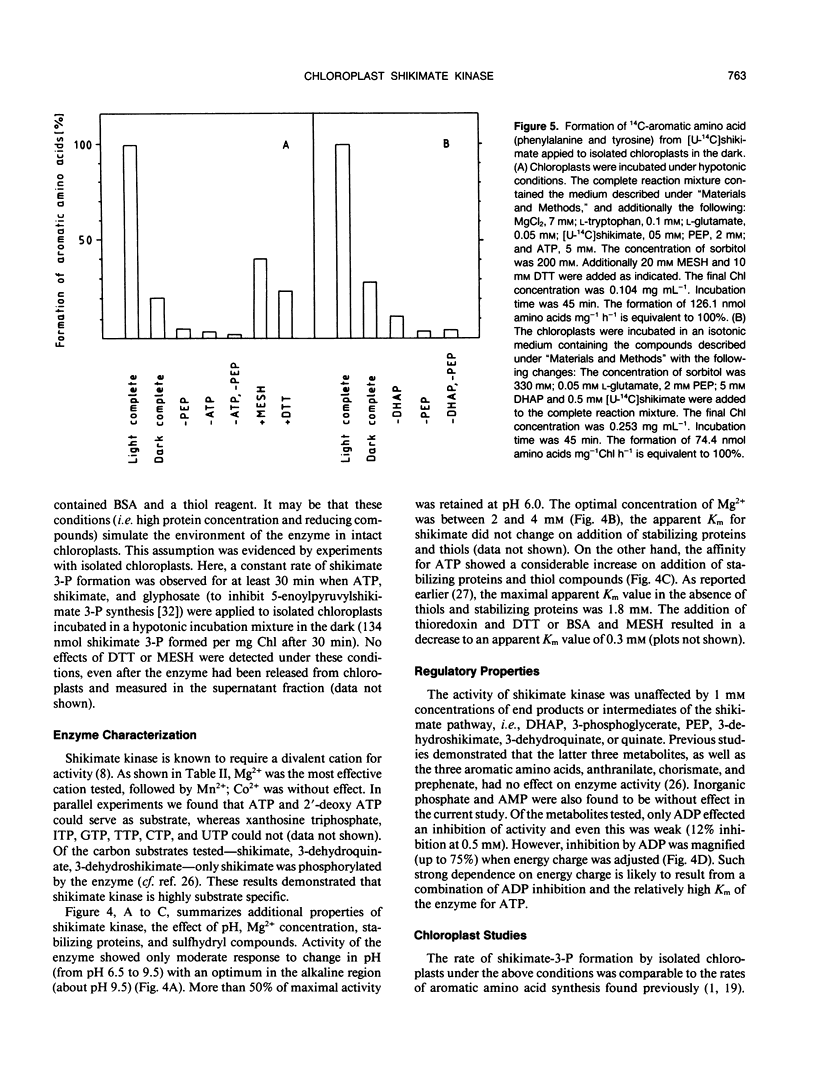
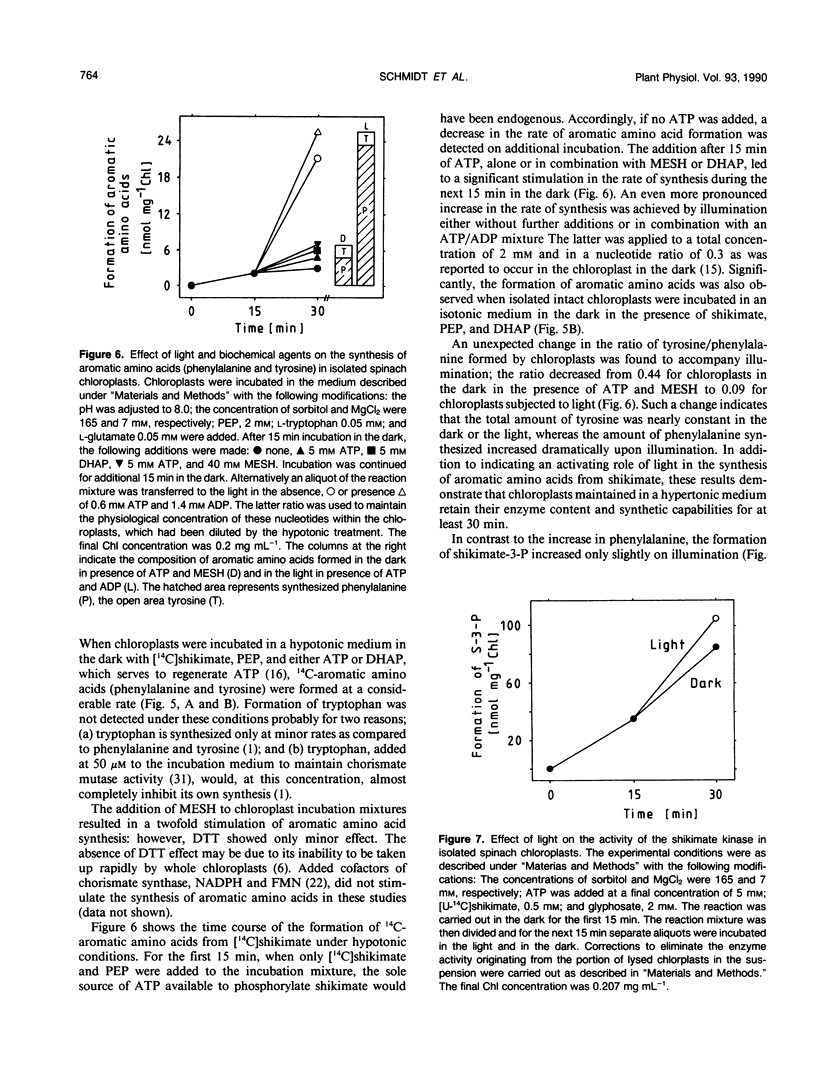
Abstract
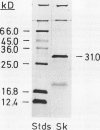
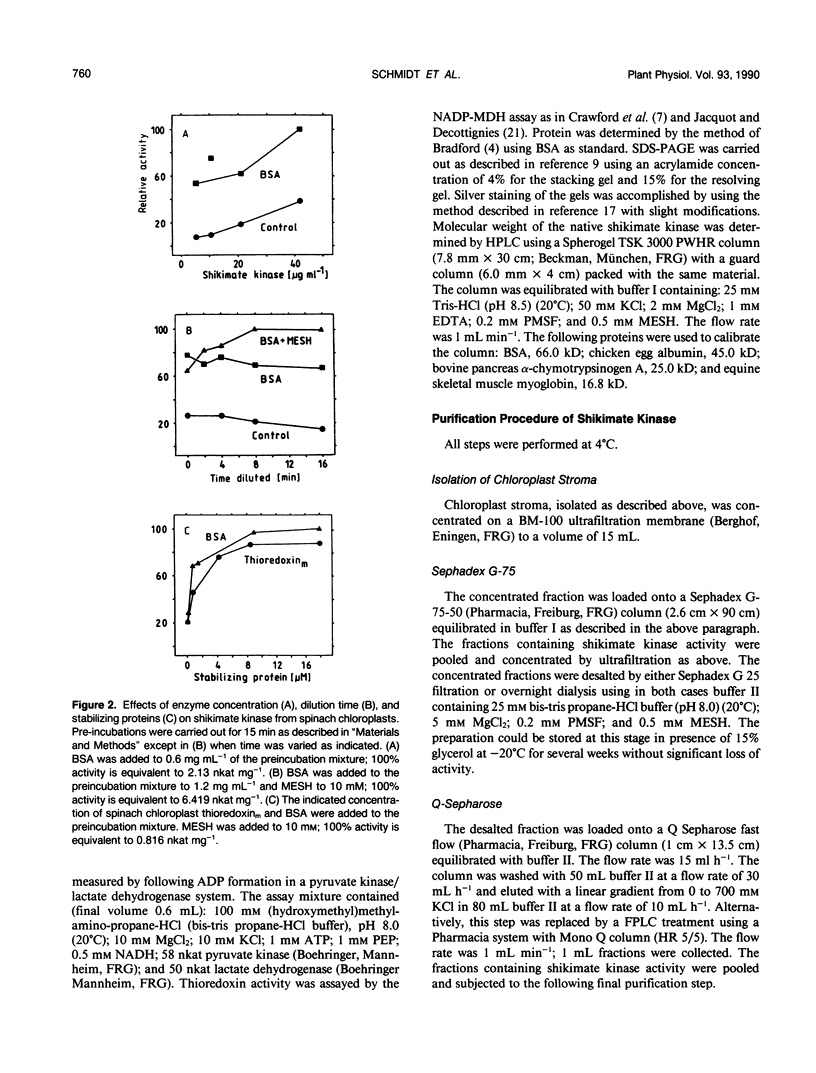
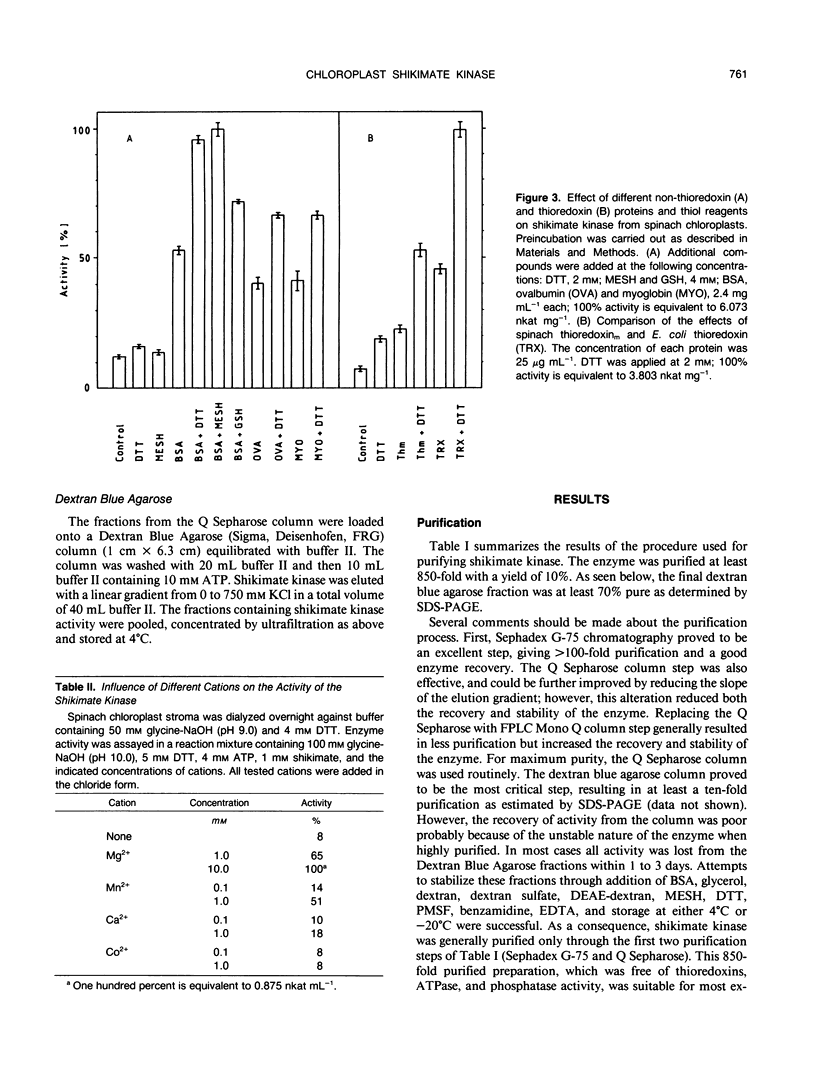
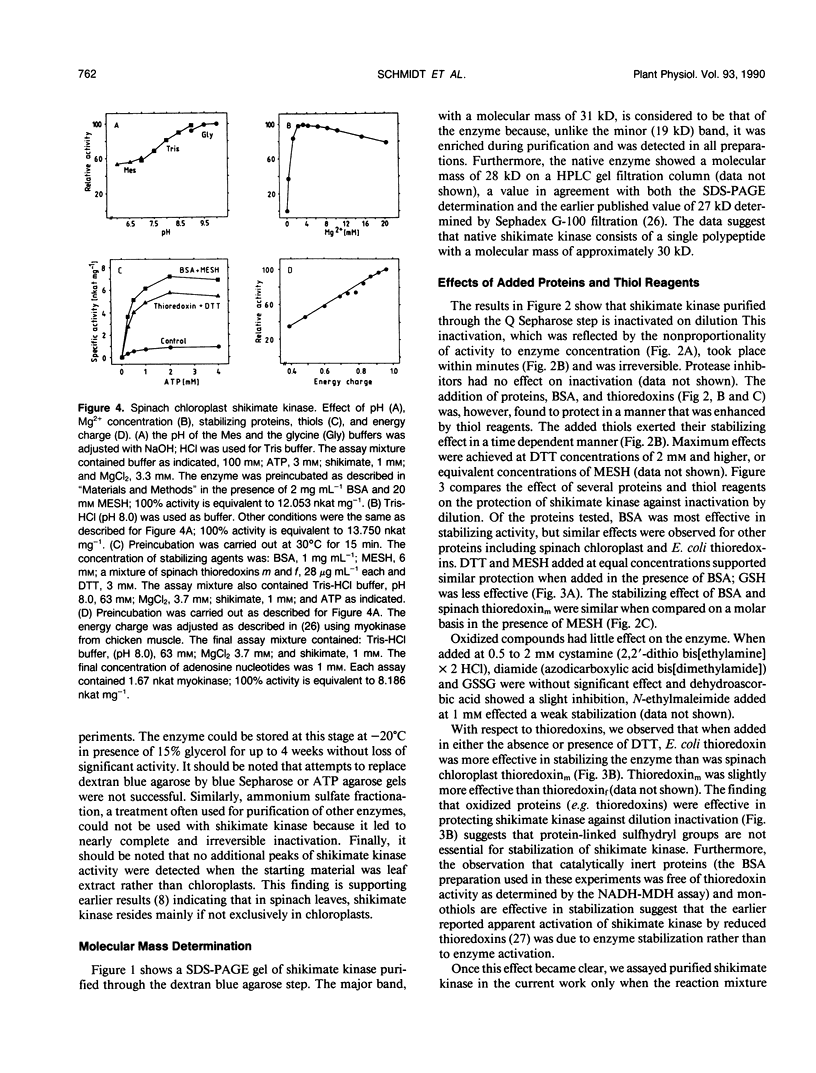
Shikimate kinase was purified to near homogenity from spinach Spinacia oleracea L. chloroplasts and found to consist of a single 31 kilodalton polypeptide. The purified enzyme was unstable, but could be stabilized by a variety of added proteins, including oxidized and reduced thioredoxins. Whereas the isolated enzyme was stimulated by mono- and dithiol reagents, the enzyme in intact chloroplasts was unaffected by added thiols and showed only minor response to dark/light transitions. These results indicate that the previously reported stimulation of shikimate kinase activity by reduced thioredoxins is due to enzyme stabilization rather than to activation. In the current study, the purified enzyme was inhibited by added ADP and showed a strong response to energy charge. When intact chloroplasts were incubated in the dark in presence of shikimate, phosphoenolpyruvate and a source of ATP (dihydroxyacetone phosphate or ATP itself under appropriate conditions), aromatic amino acids were formed: phenylalanine and tyrosine. The data indicate that energy charge plays a role in regulating shikimate kinase, thereby controlling the shikimate pathway. An unidentified enzyme of the latter part of the pathway, leading from shikimate-3-phosphate to phenylalanine, appears to be activated by light.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen J. R., Kosuge T. In vivo activity, purification, and characterization of shikimate kinase from sorghum. Plant Physiol. 1979 Sep;64(3):382–386. doi: 10.1104/pp.64.3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Connelly J. A., Conn E. E. Tyrosine biosynthesis in Sorghum bicolor: isolation and regulatory properties of arogenate dehydrogenase. Z Naturforsch C. 1986 Jan-Feb;41(1-2):69–78. doi: 10.1515/znc-1986-1-212. [DOI] [PubMed] [Google Scholar]
- Crawford N. A., Droux M., Kosower N. S., Buchanan B. B. Evidence for function of the ferredoxin/thioredoxin system in the reductive activation of target enzymes of isolated intact chloroplasts. Arch Biochem Biophys. 1989 May 15;271(1):223–239. doi: 10.1016/0003-9861(89)90273-7. [DOI] [PubMed] [Google Scholar]
- Crawford N. A., Yee B. C., Hutcheson S. W., Wolosiuk R. A., Buchanan B. B. Enzyme regulation in C4 photosynthesis: purification, properties, and activities of thioredoxins from C4 and C3 plants. Arch Biochem Biophys. 1986 Jan;244(1):1–15. doi: 10.1016/0003-9861(86)90088-3. [DOI] [PubMed] [Google Scholar]
- Fiedler E., Schultz G. Localization, purification, and characterization of shikimate oxidoreductase-dehydroquinate hydrolyase from stroma of spinach chloroplasts. Plant Physiol. 1985 Sep;79(1):212–218. doi: 10.1104/pp.79.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganson R. J., D'Amato T. A., Jensen R. A. The Two-Isozyme System of 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase in Nicotiana silvestris and Other Higher Plants. Plant Physiol. 1986 Sep;82(1):203–210. doi: 10.1104/pp.82.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L., Montoya A. L., Nester E. W. Purification and characterization of shikimate kinase enzyme activity in Bacillus subtilis. J Biol Chem. 1975 Oct 10;250(19):7675–7681. [PubMed] [Google Scholar]
- Morell H., Clark M. J., Knowles P. F., Sprinson D. B. The enzymic synthesis of chorismic and prephenic acids from 3-enolpyruvylshikimic acid 5-phosphate. J Biol Chem. 1967 Jan 10;242(1):82–90. [PubMed] [Google Scholar]
- Pinto J. E., Dyer W. E., Weller S. C., Herrmann K. M. Glyphosate Induces 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase in Potato (Solanum tuberosum L.) Cells Grown in Suspension Culture. Plant Physiol. 1988 Aug;87(4):891–893. doi: 10.1104/pp.87.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin J. L., Jensen R. A. Differentially Regulated Isozymes of 3-Deoxy-d-arabino-Heptulosonate-7-Phosphate Synthase from Seedlings of Vigna radiata [L.] Wilczek. Plant Physiol. 1985 Nov;79(3):711–718. doi: 10.1104/pp.79.3.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh B. K., Connelly J. A., Conn E. E. Chorismate mutase isoenzymes from Sorghum bicolor: purification and properties. Arch Biochem Biophys. 1985 Dec;243(2):374–384. doi: 10.1016/0003-9861(85)90514-4. [DOI] [PubMed] [Google Scholar]
- Singh B. K., Lonergan S. G., Conn E. E. Chorismate Mutase Isoenzymes from Selected Plants and Their Immunological Comparison with the Isoenzymes from Sorghum bicolor. Plant Physiol. 1986 Jul;81(3):717–722. doi: 10.1104/pp.81.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinrücken H. C., Schulz A., Amrhein N., Porter C. A., Fraley R. T. Overproduction of 5-enolpyruvylshikimate-3-phosphate synthase in a glyphosate-tolerant Petunia hybrida cell line. Arch Biochem Biophys. 1986 Jan;244(1):169–178. doi: 10.1016/0003-9861(86)90106-2. [DOI] [PubMed] [Google Scholar]
- Suzich J. A., Dean J. F., Herrmann K. M. 3-Deoxy-d-arabino-Heptulosonate 7-Phosphate Synthase from Carrot Root (Daucus carota) Is a Hysteretic Enzyme. Plant Physiol. 1985 Nov;79(3):765–770. doi: 10.1104/pp.79.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]