Abstract
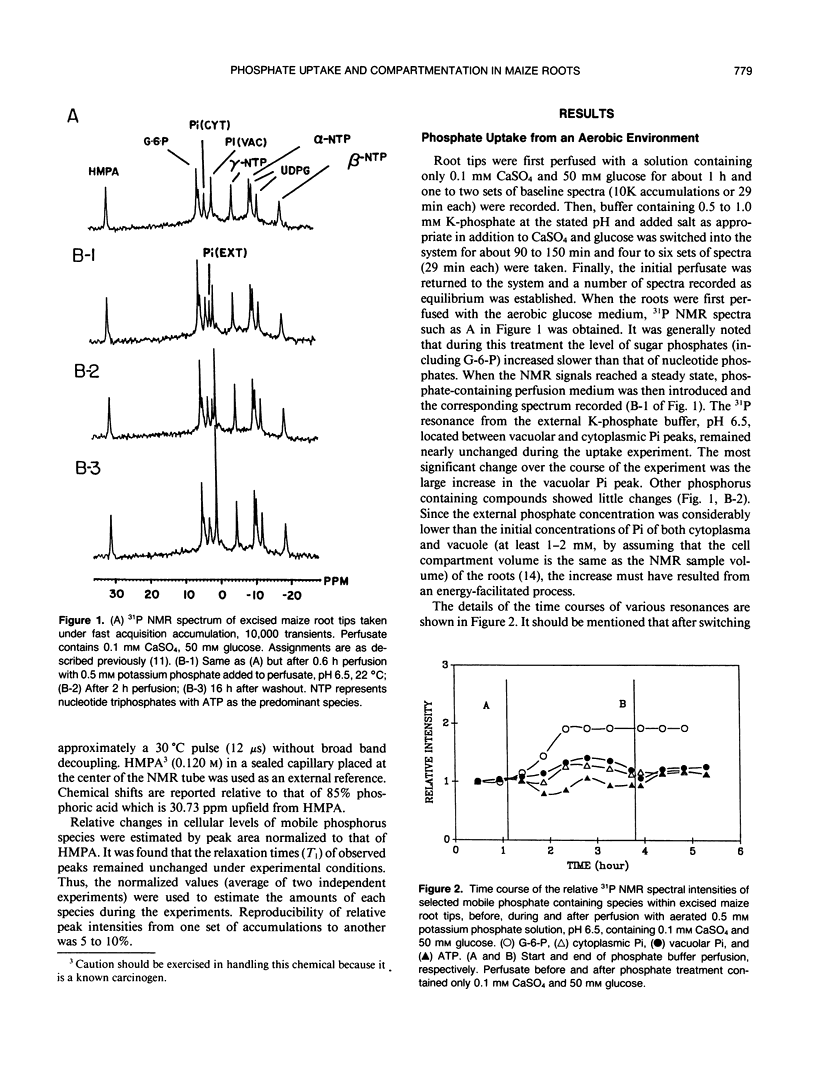
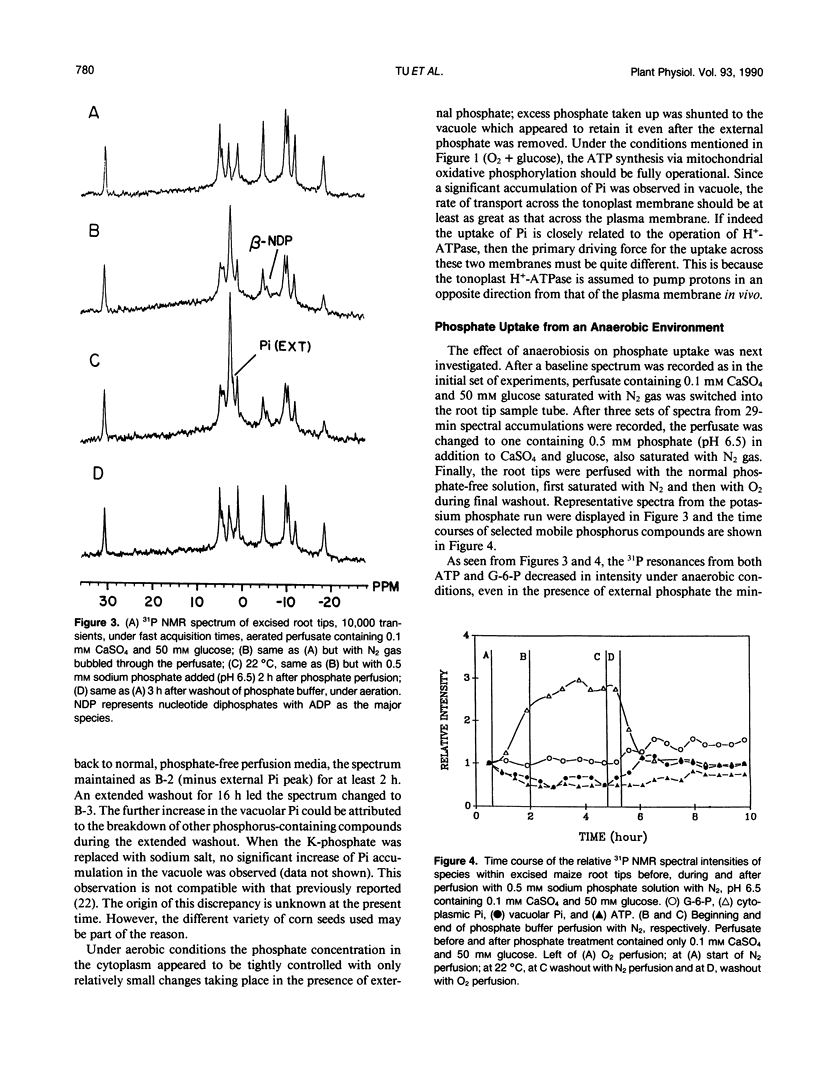
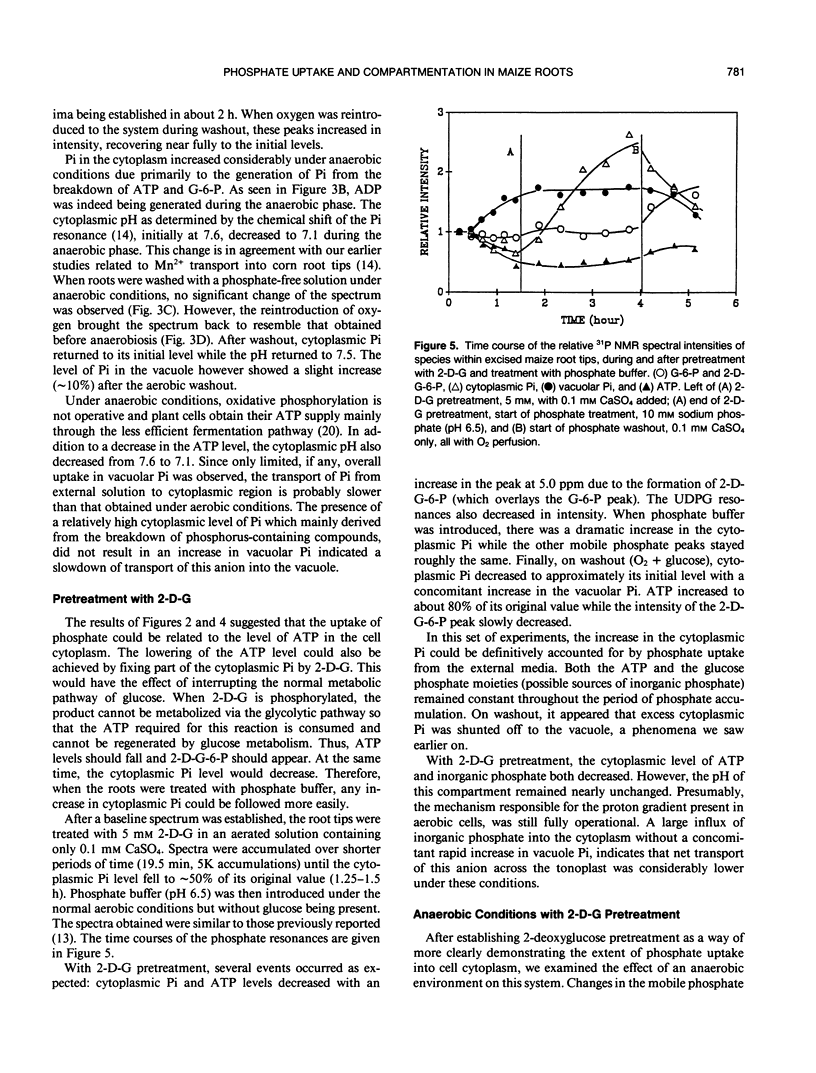
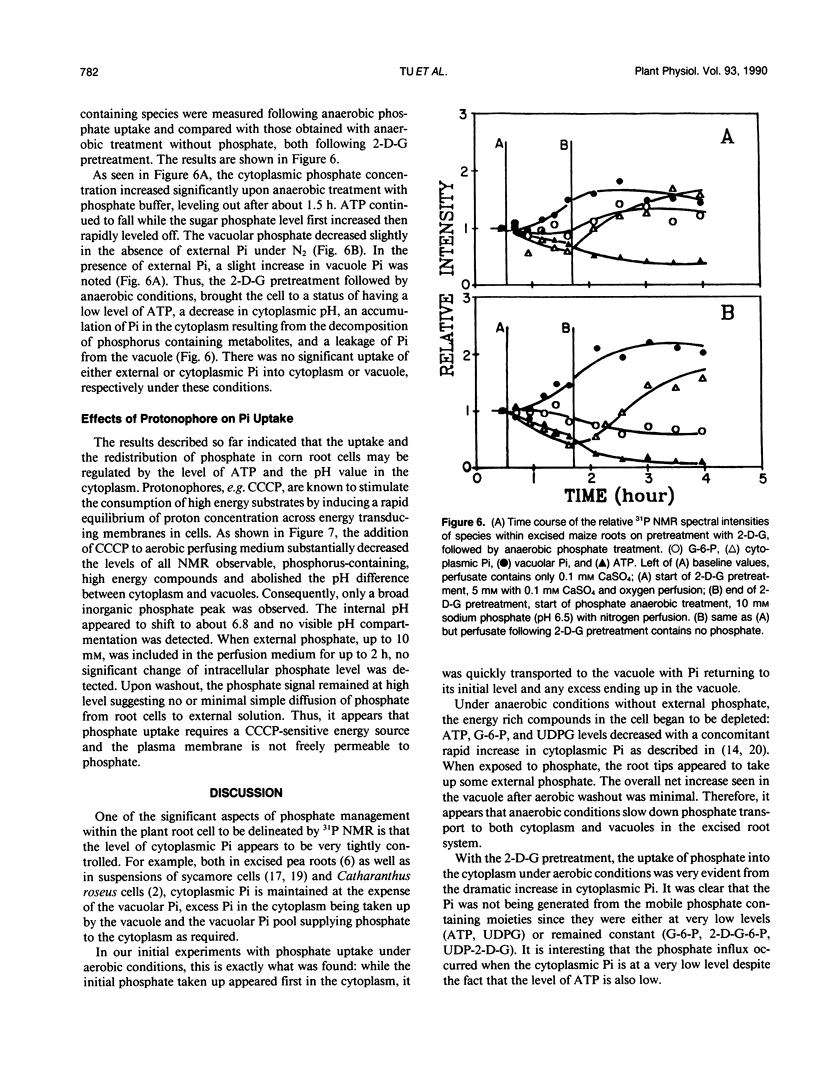
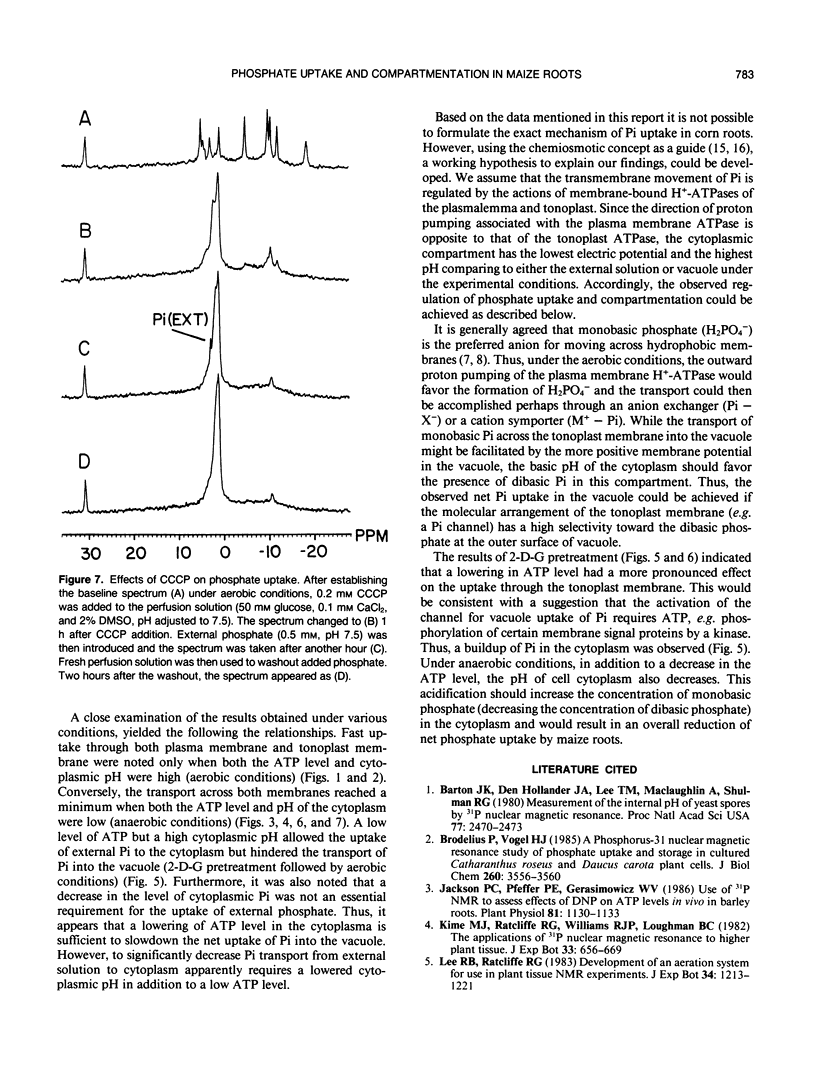
The extent of phosphate uptake measured by the relative changes in cytoplasmic Pi, vacuolar Pi, ATP, glucose-6-phosphate, and UDPG was determined using in vivo31P nuclear magnetic resonance spectroscopy. Maize (Zea mays) root tips were perfused with a solution containing 0.5 or 1.0 millimolar phosphate at pH ∼6.5 under different conditions. In the aerated state, phosphate uptake resulted in a significant increase (>80%) in vacuolar Pi, but cytoplasmic Pi only transiently increased by 10%. Under N2, the cytoplasmic Pi increased ∼150% which could be attributed to a large extent to the breakdown of ATP, sugar phosphates and UDPG. Vacuolar Pi increased but only to the extent of ∼10% of that seen under aerobic conditions. 2-deoxyglucose pretreatment was utilized to decrease the level of cytoplasmic Pi. When pretreated with the 2-deoxyglucose, the excised maize roots absorbed phosphate from the perfusate with a significant increase in the cytoplasmic Pi. The increase could only be traced to external phosphate since the concentrations of other phosphorus containing species remained constant during the uptake period. With 2-deoxyglucose pretreatment, phosphate uptake under anaerobic conditions was substantially inhibited with only the vacuolar phosphate showing a slight increase. When roots were treated with carbonyl cyanide m-chlorophenyl hydrazone, no detectable Pi uptake was found. These results were used to propose a H+-ATPase related transport mechanism for phosphate uptake and compartmentation in corn root cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton J. K., den Hollander J. A., Lee T. M., MacLaughlin A., Shulman R. G. Measurement of the internal pH of yeast spores by 31P nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1980 May;77(5):2470–2473. doi: 10.1073/pnas.77.5.2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodelius P., Vogel H. J. A phosphorus-31 nuclear magnetic resonance study of phosphate uptake and storage in cultured Catharanthus roseus and Daucus carota plant cells. J Biol Chem. 1985 Mar 25;260(6):3556–3560. [PubMed] [Google Scholar]
- Jackson P. C., Pfeffer P. E., Gerasimowicz W. V. Use of P NMR to Assess Effects of DNP on ATP Levels in Vivo in Barley Roots. Plant Physiol. 1986 Aug;81(4):1130–1133. doi: 10.1104/pp.81.4.1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Corn Root Protoplasts: ISOLATION AND GENERAL CHARACTERIZATION OF ION TRANSPORT . Plant Physiol. 1980 Oct;66(4):550–554. doi: 10.1104/pp.66.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W., Hanson J. B. Phosphate absorption rates and adenosine 5'-triphosphate concentrations in corn root tissue. Plant Physiol. 1974 Sep;54(3):250–256. doi: 10.1104/pp.54.3.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W. Potassium and Phosphate Uptake in Corn Roots: Further Evidence for an Electrogenic H/K Exchanger and an OH/Pi Antiporter. Plant Physiol. 1979 May;63(5):952–955. doi: 10.1104/pp.63.5.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P. E., Tu S. I., Gerasimowicz W. V., Cavanaugh J. R. In VivoP NMR Studies of Corn Root Tissue and Its Uptake of Toxic Metals. Plant Physiol. 1986 Jan;80(1):77–84. doi: 10.1104/pp.80.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Transport across plant roots. Q Rev Biophys. 1982 Aug;15(3):481–554. doi: 10.1017/s0033583500003437. [DOI] [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Douce R. Regulation of Pi uptake by Acer pseudoplatanus cells. Arch Biochem Biophys. 1982 Dec;219(2):371–378. doi: 10.1016/0003-9861(82)90168-0. [DOI] [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Martin J. B., Douce R. Relationship between the cytoplasm and the vacuole phosphate pool in Acer pseudoplatanus cells. Arch Biochem Biophys. 1983 Aug;225(1):143–148. doi: 10.1016/0003-9861(83)90017-6. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Jardetzky O., Walbot V., Freeling M. Cytoplasmic acidosis as a determinant of flooding intolerance in plants. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6029–6033. doi: 10.1073/pnas.81.19.6029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Callis J., Wemmer D., Walbot V., Jardetzky O. Mechanisms of cytoplasmic pH regulation in hypoxic maize root tips and its role in survival under hypoxia. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3379–3383. doi: 10.1073/pnas.81.11.3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Jardetzky O. Monitoring of cellular metabolism by NMR. Biochim Biophys Acta. 1981 Nov 9;639(1):53–76. doi: 10.1016/0304-4173(81)90005-7. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Linker C. S., Benoit A. G., Jardetzky O., Nieman R. H. Salt stimulation of phosphate uptake in maize root tips studied by p nuclear magnetic resonance. Plant Physiol. 1984 Aug;75(4):947–950. doi: 10.1104/pp.75.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. K., Wade-Jardetzky N., Jardetzky O. Intracellular pH measurements by 31P nuclear magnetic resonance. Influence of factors other than pH on 31P chemical shifts. Biochemistry. 1981 Sep 15;20(19):5389–5394. doi: 10.1021/bi00522a006. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Wemmer D., Ray P. M., Jardetzky O. Regulation of Cytoplasmic and Vacuolar pH in Maize Root Tips under Different Experimental Conditions. Plant Physiol. 1982 Jun;69(6):1344–1347. doi: 10.1104/pp.69.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu S. I., Sliwinski B. J. Mechanistic investigation on the temperature dependence and inhibition of corn root plasma membrane ATPase. Arch Biochem Biophys. 1985 Sep;241(2):348–355. doi: 10.1016/0003-9861(85)90556-9. [DOI] [PubMed] [Google Scholar]


