Abstract
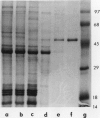
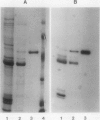
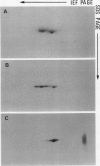
ADPglucose pyrophosphorylase has been extensively purified from potato (Solanum tuberosum L.) tuber tissue to study its structure. By employing a modified published procedure (JR Sowokinos, J Preiss [1982] Plant Physiol 69: 1459-1466) together with Mono Q chromatography, a near homogeneous enzyme preparation was obtained with substantial improvement in enzyme yield and specific activity. In single dimensional sodium dodecyl sulfate polyacrylamide gels, the enzyme migrated as a single polypeptide band with a mobility of about 50,000 daltons. Analysis by two-dimensional polyacrylamide gel electrophoresis, however, revealed the presence of two types of subunits which could be distinguished by their slight differences in net charge and molecular weight. The smaller potato tuber subunit was recognized by antiserum prepared against the smaller spinach leaf 51 kilodalton ADPglucose pyrophosphorylase subunit. In contrast, the anti-54 kilodalton raised against the spinach leaf subunit did not significantly react to the tuber enzyme subunits. The results are consistent with the hypothesis that the potato tuber ADPglucose pyrophosphorylase is not composed of a simple homotetramer as previously suggested, but is a product of two separate and distinct subunits as observed for the spinach leaf and maize enzymes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Copeland L., Preiss J. Purification of Spinach Leaf ADPglucose Pyrophosphorylase. Plant Physiol. 1981 Nov;68(5):996–1001. doi: 10.1104/pp.68.5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Hannah L. C., Nelson O. E., Jr Characterization of ADP-glucose pyrophosphorylase from shrunken-2 and brittle-2 mutants of maize. Biochem Genet. 1976 Aug;14(7-8):547–560. doi: 10.1007/BF00485834. [DOI] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser W. M., Bassham J. A. Light-Dark Regulation of Starch Metabolism in Chloroplasts: II. Effect of Chloroplastic Metabolite Levels on the Formation of ADP-Glucose by Chloroplast Extracts. Plant Physiol. 1979 Jan;63(1):109–113. doi: 10.1104/pp.63.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan H. B., Reeves C. D., Okita T. W. ADPglucose Pyrophosphorylase Is Encoded by Different mRNA Transcripts in Leaf and Endosperm of Cereals. Plant Physiol. 1986 Jun;81(2):642–645. doi: 10.1104/pp.81.2.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C. R., Preiss J. A Starch Deficient Mutant of Arabidopsis thaliana with Low ADPglucose Pyrophosphorylase Activity Lacks One of the Two Subunits of the Enzyme. Plant Physiol. 1988 Dec;88(4):1175–1181. doi: 10.1104/pp.88.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. P., Caspar T., Somerville C., Preiss J. Isolation and Characterization of a Starchless Mutant of Arabidopsis thaliana (L.) Heynh Lacking ADPglucose Pyrophosphorylase Activity. Plant Physiol. 1988 Apr;86(4):1131–1135. doi: 10.1104/pp.86.4.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignery G. A., Pikaard C. S., Hannapel D. J., Park W. D. Isolation and sequence analysis of cDNAs for the major potato tuber protein, patatin. Nucleic Acids Res. 1984 Nov 12;12(21):7987–8000. doi: 10.1093/nar/12.21.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morell M. K., Bloom M., Knowles V., Preiss J. Subunit Structure of Spinach Leaf ADPglucose Pyrophosphorylase. Plant Physiol. 1987 Sep;85(1):182–187. doi: 10.1104/pp.85.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Plaxton W. C., Preiss J. Purification and Properties of Nonproteolytic Degraded ADPglucose Pyrophosphorylase from Maize Endosperm. Plant Physiol. 1987 Jan;83(1):105–112. doi: 10.1104/pp.83.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Bacterial glycogen synthesis and its regulation. Annu Rev Microbiol. 1984;38:419–458. doi: 10.1146/annurev.mi.38.100184.002223. [DOI] [PubMed] [Google Scholar]
- Preiss J., Danner S., Summers P. S., Morell M., Barton C. R., Yang L., Nieder M. Molecular Characterization of the Brittle-2 Gene Effect on Maize Endosperm ADPglucose Pyrophosphorylase Subunits. Plant Physiol. 1990 Apr;92(4):881–885. doi: 10.1104/pp.92.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preiss J. Regulation of adenosine diphosphate glucose pyrophosphorylase. Adv Enzymol Relat Areas Mol Biol. 1978;46:317–381. doi: 10.1002/9780470122914.ch5. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- Sowokinos J. R., Preiss J. Pyrophosphorylases in Solanum tuberosum: III. PURIFICATION, PHYSICAL, AND CATALYTIC PROPERTIES OF ADPGLUCOSE PYROPHOSPHORYLASE IN POTATOES. Plant Physiol. 1982 Jun;69(6):1459–1466. doi: 10.1104/pp.69.6.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]