Abstract
To develop a nisin-producing cheese starter, Lactococcus lactis subsp. cremoris SK110 was conjugated with transposon Tn5276-NI, which codes for nisin immunity but not for nisin production. Cheese made with transconjugant SK110::Tn5276-NI as the starter was bitter. The muropeptide of the transconjugant contained a significantly greater amount of tetrapeptides than the muropeptide of strain SK110, which could have decreased the susceptibility of the cells to lysis and thereby the release of intracellular debittering enzymes.
Lactococci are used as starter bacteria in a great number of different cheeses. Enzymes of the proteolytic system of lactococci (e.g., the intracellular peptidases [22]) play a major role in the breakdown of large, casein-derived peptides into amino acids. This breakdown is considered the rate-limiting step in the maturation of cheese (10). It is generally assumed that lysis of the starter bacteria results in the release of the intracellular peptidases into the curd, which results in hydrolysis of casein-derived peptides into amino acids and, thereby, enhancement of the flavor of the cheese (1, 2, 5, 14).
Feirtag and McKay proposed that lysogenic strains could be used as starter cultures to enhance lysis of the starter cells during cheese ripening (11). It was shown for starter culture SK11 that lysis was induced by UV radiation, by mitomycin C, and by increasing the incubation temperature from 30 to 40°C for 2.5 h. When Lactococcus lactis subsp. cremoris SK110, an isolate obtained from starter culture SK11 (8), was used as a starter culture for the manufacture of Gouda cheese, a significant increase in the level of free amino acids was observed in the mature cheese when the prophage was induced during the cheese-making process (14).
Recently, a nisin-producing cheese starter containing L. lactis SK110 as the main component was developed (7). Strain SK110 was made nisin immune by conjugating it with a natural derivative of the nisin transposon Tn5276-NI, a conjugative nisin-sucrose transposon that codes for nisin immunity but not for nisin production (16). Tn5276-NI was transferred to recipient strain SK110 via an intermediate recipient strain, L. lactis MG1614. The conjugal matings were carried out on milk agar plates (19), and the transconjugants were selected as described previously (17). In cheese trials, the transconjugant strain, L. lactis SK110::Tn5276-NI, was responsible for a much more bitter flavor in cheese. This prompted us to determine the sensitivity of (induced) lysis under different growth conditions and the activity of the lytic enzymes and to analyze the peptidoglycan structures of strains SK110 and SK110::Tn5276-NI by reversed-phase high-performance liquid chromatography (RP-HPLC).
Cheese manufacture.
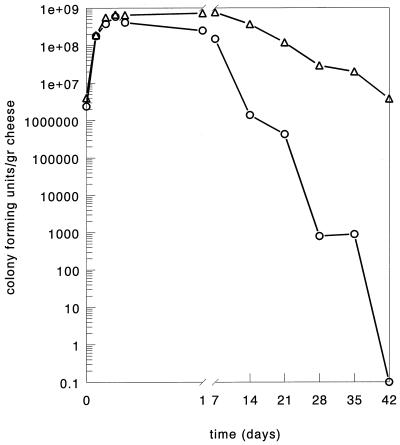
Gouda cheese was made from 200-liter portions of pasteurized (10 s, 74°C) milk by a standard procedure (23). The cheese milk was inoculated with either 0.5% strain SK110 and 0.5% L. lactis subsp. lactis biovar. diacetylactis C17 or 0.5% strain SK110::Tn5276-NI and 0.5% strain C17. The acidification rates, the salt and moisture contents, and the outgrowth 24 h after inoculation of the cheese milk with strain C17 were identical in the two cheeses (data not shown). An organoleptic evaluation of the cheese manufactured with strain SK110 cells revealed a good-quality cheese with a low bitterness score, whereas the cheese manufactured with SK110::Tn5276-NI resulted was bitter (Table 1). To determine the stability of the starter culture during ripening in the cheese environment, the number of viable cells was determined on GMA agar (13). A cheese sample was diluted 10-fold with a 2% (wt/vol) trisodium citrate solution and subsequently homogenized for 5 min with a stomacher (model 400 Lab Blender; Seward, London, United Kingdom). The results showed that after 40 days the number of starter culture SK110 cells in the cheese was about 10−4 times the number of starter culture SK110::Tn5276-NI cells (Fig. 1). The amounts of the intracellular marker enzyme lactate dehydrogenase in both cheeses were determined after 24 days (12). The results showed that the amount of lactate dehydrogenase released in cheese manufactured with strain SK110 was 1.5-fold larger than the amount released in cheese manufactured with the transconjugated strain. The larger amount of intracellular enzyme resulted in 1.3-fold-greater production of free amino acids after 8 weeks of ripening compared to the cheese manufactured with transconjugant SK110::Tn5276-NI. These data strongly suggest that there are great differences in the prophage-induced lysis process with strain SK110 and the transconjugant. We studied the biochemical background of these differences on the following three levels: (i) phage proliferation, (ii) (auto)lysin activity, and (iii) cell wall stability.
TABLE 1.
Overall flavor and bitterness scores for 4-month-old cheeses manufactured with either 0.5% SK110 and 0.5% C17 or 0.5% SK110::Tn5276-NI and 0.5% C17 and ripened at 13°C
| Starter culture | Organoleptic evaluation of the cheeses
|
|
|---|---|---|
| Bitternessa | Overallb | |
| 0.5% SK110 + 0.5% C17 | 0.4 | 6.4 |
| 0.5% SK110::Tn5276-NI + 0.5% C17 | 2.2 | 4.8 |
Bitterness on a scale from 0 (absent) to 4 (very strong).
Overall score on a scale from 4 (very poor) to 8 (excellent).
FIG. 1.
Growth and decline of lactococci in cheese manufactured with either L. lactis subsp. cremoris SK110 (○) or transconjugant SK110::Tn5276-NI (▵) as the starter.
Thermolytic response.
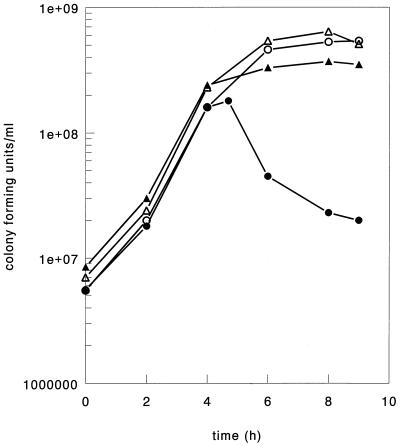
To compare sensitivities to (induced) lysis in cells of strain SK110 and the transconjugant, lysis was induced in both types of cells by raising the growth temperature of the culture from 30 to 40°C for 2.5 h in the mid-exponential phase of growth. The pH of the batch cultures was maintained at 6.3 by adding a solution containing 10% (wt/vol) NaHCO3 and 7.5% NH4OH. Transconjugant SK110::Tn5276-NI was routinely grown in medium containing 50 U of nisin per ml. The culture containing cells of strain SK110 that was subjected to the temperature shock produced the typical prophage induction profile. Compared to the increase in biomass in the non-heat-treated culture, a reduction in the number of viable cells of approximately 90% was observed (Fig. 2) (14). This reduction was a direct result of disruption of the cells, as shown by a similar decline in the optical density of the culture (data not shown). Subjecting a culture of transconjugant SK110::Tn5276-NI to a heat shock resulted in reduced outgrowth of the culture compared with the non-heat-treated culture, but did not result in any reduction in cell viability (Fig. 2).
FIG. 2.
Growth curves for cells of strain L. lactis subsp. cremoris SK110 (○ and •) and cells of transconjugant SK110::Tn5276-NI (▵ and ▴) in M17 medium under controlled pH conditions (pH 6.3). Some cultures were incubated at 30°C (open symbols). Other cultures were incubated at 30°C and then the temperature was increased to 40°C for 2.5 h beginning 4.5 h after the culture was inoculated (solid symbols).
To investigate whether the synthesis of phage particles by transconjugant SK110::Tn5276-NI cells was disturbed, we attempted to observe the temperate phage particles by transmission electron microscopy. The samples were prepared as described previously (14) and were examined with a model JEM 1200 EX transmission electron microscope (JEOL, Tokyo, Japan). In cell extracts prepared as described previously (15) from a culture containing transconjugant SK110::Tn5276-NI cells, at 0.5 h after the start of the heat treatment low numbers of incomplete isometric phage particles with an average head size of approximately 55 nm were observed. No phage particles were observed in the supernatant of the culture (data not shown). The number and size of the phage heads observed were identical to the number and size of the phage heads observed in the culture supernatant of strain SK110 when it was treated in exactly the same manner (14). This indicated clearly that phage multiplication was not affected in the transconjugant.
Lytic activity.
The difference in sensitivity to temperature-induced lysis of strain SK110 cells compared to transconjugant SK110::Tn5276-NI cells could be due to a difference in the expression of the autolytic system. Therefore, the lytic activities in cell extracts of strain SK110 and transconjugant SK110::Tn5276-NI cells were compared by using an agar diffusion bioassay (21) in which Micrococcus flavus was the indicator strain. Cells were harvested in the mid-exponential growth phase, and the cell extracts were standardized so that they contained 4 mg of protein per ml. Protein contents were determined by the Bradford method (3) by using bovine serum albumin as the standard. The results showed that the lytic activity, expressed as the size of the lytic zone on bioassay agar, was the same for both cell extracts after 48 h of incubation (data not shown). In order to compare the lytic activities of strain SK110 and transconjugant SK110::Tn5276-NI cells more quantitatively, purified cell walls from Micrococcus lysodeicticus (Sigma) were suspended in 50 mM sodium phosphate buffer (pH 6.5) at a concentration of 0.4 mg/ml and were treated with cell extracts (final protein concentration, 40 μg/ml) from both types of cells. The assay was performed at 25°C. It was observed that the decreases in absorbance at 600 nm for the two cell extracts were comparable, showing that the lytic activities resulting from prophage induction were identical in the wild type and the transconjugant (data not shown).
Cell wall hydrolysis.
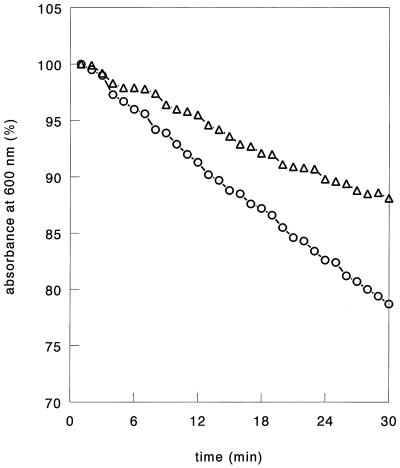
Another possible explanation for the different thermolytic responses of strain SK110 and transconjugant SK110::Tn5276-NI cells is that the sensitivities of the cell walls to lytic activity differed. Therefore, strain SK110 or transconjugant SK110::Tn5276-NI cells were harvested in the mid-exponential growth phase and resuspended in 100 ml of distilled water. Subsequently, the cells were disrupted by 40 cycles of sonication for 1 min with model XL2020 Sonicater (Heat Systems, Farmingdale, N.Y.) at maximum power with a 1.25-cm macrotip; 1 min of sonication was alternated with 2 min of cooling in ice. Each suspension was heated at 100°C for 15 min, and, after it was cooled to 37°C, DNase, RNase, and phosphate buffer (pH 6.7) (final concentrations, 5 μg/ml, 30 μg/ml, and 0.1 M, respectively) were added. After 2 h of incubation at 37°C, the cell walls were pelleted by centrifugation for 20 min at 12,000 × g. The walls were resuspended in 300 ml of 50 mM Tris-HCl buffer (pH 7.6) containing trypsin (0.6 mg/ml). Sodium dodecyl sulfate (2%, wt/vol) was added after 4 h of incubation at 37°C, and the suspension was stirred for 1 h at room temperature. The pure white cell walls were collected by centrifugation (12,000 × g, 20 min), washed three times with 0.9% NaCl and three times with distilled water, and freeze-dried. The isolated cell wall material (1.4 mg/ml) was used to study sensitivity to mutanolysin (10 U/ml; Sigma), an enzyme that hydrolyzes the N-acetylmuramyl-1,4-β-N-acetylglucosamine bonds in the peptidoglycan structure (Fig. 3). The assay was performed in 50 mM NaH2PO4 buffer (pH 6.8). Treating strain SK110 cell walls with mutanolysin resulted in a decline in the absorbance at 600 nm of the cell wall suspension that was 1.7 times faster than the decline in absorbance at 600 nm observed with the transconjugant SK110::Tn5276-NI suspension. The observed decreases in absorbance after 10 U of mutanolysin per ml was added to strain SK110 and transconjugant SK110::Tn5276-NI cell wall suspensions were 22 and 13%, respectively, after 30 min of incubation. This clearly shows that the differences in sensitivity to (induced) lysis (Fig. 1 and 2) can be explained by a difference in cell wall structure.
FIG. 3.
Relative changes in absorbance at 600 nm after preparations containing 1.4 mg of isolated cell walls of strain L. lactis SK110 (○) and transconjugant SK110::Tn5276-NI (▵) per ml were treated with mutanolysin (10 U/ml) at 37°C.
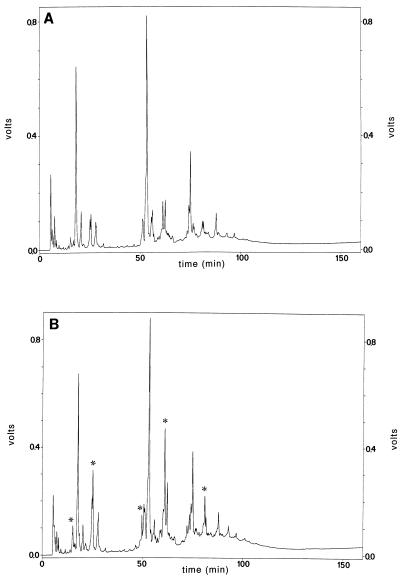
In order to obtain more detailed information about the cell wall composition and structure, isolated cell walls of strain SK110 and transconjugant SK110::Tn5276-NI were digested with muramidase, and the muropeptides were separated by RP-HPLC (9) (Fig. 4). As determined from the RP-HPLC elution profile, strain SK110 and transconjugant SK110::Tn5276-NI cells contained the same set of muropeptides. The muropeptide fraction in the peptidoglycans of transconjugant SK110::Tn5276-NI was larger than the muropeptide fraction in the peptidoglycans of strain SK110. After a comparison with the elution profile of the known muropeptides of Streptococcus faecium (6), whose elution profile is closely related to the elution profile of L. lactis, it was observed that the marked muropeptides consisted mainly of tetrapeptides.
FIG. 4.
RP-HPLC chromatograms of separated muropeptides from peptidoglycans which were obtained from strain L. lactis SK110 cells (A) and transconjugant SK110::Tn5276-NI cells (B) grown in batch cultures in M17 medium, harvested under identical conditions, and digested with muramidase. The asterisks indicate differences in the RP-HPLC patterns.
Schleifer and Kandler described the peptidoglycan structure of L. lactis as a repeating disaccharide unit composed of β(1-4)-polymerized N-acetylglucosamine and N-acetylmuramic acid (18). These peptidoglycan strands are cross-linked via linear tetrapeptides attached to the N-acetylmuramic acid, which results in a rigid cell wall structure. Therefore, the larger amount of tetrapeptides observed in the transconjugant could have a positive effect on the rigidity of the peptidoglycan and thus could alter the susceptibility of the cell wall to lytic activity. Because lysis of starter cells is a prerequisite for the release of debittering enzymes, such as aminopeptidase N (20), into the cheese matrix, the bitterness found in the cheese manufactured with the transconjugant could well be explained by the decreased sensitivity of the cells to lysis. Since, as far as we know, nothing is known about controlled peptidoglycan synthesis in lactococci, we can only speculate that the larger amount of tetrapeptides in transconjugant SK110::Tn5276-NI is caused by a decrease in the activity of ld-carboxypeptidase, which normally is active during synthesis of peptidoglycan (4).
The observed difference in peptidoglycan composition between strain SK110 and the transconjugant indicates that either a gene(s) encoded on transposon Tn5276-NI or the specific integration site of Tn5276-NI in the genome of lysogenic strain SK110 is involved in this phenomenon. Transposon Tn5276-NI is defective for the production of nisin but contains the intact genes coding for nisin immunity, nisin modification, nisin export, the sucrose operon, a bacteriophage resistance system, and, presumably, numerous unidentified functions (16, 19). So far, there have been no studies in which the peptidoglycan structure itself has been shown to play a direct role in cell defense mechanisms against nisin. This makes it impossible to identify which part of the 70-kb transposon is responsible for the observed difference in peptidoglycan composition. Future studies should include subcloning of the individual genes of the nisin operon and sequencing of the unknown areas of the transposon. These studies should provide an explanation for the bitterness obtained with the nisin starter and should also provide insight into the physiological and biochemical parameters that determine the stability of starter bacteria.
Acknowledgments
We thank Jan Wouters and Willem de Vos for practical advice and valuable discussions and Monique Slomp for preparing the transconjugants.
This work was supported by the Stichting J. Mesdag-Fonds.
REFERENCES
- 1.Bie R, Sjöström G. Autolytic properties of some lactic acid bacteria used in cheese production. I. Material and methods. Milchwissenschaft. 1975;30:653–657. [Google Scholar]
- 2.Bie R, Sjöström G. Autolytic properties of some lactic acid bacteria used in cheese production. II. Experiments with fluid substrates and cheese. Milchwissenschaft. 1975;30:739–747. [Google Scholar]
- 3.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 4.Burman L G, Park J T. Changes in the composition of Escherichia coli murein as it ages during exponential growth. J Bacteriol. 1983;155:447–453. doi: 10.1128/jb.155.2.447-453.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chapot-Chartier M-P, Deniel C, Rousseau M, Vassal L, Gripon J-C. Autolysis of two strains of Lactococcus lactis during cheese ripening. Int Dairy J. 1994;4:251–269. [Google Scholar]
- 6.de Jonge, B. Unpublished results.
- 7.Delves-Broughton J, Blackburn P, Evans R J, Hugenholtz J. Applications of the bacteriocin, nisin. Antonie Leeuwenhoek. 1996;69:193–202. doi: 10.1007/BF00399424. [DOI] [PubMed] [Google Scholar]
- 8.de Vos W M, Davies F L. Third European Congress on Biotechnology. III. Weinheim, Germany: Verlag Chemie; 1984. Plasmid DNA in lactic streptococci: bacteriophage resistance and proteinase plasmids in Streptococcus cremoris SK11; pp. 201–205. [Google Scholar]
- 9.Driehuis F, Wouters J T M. Effect of growth rate and cell shape on the peptidoglycan composition in Escherichia coli. J Bacteriol. 1987;169:97–101. doi: 10.1128/jb.169.1.97-101.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Exterkate F A, Alting A C. The role of starter peptidases in the initial proteolytic events leading to amino acids in Gouda cheese. Int Dairy J. 1995;5:15–28. [Google Scholar]
- 11.Feirtag J M, McKay L L. Thermoinducible lysis of temperature-sensitive Streptococcus cremoris strains. J Dairy Sci. 1987;70:1779–1784. [Google Scholar]
- 12.Hillier A J, Jago G R. l-Lactate dehydrogenase, FDP-activated, from Streptococcus cremoris. Methods Enzymol. 1982;89:362–367. doi: 10.1016/s0076-6879(82)89065-4. [DOI] [PubMed] [Google Scholar]
- 13.Hugenholtz J, Veldkamp H. Competition between different strains of Streptococcus cremoris. FEMS Microbiol Ecol. 1985;31:57–62. [Google Scholar]
- 14.Meijer W C. Expression and release of proteolytic enzymes of Lactococcus lactis. Ph.D. thesis. Wageningen, The Netherlands: Agricultural University of Wageningen; 1997. [Google Scholar]
- 15.Platteeuw C, Simons G, de Vos W M. Use of the Escherichia coli β-glucuronidase (gusA) gene as a reporter gene for analyzing promoters in lactic acid bacteria. Appl Environ Microbiol. 1994;60:587–593. doi: 10.1128/aem.60.2.587-593.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rauch P J G, Beerthuyzen M M, de Vos W M. Molecular analysis and evolution of conjugative transposons encoding nisin production and sucrose fermentation in Lactococcus lactis. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM; 1991. pp. 243–249. [Google Scholar]
- 17.Rauch P J G, de Vos W M. Characterization of the novel nisin-sucrose conjugative transposon Tn5276 and its insertion in Lactococcus lactis. J Bacteriol. 1992;174:1280–1287. doi: 10.1128/jb.174.4.1280-1287.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schleifer K H, Kandler O. Zur chemischen Zusammensetzung der Zellwand der Streptokokken. Arch Mikrobiol. 1967;57:335–364. [PubMed] [Google Scholar]
- 19.Steele J L, McKay L L. Partial characterization of the genetic basis for sucrose metabolism and nisin production in Streptococcus lactis. Appl Environ Microbiol. 1986;51:57–64. doi: 10.1128/aem.51.1.57-64.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan P S T, van Kessel T A J M, van de Veerdonk F L M, Zuurendonk P F, Bruins A P, Konings W N. Degradation and debittering of a tryptic digest from β-casein by aminopeptidase N from Lactococcus lactis subsp. cremoris WG2. Appl Environ Microbiol. 1993;59:1430–1436. doi: 10.1128/aem.59.5.1430-1436.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tramer J, Fowler G G. Estimation of nisin in foods. J Sci Food Agric. 1964;15:522–528. [Google Scholar]
- 22.Visser S. Proteolytic enzymes and their relation to cheese ripening and flavour: an overview. J Dairy Sci. 1993;76:329–350. [Google Scholar]
- 23.Walstra P, Noomen A, Geurts T J. Dutch-type varieties. In: Fox P F, editor. Cheese: chemistry, physics and microbiology. London, United Kingdom: Elsevier Applied Science Publishers; 1987. pp. 45–92. [Google Scholar]