculators or Formulas for Clinical Use
| Nomogram | Nomogram Link or Reference |
|---|---|
| FDA/Manufacturer3 | https://evo-ocos.staarag.ch/live/ or refer to Supplementary Table 1 for sizing chart |
| Optimized WTW3 | Supplementary Table 2 |
| Parkhurst3 | Supplementary Table 3 |
| Kang5 | http://loocus-iolcalc.ai/ |
| Kim6 | https://soo9028.github.io/iol-prediction-webpage/ |
| Rocamora7 | http://icl.emmetropia.be |
| Zheng and Zhang (ZZ)8 | www.zzcal.com |
| Reinstein9 | www.iclsizing.com |
| Kojima10 | Refer to paper formula; also available through Reinstein link (www.iclsizing.com) |
| KS11,12 | Refer to Table 3 |
| Nakamura13–15 | Refer to Table 3; also available through Reinstein link (www.iclsizing.com) |
| Shen16 | Not available |
| Kamiya17 | Not available |
| Russo18 | https://www.centrooculisticobresciano.it/download/ |
| Dougherty3 | Supplementary Table 4 |
Nomograms Requiring Topography or Biometry
Manufacturer (STAAR Surgical) Nomogram
The manufacturer’s nomogram (Supplementary Table 1) utilizes WTW, ICL power, keratometry, central corneal thickness (CCT), and ACD measurements to recommend ICL sizes. However, one study showed that use of this nomogram with instruments such as Lenstar LS900 (Haag-Streit AG, Switzerland) and Galilei G4 (Ziemer, Zürich, Switzerland) predicted oversized lenses, which could increase risk of postoperative complications such as angle closure glaucoma.3 This nomogram may not be the most optimal to use for ICL sizing. Use of this nomogram for fitting larger lens size may be inaccurate so it may be preferable to use this nomogram in the fitting of smaller lenses.
Optimized WTW Nomogram
The optimized WTW (Supplementary Table 2) is similar to the manufacturer’s nomogram with the one significant difference being that it has less propensity to predict an oversized ICL in comparison. One study found that using the optimized WTW nomogram in conjunction with devices such as the OPD III (Nidek Inc., Tokyo, Japan), Lenstar LS900 (Haag-Streit AG, Switzerland), and Galilei G4 (Ziemer, Zürich, Switzerland) resulted in larger predicted ICL size compared to using the optimized WTW nomogram with UBM.3 In this study the G4 device predicted the greatest percentage of larger ICL size compared to the other two devices.3 This combination of optimized WTW nomogram and G4 may offer benefit in patients with a low predicted postoperative vault.
Kang Nomogram (ML-derived)
Kang et al used an ML model to predict ICL size and postoperative vault using the following factors: age, sex, preoperative spherical equivalent, ICL refractive power, type of ICL (toric lens or not), WTW, angle to angle distance (ATA), ACD, anterior chamber width (ACW; equal to scleral spur to scleral spur distance [SS-SS or SP-SP]), crystalline lens rise (CLR), CCT, pupil size, and lens size.5 They used an ensemble model from XGBoost with a Korean population of 2,756 eyes and a Japanese population of 290 eyes.5 The advantage of the ensemble model is that it considers outliers of patients with incorrect lens fitting. As such this model was found to have a lower mean absolute error for ICL vault prediction compared to other ML models (single XGBoost, random forest, support vector machine) and ICL sizing nomograms (manufacturer, NK formula).5 The web version can be found here: http://loocus-iolcalc.ai/5
Kim Nomogram
Kim et al developed this nomogram with emphasis on aqueous depth (AQD), anterior chamber angle (ACA), and lens thickness with preoperative measurements obtained by ANTERION OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) using LASSO regression analysis.6 This formula was based on a dataset of 894 eyes of a South Korean population.6 The model showed a strong correlation between predicted vault and ICL sizing and the parameters (R=0.582).6
Rocamora Nomograms (ML-derived)
Rocamora et al developed this nomogram from a European population with low to moderate degree myopia.7 The nomogram is based on least absolute shrinkage and selection operator (LASSO) which is an alternative regression analysis used in machine learning which can exclude irrelevant parameters in producing a model. Three LASSO formulas were created. One used biometric data, another OCT data and the third used a full formula which combines the previous two.7 Each formula can predict the postoperative vault for a corresponding ICL size. Twenty-seven preoperative parameters were analyzed by the ML model for predictive value, but the 10 most important preoperative parameters that were determined by the ML model were included in Table 1 under the “preoperative variables”. Mean absolute error (MAE) of the predicted vs achieved postoperative vault was calculated for these three formulas and compared to other established nomograms. MAE was found to be significantly lower in the LASSO formulas compared to the other models.7 Using the LASSO biometry formula, postoperative vault could be estimated within 500 µm for 97.3% of cases and using the LASSO-OCT and LASSO-Full the same estimate could be made for 100% of cases.7
Nomograms Requiring UBM
Parkhurst and Dougherty Nomogram
The Parkhurst nomogram (Supplementary Table 3) accounts for CLR and STS measurements using UBM while the Dougherty nomogram (Supplementary Table 4) uses STS and ICL Power.3 One study found better prediction of ICL sizing utilizing the Parkhurst nomogram vs the Dougherty nomogram.3 This was likely due to extra parameters used in the Parkhurst model such as CLR in addition to more categories of STS measurements. Given these literature findings and how CLR affects ICL vault the Parkhurst nomogram may be more preferable than the Dougherty nomogram.
ZZ Nomogram
This nomogram utilizes a formula that considers horizontal and vertical STS, expected direction of ICL, ACD, axial length (AL), crystalline lens thickness (CLT), effective lens position, and surgery-induced astigmatism.8 The formula predicts vault height in addition to ICL sizing. In one study that examined the efficacy of this formula for patients that had toric ICL (TICL) implantation, the authors utilized UBM (AVISO, Quantel Medical, Clermont-Ferrand, France) video-clips to obtain real-time measurements of STS.8 The study compared TICL sizing compared to the sizing suggested by FDA nomogram.8 Results showed that the ZZ nomogram recommended ICL sizing that was greater than the FDA nomogram in 10.1% of eyes and smaller than the FDA nomogram in 16.1% of eyes.8 The predicted postoperative vault using the ZZ formula was significantly less than that predicted by the FDA nomogram in patients that were implanted with larger TICLs.8 This may lead to increased risk of postoperative complications. Given these findings it is recommended that this nomogram is avoided in patients with predicted low postoperative vault. The ZZ ICL calculator based on their formula can be found here: www.zzcal.com.8
Reinstein Nomogram
Reinstein’s nomogram utilized multivariate regression analysis of several variables including, but not limited to, WTW, ACD, STS, STS lens rise (STSL), ICL power, ATA, ciliary body inner diameter (CBID), and scotopic pupil size.9 According to his model the CBID was the most predictive of postoperative vault height.9 Key preoperative measurements were obtained using the UBM ArcScan Insight® (ArcScan Inc.,). This is a very high frequency ultrasound device (50 MHz) that provides higher resolution images of the posterior chamber that may be otherwise difficult to obtain with anterior segment optical coherence tomography (AS-OCT). The transducer is robotically controlled reducing the inconsistency of measurements in operator held UBM probes. The calculator provides the predicted vault measurements for each available size of ICL. Lens size recommendations utilizing the Dougherty, Kojima, and Nakamura formulas are also provided in the calculator.9 A study from Reinstein compared his nomogram to other established ICL sizing nomograms using achieved vault height relative to target vault height intended by the various other formulas focused on WTW and ATA measurements.9 The study found a significantly lower interquartile range (IQR) of vault height with his current formula at 131 µm compared to other formulas which have IQRs ranging from 200–300 µm.9 In addition, the study looked at the delta between predicted vault and target vault based on the formula.9 Sixty-two percent of eyes were within 100 µm of target vault and 94% were within 300 µm of target vault using the Reinstein V2 formula.9 This is in comparison to other formulas that predicted within 100 µm 18–45% and within 300 µm 51–90%.9 The calculator can be found here: www.iclsizing.com.9
Kojima Nomogram
Kojima et al utilized multiple regression analysis to develop a formula based upon ACD, STS, and STSL with preoperative measurements obtained through UBM.10 This formula was based on a dataset of 47 eyes from Japanese patients.10 The 3-month postoperative vault distribution with this formula was compared to the predicted vaults of the manufacturers and Dougherty’s nomograms and it was found that Kojima formula was superior in selecting for moderate sized vaults (150–1,000 µm).10 The mean vault error (postoperative vault-predicted vault) was 0.06 ± 0.29 mm.10 Kojima formula:
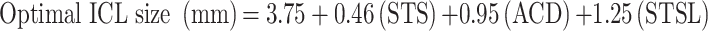 |
KS Nomogram
Igarashi et al developed a formula based on ATA distance in a Japanese population.11,12 The formula has two iterations (Table 3). The First version (KS V1) relied upon ATA and WTW measurement covering 44 eyes of Japanese patients.11 Significant correlation between ICL size, postoperative vault, and ATA was found with Spearman rank correlation coefficient r=0.59.11 The second version (KS V2) was created because it was determined that the ICL size influenced vault prediction error (postoperative vault-predicted vault).12 For example, at ICL size 12.1 mm the postop vault was smaller than predicted by KS V1 formula and postop vault for 13.2 and 13.7 mm was larger than predicted by KS formula.11,12
Table 3.
Nomogram Formulas for KS Versions 1, 2 and Nakamura Version 3
| Nomogram Version | Formula | |
|---|---|---|
| KS V111 | Predicted postoperative vault= 660.9 × (ICL size[mm]-ATA[mm]) + 86.611 | |
| KS V212 | ICL Size | Multiply KS V1 value by: |
| 12.1 mm | 0.8 | |
| 13.2 mm, 13.7 mm | 1.3 | |
| 12.6 mm | No scaling needed; use KS V1 | |
| Nakamura Version 3 Formula Optimal ICL Size (mm) | 5.307 + 0.617 (ACW [mm]) + 0.501 (CLR[mm])12 | |
| Nakamura Version 3 Formula Predicted vault (mm) | Predicted vault (mm)= 0.5 + 0.729 × (Implanted ICL size-optimal ICL size)12 | |
Nakamura Nomogram (NK V1–V3)
The Nakamura (NK) formula focuses on preoperative measurements such as STS, STSL, and ACD.13–15 It has three iterations. In its first iteration the authors state that the ICL size selected for implantation was too large leading to an overrepresentation of high vault.13 The formula was amended to NK version 2 (NK V2) which led to more accurate selection of ICL size and less high vault.14 The formula was based upon the theory that horizontal compression of the ICL led to increased vault which was tested in vitro using a specific device that could apply compression to measure corresponding vault increase.13,14 As a result they developed a horizontal compression coefficient of 1.1 reflecting that 1.0 mm of horizontal compression leads to an increased vault of 1.1 mm.13,14 This coefficient was utilized in their previous equations. To better reflect physiological conditions, they performed stepwise multiple regression analysis over a sample of 115 eyes to devise the third iteration (NK V3) of the formula which uses variables such as anterior chamber width (ACW equal to SS-SS or SP-SP) and CLR (Table 3).15 From this analysis a smaller horizontal compression coefficient of 0.729 was calculated.15 When compared to the second iteration NK V2 the NK V3 showed less error between predicted and achieved vault.15
Shen Nomogram (ML-derived)
Shen et al used various models of machine learning (ML) to predict ICL sizing and vault measurement.16 This was based on a cross-sectional study of patients who underwent ICL implantation in China from 2015–2020 covering 6297 eyes and 3536 patients.16 To predict ICL sizing and vault measurement 18 parameters were plugged into various machine learning models.16 Important parameters include pupil size, ACA, AL, corneal thickness (CT), etc. (Only the top 10 parameters of importance included; refer to Table 1.) ML models had high predictive accuracy for ICL sizing including random forest (82.2%), gradient boosting (81.5%), and XGBoost (81.8%).16 These three models of ML demonstrated similar accuracies when predicting ICL sizes 12.6 mm and 13.2 mm with XGBoost showing highest accuracy in prediction of 12.1 mm size compared to the other two models.16
Kamiya Nomogram (ML-Derived)
Kamiya et al developed an ML model using several methods such as support vector, random forest, gradient boost, and linear regression to predict the postoperative vault using preoperative parameters such as ICL size, WTW, ACD, ATA, CLR, ACW (equal to SS-SS or SP-SP), lens vault (LV), etc.17 The sample consisted of 1,745 eyes.17 One-month postop vault was compared to predicted vault. All the ML methods that predicted postop vault showed significantly less mean absolute error (MAE) and greater proportion of eyes within 50–200 µm of target ICL vault compared to the manufacturer’s nomogram.17 Random forest had the lowest MAE out of all the ML methods.17
Russo Nomogram
Russo et al looked at the accuracy of ML to compare predicted vault vs actual vault in a sample of European participants covering 561 eyes using AS-OCT measurements.18 Correlations for predicted vault vs actual vault with various ML models used in their study were high with random forest (R2=0.57), gradient boosting (R2=0.68), and XGBoost (R2=0.63).18
The random forest model predicted actual vault within ±250 µm in 90% of test eyes while extra tree predicted ±250 µm in 94% of test eyes.18 This is compared to the manufacturer’s nomogram which predicted within ±250 µum for 72% of test eyes.18 In addition, classifiers were used with the extra tree model to train the model on two different subsets of sample eyes to achieve better accuracy.18 The model was trained on sample of eyes within ideal range of 250–750 µm with the model achieving 98% accuracy of predicting actual vault.18 It was then trained on a subset of sample eyes with extreme vaults either below 250 µm or above 750 µm with the model achieve 90% accuracy.18 The model can be found here: https://www.centrooculisticobresciano.it/download/18
Recommendations
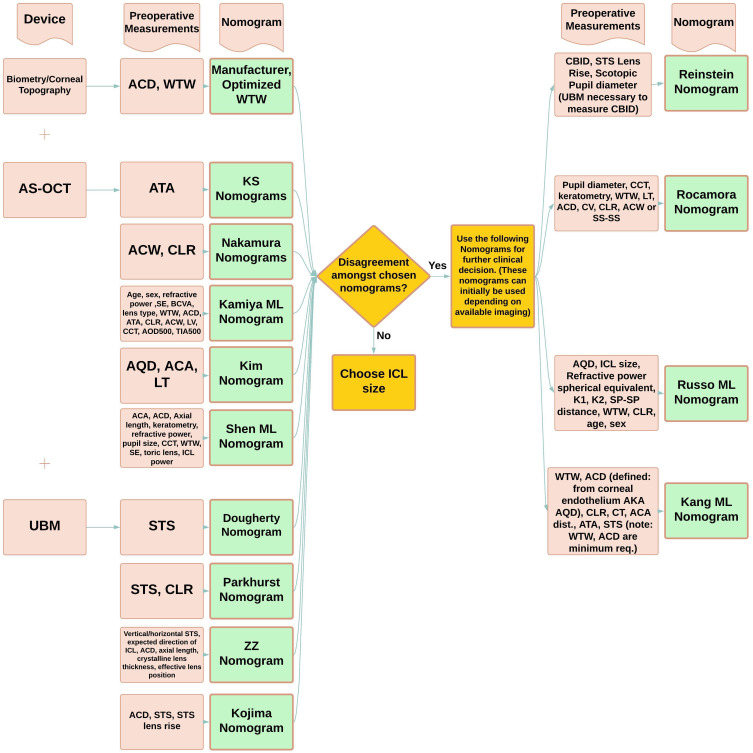
Based upon the literature there are several nomograms that will allow clinicians to focus on optimizing ICL sizing given their available imaging tools and preoperative measurements at disposal. A ICL nomogram flowchart has been developed (Figure 4) to help guide clinicians in their decision.
Figure 4.
Nomogram Decision Flowchart. Peach boxes refer to theme (device, preoperative measurements, nomogram), device type (biometry, corneal topography, AS-OCT, UBM), or specific preoperative measurements. Green boxes refer to specific nomograms. Yellow boxes refer to instructions to guide decision-making in selecting a nomogram.
Abbreviations: ACA, anterior chamber angle; ACW, anterior chamber width; ACD, anterior chamber depth; AQD, aqueous depth; ATA, angle to angle distance; BCVA, best corrected visual acuity; CBID, ciliary body inner diameter; CCT, central corneal thickness; CLR, crystalline lens rise; K1, flat keratometry; K2, steep keratometry; LV, lens vault; ICL, implantable collamer lens; SE, spherical equivalent; SP-SP, scleral spur to scleral spur; STS, sulcus-to-sulcus; UBM, ultrasound biomicroscopy; WTW, white-to-white; AOD500, angle of opening distance at 500 µm; TIA500, trabecular iris angle at 500 µm.
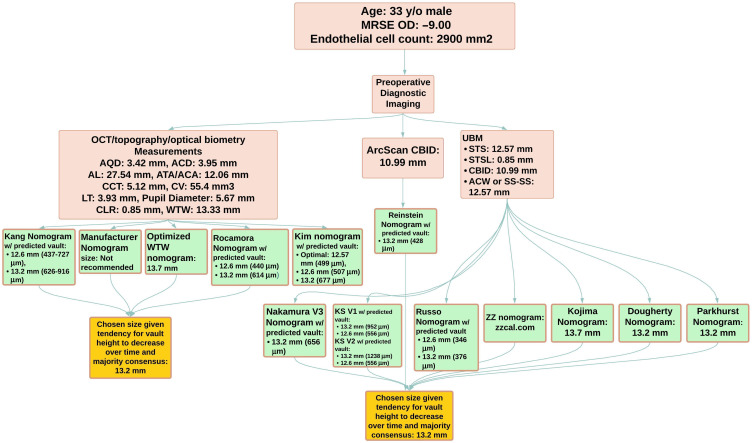
For example, most clinicians performing pIOL implantation will likely have a biometry and topography device with applications for measuring WTW, ACD, AQD, CLR, keratometry parameters, ATA, or ACA distance (ATA distance=ACA distance), ACW (ACW equal to scleral spur to scleral spur distance [SS-SS or SP-SP]), AL, CT, lens thickness, corneal volume (CV), and pupil diameter. Given these preoperative measurements the clinician can use the manufacturer, optimized WTW, Kang, Kim, and Rocamora nomograms (Figure 5). If a clinician has a UBM then one can expand upon the preoperative measurements by adding STS (both horizontal and vertical distance), STSL, CBID. In this scenario the physician can utilize all available nomograms that require UBM (Figure 5) cited in this review to weigh several ICL sizing options. If there is agreement among selected nomograms, then the physician can proceed with choosing a size. However, there may be disagreement among the nomograms which would require further clinical judgement. Part of this judgement is deciding what is the majority size recommendation among the nomograms and which predicted postoperative vault for each size will be safe for patients. The Kang, Rocamora, Russo, Kim, Nakamura, KS, ZZ, and Reinstein nomograms will provide a predicted postoperative vault based on ICL size for this purpose (Figure 5).
Figure 5.
An Example of a Clinical Case Using Existing Nomograms. Peach boxes refer to optical device measurements of a case patient. Green boxes refer to specific nomograms with their predicted implantable collamer lens sizes and predicted vault height. Yellow boxes refer to the final chosen size of the implantable collamer lens based on this patient case.
Abbreviations: ACA, anterior chamber angle; ACD, anterior chamber depth; ACW, anterior chamber width; AL, axial length; AQD, aqueous depth; ATA, angle to angle distance; CBID, ciliary body inner diameter; CCT, central corneal thickness; CLR, crystalline lens rise; CV, corneal volume; LT, lens thickness; STS, sulcus-to-sulcus; STSL, sulcus-to-sulcus lens rise; SS-SS, scleral spur to scleral spur; UBM, ultrasound biomicroscopy; WTW, white-to-white.
Discussion
ICL sizing has become the focus of pIOL implantation as it affects vault height and subsequent postoperative outcomes. If vault height is too low there is risk of contact between implanted lens and crystalline lens leading to cataracts. If vault height is too high there is risk of angle-closure glaucoma, endothelial cell damage, and pigment dispersion. Literature suggests that clinicians have implanted lenses with vaults lower than 250 and higher than 750 without postoperative complications.21 However, it is widely considered that 250–750 µm is a safe target for postoperative value.21,22 Clinical judgement is still a major factor in making these decisions as performing a lens exchange on a patient that is asymptomatic may not be reasonable.
Surgeons may differ in their comfort level with an acceptable postoperative vault height, taking into consideration that postoperative vault height may shift over time. Literature suggests that postoperative vault height generally decreases over time in the first 3–6 months and stabilizes within 6–12 months.21,23–25 One study that utilized ML to understand which factors affect postoperative vault height showed orientation of implantation to be a significant factor.26 In this study they found that a vertical orientation of implantation led to vault height decreases of 59.35 µm and 161.99 µm in the 12.6 mm and 13.2 mm ICLs respectively.26 Given these factors the surgeon may consider a larger ICL size to compensate for this decrease over time.
Another factor that may affect vault height is the light intensity.27,28 Theoretically, in photopic conditions miosis occurs and the iris pushes down on the ICL leading to increased chamber angle, ATA, and ACD with compensatory increase in lens rise. A study by Gonzalez-Lopez et al found that mean vault height under maximum miosis was 374 ± 208 um and 540 ± 252 µm under maximum mydriasis.29 The mean vault range (VR: difference between vault under maximal photopic and scotopic conditions) was 167 ± 70 µm. It was also found that that ICL tends to move more under higher vault conditions with mean VR 211 ± 77 µm compared to lower vault conditions with mean VR 122 ± 52 µm.29 Thus, clinicians should consider how lighting conditions affect vault height measurement.
Intraoperative measurement of vault during ICL implantation may be a useful tool to allow a more desirable postoperative vault. For example, an observational cohort study by Torbey et al examined 45 eyes of 26 patients undergoing ICL implantation and found that OCT intraoperative vault measurement of these patients had a high predictability of postoperative vault at 3 months (R2 = 0.81, P<0.001).30 ICL implantation of one eye may also inform postoperative vault outcome of the second eye allowing the clinician to make an informed decision as to whether to bilaterally implant an ICL. A study by Martinez-Plaza et al examining 40 eyes of 20 patients undergoing bilateral EVO+ ICL implantation found the vault of the first eye implanted at postop day 1 of these patients highly predicted the vault of the second implanted eye (R2 = 0.87 and P<0.001) with mean inter-eye difference of −0.95 µm.31 These evidence-based considerations can aid the clinician in making decisions on further ICL implantation or reimplantation given a particular vault height.
The initial nomogram developed by the manufacturer of the EVO+-ICL (STAAR surgical, Monrovia, CA, USA) is commonly used in clinical practice, but its sizing accuracy may be poor with the 13.7 mm size.3 To improve sizing accuracy other nomograms have been developed with linear regression, multivariate regression, and various models of ML through artificial intelligence. These nomograms not only indicate appropriate size, but also calculate a predicted vault height, which has been validated against postoperative vault measurements. Clinical application of these nomograms depends on the preoperative measurements that can be obtained by the physician. Clinicians must consider which diagnostic devices are available within practice as this will determine which nomograms are available for use.
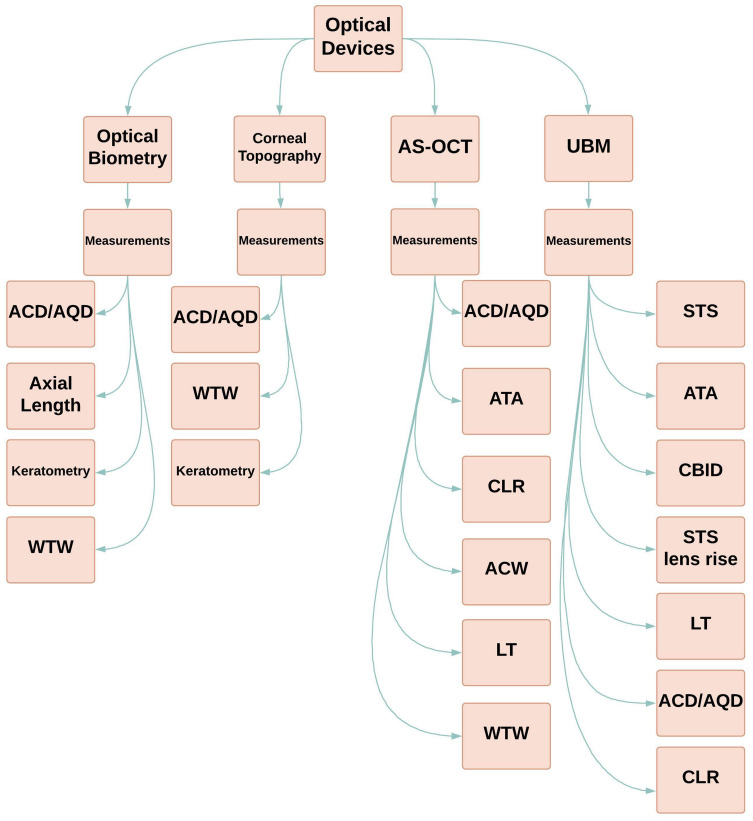
In general, most clinicians can obtain preoperative measurements required by many nomograms through use of either biometry or AS-OCT (Figure 6). It is important to note limitations of the AS-OCT in detecting more posterior measurements and structures such as ciliary body, STS, and scleral spur. In contrast, UBM utilizes very high-frequency ultrasound (35–100 MHz) allowing these measurements.32 However, UBM measurement is subject to operator error with holding the transducer.
Figure 6.
Optical Devices and their Obtainable Preoperative Measurements.
Abbreviations: ACD, anterior chamber depth; ACW, anterior chamber width; AQD, aqueous depth; AS-OCT, anterior segment optical coherence tomography; ATA, angle-to-angle distance; CBID, ciliary body inner diameter; CLR, crystalline lens rise; LT, lens thickness; STS, sulcus-to-sulcus; UBM, ultrasound biomicroscopy; WTW, white-to-white.
There can also be discordant preoperative measurements between different devices.33,34 Discussion of this topic is beyond the scope of this paper. However, one limitation of utilizing these nomograms is that each nomogram was derived from preoperative measurements using different devices which may not agree. It may be prudent to average preoperative measurements within each individual device and/or across devices depending upon which type of optical equipment is available.
Another limitation to consider is the race of the sample population by which each nomogram was developed. Most nomograms were based on Asian populations while four were based on Caucasian populations. More studies are needed to reflect a racially diverse population. The limited option of only four ICL sizes evokes the idea that perhaps a diverse patient population may benefit from other intermediate sizes. Other proposed intermediate sizes may potentially include 12.3, 12.9, and 13.4 mm.
In conclusion, ICL sizing and its effect on vault height has become important to clinicians as they consider ICL implantation and the risk of postoperative complications. Nomograms are a valuable tool in deciding the best ICL size and are based on several preoperative measurements that are not universally agreed upon as necessary. Both traditional statistical models and ML have been utilized in the development of these nomograms and each have their strengths and weaknesses.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Hirose F, Hata M, Ito S, Matsuki T, Kurimoto Y. Light–dark changes in iris thickness and anterior chamber angle width in eyes with occludable angles. Graefe’s Arch Clin Exper Ophthalmol. 2013;251(10):2395–2402. doi: 10.1007/s00417-013-2378-4 [DOI] [PubMed] [Google Scholar]
- 2.Packer M. Evaluation of the EVO/EVO+ Sphere and Toric Visian ICL: six month results from the United States food and drug administration clinical trial. Clin Ophthalmol. 2022;16:1541–1553. PMID: 35645557; PMCID: PMC9132105. doi: 10.2147/OPTH.S369467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moshirfar M, Placide J, Neves da Silva HV, et al. Assessing the efficacy of four diagnostic devices and four nomograms in posterior chamber phakic intraocular lens size selection. J Refract Surg. 2022;38(2):106–111. PMID: 35156462. doi: 10.3928/1081597X-20211109-01 [DOI] [PubMed] [Google Scholar]
- 4.Niu L, Miao H, Han T, Ding L, Wang X, Zhou X. Visual outcomes of Visian ICL implantation for high myopia in patients with shallow anterior chamber depth. BMC Ophthalmol. 2019;19(1):121. doi: 10.1186/s12886-019-1132-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kang EM, Ryu IH, Lee G, et al. Development of a web-based ensemble machine learning application to select the optimal size of posterior chamber phakic intraocular lens. Trans Vis Sci Tech. 2021;10:5. doi: 10.1167/tvst.10.6.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim T, Kim SJ, Lee BY, et al. Development of an implantable collamer lens sizing model: a retrospective study using ANTERION swept-source optical coherence tomography and a literature review. BMC Ophthalmol. 2023;23(23):59. doi: 10.1186/s12886-023-02814-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rocamora L, Orlando JI, Lwowski C, Kohnen T, Mertens E, Van Keer K. Postoperative vault prediction for phakic implantable collamer lens surgery: LASSO formulas. J Cataract Refract Surg. 2023;49(2):126–132. PMID: 36255226; PMCID: PMC9872858. doi: 10.1097/j.jcrs.0000000000001079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J, Shao J, Zheng L, Zhao X, Chen S. Implantable collamer lens sizing based on measurement of the sulcus-to-sulcus distance in ultrasound biomicroscopy video clips and ZZ ICL formula. BMC Ophthalmol. 2022;22(1):363. PMID: 36071422; PMCID: PMC9454160. doi: 10.1186/s12886-022-02583-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reinstein DZ, Archer TJ, Vida RS, Piparia V, Potter JG. New sizing parameters and model for predicting postoperative vault for the implantable collamer lens posterior chamber phakic intraocular lens. J Refract Surg. 2022;38(5):272–279. PMID: 35536711. doi: 10.3928/1081597X-20220302-01 [DOI] [PubMed] [Google Scholar]
- 10.Kojima T, Yokoyama S, Ito M, et al. Optimization of an implantable collamer lens sizing method using high-frequency ultrasound biomicroscopy. Am J Ophthalmol Elsevier. 2012;153:632–637e1. doi: 10.1016/j.ajo.2011.06.031 [DOI] [PubMed] [Google Scholar]
- 11.Igarashi A, Shimizu K, Kato S, Kamiya K. Predictability of the vault after posterior chamber phakic intraocular lens implantation using anterior segment optical coherence tomography. J Cataract Refractive Surg. 2019;45:1099–1104. doi: 10.1016/j.jcrs.2019.02.020 [DOI] [PubMed] [Google Scholar]
- 12.Igarashi A, Shimizu K, Kato S. Assessment of the vault after implantable collamer lens implantation using the KS formula. J Refract Surg. 2021;37(9):636–641. PMID: 34506239. doi: 10.3928/1081597X-20210610-06 [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Implantable collamer lens sizing method based on swept-source anterior segment optical coherence tomography. Am J Ophthalmol. 2018;187:99–107. doi: 10.1016/j.ajo.2017.12.015 [DOI] [PubMed] [Google Scholar]
- 14.Nakamura T, Isogai N, Kojima T, Yoshida Y, Sugiyama Y. Optimization of implantable collamer lens sizing based on swept-source anterior segment optical coherence tomography. J Cataract Refract Surg. 2020;46(5):742–748. PMID: 32358270. doi: 10.1097/j.jcrs.0000000000000134 [DOI] [PubMed] [Google Scholar]
- 15.Nakamura T, Nishida T, Isogai N, Kojima T, Sugiyama Y, Yoshida Y. Evaluation of implantable collamer lens sizing developed by reviewing the horizontal compression-vault coefficient. J Cataract Refract Surg. 2023;49(5):525–530. PMID: 36700937. doi: 10.1097/j.jcrs.0000000000001140 [DOI] [PubMed] [Google Scholar]
- 16.Shen Y, Wang L, Jian W, et al. Big-data and artificial-intelligence-assisted vault prediction and EVO-ICL size selection for myopia correction. Br J Ophthalmol. 2023;107:201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kamiya K, Ryu IH, Yoo TK, et al. Prediction of phakic intraocular lens vault using machine learning of anterior segment optical coherence tomography metrics. Am J Ophthalmol. 2021;226:90–99. doi: 10.1016/j.ajo.2021.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Russo A, Filini O, Savini DG, et al. Predictability of the vault after implantable collamer lens implantation using OCT and artificial intelligence in caucasian eyes. J Cataract Refract Surg. 2023;49:724–731. PMID: 36913536. doi: 10.1097/j.jcrs.0000000000001182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin F, Wang Y, Liu Y, Qu X, Zhou X. the influence of 0.5% tropicamide on anterior segment parameters with CASIA2 in emmetropic, myopic, and hyperopic eyes. Front Physiol. 2022;13:957097. PMID: 35903064; PMCID: PMC9315225. doi: 10.3389/fphys.2022.957097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruan X, Liang C, Xia Z, et al. In-vivo lens biometry using the novel ultrasound biomicroscopy. Front Med. 2022;9:777645. PMID: 35237620; PMCID: PMC8882853. doi: 10.3389/fmed.2022.777645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Packer M. Meta-analysis and review: effectiveness, safety, and central port design of the intraocular collamer lens. Clin Ophthalmol. 2016;10:1059–1077. PMID: 27354760; PMCID: PMC4907705. doi: 10.2147/OPTH.S111620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deshpande K, Shroff R, Biswas P, et al. Phakic intraocular lens: getting the right size. Indian J Ophthalmol. 2020;68(12):2880–2887. doi: 10.4103/ijo.IJO_2326_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li B, Chen X, Cheng M, et al. Long-term vault changes in different levels and factors affecting vault change after implantation of implantable collamer lens with a central hole. Ophthalmo Ther. 2023;12:251–261. doi: 10.1007/s40123-022-00606-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan Z, Miao H, Zhao F, et al. Two-year outcomes of Visian implantable collamer lens with a central hole for correcting high myopia. J Ophthalmol. 2018;2018:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidinger G, Lackner B, Pieh S, Skorpik C. Long-term changes in posterior chamber phakic intraocular collamer lens vaulting in myopic patients. Ophthalmology. 2010;117:1506–1511. doi: 10.1016/j.ophtha.2009.12.013 [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Li F, Li L, Zhang J. A quantitative study of the effect of ICL orientation selection on post-operative vault and model-assisted vault prediction. Front Neurol. 2023;14:1136579. PMID: 36937516; PMCID: PMC10020497. doi: 10.3389/fneur.2023.1136579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Lopez F, Mompean B, Bilbao-Calabuig R, Luezas J, Ortega-Usobiaga J, Druchkiv V. Predicting implantable collamer lens sizing. J Cataract Refract Surg. 2020;46(12):1692–1693. doi: 10.1097/j.jcrs.0000000000000433 [DOI] [PubMed] [Google Scholar]
- 28.Kato S, Shimizu K, Igarashi A. Vault changes caused by light-induced pupil constriction and accommodation in eyes with an implantable collamer lens. Cornea. 2019;38(2):217–220. PMID: 30371566; PMCID: PMC6344073. doi: 10.1097/ICO.0000000000001785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Lopez F, Mompean B, Bilbao-Calabuig R, Vila-Arteaga J, Beltran J, Baviera J. Dynamic assessment of light-induced vaulting changes of implantable collamer lens with central port by swept-source OCT: pilot study. Transl Vis Sci Technol. 2018;7(3). doi: 10.1167/tvst.7.3.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torbey J, Mehanna CJ, Abdul Fattah M, Awwad ST. Comparison of intraoperative vs postoperative optical coherence tomography measurement of implantable collamer lens vaulting. J Cataract Refract Surg. 2020;46(5):737–741. doi: 10.1097/j.jcrs.0000000000000119 [DOI] [PubMed] [Google Scholar]
- 31.Martínez-Plaza E, López-Miguel A, López-de la Rosa A, et al. Inter-eye and postoperative prediction of vault after implantation of EVO + Visian phakic implantable collamer lens. Int Ophthalmol. 2023;43:1501–1510. doi: 10.1007/s10792-022-02546-5 [DOI] [PubMed] [Google Scholar]
- 32.He M, Wang D, Jiang Y. Overview of Ultrasound Biomicroscopy. J Curr Glaucoma Pract. 2012;6(1):25–53. PMID: 27990069; PMCID: PMC5159457. doi: 10.5005/jp-journals-10008-1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ang RET, Reyes EKF, Ayuyao FAJ, et al. Comparison of white-to-white measurements using four devices and their determination of ICL sizing. Eye Vis. 2022;9(36):10.1186/s40662-022-00308–z. doi: 10.1186/s40662-022-00308-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tañá-Rivero P, Aguilar-Córcoles S, Rodríguez-Prats JL, Montés-Micó R, Ruiz-Mesa R. Agreement of white-to-white measurements with swept-source OCT, Scheimpflug and color LED devices. Int Ophthalmol. 2021;41(1):57–65. PMID: 32860152. doi: 10.1007/s10792-020-01552-9 [DOI] [PubMed] [Google Scholar]