Abstract
Previous studies show that COVID-19 survivors have elevated muscle sympathetic nerve activity (MSNA), endothelial dysfunction, and aortic stiffening. However, the neurovascular responses to mental stress and exercise are still unexplored. We hypothesized that COVID-19 survivors, compared with age- and body mass index (BMI)-matched control subjects, exhibit abnormal neurovascular responses to mental stress and physical exercise. Fifteen severe COVID-19 survivors (aged: 49 ± 2 yr, BMI: 30 ± 1 kg/m2) and 15 well-matched control subjects (aged: 46 ± 3 yr, BMI: 29 ± 1 kg/m2) were studied. MSNA (microneurography), forearm blood flow (FBF), and forearm vascular conductance (FVC, venous occlusion plethysmography), mean arterial pressure (MAP, Finometer), and heart rate (HR, ECG) were measured during a 3-min mental stress (Stroop Color-Word Test) and during a 3-min isometric handgrip exercise (30% of maximal voluntary contraction). During mental stress, MSNA (frequency and incidence) responses were higher in COVID-19 survivors than in controls (P < 0.001), and FBF and FVC responses were attenuated (P < 0.05). MAP was similar between the groups (P > 0.05). In contrast, the MSNA (frequency and incidence) and FBF and FVC responses to handgrip exercise were similar between the groups (P > 0.05). MAP was lower in COVID-19 survivors (P < 0.05). COVID-19 survivors exhibit an exaggerated MSNA and blunted vasodilatory response to mental challenge compared with healthy adults. However, the neurovascular response to handgrip exercise is preserved in COVID-19 survivors. Overall, the abnormal neurovascular control in response to mental stress suggests that COVID-19 survivors may have an increased risk to cardiovascular events during mental challenge.
Keywords: exercise, mental stress, peripheral blood flow, sympathetic activity, vascular conductance
INTRODUCTION
Although most patients have fully recovered from the coronavirus disease (COVID-19), some have reported persistent symptoms after the acute infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), such as fatigue, breathlessness, chest pain, elevated blood pressure (BP), and exercise intolerance (1–4). Recently, we showed that severe COVID-19 survivors (5) have sympathetic neural overdrive, reduced endothelial function, and aortic stiffening, which may help to explain, at least in part, these clinical manifestations. However, we evaluated the neurovascular control in these patients at rest, and how these responses occur during sympathoexcitatory stimuli like mental stress and isometric handgrip exercise are still unexplored.
Acute exposure to mental stress activates the sympathetic nervous system and the hypothalamic-pituitary-adrenal axis by central mechanisms in the brain (6, 7). This activation leads to the secretion of hormones and neurotransmitters (e.g., cortisol, epinephrine, norepinephrine, and nitric oxide), evoking increases in muscle sympathetic nerve activity (MSNA), heart rate (HR), mean arterial pressure (MAP), and forearm blood flow (FBF) in healthy humans (6–10). On the other hand, these physiological responses are altered in many disease states, and the magnitude of these changes is broadly related to morbidity and mortality in these populations (11, 12). Consistent evidence indicates that patients with heart failure and reduced ejection fraction, compared with control subjects, have exaggerated MSNA and reduced muscle vasodilation to a mental stress test (i.e., the Stroop Color-Word Test; 13). Because severe COVID-19 survivors exhibit excessive sympathetic neural outflow and endothelial dysfunction, it is expected that sympathetic activity in these patients is dramatically augmented during the stressor stimulus, resulting in an attenuated peripheral blood flow response and augmented blood pressure (BP) response. Thus, it is clinically relevant to assess the neurovascular and hemodynamic responses during mental challenges in patients who required hospitalization to treat the acute infection of COVID-19.
During physical exercise, sympathetic neural outflow is mediated via the integration of central and peripheric reflex controls (14, 15). In particular, the activation of skeletal muscle mechanoreceptors (myelinated fibers of group III, sensitive to mechanical stimuli) and metaboreceptors (unmyelinated fibers of group IV, sensitive to ischemic metabolites) contributes to the regulation of the sympathetic outflow during exercise (14–16). As resting MSNA is augmented in severe COVID-19 survivors, it is expected that neural responses to skeletal muscular beds would be markedly increased during exercise due to mechano-metaboreceptor sensitivity alterations. This excessive sympathetic activity may, in turn, impair the local hemodynamic forces within the vascular beds (e.g., attenuated increases in shear stress), leading to reduced muscle vasodilatory responses to exercise in these patients. However, whether severe COVID-19 survivors have an abnormal sympathetic and vasodilatory responses to exercise is unknown.
Accordingly, we sought to determine whether severe COVID-19 survivors exhibit abnormal neurovascular and pressure responses to sympathoexcitatory maneuvers. We tested the hypothesis that severe COVID-19 survivors, compared with healthy control subjects, would have a greater MSNA, reduced muscle blood flow, and elevated BP to both mental challenges and isometric handgrip exercise.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. All experimental procedures and measurements were conducted in accordance with the Declaration of Helsinki and were approved by the Research Committee of the D’Or Institute for Research and Education (IDOR, CAAE 31468020.1.0000.524). The nature, benefits, and risks of all study procedures were explained to participants, and their written informed consent was obtained before participation in the study.
Participants
COVID-19 survivors who required hospitalization (wards or intensive care units) to treat the acute SARS-CoV-2 infection were included in this study. All patients 1) were aged ≥18 yr, 2) had a laboratory-confirmed diagnosis of COVID-19 by reverse transcription-polymerase chain reaction (RT-PCR), 3) had chest computed tomography pneumonia (≥25%), 4) had oxygen saturation () ≤93% in room air at sea level, 5) received oxygen supply from a device, and 6) were discharged from the hospital after 1–6 mo of treatment. COVID-19 survivors completed a symptom survey on the day of the study. Our study was performed between September 2020 and October 2021, in which the predominant variants in Brazil were β and γ. Well-matched control subjects without a history of SARS-CoV-2 infection were included in the study. All control subjects had a nonreactive serological test for the antibodies immunoglobulin G (IgG) and M (IgM) and had not been immunized against COVID-19. Both groups have not been vaccinated against COVID-19. In addition, they were free of cardiovascular or kidney diseases, and the female participants were not pregnant or breastfeeding. The individuals of the present study also participated in a study described in our recently published paper (5).
Measurements
Muscle sympathetic nerve activity.
MSNA was directly measured by means of microneurography technique (5, 17). In brief, external electrical stimulation was used to locate the peroneal nerve of the lower leg. Then, a tungsten microelectrode with a tip diameter of ∼5 µm and a shaft diameter of ∼100–200 µm and 3–4 cm was inserted in the nerve to provide multiunit postganglionic muscle sympathetic nerve recordings. The microelectrode was epoxy-insulated except for the tip, which was not insulated. Thereafter, a reference tungsten microelectrode was inserted subcutaneously 1–3 cm from the recording electrode. Electrodes were connected to a preamplifier (gain: 1,000) and amplifier (variable gain: 30–80). Signals were amplified by a factor of 50,000–100,000 and band-pass filtered (700–2,000 Hz). Nerve activity was rectified and integrated (time constant 0.1 s) to obtain a mean voltage display of sympathetic nerve activity. Muscle sympathetic burst was identified by the following criteria: 1) a signal-to-noise ratio greater than 3:1, 2) a pulse-synchronized burst pattern with a burst occurring 1.2–1.3 s after the R wave in the ECG, 3) a typical sympathetic efferent nerve, and 4) a good nerve quality. The nerve burst was confirmed by a voluntary expiratory apnea, which activates chemoreflex control of the MSNA. Two investigators manually count the recorded nerve bursts. In case of disagreement, a third investigator was asked to analyze the nerve. MSNA was expressed as burst frequency (bursts per minute) and burst incidence (bursts per 100 heartbeats).
Forearm Blood Flow
FBF was measured simultaneously by venous occlusion plethysmography (17). The resting right arm was elevated above heart level to ensure adequate venous drainage. A mercury-filled Silastic tube attached to a low-pressure transducer was placed around the forearm, 5 cm from the humeroradial joint, and connected to plethysmography (AI6; D. E. Hokanson, Inc., Bellevue, WA). A wrist cuff was continuously inflated to 220 mmHg to occlude blood flow to the hand, whereas an upper arm cuff was inflated to 60 mmHg for 8 s and then deflated for 7 s (i.e., 15-s blood flow intervals). FBF was expressed in mL/min/100 mL and forearm vascular conductance (FVC) was expressed in units.
Hemodynamic Responses
A finger photoplethysmograph (Finometer Pro, Finapress Medical Systems, Amsterdam, The Netherlands) was used to continually assess BP. HR was measured by means of electrocardiogram (18).
Mental Stress Testing
Mental stress was elicited over a 3-min period using an adapted, computerized version of the Stroop Color-Word Test (7). The test consisted of a slideshow projected on the ceiling in front of the subjects. The slides changed every 2 s. Auditory conflicts were continuously delivered via earphones using a standardized audio clip integrated into the slideshow. Perceived stress level was recorded after each test using a 5-point scale, as follows: 0 (not stressful), 1 (somewhat stressful), 2 (stressful), 3 (very stressful), and 4 (extremely stressful).
Isometric Handgrip Exercise
After the maximal voluntary contraction (MVC; average of three attempts) was obtained, handgrip isometric exercise was performed at 30% of MVC with the left arm using a handgrip dynamometer for 3 min. The participants were instructed to breathe normally during exercise to avoid the Valsalva maneuver.
Experimental Protocol
Neurovascular and hemodynamic variables were measured simultaneously with the patients in the supine position in a quiet, temperature-controlled (21°C) room. After the electrodes were placed on the chest, the right arm was positioned for venous occlusion plethysmography. On the right leg, a tungsten microelectrode was inserted into the peroneal nerve. After the patients rested for 20 min, MSNA, FBF, HR, and BP were continually recorded for 3 min at baseline, 3 min of mental stress, and 3 min of recovery. After 10 min at rest, the patients performed the handgrip protocol at 30% MVC. MSNA, FBF, and HR were recorded for 3 min at baseline, 3 min of isometric handgrip exercise, and 3 min of recovery. During the handgrip protocol, BP was measured each minute in the leg.
Data Analysis
MSNA, FBF, BP, and HR were calculated as mean values of the 3-min recorded values during baseline and recovery and throughout the mental stress test and isometric handgrip exercise. MSNA bursts were identified and confirmed through visual inspection by two independent experienced investigators (D.F. and A.R.K.S.), who were blinded to the study patients. MAP and FVC were calculated as [2(diastolic BP) + (systolic BP)]/3 and MAP/FBF, respectively.
Statistical Analysis
The Shapiro–Wilk test was used to verify data distribution, and the Mauchly test was used to verify sphericity. The normality and sphericity assumptions were not violated. Mann–Whitney U test or nonmatched t test was used to test differences in clinical and physical characteristics between groups. Two-way ANOVA was used to test differences in neurovascular and hemodynamic changes during mental stress and isometric handgrip exercise among groups, followed by Fisher’s exact posthoc test. Pearson coefficient was used to assess the relationships between variables. Absolute delta was calculated as peak variable − baseline variable. Data are presented as means or delta ± SE. Significance was set at P < 0.05. All the figures and analyses were performed in GraphPad Prism 8.0 and Statistical 12.0, respectively.
RESULTS
Physical and Clinical Characteristics
The physical and clinical characteristics are reported in Table 1. Thirty participants (15 controls and 15 severe COVID-19 survivors) were included in the study. The groups had no differences in age, BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), HR, total cholesterol and fractions, glucose, urea, sodium, potassium, pro-brain natriuretic peptide (pro-BNP), and troponin I (P > 0.05 for all variables). COVID-19 survivors were tested ∼3 mo after hospital discharge. All control subjects had negative serology for IgG and IgM and were free of signs and symptoms of COVID-19.
Table 1.
Clinical and physical characteristics in control subjects and COVID-19 survivors
| Variables | Control Subjects (n = 15) | COVID-19 Survivors (n = 15) | P |
|---|---|---|---|
| Male sex, n (%) | 9 (60) | 10 (66) | 0.48 |
| Age, yr | 44.00 ± 2.00 | 49.00 ± 2.00 | 0.14 |
| BMI, kg/m2 | 29.00 ± 1.00 | 30.00 ± 1.00 | 0.20 |
| SBP, mmHg | 127.60 ± 3.50 | 130.50 ± 2.70 | 0.14 |
| DBP, mmHg | 77.10 ± 2.20 | 7.20 ± 2.10 | 0.44 |
| HR, beats/min | 67.00 ± 2.00 | 74.00 ± 3.00 | 0.06 |
| Arterial hypertension, n (%) | 1 (6) | 1 (6) | 0.50 |
| Clinical parameters at hospitalization | |||
| Length of hospitalization, days | 12.00 ± 7.00 | ||
| Oxygen saturation, % | 87 ± 2.6 | ||
| Pneumonia | |||
| 0%–25%, n (%) | 2 (15) | ||
| 25%–50%, n (%) | 9 (60) | ||
| 50%–75%, n (%) | 4 (25) | ||
| Mechanical ventilation, n (%) | 3 (20) | ||
| Oxygen therapy, n (%) | 12 (80) | ||
| Clinical parameters at study | |||
| Hospital discharge, days | 74.00 ± 15.00 | ||
| Troponin I—hs, ng/L | |||
| <2.5, n (%) | 11 (73.3) | 4 (26.6) | 0.09 |
| >2.5, n (%) | 6 (40.0) | 9 (60.0) | |
| Pro-BNP, pg/mL | 14.92 ± 3.70 | 15.80 ± 2.90 | 0.87 |
| Glucose, mg/dL | 98.84 ± 13.19 | 98.47 ± 13.37 | 0.92 |
| Total cholesterol, mg/dL | 210.10 ± 34.50 | 239.22 ± 58.20 | 0.15 |
| HDL cholesterol, mg/dL | 55.3 ± 4.32 | 52.40 ± 4.00 | 0.66 |
| LDL cholesterol, mg/dL | 133.00 ± 7.75 | 151.15 ± 11.60 | 0.24 |
| Triglycerides, mg/dL | 110.50 ± 13.12 | 224.40 ± 67.70 | 0.15 |
| Urea, mg/dL | 32.50 ± 1.60 | 31.29 ± 2.58 | 0.85 |
| Creatinine, μmol/L | 0.95 ± 0.03 | 1.08 ± 0.06 | 0.09 |
| Sodium, mEq/L | 141.00 ± 2.92 | 141.41 ± 1.87 | 0.62 |
| Potassium, mEq/L | 4.38 ± 0.37 | 4.37 ± 0.52 | 0.95 |
| IgM, UA/mL | 0.39 ± 0.08 | ||
| IgG, UA/mL | 0.19 ± 0.08 | ||
Data are presented as means ± SE. BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; pro-BNP, pro-B-type natriuretic peptide; HDL cholesterol, high-density lipoprotein cholesterol; LDL cholesterol, low-density lipoprotein cholesterol; IgM, immunoglobulin M; IgG, immunoglobulin G.
Sympathetic Neural Responses to Mental Stress
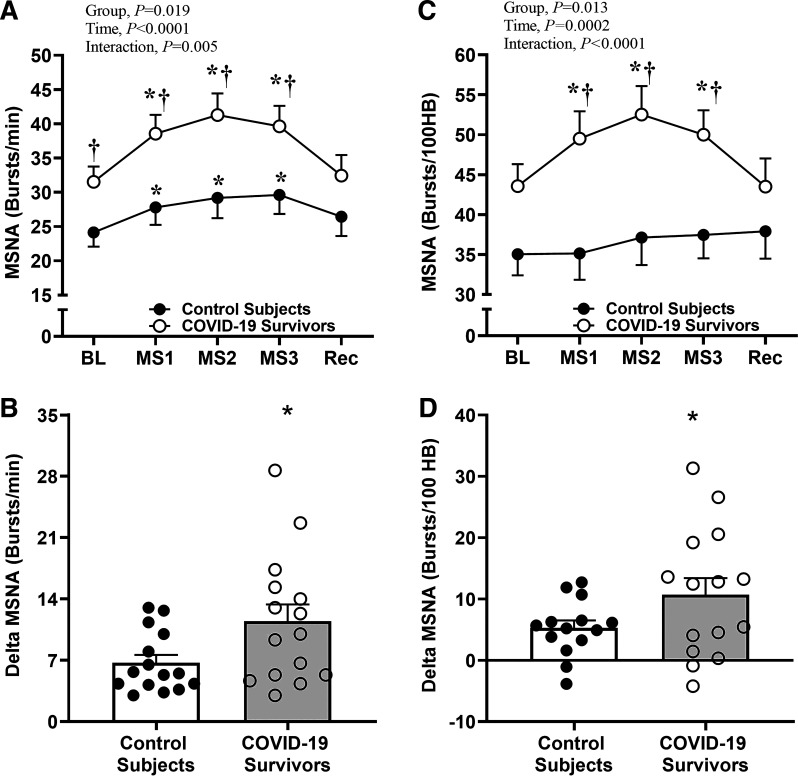
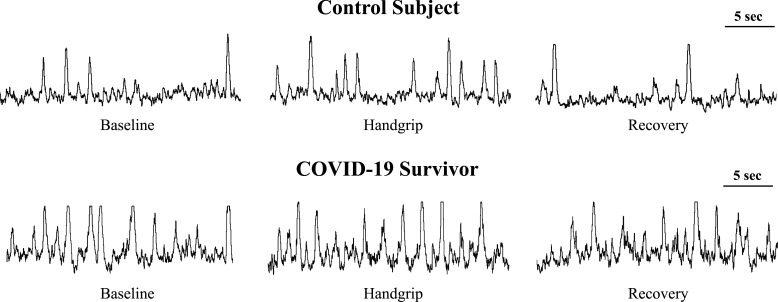
To investigate whether COVID-19 survivors exhibit exaggerated sympathetic neural activity during central activation via stress challenge, we evaluated MSNA by microneurography. Examples of original nerve recordings during the protocol from one control subject and one COVID-19 survivor are shown in Fig. 1. At rest, MSNA burst frequency was greater in COVID-19 survivors than in controls (P < 0.05, Fig. 2A). Mental stress increased MSNA burst frequency during the 3 min of stress stimulus in both groups, but this response was clearly higher in COVID-19 survivors compared with that of controls (Interaction effect, P = 0.005, Fig. 2A). Delta analysis showed that the magnitude of increase in the MSNA burst frequency was ∼65% greater in COVID-19 survivors versus controls (P = 0.04, Fig. 2B).
Figure 1.
Original recordings of muscle sympathetic neve activity during mental stress in one control subject and in one COVID-19 survivor.
Figure 2.
Sympathetic neural responses to mental stress in COVID-19 survivors. Muscle sympathetic nerve activity (MSNA) burst frequency during mental stress (MS) in control subjects and in severe COVID-19 survivors (A); delta MSNA burst frequency (B); MSNA burst incidence (C); and delta MSNA burst incidence (D). BL, baseline; Rec, recovery. *P < 0.05 vs. BL; †P < 0.05 vs. control subjects.
To our surprise, the mental stress increased MSNA burst incidence only in COVID-19 survivors (Time effect, P = 0.0002, Fig. 2C). Furthermore, MSNA burst incidence during the 3 min of stress was greater in COVID-19 survivors than in controls (Interaction effect, P < 0.0001, Fig. 2C). Delta analysis revealed that the magnitude of increase in the MSNA burst incidence was ∼128% higher in COVID-19 survivors versus controls (P = 0.05, Fig. 2D).
Vascular Responses to Mental Stress
To assess vascular responses to mental stress, we measured both FBF and FVC. Mental stress increased FBF during minutes 1, 2, and 3 of the mental stress stimulus in the control participants; in the COVID-19 survivors, this increase was observed only during minutes 2 and 3 of the mental stress stimulus (Time effect, P < 0.0001, Fig. 3A). Importantly, FBF during the 3 min of stress was significantly lower in COVID-19 survivors versus controls (Interaction effect, P = 0.03, Fig. 3A). Delta analysis showed that the FBF response was ∼115% smaller in COVID-19 survivors compared with that of controls (P = 0.004, Fig. 3B).
Figure 3.
Vascular responses to mental stress in COVID-19 survivors. Forearm blood flow (FBF) during mental stress (MS) in control subjects and in severe COVID-19 survivors (A); delta FBF (B); forearm vascular conductance (FVC) (C); and delta FVC (D). BL, baseline; Rec, recovery. *P < 0.05 vs. BL; †P < 0.05 vs. control subjects.
Likewise, mental stress increased FVC during minutes 1, 2, and 3 of the mental stress stimulus in the controls, but in the COVID survivors it increased only during minutes 2 and 3 (Time effect, P < 0.0001, Fig. 3C). Also, FVC in response to mental stress was significantly lower in COVID-19 survivors than in controls during minutes 1 and 3 (Interaction effect, P = 0.03, Fig. 3C). Delta analysis showed that the FVC response was ∼120% blunted in COVID-19 survivors compared with that of controls (P = 0.004, Fig. 3D).
Cardiovascular Responses to Mental Stress
Mental stress similarly increased SBP, DBP, MAP, and HR during the 3 min of mental stress in COVID-19 survivors and in controls (Time effect, P < 0.0001 for all variables, Fig. 4, A, C, E, and G, respectively). Delta analysis revealed that the DBP response to mental stress was attenuated in COVID-19 survivors versus controls (P = 0.04, Fig. 4D), but SBP, MAP, and HR responses did not differ between the groups (P > 0.05 for all variables, Fig. 4, B, F, and H, respectively). To our surprise, no association existed between Δ frequency MSNA with ΔSBP (r = −0.17, P = 0.36), ΔDBP (r = −0.01, P = 0.95), or ΔMAP (r = −0.09, P = 0.61)
Figure 4.
Pressure responses to mental stress in COVID-19 survivors. Systolic blood pressure (SBP) during mental stress (MS) in control subjects and in severe COVID-19 survivors (A); delta SBP (B); diastolic blood pressure (DBP) (C); delta DBP (D); mean arterial pressure (MAP) (E); delta MAP (F); heart rate (HR) (G); and delta HR (H). BL, baseline; Rec, recovery. *P < 0.05 vs. control subjects.
The perceived stress level during mental stress testing was similar between the groups (COVID-19 survivors: 2.0 ± 1.0 vs. controls: 2.0 ± 1.5, P = 0.23).
Sympathetic Neural Responses to Isometric Handgrip Exercise
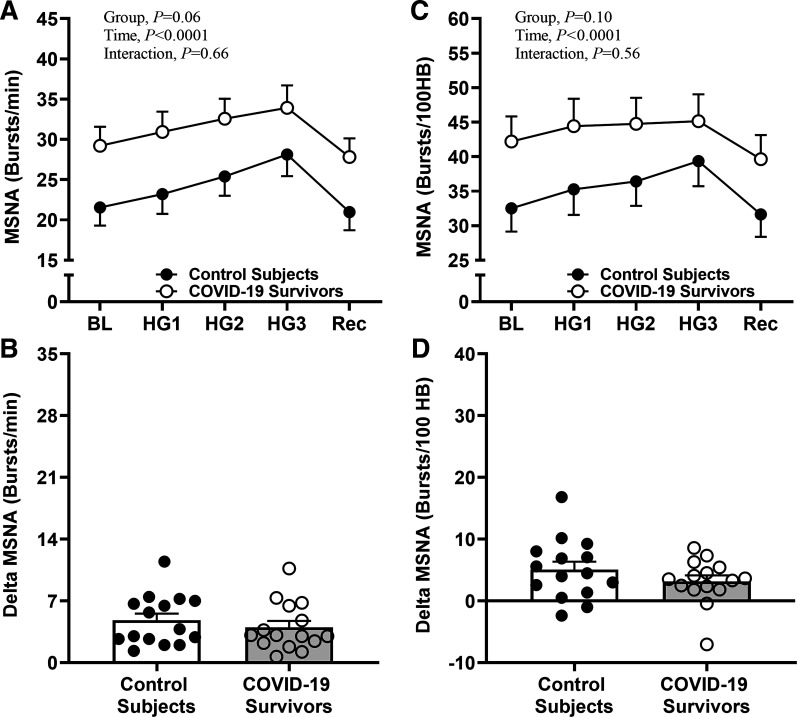
To investigate the role of peripheral mechanisms in the sympathetic neural activity control, the participants performed an isometric handgrip exercise at moderate intensity (30% MVC). Examples of original nerve recordings during the isometric handgrip exercise protocol from one control subject and one COVID-19 survivor are shown in Fig. 5. MSNA burst frequency increased during the 3 min of exercise in COVID-19 survivors and in controls (Time effect, P < 0.0001, Fig. 6A). Although the ANOVA analysis revealed that MSNA burst frequency behavior during the handgrip tended to be greater in COVID-19 survivors versus controls (Group effect, P = 0.06, Fig. 6A), delta analysis showed that the magnitude of increase in this variable was equal among both groups (P = 0.43, Fig. 6B).
Figure 5.
Original recordings of muscle sympathetic neve activity during isometric handgrip exercise in one control and in one COVID-19 survivor.
Figure 6.
Sympathetic neural responses to isometric handgrip exercise in COVID-19 survivors. Muscle sympathetic nerve activity (MSNA) burst frequency during isometric handgrip exercise (HG) in control subjects and in severe COVID-19 survivors (A); delta MSNA burst frequency (B); MSNA burst incidence (C); and delta burst MSNA incidence (D). BL, baseline; Rec, recovery.
Furthermore, the increases in MSNA burst incidence during the 3-min handgrip exercise were similar between the groups (Group effect, P = 0.56, Fig. 6C). Delta analysis also did not show differences in MSNA burst incidence responses between the groups (P > 0.05, Fig. 6D).
Vascular Responses to Isometric Handgrip Exercise
To assess the vascular reactivity to the handgrip exercise, we measured both FBF and FVC. Both FBF and FVC during exercise were similar between the groups (Interaction effect, P > 0.05 to both variables, Fig. 7, A and C, respectively). Delta analysis showed that the magnitude of increases in both FBF and FVC was not different between the groups (P > 0.05, Fig. 7, B and D).
Figure 7.
Vascular responses to isometric handgrip exercise in COVID-19 survivors. Forearm blood flow (FBF) during isometric handgrip exercise (HG) in control subjects and severe COVID-19 survivors (A); delta FBF (B); forearm vascular conductance (FVC) (C); and delta FVC (D). BL, baseline; Rec, recovery.
Cardiovascular Responses to Handgrip Exercise
Although the increases in DBP and MAP during handgrip exercise were attenuated in COVID-19 survivors versus controls (Interaction effect, P < 0.01 for all variables, Fig. 8, A, C, and E, respectively), the increases in SBP and HR were similar between the groups (Interaction effect, P < 0.01, Fig. 8, A and G). Delta analysis showed that the magnitude of increases in DBP and MAP was attenuated in COVID-19 versus controls (P < 0.05 for both variables, Fig. 8, D and F). No differences were observed in SBP and HR responses (P > 0.05 for both variables, Fig. 8, B and H). Also, no association existed between Δfrequency MSNA with ΔSBP (r = 0.21, P = 0.24), ΔDBP (r = 0.05, P = 0.77), or ΔMAP (r = 0.14, P = 0.44)
Figure 8.
Pressure responses to isometric handgrip exercise in COVID-19 survivors. Systolic blood pressure (SBP) during isometric handgrip exercise (HG) in control subjects and severe COVID-19 survivors (A); delta SBP (B); diastolic blood pressure (DBP) (C); delta DBP (D); mean arterial pressure (MAP) (E); delta MAP (F); heart rate (HR) (G); and delta HR (H). BL, baseline; Rec, recovery. *P < 0.05 vs. control subjects.
DISCUSSION
The findings of the current study reveal for the first time that COVID-19 survivors have an exaggerated increase in sympathetic neural activity and an attenuated vasodilatory muscle response to mental stress testing. In contrast, the neurovascular responses during handgrip exercise are preserved in COVID-19 survivors, but pressure responses are attenuated.
Neurovascular and Cardiovascular Responses to Mental Challenge
Previous evidence has shown that COVID-19 survivors exhibited resting sympathetic neural overdrive and vascular dysfunction (5, 19, 20). In the present study, we also observed a greater resting MSNA in COVID-19 survivors compared with control subjects and extended these findings to physiological maneuvers. Mental challenge is a unique condition to study the neurovascular reactivity in humans during a stressful condition. Mental stress provokes an intense increase in sympathetic outflow and muscle blood flow (10, 21). Remarkably, these responses to stress are highly reliable using a test-retest design (22). Herein, we described that MSNA levels during mental stress are increased in COVID-19 survivors compared with control subjects. Moreover, our findings show that the augmented MSNA levels during mental challenges are not simply a consequence of greater baseline in the COVID-19 survivors. In fact, the changes in MSNA above baseline during mental stress were significantly greater in COVID-19 survivors. These findings highlight that the MSNA responsiveness to mental stress is remarkably increased after infection of SARS-CoV-2. These findings may be critical to COVID-19 survivors because abnormal neural reactivity to mental stress has been linked to several cardiovascular diseases (8, 11, 12).
The mechanisms involved in the exaggerated MSNA responses to mental stress in COVID-19 are out the scope of our study. However, it is possible that infection with SARS-CoV-2 provokes alterations in the central nervous system (23). The increase in sympathetic nerve activity is highly mediated by the central command (24, 25). In fact, postmortem reports show related neuropathological changes with pronounced neuroinflammation in the brainstem of patients with COVID-19(26).
Notably, FBF and FVC responses to mental stress were attenuated in COVID-19 survivors compared with control subjects. Our study does not clarify the mechanisms related to blunted peripheral vasodilatory responses in COVID-19 survivors. However, it is possible that the blunted peripheral vasodilatory responses are associated with the disequilibrium between vasoconstrictor and vasodilatation forces. The increased MSNA during mental stress may have favored the muscle vasoconstrictor forces. Of course, we cannot rule out that the endothelial dysfunction plays a role in this matter. There is evidence that the muscle vasodilatory responses in response to mental challenge are mediated, in part, by endothelial function (13, 27–29). We and others showed that vascular dysfunction is present in severe COVID-19 survivors (5).
The HR responses to mental challenge were not different between COVID-19 survivors and control subjects. This finding is in line with our recent observation that showed similar resting HR and HR variability in COVID-19 and control subjects (1). Likewise, MAP equally increased in both COVID-19 and control subjects.
The findings of the current study revealed that the increased MSNA response to mental stress in COVID-19 survivors was not accompanied by an exacerbated BP response. Also, we did not observe relationships between changes in MSNA and changes in BP regardless of the COVID-19 status. This is consistent across different populations, such as individuals with a familial history of hypertension and healthy controls (30, 31), where there is a marked dissociation between MSNA and BP responsiveness. Thus, other mechanisms seem to explain the pressor response to mental stressor.
Neurovascular and Cardiovascular Responses to Handgrip Exercise
Sympathetic nerve activation during exercise is initially mediated by muscle mechanoreceptors and sustained by metaboreceptors, which are activated due to the increase of metabolites in the skeletal muscle (14, 15). Our findings showed differences in MSNA burst frequency and incidence during the handgrip exercise among COVID-19 survivors and control subjects. Thus, these findings suggest that mechanoreflex and metaboreflex controls of sympathetic nerve activity during exercise seem to be preserved in COVID-19 survivors.
Previous studies demonstrate that during the exercise, part of the cardiac output is shifted to the active muscle due to an increase in local metabolic demand, leading to an attenuation of the arteriolar constriction and adequate perfusion to the contracting muscle (i.e., functional sympatholysis; 32) There is also evidence that blood flow increases in the inactive vascular beds (6, 33). This response increases the shear stress and circumferential stretch in the vascular wall, stimulating the endothelium to increase the production of vasoactive substances (e.g., nitric oxide) and, in consequence, evoking the muscle vasodilation (6, 33). Our findings revealed that FBF and FVC responses in the contralateral forearm during handgrip exercise were similar between COVID-19 survivors and control subjects, suggesting that the mechanisms of blood flow control in the inactive vascular bed are preserved in COVID-19 survivors.
The HR levels during exercise were not different in COVID-19 and control subjects. In contrast, pressure responses tended to be lower in COVID-19 survivors. We have no definitive explanation for these findings. However, it is possible to suspect that the cardiac responses to stress tasks are impaired in COVID-19 survivors. Some reported that COVID-19 survivors have attenuated increases in cardiac output during submaximal and maximum exercise echocardiography (34, 35). Since HR responses to exercise were preserved in COVID-19, it could attribute the lowered BP responses to an attenuation in stroke volume in the COVID-19 survivors. It is unlikely that the attenuated blood pressure responses were due to the increased peripheral resistance because the sympathetic outflow was not altered in COVID-19 survivors.
LIMITATIONS
We recognize limitations in this study. First, the alterations in the neurovascular control may not be exclusive of SARS-CoV-2 infection as other viral infections (e.g., influenza) have also been associated with cardiovascular dysfunction (3, 36). However, previous studies demonstrated that cardiovascular sequelae are more evident in COVID-19 than in influenza (3, 36). Second, our study was conducted in patients who manifest severe COVID-19. Thus, we do not know if nonhospitalized adults who recovered from a mild SARS-CoV-2 infection have the abnormal neurovascular and hemodynamic responses to mental stress and handgrip exercise. Third, we did not assess the mental health status of the participants of the present study. It is possible that COVID-19 survivors have a greater neural reactivity to mental stress due to psychological disorders. However, perceived stress levels were not different between the groups. Fourth, the participants of the current study performed an isometric handgrip exercise. With this physiological maneuver, it is not possible to study the isolated contribution of each reflex (mechanoreflex and metaboreflex) on sympathetic exacerbation (14) in COVID-19 survivors. Also, we did not test other reflexes, such as peripheral chemoreflex and cardiopulmonary reflex, which may contribute to increased sympathetic activity in this population. Thus, this topic needs to be addressed in future studies. Finally, the cardiovascular adjustments that occur in response to isometric handgrip exercise differ from those occurring in response to aerobic exercise. Therefore, it is unknown whether COVID-19 survivors have a preserved neurovascular response to conventional aerobic exercise or not.
Perspectives and Significance
Herein, we demonstrated that COVID-19 survivors exhibit an exaggerated MSNA and blunted vasodilatory response to mental challenge compared with control adults. However, the neurovascular response to exercise is preserved in COVID-19 survivors. Overall, the abnormal neurovascular control in response to mental stress suggests that COVID-19 survivors may have an increased risk to cardiovascular events during mental challenge.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
A.R.K.S. is supported by Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ, E-26/211.526/2021) and D’Or Institute for Research and Education. C.E.N. is supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, no. 304697/2020-6). M.J.R. is supported by United States National Institutes of Health Award K01DK115524. D.H.C. is supported by National Institutes of Health Award K01HL153326.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.F., R.M.-B., E.C.R., J.M.M, F.R.S., T.S.R., V.M.C.S., K.D.A., D.H.C., M.J.R., L.A.B., F.M.C.-C., M.C.C.I., D.R.S., C.E.N., and A.R.K.S. conceived and designed research; D.F., M.J.N.N.A., B.E.O., V.M.C.S., C.P.J., D.H.C., and A.R.K.S. performed experiments; D.F., R.M.-B., C.M.V.M., E.C.R., J.E.I., T.S.R., V.M.C.S., K.D.A., D.H.C., M.J.R., F.M.C.-C., M.C.C.I., D.R.S., C.E.N., and A.R.K.S. analyzed data; D.F., R.M.-B., L.T., C.M.V.M., E.C.R., J.M.M., F.R.S., M.J.N.N.A., B.E.O., J.E.I., A.O.S., V.M.C.S., C.P.J., K.D.A., D.H.C., M.J.R., F.M.C.-C., M.C.C.I., D.R.S., C.E.N., and A.R.K.S. interpreted results of experiments; D.F. and A.R.K.S. prepared figures; D.F., R.M.-B., L.T., C.M.V.M., E.C.R., J.M.M., F.R.S., M.J.N.N.A., B.E.O., J.E.I., A.O.S., T.S.R., V.M.C.S., C.P.J., K.D.A., D.H.C., M.J.R., L.A.B., F.M.C.-C., M.C.C.I., D.R.S., C.E.N., and A.R.K.S.drafted manuscript; D.F., R.M.-B., L.T., C.M.V.M., E.C.R., J.M.M., F.R.S., M.J.N.N.A., B.E.O., J.E.I., A.O.S., T.S.R., V.M.C.S., C.P.J., K.D.A., D.H.C., M.J.R., L.A.B., F.M.C.-C., M.C.C.I., D.R.S., C.E.N., and A.R.K.S. edited and revised manuscript; D.F., R.M.-B., L.T., C.M.V.M., E.C.R., J.M.M., F.R.S., M.J.N.N.A., B.E.O., J.E.I., A.O.S., T.S.R., V.M.C.S., C.P.J., K.D.A., D.H.C., M.J.R., L.A.B., F.M.C.-C., M.C.C.I., D.R.S., C.E.N., and A.R.K.S. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors are grateful for the time and effort of all participants. We thank Elaine Lagonegro, Fabiana Panham, Ana Lopes, and the NAPE Team (IDOR) for the regulatory and administrative support of this work.
REFERENCES
- 1. Satterfield BA, Bhatt DL, Gersh BJ. Cardiac involvement in the long-term implications of COVID-19. Nat Rev Cardiol 19: 332–341, 2022. [Erratum in Nat Rev Cardiol 19: 342, 2021]. doi: 10.1038/s41569-021-00631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wang W, Wang CY, Wang SI, Wei JC. Long-term cardiovascular outcomes in COVID-19 survivors among non-vaccinated population: a retrospective cohort study from the TriNetX US collaborative networks. EClinicalMedicine 53: 101619, 2022. [Erratum in EClinicalMedicine 59: 101968, 2023]. doi: 10.1016/j.eclinm.2022.101619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Xie Y, Choi T, Al-Aly Z. Risk of death in patients hospitalized for COVID-19 vs seasonal influenza in fall-winter 2022-2023. JAMA 329: 1697–1699, 2023. doi: 10.1001/jama.2023.5348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bowe B, Xie Y, Al-Aly Z. Acute and postacute sequelae associated with SARS-CoV-2 reinfection. Nat Med 28: 2398–2405, 2022. doi: 10.1038/s41591-022-02051-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Faria D, Moll-Bernardes RJ, Testa L, Moniz CMV, Rodrigues EC, Rodrigues AG, Araujo A, Alves MJNN, Ono BE, Izaias JE, Salemi VMC, Jordão CP, Amaro-Vicente G, Rondon MUPB, Ludwig KR, Craighead DH, Rossman MJ, Consolim-Colombo FM, De Angelis K, Irigoyen MCC, Seals DR, Negrão CE, Sales ARK. Sympathetic neural overdrive, aortic stiffening, endothelial dysfunction, and impaired exercise capacity in severe COVID-19 survivors: a mid-term study of cardiovascular sequelae. Hypertension 80: 470–481, 2023. doi: 10.1161/HYPERTENSIONAHA.122.19958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sales AR, Fernandes IA, Rocha NG, Costa LS, Rocha HN, Mattos JD, Vianna LC, Silva BM, Nóbrega AC. Aerobic exercise acutely prevents the endothelial dysfunction induced by mental stress among subjects with metabolic syndrome: the role of shear rate. Am J Physiol Heart Circ Physiol 306: H963–H971, 2014. doi: 10.1152/ajpheart.00811.2013. [DOI] [PubMed] [Google Scholar]
- 7. Macefield VG, James C, Henderson LA. Identification of sites of sympathetic outflow at rest and during emotional arousal: concurrent recordings of sympathetic nerve activity and fMRI of the brain. Int J Psychophysiol 89: 451–459, 2013. doi: 10.1016/j.ijpsycho.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 8. Carter JR, Goldstein DS. Sympathoneural and adrenomedullary responses to mental stress. Compr Physiol 5: 119–146, 2015. doi: 10.1002/cphy.c140030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Esler M, Eikelis N, Schlaich M, Lambert G, Alvarenga M, Kaye D, El-Osta A, Guo L, Barton D, Pier C, Brenchley C, Dawood T, Jennings G, Lambert E. Human sympathetic nerve biology: parallel influences of stress and epigenetics in essential hypertension and panic disorder. Ann NY Acad Sci 1148: 338–348, 2008. doi: 10.1196/annals.1410.064. [DOI] [PubMed] [Google Scholar]
- 10. Carter JR, Kupiers NT, Ray CA. Neurovascular responses to mental stress. J Physiol 564: 321–327, 2005. doi: 10.1113/jphysiol.2004.079665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lambert G, Schlaich M, Lambert E, Dawood T, Esler M. Stress reactivity and its association with increased cardiovascular risk: a role for the sympathetic nervous system? Hypertension 55: e20, 2010. doi: 10.1161/HYPERTENSIONAHA.110.153841. [DOI] [PubMed] [Google Scholar]
- 12. Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 55: 1026–1032, 2010. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- 13. Middlekauff HR, Nguyen AH, Negrao CE, Nitzsche EU, Hoh CK, Natterson BA, Hamilton MA, Fonarow GC, Hage A, Moriguchi JD. Impact of acute mental stress on sympathetic nerve activity and regional blood flow in advanced heart failure: implications for 'triggering' adverse cardiac events. Circulation 96: 1835–1842, 1997. doi: 10.1161/01.cir.96.6.1835. [DOI] [PubMed] [Google Scholar]
- 14. Teixeira AL, Vianna LC. The exercise pressor reflex: an update. Clin Auton Res 32: 271–290, 2022. doi: 10.1007/s10286-022-00872-3. [DOI] [PubMed] [Google Scholar]
- 15. Smith SA, Mitchell JH, Garry MG. The mammalian exercise pressor reflex in health and disease. Exp Physiol 91: 89–102, 2006. doi: 10.1113/expphysiol.2005.032367. [DOI] [PubMed] [Google Scholar]
- 16. Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest 79: 508–516, 1987. doi: 10.1172/JCI112841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sales ARK, Negrão MV, Testa L, Ferreira-Santos L, Groehs RVR, Carvalho B, Toschi-Dias E, Rocha NG, Laurindo FRM, Debbas V, Rondon MUPB, Mano MS, Hajjar LA, Hoff PMG, Filho RK, Negrão CE. Chemotherapy acutely impairs neurovascular and hemodynamic responses in women with breast cancer. Am J Physiol Heart Circ Physiol 317: H1–H12, 2019. doi: 10.1152/ajpheart.00756.2018. [DOI] [PubMed] [Google Scholar]
- 18. Bogert LW, van Lieshout JJ. Non-invasive pulsatile arterial pressure and stroke volume changes from the human finger. Exp Physiol 90: 437–446, 2005. doi: 10.1113/expphysiol.2005.030262. [DOI] [PubMed] [Google Scholar]
- 19. Stute NL, Stickford JL, Province VM, Augenreich MA, Ratchford SM, Stickford ASL. COVID-19 is getting on our nerves: sympathetic neural activity and haemodynamics in young adults recovering from SARS-CoV-2. J Physiol 599: 4269–4285, 2021. doi: 10.1113/JP281888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ratchford SM, Stickford JL, Province VM, Stute N, Augenreich MA, Koontz LK, Bobo LK, Stickford ASL. Vascular alterations among young adults with SARS-CoV-2. Am J Physiol Heart Circ Physiol 320: H404–H410, 2021. doi: 10.1152/ajpheart.00897.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yang H, Drummer TD, Carter JR. Sex differences in sympathetic neural and limb vascular reactivity to mental stress in humans. Am J Physiol Heart Circ Physiol 304: H436–H443, 2013. doi: 10.1152/ajpheart.00688.2012. [DOI] [PubMed] [Google Scholar]
- 22. Fonkoue IT, Carter JR. Sympathetic neural reactivity to mental stress in humans: test-retest reproducibility. Am J Physiol Regul Integr Comp Physiol 309: R1380–R1386, 2015. doi: 10.1152/ajpregu.00344.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sarkar S, Karmakar S, Basu M, Ghosh P, Ghosh MK. Neurological damages in COVID-19 patients: mechanisms and preventive interventions. MedComm (2020) 4: e247, 2023. doi: 10.1002/mco2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Esler M, Lambert G, Brunner-La Rocca HP, Vaddadi G, Kaye D. Sympathetic nerve activity and neurotransmitter release in humans: translation from pathophysiology into clinical practice. Acta Physiol Scand 177: 275–284, 2003. doi: 10.1046/j.1365-201X.2003.01089.x. [DOI] [PubMed] [Google Scholar]
- 25. Hajduczok G, Hade JS, Mark AL, Williams JL, Felder RB. Central command increases sympathetic nerve activity during spontaneous locomotion in cats. Circ Res 69: 66–75, 1991. doi: 10.1161/01.res.69.1.66. [DOI] [PubMed] [Google Scholar]
- 26. Agrawal S, Farfel JM, Arfanakis K, Al-Harthi L, Shull T, Teppen TL, Evia AM, Patel MB, Ely EW, Leurgans SE, Bennett DA, Mehta R, Schneider JA. Brain autopsies of critically ill COVID-19 patients demonstrate heterogeneous profile of acute vascular injury, inflammation and age-linked chronic brain diseases. Acta Neuropathol Commun 10: 186, 2022. doi: 10.1186/s40478-022-01493-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santos AC, Alves MJ, Rondon MU, Barretto AC, Middlekauff HR, Negrão CE. Sympathetic activation restrains endothelium-mediated muscle vasodilatation in heart failure patients. Am J Physiol Heart Circ Physiol 289: H593–H599, 2005. doi: 10.1152/ajpheart.01240.2004. [DOI] [PubMed] [Google Scholar]
- 28. Halliwill JR, Lawler LA, Eickhoff TJ, Dietz NM, Nauss LA, Joyner MJ. Forearm sympathetic withdrawal and vasodilatation during mental stress in humans. J Physiol 504: 211–220, 1997. doi: 10.1111/j.1469-7793.1997.211bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cardillo C, Kilcoyne CM, Quyyumi AA, Cannon RO 3rd, Panza JA. Role of nitric oxide in the vasodilator response to mental stress in normal subjects. Am J Cardiol 80: 1070–1074, 1997. doi: 10.1016/s0002-9149(97)00605-x. [DOI] [PubMed] [Google Scholar]
- 30. Fonkoue IT, Wang M, Carter JR. Sympathetic neural reactivity to mental stress in offspring of hypertensive parents: 20 years revisited. Am J Physiol Heart Circ Physiol 311: H426–H432, 2016. doi: 10.1152/ajpheart.00378.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Carter JR, Ray CA. Sympathetic neural responses to mental stress: responders, nonresponders and sex differences. Am J Physiol Heart Circ Physiol 296: H847–H853, 2009. doi: 10.1152/ajpheart.01234.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Thomas GD. Functional sympatholysis in hypertension. Auton Neurosci 188: 64–68, 2015. doi: 10.1016/j.autneu.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Padilla J, Simmons GH, Bender SB, Arce-Esquivel AA, Whyte JJ, Laughlin MH. Vascular effects of exercise: endothelial adaptations beyond active muscle beds. Physiology (Bethesda) 26: 132–145, 2011. doi: 10.1152/physiol.00052.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Szekely Y, Lichter Y, Sadon S, Lupu L, Taieb P, Banai A, Sapir O, Granot Y, Hochstadt A, Friedman S, Laufer-Perl M, Banai S, Topilsky Y. Cardiorespiratory abnormalities in patients recovering from coronavirus disease 2019. J Am Soc Echocardiogr 34: 1273–1284.e9, 2021. doi: 10.1016/j.echo.2021.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baratto C, Caravita S, Faini A, Perego GB, Senni M, Badano LP, Parati G. Impact of COVID-19 on exercise pathophysiology: a combined cardiopulmonary and echocardiographic exercise study. J Appl Physiol (1985) 130: 1470–1478, 2021. doi: 10.1152/japplphysiol.00710.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med 28: 583–590, 2022. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.