Abstract
Background:
Previous studies have identified variability in molecular markers correlating with poorer survival outcomes in patients with right colon cancer (RCC) compared to left colon cancer (LCC). However, several studies have shown conflicting results when examined stage for stage. We examined RCC and LCC to assess for differences in histopathologic features and overall survival (OS)
Materials and Methods:
The National Cancer Data Base was used to identify patients with RCC and LCC from 2004 to 2013. A propensity adjusted analysis evaluating the association between primary site and OS was performed.
Results:
Of the 422,443 patients identified, 54.7% had RCC and 45.3% had LCC. For all stages, patients with RCC were older, had more poorly differentiated tumors, and a higher degree of microsatellite instability (MSI) than LCC. Patients with RCC also had more KRAS mutations than LCC. RCC patients had poorer 3- and 5-year OS at all stages, particularly stage 3 (62% vs. 73% and 50% vs. 62%, respectively, p<0.001). Median OS was 77.5 months for LCC compared to 62.3 months for RCC (p<0.001).
Conclusion:
This is one of the largest studies demonstrating that RCC and LCC are different biological entities. RCC had significantly higher rates of MSI for all stages, which has been previously shown to be prognostically advantageous. However, this study showed poorer OS at every stage of disease for RCC over LCC. These factors have important implications for the further use of targeted therapies in the treatment of advanced colon cancer.
Keywords: Colon Cancer, Tumor Sidedness, Molecular Markers
MicroAbstract
This is a large retrospective study of the National Cancer Database to investigate the possible underlying factors associated with poorer survival seen in right colon cancer compared to left. Right colon cancer patients were older, had more biologically aggressive tumors, more microsatellite instability and KRAS mutations; which more profoundly impacted survival especially at later stages of disease.
Introduction
Colon cancer is the second most frequent cause of cancer related death in the United States.1 Previous studies using the Surveillance, Epidemiology and End Results (SEER) and large single institution databases have noted variability in presentation, pathologic molecular markers, and survival outcomes between cancers in the right (RCC) and left colon (LCC).1–3 Several studies have demonstrated an increasing prevalence of RCC with poorer overall survival (OS) in these patients.2 However, multiple reports have demonstrated conflicting results when examining survival when stratified by stage. Some studies have shown significantly poorer OS of patients with RCC than LCC at stage 3 and stage 4 disease, while others found improved OS and cancer specific survival (CSS) for stage 1 and 2 RCC patients compared with LCC.1,4
The variation in histopathology between RCC and LCC is likely secondary to the different embryological origins of the proximal and distal colonic segments, namely the right colon from the midgut and the left colon and rectum from the hindgut.5 RCCs are more likely to be bulky and exophytic, present with anemia, and have diploid, poorly differentiated or mucinous histology.2 LCCs are often infiltrating, circumferentially constricting lesions that present with obstructive symptoms.2,5
Molecular biologic patterns also differ widely between RCC and LCC. RCCs have been shown to have higher rates of microsatellite instability (MSI), higher KRAS mutation rates, and greater expression of epidermal growth factor receptor (EGFR).2, 6, 7 LCCs are found to have more chromosomal instability and p53 tumor suppressor mutations.2,5 We sought to investigate the variability between RCC and LCC using a large cohort of patients within the National Cancer Data Base and assess the association of these differences on OS.
Materials and Methods
The Commission on Cancer’s (COC), National Cancer Data Base (NCDB) was used to identify patients from 2004– 2013 with a diagnosis of colon adenocarcinoma. This study was deemed exempt by our Institutional Review Board (IRB). Patients were queried from the colon Participant User Files (PUF) of the NCDB. Patients included had histologic colon adenocarcinoma. These patients were divided into those with right and left colon cancer via ICDO-3 (International Classification of Diseases for Oncology, Third Edition) topography codes. RCCs were those found in the cecum (C18.0), ascending colon (C18.2), and hepatic flexure (C18.3). LCCs were found within the splenic flexure (C18.5), descending colon (C18.6), and sigmoid (C18.7). Those with appendiceal, transverse colon, rectosigmoid, and rectal cancer were excluded from analysis.
Data collected included patient demographic variables (age, gender, race, insurance status, income, education level and distance from the hospital), tumor variables [tumor size, grade, clinical and pathologic TNM stage, pre-operative carcinoembryonic antigen (CEA), lymphovascular invasion (LVI), perineural invasion (PNI), microsatellite instability (MSI) status, KRAS mutation status, loss of heterozygosity (LOH), and resection margins]. Treatment variables such as facility location and type, surgical procedure as well as administration of chemotherapy, were also included. Comorbidities are recorded as the Charlson-Deyo Comorbidity Score, derived from the sum of scores for each comorbid condition listed in the Charlson Comorbidity Score Mapping Table.
Outcomes examined were divided into short and long term outcomes and stratified by stage. Short term outcomes analyzed were length of stay (LOS), rates of unplanned 30-day readmission, 30-day and 90-day mortality. Long term outcomes were 3- and 5-year OS rates as well as median OS.
Statistical Analysis
Patient characteristics were reported by primary site (RCC vs. LCC), with comparisons were made using the Mann-Whitney U and Pearson chi-square tests for continuous and categorical variables, respectively. OS was summarized by primary site using standard Kaplan-Meier methods, where estimates of median OS and 3/5-year OS rates were obtained with 95% confidence intervals. Comparisons were made using the log-rank test.
A propensity adjusted (PA-) analysis was conducted to evaluate the association between primary site and outcomes while adjusting for patient characteristics. The propensity scores for primary site were obtained using a logistic regression model based on patient demographic/clinical characteristics. For short term outcomes, the propensity score is based on facility type and location, age, gender, race, insurance status, income, education, urban/rural setting, hospital distance, Charlson-Deyo score, primary or subsequent malignancy, tumor grade, tumor size, pathologic stage, circumferential resection margin (CRM) status, surgical procedure, and radiation/chemotherapy treatment sequence. The OS analysis adjusted for these variables and regional nodes examined and positive, metastatic disease at evaluation, surgical margins, and unplanned hospital readmission. The other variables listed in Tables 1 or 2 were excluded due to missing data.
Table 1.
Demographic, Clinicopathologic and Treatment Information for Patients with Right vs. Left Sided Colon Cancer
| Right | Left | Overall | P-value | ||
|---|---|---|---|---|---|
| Overall | N | 231,026 (54.7) | 191,417 (45.3) | 422,443 (100%) | |
| Age (years) | Mean/Std | 71.3/12.7 | 65.9/13.6 | 68.8/13.4 | <.001 |
| Gender | Male | 104,083 (45.1%) | 102,395 (53.5%) | 206,478 (48.9%) | <.001 |
| Female | 126,943 (54.9%) | 89,022 (46.5%) | 215,965 (51.1%) | ||
| Race | White | 194,665 (85.0%) | 156,679 (82.7%) | 351,344 (83.9%) | <.001 |
| Black | 28,288 (12.3%) | 23,991 (12.7%) | 52,279 (12.5%) | ||
| Asian | 4,482 (2.0%) | 6,993 (3.7%) | 11,475 (2.7%) | ||
| Other | 1,644 (0.7%) | 1,892 (1.0%) | 3,536 (0.8%) | ||
| Insurance | Not Insured | 6,203 (2.7%) | 7,778 (4.1%) | 13,981 (3.4%) | <.001 |
| Private | 63,153 (27.8%) | 72,540 (38.6%) | 135,693 (32.7%) | ||
| Government | 157,954 (69.5%) | 107,692 (57.3%) | 265,646 (64.0%) | ||
| Setting | Metro counties | 188,595 (84.5%) | 156,372 (84.6%) | 344,967 (84.5%) | 1.000 |
| Urban | 30,160 (13.5%) | 24,962 (13.5%) | 55,122 (13.5%) | ||
| Rural | 4,341 (1.9%) | 3,599 (1.9%) | 7,940 (1.9%) | ||
| Facility Type | CCP | 34,448 (15.1%) | 28,634 (15.4%) | 63,082 (15.2%) | <.001 |
| Comprehensive CCP | 118,732 (52.1%) | 93,843 (50.5%) | 212,575 (51.4%) | ||
| Academic/Research Program | 58,217 (25.5%) | 49,689 (26.7%) | 107,906 (26.1%) | ||
| Integrated Network Cancer Program | 16,260 (7.1%) | 13,386 (7.2%) | 29,646 (7.2%) | ||
| Other | 279 (0.1%) | 254 (0.1%) | 533 (0.1%) | ||
| Charlson Deyo Score | 0 | 155,255 (67.2%) | 138,034 (72.1%) | 293,289 (69.4%) | <.001 |
| 1 | 54,128 (23.4%) | 39,506 (20.6%) | 93,634 (22.2%) | ||
| 2 | 21,643 (9.4%) | 13,877 (7.2%) | 35,520 (8.4%) | ||
| Tumor Size (cm) | No tumor | 31 (0.0%) | 56 (0.0%) | 87 (0.0%) | <.001 |
| < 1 | 5,907 (2.9%) | 6,437 (4.1%) | 12,344 (3.4%) | ||
| 1–2 | 12,325 (6.1%) | 12,988 (8.2%) | 25,313 (7.0%) | ||
| 2–3 | 25,467 (12.5%) | 22,075 (13.9%) | 47,542 (13.1%) | ||
| 3–4 | 36,231 (17.8%) | 31,049 (19.6%) | 67,280 (18.6%) | ||
| 4–5 | 36,734 (18.0%) | 30,462 (19.2%) | 67,196 (18.6%) | ||
| >= 5 | 86,854 (42.7%) | 55,583 (35.0%) | 142,437 (39.3%) | ||
| Clinical T Stage | 0 | 2,870 (3.5%) | 2,993 (4.3%) | 5,863 (3.9%) | <.001 |
| 1 | 19,284 (23.8%) | 20,317 (29.0%) | 39,601 (26.2%) | ||
| 2 | 12,524 (15.5%) | 9,166 (13.1%) | 21,690 (14.4%) | ||
| 3 | 35,107 (43.4%) | 27,652 (39.4%) | 62,759 (41.5%) | ||
| 4 | 11,139 (13.8%) | 9,993 (14.3%) | 21,132 (14.0%) | ||
| Clinical N Stage | 0 | 91,522 (76.6%) | 78,603 (79.2%) | 170,125 (77.7%) | <.001 |
| 1 | 18,310 (15.3%) | 13,960 (14.1%) | 32,270 (14.7%) | ||
| 2 | 9,725 (8.1%) | 6,702 (6.8%) | 16,427 (7.5%) | ||
| Clinical M Stage | 0 | 192,975 (87.3%) | 156,618 (85.4%) | 349,593 (86.4%) | <.001 |
| 1 | 28,123 (12.7%) | 26,787 (14.6%) | 54,910 (13.6%) | ||
| Clinical Stage | 0 | 2,452 (2.5%) | 2,663 (3.1%) | 5,115 (2.8%) | <.001 |
| 1 | 26,924 (27.6%) | 24,973 (29.3%) | 51,897 (28.4%) | ||
| 2 | 23,247 (23.9%) | 18,052 (21.2%) | 41,299 (22.6%) | ||
| 3 | 16,283 (16.7%) | 12,384 (14.5%) | 28,667 (15.7%) | ||
| 4 | 28,541 (29.3%) | 27,178 (31.9%) | 55,719 (30.5%) | ||
| CEA | Elevated | 59,429 (45.9%) | 51,648 (49.0%) | 111,077 (47.3%) | <.001 |
| Normal | 70,110 (54.1%) | 53,654 (51.0%) | 123,764 (52.7%) | ||
| Surgical Approach | None | 15,367 (16.8%) | 14,353 (19.5%) | 29,720 (18.0%) | <.001 |
| Open/Converted | 46,195 (50.4%) | 36,880 (50.0%) | 83,075 (50.2%) | ||
| Laparoscopic | 28,638 (31.2%) | 21,115 (28.6%) | 49,753 (30.1%) | ||
| Robot | 1,538 (1.7%) | 1,398 (1.9%) | 2,936 (1.8%) | ||
| Chemotherapy | No | 145,971 (66.0%) | 108,156 (59.0%) | 254,127 (62.8%) | <.001 |
| Yes | 75,187 (34.0%) | 75,065 (41.0%) | 150,252 (37.2%) | ||
| Chemotherapy Sequence | None | 126,305 (70.0%) | 94,216 (63.5%) | 220,521 (67.1%) | <.001 |
| Neoadjuvant | 1,791 (1.0%) | 3,011 (2.0%) | 4,802 (1.5%) | ||
| Adjuvant | 52,312 (29.0%) | 51,113 (34.4%) | 103,425 (31.5%) | ||
Government Insurance- Medicare or Medicaid, Metro Counties- counties in metro areas of < 250,000 to > 1 million population, Urban- 2500 - >20,000 population, Rural- < 2500 population, CCP-Community Cancer Program, Academic Research Programs (includes NCI-designated comprehensive cancer centers). P-values are adjusted using the Holm-Bonferroni method.
Table 2.
Univariate analysis of operative, pathologic and oncologic factors as well as molecular markers and short and long term outcomes of RCC compared to LCC
| Right Colon Cancer No. patients |
RCC Total Available (%) | Left Colon Cancer No. patients |
LCC Total Available (%) | P value | ||
|---|---|---|---|---|---|---|
| Total Number (%) | 231,026 | 54.7 | 191,417 | 45.3 | ||
| Operative Factors | Positive Surgical Margins | 13,811 | 203,882 (6.8) | 12,181 | 165,967 (7.3) | <.001 |
| Positive CRM | 5,866 | 207,356 (2.8) | 4,783 | 169,897 (2.8) | 1.00 | |
| Nodes Examined (> 10) | 174,942 | 227,740 (76.8) | 119,460 | 188,243 (63.4) | <.001 | |
| Pathologic Factors | Positive Lymph nodes | 86,210 | 203,863 (42.3) | 70,784 | 161,210 (43.9) | <.001 |
| Well Differentiated Tumors | 19,276 | 215,929 (8.9) | 17,909 | 174,995 (10.2) | <.001 | |
| Moderately Differentiated | 142,864 | 215,929 (66) | 131,977 | 174,995 (75) | <.001 | |
| Poorly Differentiated Tumors | 48,639 | 215,929 (22.5) | 22,905 | 174,995 (13.1) | <.001 | |
| Undifferentiated Tumors | 5,119 | 215,929 (2.4) | 2,148 | 174,995 (1.2) | <.001 | |
| LVI | 23,809 | 75,161 (31.7) | 17,048 | 57,569 (29.6) | <.001 | |
| PNI | 9,141 | 76,079 (12.0) | 8,190 | 59,153 (13.8) | <.001 | |
| Oncologic Factors | Metastases at Diagnosis | 42,325 | 225,815 (18.7) | 38,964 | 186,774 (20.9) | <.001 |
| Liver Metastases | 13,039 | 90,479 (14.4) | 12,714 | 72,627 (17.5) | <.001 | |
| Lung Metastases | 3,354 | 90,261 (3.7) | 3,407 | 72,409 (4.7) | <.001 | |
| Brain Metastases | 282 | 90,295 (0.3) | 195 | 72,426 (0.3) | 0.44 | |
| Tumor Deposits | 4,741 | 71,680 (6.6) | 4,572 | 55,590 (8.2) | <.001 | |
| Molecular Markers | MSI | 5,871 | 18,676 (31.4) | 2,413 | 14,443 (16.7) | <.001 |
| KRAS Mutations | 5,183 | 11,512 (45.0) | 3,439 | 10,216 (33.7) | <.001 | |
| Outcomes | LOS > 1 week | 56,233 | 191,335 (29.4) | 47,023 | 155,095 (30.3) | <.001 |
| Unplanned Readmission | 12,052 | 224,655 (5.4) | 9,064 | 185,758 (4.9) | <.001 | |
| 30 Day Mortality | 7,794 | 184,206 (4.2) | 5,438 | 151,751 (3.6) | <.001 | |
| 90 Day Mortality | 14,322 | 183,254 (7.8) | 9,385 | 150,919 (6.2) | <.001 | |
| Three- year Survival (%) | 61.0 | 67.0 | <.001 | |||
| Five- Year Survival (%) | 51.0 | 56.0 | <.001 | |||
| Median Overall Survival (months) | 62.3 | 77.5 | <.001 | |||
CRM- Circumferential Resection Margin, LVI- Lymphovascular invasion, PNI- Perineural invasion, MSI- Microsatellite Instability, LOH- Loss of Heterozygosity, LOS- Length of Stay, P-values are adjusted using the Holm-Bonferroni method.
Multivariable logistic and Cox regression models were then fit for each outcome using primary site and propensity score as predictor variables, with adjusted odds/hazard ratios (OR/HRs) for primary site and corresponding 95% confidence intervals obtained from model estimates.
All analyses were conducted in the overall sample and within each clinical stage using SAS v9.4 (Cary, NC) at a nominal significance level of 0.05. The Holm-Bonferroni method was used to control the family-wise error rate within each set of analyses.
Results
There were 422,443 patients identified in the NCDB colon database from 2004 to 2013. Of these, 54.7% of patients had RCC and 45.3% had LCC (Table 1). Patients with RCC were significantly more likely to be female (54.9% vs. 46.5%, p<0.001), older (71.7% over age 65 compared to 55.2% for LCC) and have more co-morbidities. In this study, patients with RCC were more likely to be white and more patients with LCC were black and Asian. RCC patients more frequently had government rather than private insurance, however, more LCC patients were uninsured.
Pathologic grade significantly differed between RCC and LCC, with LCC having a higher proportion of well differentiated tumors and RCC having more poorly differentiated and undifferentiated tumors (Table 2). At all stages, RCC had more nodes examined than left, with 76.8% patients having more than 10 lymph nodes collected, compared with 63.4% in LCC, p<0.001. LCC had more positive nodes overall (43.9% vs. 42.3%, p<0.001) as well as more patients with tumor deposits (8.2% vs. 6.6%, p<0.001). On univariate analysis, LCC had a higher incidence of metastatic disease at diagnosis (20.9% vs. 18.7, p<0.001) as well as more patients with liver and lung metastases (Table 2). PA-analysis (Table 3) showed that RCC more commonly had metastatic disease to the brain than LCC; but affected a small number of patients overall (OR 2.14, CI 1.12–4.07, p=0.009).
Table 3-.
Multivariate Analysis of Short and Long-Term Outcomes of Right (RCC) vs Left Colon Cancer (LCC)
| Univariate Analysis | Propensity Adjusted Analysis | |||||
|---|---|---|---|---|---|---|
| Outcome | RCC vs LCC OR/HR (95% CI) | P-value | N | RCC vs LCC OR/HR (95% CI) | P-value | N |
| Positive Surgical Margins | 0.92 (0.89, 0.94) | <.001 | 369,849 | 0.94 (0.89, 0.99) | 0.009 | 237,748 |
| Nodes Examined > 10 | 1.91 (1.88, 1.93) | <.001 | 415,983 | 1.90 (1.83, 1.97) | <.001 | 238,713 |
| Positive Lymph Nodes | 0.94 (0.92, 0.95) | <.001 | 365,073 | 1.01 (0.98, 1.03) | 0.97 | 238,107 |
| LVI | 1.10 (1.08, 1.13) | <.001 | 132,730 | 1.04 (0.99, 1.10) | 0.13 | 85,593 |
| Metastases at Diagnosis | 0.88 (0.86, 0.89) | <.001 | 412,589 | 0.99 (0.97, 1.02) | 0.97 | 238,493 |
| Liver Metastases | 0.79 (0.77, 0.82) | <.001 | 163,106 | 0.95 (0.88, 1.02) | 0.25 | 91,827 |
| Lung Metastases | 0.78 (0.74, 0.82) | <.001 | 162,670 | 0.92 (0.80, 1.07) | 0.84 | 91,731 |
| Brain Metastases | 1.16 (0.96, 1.40) | 0.44 | 162,721 | 2.14 (1.12, 4.07) | 0.009 | 91,763 |
| LOS > 1 week | 0.96 (0.94, 0.97) | <.001 | 346,430 | 0.75 (0.73, 0.78) | <.001 | 221,455 |
| Readmission | 1.11 (1.08, 1.14) | <.001 | 410,413 | 1.02 (0.97, 1.08) | 0.94 | 234,702 |
| 30-Day Mortality | 1.19 (1.15, 1.23) | <.001 | 335,957 | 0.85 (0.79, 0.92) | <.001 | 212,159 |
| 90-Day Mortality | 1.28 (1.24, 1.31) | <.001 | 334,173 | 0.92 (0.87, 0.97) | <.001 | 211,184 |
| Overall Survival | 1.20 (1.18, 1.21)) | <.001 | 379,785 | 1.06 (1.04, 1.09) | <.001 | 204,825 |
LVI- Lymphovascular invasion, PNI- Perineural invasion, LOS- Length of Stay, N-number of observations included in the analysis, P-values are adjusted using the Holm-Bonferroni method.
RCCs had more patients with LVI (31.7 vs. 29.6%, p<0.001) than LCC but this trend was only observed in advanced stages. RCC had a higher incidence of LVI than LCC for stage 3 (52% vs. 44.3%, p<0.001) and stage 4 (61.7% vs. 54.9%, p<0.001) cancers. Conversely, there were fewer RCC patients with PNI than LCC (12.0% vs. 13.8%, p<0.001). However, this was not a statistically significant trend stage for stage, except in stage 2 (9.1% vs. 11%, p<0.001).
Overall, 31.4% of 18,676 RCC patients with MSI data collected were microsatellite unstable compared to 16.7% of 14,443 LCC patients, p<0.001. This was consistent when examining all stages of colon cancer. Similarly, KRAS was mutated more commonly in RCC than LCC (45.0% vs. 33.7%, p<0.001). This was the case in all stages, but only reached statistical significance in stage 3 (42.4% vs. 32.0%, p<0.001) and stage 4 (53.4% vs. 35.4%, p<0.001).
There also appeared to be some treatment differences between RCC and LCC. Open and laparoscopic surgery were more commonly performed in RCC with more robotic operations in LCC. RCC patients had fewer positive surgical margins than LCC (6.8% vs. 7.3%, p<0.001) with no difference in the CRM rate between the two groups. LCC patients were more likely to receive chemotherapy at all stages, especially in stage 3 (71.5% vs. 62.0%, p<0.001) (Table 1).
Differences in short and long-term outcomes between RCC and LCC were also investigated. Patients with RCC were less likely to stay longer than 7 days for every stage of disease (OR 0.75, CI 0.73–0.78, p<0.001). 30- and 90-day mortality were also lower in RCC compared to LCC overall (OR 0.85, CI 0.79–0.92 and OR 0.92, CI 0.87–0.97 respectively, p<0.001). However, in a stage specific analysis, only 30-day mortality for stage 2 cancers reached statistical significance (OR 0.77, CI 0.62–0.97, p=0.011).
There was a total of 379,785 patients were included in the OS analysis, with 171,321 deaths observed and median follow-up times (in months) of 61.7 (range: 0.04 – 133.6) and 62.5 (range: 0.04 – 131.6) for RCC and LCC, respectively. In contrast to short term outcomes, 3-year (61% vs. 67%) and 5-year (51% vs. 56%) OS were poorer for RCC compared to LCC (p<0.001). This was true for all stages and more pronounced for stage 3 and 4 patients. In PA-analysis, improved OS was observed for LCC compared with RCC (HR 1.06, CI 1.04–1.09, p<0.001). When examined stage for stage, Kaplan-Meier curves demonstrated poorer OS of RCC compared to LCC at all stages (Figure 1). However, only stage 3 (HR 1.19, CI 1.09– 1.31, p<0.001) and stage 4 (HR 1.26, CI 1.18–1.35, p<0.001) cancers showed a statistically significant difference. In examining the prognostic effect of chemotherapy, we found that it slightly improved median OS in the RCC group compared to patients not treated with chemotherapy (76 vs. 75.5 months, p<0.001). However, in LCC patients, the chemotherapy group had poorer median OS than the non-chemotherapy group (55.8 vs. 63.1 months, p<0.001), with these patients more likely to have positive surgical margins, node positivity and metastatic disease than RCC.
Figure 1-.
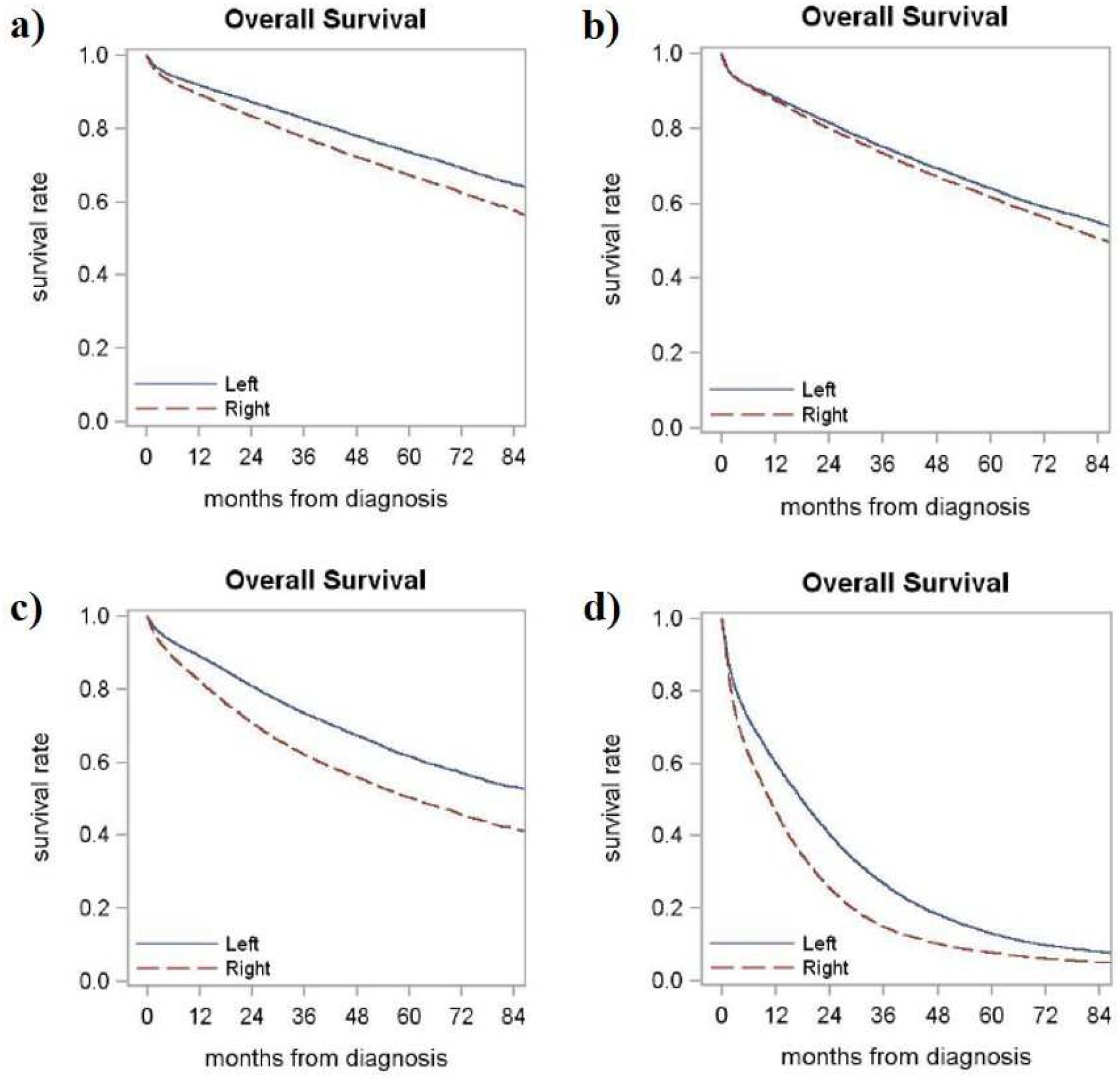
Kaplan Meier (KM) curves demonstrating poorer overall survival (OS) of right compared to left colon cancer at a) Stage 1 (median OS 101.2 vs. 129.3 months, p < 0.001), b) Stage 2 (median OS 85.5 vs. 98.9 months, p < 0.001), c) Stage 3 (median OS 60.8 vs. 94.3 months, p < 0.001) and d) Stage 4 (median OS 10.7 vs. 17.8 months, p < 0.001).
Discussion
This is the largest retrospective database analysis showing that RCC and LCC are likely different biological entities. They appear to have variable histopathologic features and molecular markers as well as differing presentations and survival outcomes. RCC has poorer OS at all stages; however, there is some disparity between studies when examining survival by stage. We found on univariate analysis that OS was significantly poorer for RCC compared to LCC for all stages of disease. However, on multivariate analysis, only stage 3 and stage 4 disease showed poorer survival for RCC vs. LCC. This stage based disparity has been noted in other studies with variable results. Weiss et al. found that RCC had poorer survival for all stages except Stage 2.1 Warschkow et al. demonstrated better OS and CSS for stage 1 and 2 of RCC over LCC as well as similar OS and CSS between both sides for stage 3 cancers.4 Benedix et al. in their study of 17,641 patients also found that 5-year OS was significantly higher for patients with LCC compared to RCC (71% vs. 67%, p<0.01).2 Similarly, a meta-analysis showed that LCC was significantly associated with a 19% reduced risk of death with a less pronounced difference demonstrated in early stages compared with advanced disease.8
Despite the poorer long-term outcomes of RCCs, they had improved operative and short-term outcomes compared to LCCs. The RCC cohort had more negative margins at resection and more than 10 pathologic nodes examined likely denoting more successful operations. Similarly, RCC had shorter hospital LOS and superior 30- and 90-day mortality than LCC. Patients with LCC also had higher incidence of liver and lung metastases and were also more likely to have node positive cancers.9 Even after controlling for age and co-morbidities, intrinsic pathologic differences may have contributed to poorer prognoses. These included the presence of more poorly differentiated and undifferentiated tumors as well as LVI, particularly in stage 3 and stage 4 disease. This may underscore the significantly poorer long-term survival of RCC compare to LCC at later stages of disease.
Patterns of molecular markers have also been found to differ between RCC and LCC.10 In this analysis, RCC correlated with higher proportion of tumoral MSI compared to LCC, despite being recorded in a minority of institutions within the NCDB. The right sided predilection for microsatellite unstable tumors has been demonstrated in other studies and is associated with being a positive prognostic factor for colon cancer especially in stage 2 and stage 3 patients.11, 12 Sinicrope et al. found that in the subgroup of patients with MSI, favorable disease-free survival (DFS) was observed in RCC but not LCC, with inferior DFS for RCC patients with proficient mismatch repair compared to LCC.13 Yaeger et al. also found that microsatellite stable RCC patients had poorer survival, older age at diagnosis and increased oncogenic mutations.14 MSI status did not appear to convey a survival advantage to RCC in our analysis, however, only OS rather than DFS was recorded. RCCs have also been associated with an increase in BRAF mutations which are closely correlated with MSI in colorectal cancers and carry a poor prognosis.6, 15–18 This, however, is not demonstrable in this study due to lack of documentation of BRAF status within the NCDB. The propensity for MSI-High RCC’s may also allow for the preferred treatment of these tumors with anti-PD-1 therapy which has demonstrated greater efficacy in MMR deficient colorectal cancers.19, 20
This study also correlated with a significantly higher rate of mutated KRAS oncogene in RCC compared with LCC. This difference may likewise have contributed to the poorer OS of RCC. Other studies have also shown significantly more mutations in codon 12/13 of KRAS; however, this has not necessarily been demonstrated to be associated with poor prognosis in RCC, unlike in LCC.16, 21 Knowing the mutational status in combination with the tumor location may allow providers to individualize treatment for patients. For instance, analysis of the NCIC CTG CO.17 trial showed that in chemotherapy refractory patients with wild-type KRAS, the addition of Cetuximab (monoclonal IgG approved for treatment of metastatic colorectal cancer with wild-type KRAS) improved PFS in patients with metastatic LCC but not RCC.21–23 In addition, the evaluation of tumor sidedness has recently been added to the NCCN guidelines with evidence from the phase III CALGB/SWOG 80405 trial demonstrating that in patients with metastatic colon cancer, those with LCC had improved OS with cetuximab treatment.24 An analysis of the TRIBE trial examining the benefit of FOLFOXIRI plus bevacizumab compared to FOLFIRI plus bevacizumab in metastatic colorectal cancer demonstrated more relative benefit from treating RCCs with trimodal therapy in terms of PFS and OS independent of RAS and BRAF status.25 We also found a small improvement in OS in RCC patients treated with chemotherapy than LCC, without being able to discern the exact treatment regimens administered.
There are several limitations to this study. This is a retrospective review of pooled data from the NCDB with inherent variability between institutions in the information collected. Molecular marker data has not been reported in a large percentage of cases and selection bias may have contributed to the high number of MSI tumors in advanced stage cancers. Other molecular information missing from the NCDB included whether tumors were MSI high or low, BRAF status and specific KRAS codon mutation. Other under reported factors within this database included MSI and KRAS mutational status, pathologic M (metastasis) stage and evidence of perineural invasion. The NCDB also reports OS as a prognostic outcome but does not report either CSS or DFS which have been demonstrated in other studies have variable outcomes. This database also does not specify type of chemotherapy, operative technique or post-operative complications.
Conclusion
Using the NCDB, we were able to produce the largest series illustrating the intrinsic biological differences between RCC and LCC. These variations appeared to become more marked at stage 3 and stage 4 disease demonstrating that the poorer biology (higher grade tumors, more LVI, higher proportion of KRAS mutations) of RCCs worsened the prognoses of patients who already had nodal and distant metastases. The variability in the presentation of mutated molecular markers between RCC and LCC may encourage the use of more aggressive chemotherapeutic regimens for RCCs as well as targeted therapies which may improve survival in these patients, especially those with late stage cancers.
Clinical Practice Points.
Right sided colon cancer has been shown to have inferior survival compared to left sided colon cancer. Previous studies have shown inconsistent results stage for stage. In this large patient study of the National Cancer Database we found 3-year and 5-year overall survival were poorer for right colon cancer which was true for all stages and more pronounced for stage 3 and 4 patients. We were able to demonstrate intrinsic biological differences between right and left colon cancer. These variations appeared to become more marked at stage 3 and stage 4 disease demonstrating that the poorer biology (higher grade tumors, more LVI, higher proportion of KRAS mutations) of RCCs worsened the prognoses of patients who already had nodal and distant metastases.
Acknowledgments
This work was supported by National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Cancer Institute’s Bioinformatics and Biostatistics Shared Resources.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors of this study have no conflicts of interest.
References
- 1.Weiss JM, Pfau PR, O’Connor ES, et al. Mortality by stage for right- versus left-sided colon cancer: analysis of surveillance, epidemiology, and end results--Medicare data. J Clin Oncol. 2011;29:4401–4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benedix F, Kube R, Meyer F, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. 2010;53:57–64. [DOI] [PubMed] [Google Scholar]
- 3.Meguid RA, Slidell MB, Wolfgang CL, Chang DC, Ahuja N. Is there a difference in survival between right- versus left-sided colon cancers? Ann Surg Oncol. 2008;15:2388–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warschkow R, Sulz MC, Marti L, et al. Better survival in right-sided versus left-sided stage I - III colon cancer patients. BMC Cancer. 2016;16:554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yahagi M, Okabayashi K, Hasegawa H, Tsuruta M, Kitagawa Y. The Worse Prognosis of Right-Sided Compared with Left-Sided Colon Cancers: a Systematic Review and Meta-analysis. J Gastrointest Surg. 2016;20:648–655. [DOI] [PubMed] [Google Scholar]
- 6.Li P, Xiao Z, Braciak TA, Ou Q, Chen G, Oduncu FS. A relationship to survival is seen by combining the factors of mismatch repair status, tumor location and age of onset in colorectal cancer patients. PLoS One. 2017;12:e0172799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmol I, Sanchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int J Mol Sci. 2017;18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petrelli F, Tomasello G, Borgonovo K, et al. Prognostic Survival Associated With Left-Sided vs Right-Sided Colon Cancer: A Systematic Review and Meta-analysis. JAMA Oncol. 2016. [DOI] [PubMed] [Google Scholar]
- 9.Amri R, Bordeianou LG, Sylla P, Berger DL. Variations in Metastasis Site by Primary Location in Colon Cancer. J Gastrointest Surg. 2015;19:1522–1527. [DOI] [PubMed] [Google Scholar]
- 10.Glebov OK, Rodriguez LM, Nakahara K, et al. Distinguishing right from left colon by the pattern of gene expression. Cancer Epidemiol Biomarkers Prev. 2003;12:755–762. [PubMed] [Google Scholar]
- 11.Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saridaki Z, Souglakos J, Georgoulias V. Prognostic and predictive significance of MSI in stages II/III colon cancer. World J Gastroenterol. 2014;20:6809–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sinicrope FA, Mahoney MR, Smyrk TC, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol. 2013;31:3664–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yaeger R, Chatila WK, Lipsyc MD, et al. Clinical Sequencing Defines the Genomic Landscape of Metastatic Colorectal Cancer. Cancer Cell. 2018;33:125–136 e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eklof V, Wikberg ML, Edin S, et al. The prognostic role of KRAS, BRAF, PIK3CA and PTEN in colorectal cancer. Br J Cancer. 2013;108:2153–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith CG, Fisher D, Claes B, et al. Somatic profiling of the epidermal growth factor receptor pathway in tumors from patients with advanced colorectal cancer treated with chemotherapy +/−cetuximab. Clin Cancer Res. 2013;19:4104–4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen W, Swanson BJ, Frankel WL. Molecular genetics of microsatellite-unstable colorectal cancer for pathologists. Diagn Pathol. 2017;12:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bupathi M, Wu C. Biomarkers for immune therapy in colorectal cancer: mismatch-repair deficiency and others. J Gastrointest Oncol. 2016;7:713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Green AR, Aleskandarany MA, Ali R, et al. Clinical Impact of Tumor DNA Repair Expression and T-cell Infiltration in Breast Cancers. Cancer Immunol Res. 2017;5:292–299. [DOI] [PubMed] [Google Scholar]
- 21.Wang F, Bai L, Liu TS, et al. Right-sided colon cancer and left-sided colorectal cancers respond differently to cetuximab. Chin J Cancer. 2015;34:384–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brule SY, Jonker DJ, Karapetis CS, et al. Location of colon cancer (right-sided versus left-sided) as a prognostic factor and a predictor of benefit from cetuximab in NCIC CO.17. Eur J Cancer. 2015;51:1405–1414. [DOI] [PubMed] [Google Scholar]
- 23.Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and Predictive Relevance of Primary Tumor Location in Patients With RAS Wild-Type Metastatic Colorectal Cancer: Retrospective Analyses of the CRYSTAL and FIRE-3 Trials. JAMA Oncol. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Venook Alan P. N D, Lenz Heinz-Josef, Innocenti Federico, Mahoney Michelle R., O’Neil Bert H., Shaw James Edward, Polite Blase N., Hochster Howard S., Atkins James Norman, Goldberg Richard M., Mayer Robert J., Schilsky Richard L., Bertagnolli Monica M., Blanke Charles David, Cancer and Leukemia Group B (Alliance), SWOG, and ECOG. CALGB/SWOG 80405: Phase III trial of irinotecan/5-FU/leucovorin (FOLFIRI) or oxaliplatin/5-FU/leucovorin (mFOLFOX6) with bevacizumab (BV) or cetuximab (CET) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). ASCO Meeting Abstracts 2016:3504. [Google Scholar]
- 25.Cremolini C, Antoniotti C, Lonardi S, et al. Primary tumor sidedness and benefit from FOLFOXIRI plus bevacizumab as initial therapy for metastatic colorectal cancer. Retrospective analysis of the TRIBE trial by GONO. Ann Oncol. 2018. [DOI] [PubMed] [Google Scholar]


