Keywords: anosmia, COVID-19, neuroinflammation, olfaction, SARS-CoV-2
Abstract
Anosmia, the loss of the sense of smell, is one of the main neurological manifestations of COVID-19. Although the SARS-CoV-2 virus targets the nasal olfactory epithelium, current evidence suggests that neuronal infection is extremely rare in both the olfactory periphery and the brain, prompting the need for mechanistic models that can explain the widespread anosmia in COVID-19 patients. Starting from work identifying the non-neuronal cell types that are infected by SARS-CoV-2 in the olfactory system, we review the effects of infection of these supportive cells in the olfactory epithelium and in the brain and posit the downstream mechanisms through which sense of smell is impaired in COVID-19 patients. We propose that indirect mechanisms contribute to altered olfactory system function in COVID-19-associated anosmia, as opposed to neuronal infection or neuroinvasion into the brain. Such indirect mechanisms include tissue damage, inflammatory responses through immune cell infiltration or systemic circulation of cytokines, and downregulation of odorant receptor genes in olfactory sensory neurons in response to local and systemic signals. We also highlight key unresolved questions raised by recent findings.
CLINICAL HIGHLIGHTS.
Anosmia is one of the major neurological manifestations of COVID-19.
SARS-CoV-2 infects non-neuronal cell types, induces tissue damage in the olfactory epithelium, and prompts inflammation in both the olfactory epithelium and the brain.
Inflammation likely affects neuronal functions through local and systemic cytokine signals.
Persistent hyposmia and anosmia could relate to aberrant regeneration and persistent inflammation.
Reducing inflammation after infection could improve anosmia.
The causal agent in COVID-19, SARS-CoV-2, has infected hundreds of millions of people, has killed millions of them, and is associated with a wide variety of symptoms across organs, including pneumonia and anosmia (loss of the sense of smell). SARS-CoV-2 is a beta-coronavirus and is closely related to the highly pathogenic strain SARS-CoV. Like SARS-CoV, SARS-CoV-2 uses the binding of its spike proteins with a receptor ACE2 and the proteolytic processing of spike proteins for cell entry. After binding to the respiratory tract, SARS-CoV-2 migrates down to the airways and enters alveolar epithelial cells in the lungs and induces an inflammatory response. Cytokine storm and its associated respiratory failures are considered a primary cause of death in COVID-19 (1).
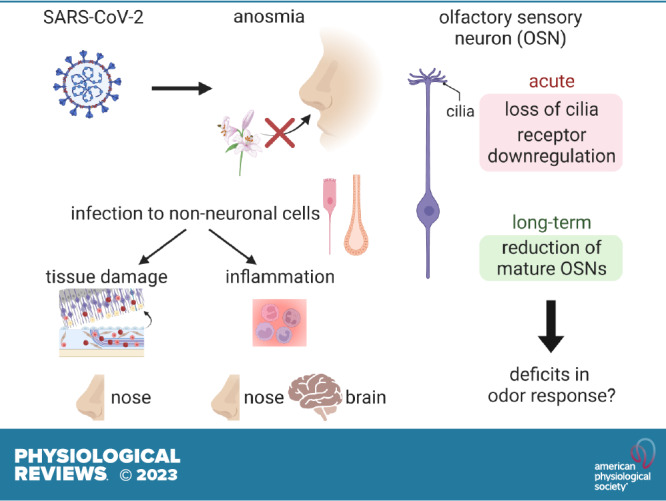
Although most of the symptoms associated with COVID-19 are relatively acute, some patients have shown long-lasting symptoms referred to as postacute sequelae of COVID-19 (PASC) or long COVID. Olfactory impairment is one of the most common symptoms of COVID-19 patients and is the best predictor of SARS-CoV-2 infection (2, 3), especially because these impairments often manifest without rhinorrhea or conductive olfactory loss, the forms of olfactory impairment typically observed in nasal infections like the common cold. In addition, persistent hyposmia (weakened sense of smell) or parosmia (aberrant sense of smell) have been reported in PASC patients (4). Both acute and chronic impairments of olfactory function significantly lower the quality of life (5). Although the clinical presentation of COVID-19-associated anosmia has been extensively documented and summarized in past reviews (6–8), the precise molecular mechanisms through which infection with SARS-CoV-2 leads to olfactory symptoms are poorly understood. Recent studies have shown that infection of non-neuronal cell types results in tissue damage, immune cell infiltration, and secretion of signaling molecules, which indirectly affect the function of olfactory sensory neurons (OSNs) and may contribute to impaired olfactory perception. Here, we review this work with the hope that a better understanding of the unique mechanisms through which SARS-CoV-2 infection leads to olfactory dysfunctions may guide the development of treatments to help COVID-19 patients recover their normal sense of smell.
For most animals, olfaction is fundamental to life, enabling animals to avoid predators, find food or mates, and learn associations between specific smells and outcomes. The first step of olfaction begins with the detection of odors via odorant receptors (ORs) in OSNs housed in the olfactory epithelium (9, 10). ORs are G protein-coupled receptors (GPCRs), and mammalian genomes encode a large number of ORs, with ∼400 in humans and >1,000 in mice. Each OSN expresses a single OR and projects its axon through the cribiform plate directly to the olfactory bulb in the brain. Axons of OSNs expressing the same OR coalesce in one or two pairs of glomeruli, which are insular neuropil structures in the olfactory bulb where OSNs make synaptic connections with neurons that project olfactory information to the brain. These circuit features convert the pattern of neuronal activity across OSNs into spatial patterns of glomerular activity, which are sent by projection neurons to downstream olfactory cortical and striatal brain areas (11). Although humans are generally believed to have less well developed sense of smell than, e.g., dogs and rodents, humans are actually quite adept at detecting and distinguishing between odorants, and olfaction has a strong impact on various aspects of human behaviors, including memory, emotions, and social communications (12). Because olfaction imbues everyday life, patients with olfactory dysfunctions frequently report symptoms of depression (5, 13).
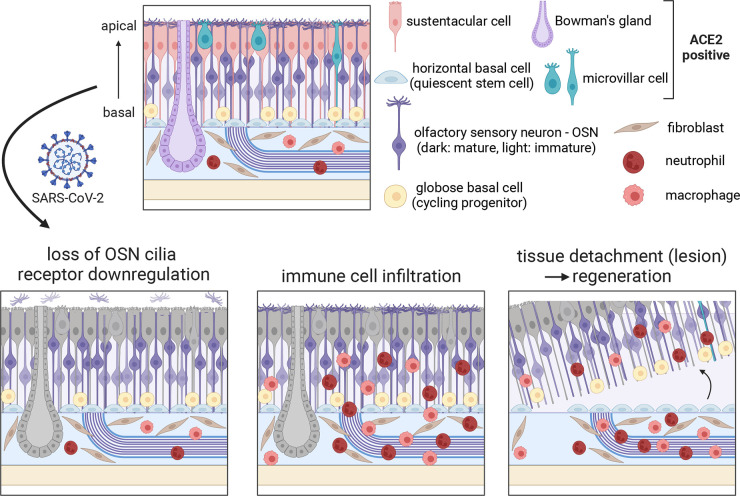
To understand how SARS-CoV-2 affects the olfactory system, several studies have looked for potential target cell types in the olfactory epithelium and the brain based on the expression of the SARS-CoV-2 receptor ACE2 and other genes crucial to viral entry like TMPRSS2. These studies reveal that ACE2 expression, at both the gene and protein levels, is restricted to non-neuronal cell types including sustentacular cells, mucus-secreting Bowman’s gland cells, stem cells like horizontal basal cells (HBCs), and vascular cells (FIGURE 1) (14–16). In contrast, ACE2 was not detected in OSNs, suggesting that they are not permissive for SARS-CoV-2 infection. Although OSNs do express NRP1, which has been suggested to augment the role of ACE2 and TMPRSS2 to facilitate SARS-COV-2 entry into OSNs (17), both model organisms and autopsies from COVID-19 patients have convincingly demonstrated that the primary target of SARS-CoV-2 in the olfactory neuroepithelium is sustentacular cells (18, 19), and direct infection of OSNs is either not detected or rarely observed (20). Therefore, although viruses like Herpes simplex virus transsynaptically transduce neurons in the brain via the OSNs (21), the lack of OSN infection makes neuroinvasion into the brain via OSN axons unlikely. Consistent with this, infection of neurons in the brain has only rarely been observed (22). These results strongly suggest that neither neuronal infection nor neuroinvasion accounts for anosmia in COVID-19 patients.
FIGURE 1.
Cell types in the olfactory epithelium and potential mechanisms of COVID-19-induced anosmia. Top: schematic of the olfactory epithelium with major cell types. Bottom: 3 primary effects of SARS-CoV-2 infection: loss of OSN cilia and receptor downregulation, immune cell infiltration, and tissue detachment (complete lesion), which activates horizontal basal cells (HBCs) and triggers regeneration. Sustentacular cells, Bowman’s gland, and microvillar cells (colored in gray at bottom) are the primary targets of SARS-CoV-2. Image created with BioRender.com, with permission.
Additionally, although multiple cell types in the olfactory epithelium express ACE2, data from human autopsies suggests that not all cell types are equally permissive to SARS-CoV-2 infection (18). Similar to these findings, recent work in nasal epithelial organoid cultures suggests a model that depends on initial infection of cells with motile cilia and subsequent viral egress via microvilli (both of which are present in olfactory support cells) (23). However, it remains to be seen whether these results will also generalize to the olfactory epithelium or whether there exist other molecular or biophysical features that can account for the differential susceptibility to SARS-CoV-2 infection across olfactory cell and tissue types (24). Additionally, multiple SARS-CoV-2 variants have emerged with different degrees of chemosensory alterations (25). Consistent with clinical observations, a recent study in hamsters revealed that the omicron variant causes less severe damage compared with the gamma and delta variants in both the olfactory epithelium and the lung (26). Similarly, work in human explant cultures found that omicron replicated faster in the bronchi but less efficiently in the lung (27). However, there is little data on omicron infection of the human olfactory epithelium, and further investigations are required to dissect the mechanisms of variant-specific phenotypes. Furthermore, although previously vaccinated individuals with breakthrough cases from the delta variant can still present with olfactory impairments (28), the relationship between vaccination and anosmia remains understudied. Recent work in animal models suggests that intranasal delivery of vaccines and unadjuvanted spike proteins can reduce viral burdens in the nasal turbinate (29), but it remains to be seen whether such strategies can also help prevent COVID-19-associated olfactory dysfunctions.
The observation that SARS-CoV-2 likely does not infect neurons in the nose and the brain constrains the possible mechanisms for olfactory dysfunction observed in COVID-19 patients. Recent studies have identified three potential causes: 1) tissue damage and immune responses in the olfactory epithelium, 2) altered gene expression in OSNs via cell-nonautonomous mechanisms, and 3) inflammatory responses in the brain. Mechanistic insight into the sources of olfactory dysfunction has largely benefited from work in animal models. However, since mouse ACE2 is not sufficient for viral entry, work in mouse models has required either transgenic models expressing human ACE2 or engineered mouse-adapted SARS-CoV-2 variants (30–32), both of which may not accurately reflect the biology and pathophysiology in vivo in humans. In contrast, hamsters are efficiently infected by SARS-CoV-2 (26, 33), and recent studies in hamster models have unveiled the time course of phenotypes that ensue after SARS-CoV-2 infection (FIGURE 2). Studies from human autopsies have also provided definite evidence to help arbitrate between possible models, but such samples and studies are limited. In addition, work in human COVID-19 patients with anosmia or hyposmia can directly link objective measures of olfactory system function with biological samples. Although the three potential causes mentioned above are neither exhaustive nor mutually exclusive, below we review these three mechanisms individually and summarize key outstanding questions.
FIGURE 2.
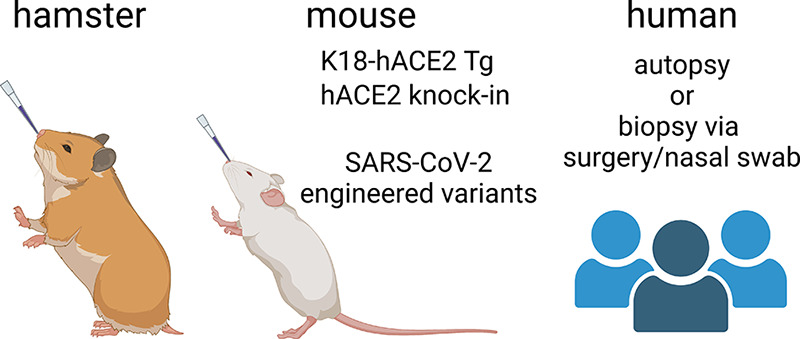
Experimental models used in COVID-19-related studies. Hamsters have been used as an animal model because their ACE2 can bind to SARS-CoV-2 spike protein and wild-type animals can be efficiently infected with SARS-CoV-2. Mice have also been used but researchers need to use either 1) transgenic animals that express human ACE2 from KRT18 promoters (active in epithelial cells) or from endogenous mouse Ace2 promoter or 2) SARS-CoV-2 variants that have mutations in the spike protein and other viral genes that allow for binding to mouse ACE2 and facilitate cell entry. Human autopsies and biopsies have also been used, but samples from anosmic COVID-19 patients are limited. Image created with BioRender.com, with permission.
Work in hamsters has provided a detailed account of the downstream consequences of sustentacular cell infection by SARS-CoV-2. Bryche et al. (34) revealed that SARS-CoV-2 infection of sustentacular cells led to massive tissue damage in the olfactory epithelium; similar findings have also been observed in other studies (35, 36). Damage to the olfactory epithelium was observed as early as 2 days postinfection (DPI), with most of the olfactory epithelium disappearing by 4 DPI. At 14 DPI the olfactory epithelium partially recovered, but complete regeneration took a month or longer. Interestingly, like SARS-CoV-2, the antithyroid drug methimazole also targets sustentacular cells and Bowman’s gland cells and causes severe damage to the olfactory epithelium that regenerates at roughly the same time course (37), but whether methimazole recapitulates the precise epithelial response to SARS-CoV-2 remains uncertain. Prior work has shown that ACE2 is expressed in activated stem cells (HBCs) during methimazole-induced regeneration (14), suggesting the possibility that infection of those stem cells could affect the pattern of regeneration and contribute to long-term or permanent smell loss. Especially for the subset of human COVID-19 patients who report parosmia or other distortions to their sense of smell, it is possible that tissue regeneration is either incomplete or altered in a way that perturbs the spatial activity patterns in olfactory bulb glomeruli in the brain that are evoked by triggering odors (38).
Hamsters have significant anosmia even at 2 DPI, suggesting that anosmia might occur either before or in parallel with the gross desquamation of the olfactory epithelium. Interestingly, immunohistological and ultrastructural studies at these time points demonstrate that SARS-CoV-2 infection can result in the pervasive loss of OSN cilia (34, 39, 40), even when the overall tissue structure is largely normal. The vast majority of key signaling components of OSN signal transduction such as ORs, G proteins, adenylyl cyclase (Adcy3), and cyclic nucleotide-gated ion channels (Cnga2/4) are localized to the cilia, and OSNs cannot detect odors and transduce signals without their cilia (10). Therefore, one possible model is that loss of OSN cilia contributes to acute olfactory dysfunctions in COVID-19 patients, even in cases without large-scale tissue damage (7). Similarly, local disruption to sustentacular cells and Bowman’s gland cells could indirectly affect OSN function, as infection of sustentacular cells could lead to accumulation of toxic substances, insufficient glucose levels in OSNs, and/or aberrant local ionic balance (41–43), and infection of Bowman’s gland cells could also lead to abnormal composition of the mucus (44). As the relationships between support cells and OSNs are not well understood, the contribution of their interactions to olfactory system function and pathophysiology is a fruitful avenue for future research.
OSNs also induce a wide range of cellular changes in response to local infection of non-neuronal cells, despite not being directly infected by SARS-CoV-2. Zazhytska et al. (19) examined the effects of SARS-CoV-2 infection on OSN gene expression and chromosomal organization and observed large-scale reconfiguration of OSN transcriptomes in infected hamsters. For example, at 3 DPI, antiviral response genes such as Igs15 and Irf7/9 were strongly induced in OSNs, whereas many genes relevant to odor signal transduction such as ORs and Adcy3 were downregulated. Although most antiviral response genes went back to baseline levels at 10 DPI, the changes in odor signal transduction genes were more persistent. Consistent with these changes, the authors also observed changes in nuclear organization. OR gene clusters from multiple chromosomes are known to form OSN-specific genomic compartments that are crucial for stable and singular OR expression (45). Interestingly, SARS-CoV-2 infection weakened chromosomal interactions between OR clusters, which was observed as early as 1 DPI and persisted at 10 DPI. Deficits in nuclear organization preceded OR gene expression changes, suggesting a causal relationship that may be important for subsequent olfactory dysfunction; consistent with this possibility, olfactory epithelial tissues from COVID-19 patients exhibited both OR downregulation and reduced interactions between OR gene clusters. Importantly, this study also showed that irradiated serum from infected hamsters at 3 DPI can disrupt nuclear organization, which provides support for a model in which secretory signals like cytokines induced by SARS-CoV-2 infection of non-neuronal cells induce widespread gene expression changes in OSNs. Interestingly, downregulation of ORs and signal transduction factors has also been observed after SARS-CoV-2 infection in mouse and zebrafish models (30, 40, 46). However, it remains unclear what kind of cytokines are detected by OSNs, which signaling pathways play key roles in disrupting nuclear architecture, and if, how, and when OR gene expression recovers to normal levels.
SARS-CoV-2 infection in both hamsters and humans dramatically increases the number of immune cells like monocytes/macrophages and neutrophils in the olfactory epithelium (19, 34, 47). Infiltration of immune cells and increased cytokine levels could affect the OSN functions in an indirect fashion or could ultimately lead to the gross tissue damage observed in hamster models. Although PASC-associated anosmia has been understudied compared with acute anosmia, Finlay et al. (48) examined the olfactory epithelium in PASC patients reporting olfactory dysfunction persisting for at least 4 months since the onset of COVID-19, at a time point when viral RNAs were undetected. This study revealed that resident CD8+ T cells were highly enriched in patients with hyposmia. This T-cell subtype expressed an inflammatory cytokine interferon-gamma, whose receptors were expressed in both sustentacular cells and OSNs. Additionally, the authors also observed changes in the composition of other myeloid cell types, including an enrichment of CD207+ dendritic cells and a reduction of CD162+ M2 macrophages. Furthermore, sustentacular cells showed a sustained transcriptional immune response phenotype, and the total number of mature OSNs was reduced. In contrast, despite the overall reduction in the number of OSNs, OSN gene expression patterns in these patients were considerably more modest, with roughly normal levels of ORs and signaling genes. Together, this study suggests that PASC-associated olfactory dysfunctions are the combined consequence of persistent immune cell infiltration and cytokine release, which alter gene expression patterns in sustentacular cells and HBCs and cause an overall reduction of mature OSNs. However, the precise mechanisms through which the persistent presence of immune cells and cytokines affects OSN function remains unclear.
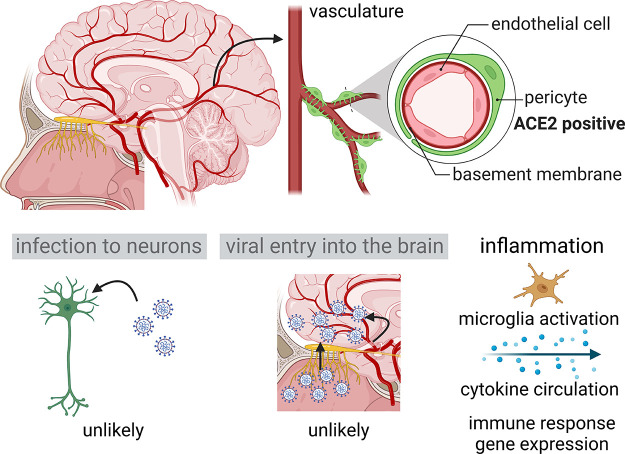
Multiple lines of evidence in humans and model organisms demonstrate that SARS-CoV-2 targets the olfactory epithelium, but the extent to which COVID-19-associated anosmia is exclusively a result of peripheral effects is less well understood. Although most studies suggest that direct neuronal infection is extremely rare in the brain, as it is for OSNs in the olfactory epithelium, it remains possible that altered neural functions in the brain via cell-nonautonomous mechanisms like inflammation might contribute to olfactory dysfunctions (49, 50) (FIGURE 3). SARS-CoV-2 mainly targets ACE2-expressing vascular cell types like pericytes (51), which express ACE2 and may therefore still invade into the brain parenchyma through blood vessels (FIGURE 3). However, by using a postmortem bedside surgical procedure that allows for the collection of tissue and fluid samples from deceased COVID-19 patients right after death, Khan et al. (52) presented strong evidence that SARS-CoV-2 is not detected in the parenchyma of the olfactory bulb, in the frontal lobe, or in cerebrospinal fluid (CSF). This study further revealed that perineurial olfactory nerve fibroblasts, which enwrap OSN axons and olfactory ensheathing cells, form an anatomical barrier that blocks the entry of SARS-CoV-2 through vulnerable interfaces like the cribriform plate. In hamster models, despite the anosmia symptoms and gross damage to the olfactory epithelium, most studies have not detected any viral RNAs in the olfactory bulb (34). Therefore, although other studies have identified gene expression signatures of persistent inflammation in the olfactory bulb and several other brain regions of hamsters intranasally inoculated with SARS-CoV-2 (53–55), such signatures are likely the result of systemic signals either diffusing through the cribriform plate or circulating in the blood that can induce inflammation phenotypes in the brain (56) (FIGURE 3). Large-scale neuroimaging studies have also observed changes in brain structure in the olfactory cortex (57), but whether these structural changes are a cause or a consequence of olfactory dysfunction and persistent inflammation in the brain is still unclear. Further studies are required to understand the link between changes in cellular states in the brain and COVID-19-induced anosmia, as well as the relationship between anosmia and other neurological phenotypes like the “brain fog” observed in patients with PASC (58–60). Interestingly, a recent study in hamsters revealed that SARS-CoV-2, but not influenza A, induces a persistent inflammatory gene expression signature in several brain areas (53), suggesting that some of these neurological phenotypes might be unique to SARS-CoV-2.
FIGURE 3.
Effects of SARS-CoV-2 infection in the brain. Top: schematic of human brain with vasculature, with the olfactory bulb highlighted in yellow and the structure and cell types of the vasculature depicted on right. Bottom: 3 possible central mechanisms that may contribute to COVID-19-associated anosmia. Although both direct infection to neurons and viral invasion to the brain are unlikely based on recent studies, inflammatory responses due to microglial activation and cytokine circulation are suggested to affect neurological functions in COVID-19 patients. Image created with BioRender.com, with permission.
Altogether, these recent studies have provided new insight into possible mechanisms through which SARS-CoV-2 infection leads to COVID-19-associated anosmia. Although it is clear that direct infection of neurons in the nose or brain is rare and unlikely to account for the widespread anosmia in COVID-19 patients, the relative contributions of tissue damage and immune responses in the olfactory epithelium, altered gene expression in OSNs, and inflammatory responses in the brain to olfactory dysfunctions require future investigation. Moreover, as there are still significant numbers of COVID-19 and PASC patients who suffer from both short-term and more persistent olfactory dysfunction, future work is required to understand the commonalties and differences in the mechanisms that account for such acute and chronic symptoms. To date, however, there have been relatively few studies with primary data from anosmic or parosmic COVID-19 patients. Finally, olfactory dysfunctions are a hallmark of many neurological disorders including Alzheimer’s disease and Parkinson’s disease (61), which, coincidentally, are also associated with inflammation. Therefore, future studies to elucidate mechanisms of COVID-19 may provide general insight into the neuroimmunological basis of other disorders affecting the sense of smell.
GRANTS
S.R.D. and T.T. are supported by the Tan-Yang Center at Harvard Medical School. S.R.D. is supported by NIH Grant U19 NS112953 and by grants from the Simons Collaboration on the Global Brain, the Brain Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.T. and D.H.B. drafted manuscript; S.R.D. edited and revised manuscript; T.T., D.H.B., and S.R.D. approved final version of manuscript.
ACKNOWLEDGMENTS
Figures were created with BioRender.com, with permission.
REFERENCES
- 1. Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19: 141–154, 2021. doi: 10.1038/s41579-020-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gerkin RC, Ohla K, Veldhuizen MG, Joseph PV, Kelly CE, Bakke AJ, , et al. Recent smell loss is the best predictor of COVID-19 among individuals with recent respiratory symptoms. Chem Senses 46: bjaa081, 2021. doi: 10.1093/chemse/bjaa081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA, Ganesh S, Varsavsky T, Cardoso MJ, El-Sayed Moustafa JS, Visconti A, Hysi P, Bowyer RC, Mangino M, Falchi M, Wolf J, Ourselin S, Chan AT, Steves CJ, Spector TD. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 26: 1037–1040, 2020. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ohla K, Veldhuizen MG, Green T, Hannum ME, Bakke AJ, Moein ST, , et al. A follow-up on quantitative and qualitative olfactory dysfunction and other symptoms in patients recovering from COVID-19 smell loss. Rhinology 60: 207–217, 2022. doi: 10.4193/Rhin21.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Croy I, Nordin S, Hummel T. Olfactory disorders and quality of life—an updated review. Chem Senses 39: 185–194, 2014. doi: 10.1093/chemse/bjt072. [DOI] [PubMed] [Google Scholar]
- 6. Meng X, Pan Y. COVID-19 and anosmia: the story so far. Ear Nose Throat J 2021: 1455613211048998, 2021. doi: 10.1177/01455613211048998. [DOI] [PubMed] [Google Scholar]
- 7. Cooper KW, Brann DH, Farruggia MC, Bhutani S, Pellegrino R, Tsukahara T, Weinreb C, Joseph PV, Larson ED, Parma V, Albers MW, Barlow LA, Datta SR, Di Pizio A. COVID-19 and the chemical senses: supporting players take center stage. Neuron 107: 219–233, 2020. doi: 10.1016/j.neuron.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Killingley B, Mann AJ, Kalinova M, Boyers A, Goonawardane N, Zhou J, , et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med 28: 1031–1041, 2022. doi: 10.1038/s41591-022-01780-9. [DOI] [PubMed] [Google Scholar]
- 9. Brann DH, Datta SR. Finding the brain in the nose. Annu Rev Neurosci 43: 277–295, 2020. doi: 10.1146/annurev-neuro-102119-103452. [DOI] [PubMed] [Google Scholar]
- 10. Manzini I, Schild D, Natale CD. Principles of odor coding in vertebrates and artificial chemosensory systems. Physiol Rev 102: 61–154, 2022. doi: 10.1152/physrev.00036.2020. [DOI] [PubMed] [Google Scholar]
- 11. Mori K, Sakano H. How is the olfactory map formed and interpreted in the mammalian brain? Annu Rev Neurosci 34: 467–499, 2011. doi: 10.1146/annurev-neuro-112210-112917. [DOI] [PubMed] [Google Scholar]
- 12. McGann JP. Poor human olfaction is a 19th-century myth. Science 356: eaam7263, 2017. doi: 10.1126/science.aam7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kohli P, Soler ZM, Nguyen SA, Muus JS, Schlosser RJ. The association between olfaction and depression: a systematic review. Chem Senses 41: 479–486, 2016. doi: 10.1093/chemse/bjw061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brann DH, Tsukahara T, Weinreb C, Lipovsek M, Van den Berge K, Gong B, Chance R, Macaulay IC, Chou HJ, Fletcher RB, Das D, Street K, de Bezieux HR, Choi YG, Risso D, Dudoit S, Purdom E, Mill J, Hachem RA, Matsunami H, Logan DW, Goldstein BJ, Grubb MS, Ngai J, Datta SR. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci Adv 6: eabc5801, 2020. doi: 10.1126/sciadv.abc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, Egervari K, Lobrinus JA, Landis BN, Carleton A, Rodriguez I. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience 23: 101839, 2020. doi: 10.1016/j.isci.2020.101839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci 11: 1555–1562, 2020. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cantuti-Castelvetri L, Ojha R, Pedro LD, Djannatian M, Franz J, Kuivanen S, van der Meer F, Kallio K, Kaya T, Anastasina M, Smura T, Levanov L, Szirovicza L, Tobi A, Kallio-Kokko H, Österlund P, Joensuu M, Meunier FA, Butcher SJ, Winkler MS, Mollenhauer B, Helenius A, Gokce O, Teesalu T, Hepojoki J, Vapalahti O, Stadelmann C, Balistreri G, Simons M. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370: 856–860, 2020. doi: 10.1126/science.abd2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Khan M, Yoo SJ, Clijsters M, Backaert W, Vanstapel A, Speleman K, Lietaer C, Choi S, Hether TD, Marcelis L, Nam A, Pan L, Reeves JW, Van Bulck P, Zhou H, Bourgeois M, Debaveye Y, De Munter P, Gunst J, Jorissen M, Lagrou K, Lorent N, Neyrinck A, Peetermans M, Thal DR, Vandenbriele C, Wauters J, Mombaerts P, Van Gerven L. Visualizing in deceased COVID-19 patients how SARS-CoV-2 attacks the respiratory and olfactory mucosae but spares the olfactory bulb. Cell 184: 5932–5949.e15, 2021. doi: 10.1016/j.cell.2021.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zazhytska M, Kodra A, Hoagland DA, Frere J, Fullard JF, Shayya H, McArthur NG, Moeller R, Uhl S, Omer AD, Gottesman ME, Firestein S, Gong Q, Canoll PD, Goldman JE, Roussos P, tenOever BR, Jonathan BO, Lomvardas S. Non-cell-autonomous disruption of nuclear architecture as a potential cause of COVID-19-induced anosmia. Cell 185: 1052–1064.e12, 2022. doi: 10.1016/j.cell.2022.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, , et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 24: 168–175, 2021. doi: 10.1038/s41593-020-00758-5. [DOI] [PubMed] [Google Scholar]
- 21. Mori I. Transolfactory neuroinvasion by viruses threatens the human brain. Acta Virol 59: 338–349, 2015. doi: 10.4149/av_2015_04_338. [DOI] [PubMed] [Google Scholar]
- 22. Solomon IH, Normandin E, Bhattacharyya S, Mukerji SS, Keller K, Ali AS, Adams G, Hornick JL, Padera RF, Sabeti P. Neuropathological features of Covid-19. N Engl J Med 383: 989–992, 2020. doi: 10.1056/NEJMc2019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wu CT, Lidsky PV, Xiao Y, Cheng R, Lee IT, Nakayama T, Jiang S, He W, Demeter J, Knight MG, Turn RE, Rojas-Hernandez LS, Ye C, Chiem K, Shon J, Martinez-Sobrido L, Bertozzi CR, Nolan GP, Nayak JV, Milla C, Andino R, Jackson PK. SARS-CoV-2 replication in airway epithelia requires motile cilia and microvillar reprogramming. Cell 186: 112–130.e20, 2023. doi: 10.1016/j.cell.2022.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, , et al. SARS-CoV-2 Reverse Genetics Reveals a Variable Infection Gradient in the Respiratory Tract. Cell 182: 429–446.e14, 2020. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Coelho DH, Reiter ER, French E, Costanzo RM. Decreasing incidence of chemosensory changes by COVID-19 variant. Otolaryngol Head Neck Surg 168: 704–706, 2023. doi: 10.1177/01945998221097656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armando F, Beythien G, Kaiser FK, Allnoch L, Heydemann L, Rosiak M, Becker S, Gonzalez-Hernandez M, Lamers MM, Haagmans BL, Guilfoyle K, van Amerongen G, Ciurkiewicz M, Osterhaus A, Baumgärtner W. SARS-CoV-2 Omicron variant causes mild pathology in the upper and lower respiratory tract of hamsters. Nat Commun 13: 3519, 2022. doi: 10.1038/s41467-022-31200-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hui KP, Ho JC, Cheung MC, Ng KC, Ching RH, Lai KL, Kam TT, Gu H, Sit KY, Hsin MK, Au TW, Poon LL, Peiris M, Nicholls JM, Chan MC. SARS-CoV-2 Omicron variant replication in human bronchus and lung ex vivo. Nature 603: 715–720, 2022. doi: 10.1038/s41586-022-04479-6. [DOI] [PubMed] [Google Scholar]
- 28. Man K, Simons CT, Mohamed-Osman A, Travers SP, Zhao K. Chemosensory losses in past and active likely delta variant break-through COVID-19 cases. Med 3: 450–451, 2022. doi: 10.1016/j.medj.2022.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mao T, Israelow B, Peña-Hernández MA, Suberi A, Zhou L, Luyten S, Reschke M, Dong H, Homer RJ, Saltzman WM, Iwasaki A. Unadjuvanted intranasal spike vaccine elicits protective mucosal immunity against sarbecoviruses. Science 378: eabo2523, 2022. doi: 10.1126/science.abo2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ye Q, Zhou J, He Q, Li RT, Yang G, Zhang Y, Wu SJ, Chen Q, Shi JH, Zhang RR, Zhu HM, Qiu HY, Zhang T, Deng YQ, Li XF, Liu JF, Xu P, Yang X, Qin CF. SARS-CoV-2 infection in the mouse olfactory system. Cell Discov 7: 49, 2021. doi: 10.1038/s41421-021-00290-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, Leidinger MR, Knudson CM, Meyerholz DK, McCray PB Jr, Perlman S. COVID-19 treatments and pathogenesis including anosmia in K18-hACE2 mice. Nature 589: 603–607, 2021. doi: 10.1038/s41586-020-2943-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leist SR, Dinnon KH 3rd, Schäfer A, Tse LV, Okuda K, Hou YJ, West A, Edwards CE, Sanders W, Fritch EJ, Gully KL, Scobey T, Brown AJ, Sheahan TP, Moorman NJ, Boucher RC, Gralinski LE, Montgomery SA, Baric RS. A mouse-adapted SARS-CoV-2 induces acute lung injury and mortality in standard laboratory mice. Cell 183: 1070–1085.e12, 2020. doi: 10.1016/j.cell.2020.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muñoz-Fontela C, Dowling WE, Funnell SG, Gsell PS, Riveros-Balta AX, Albrecht RA, , et al. Animal models for COVID-19. Nature 586: 509–515, 2020. doi: 10.1038/s41586-020-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bryche B, St Albin A, Murri S, Lacôte S, Pulido C, Ar Gouilh M, Lesellier S, Servat A, Wasniewski M, Picard-Meyer E, Monchatre-Leroy E, Volmer R, Rampin O, Le Goffic R, Marianneau P, Meunier N. Massive transient damage of the olfactory epithelium associated with infection of sustentacular cells by SARS-CoV-2 in golden Syrian hamsters. Brain Behav Immun 89: 579–586, 2020. doi: 10.1016/j.bbi.2020.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Reyna RA, Kishimoto-Urata M, Urata S, Makishima T, Paessler S, Maruyama J. Recovery of anosmia in hamsters infected with SARS-CoV-2 is correlated with repair of the olfactory epithelium. Sci Rep 12: 628, 2022. doi: 10.1038/s41598-021-04622-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Urata S, Maruyama J, Kishimoto-Urata M, Sattler RA, Cook R, Lin N, Yamasoba T, Makishima T, Paessler S. Regeneration profiles of olfactory epithelium after SARS-CoV-2 infection in golden Syrian hamsters. ACS Chem Neurosci 12: 589–595, 2021. doi: 10.1021/acschemneuro.0c00649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Suzukawa K, Kondo K, Kanaya K, Sakamoto T, Watanabe K, Ushio M, Kaga K, Yamasoba T. Age-related changes of the regeneration mode in the mouse peripheral olfactory system following olfactotoxic drug methimazole-induced damage. J Comp Neurol 519: 2154–2174, 2011. doi: 10.1002/cne.22611. [DOI] [PubMed] [Google Scholar]
- 38. Parker JK, Kelly CE, Gane SB. Insights into the molecular triggers of parosmia based on gas chromatography olfactometry. Commun Med (Lond) 2: 58, 2022. doi: 10.1038/s43856-022-00112-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. de Melo GD, Lazarini F, Levallois S, Hautefort C, Michel V, Larrous F, Verillaud B, Aparicio C, Wagner S, Gheusi G, Kergoat L, Kornobis E, Donati F, Cokelaer T, Hervochon R, Madec Y, Roze E, Salmon D, Bourhy H, Lecuit M, Lledo PM. COVID-19-related anosmia is associated with viral persistence and inflammation in human olfactory epithelium and brain infection in hamsters. Sci Transl Med 13: eabf8396, 2021. doi: 10.1126/scitranslmed.abf8396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Verma AK, Zheng J, Meyerholz DK, Perlman S. SARS-CoV-2 infection of sustentacular cells disrupts olfactory signaling pathways. JCI Insight 7: e160277, 2022. doi: 10.1172/jci.insight.160277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suzuki Y, Takeda M, Farbman AI. Supporting cells as phagocytes in the olfactory epithelium after bulbectomy. J Comp Neurol 376: 509–517, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- 42. Vogalis F, Hegg CC, Lucero MT. Ionic conductances in sustentacular cells of the mouse olfactory epithelium. J Physiol 562: 785–799, 2005. doi: 10.1113/jphysiol.2004.079228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harkema JR, Carey SA, Wagner JG. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol 34: 252–269, 2006. doi: 10.1080/01926230600713475. [DOI] [PubMed] [Google Scholar]
- 44. Solbu TT, Holen T. Aquaporin pathways and mucin secretion of Bowman’s glands might protect the olfactory mucosa. Chem Senses 37: 35–46, 2012. doi: 10.1093/chemse/bjr063. [DOI] [PubMed] [Google Scholar]
- 45. Bashkirova E, Lomvardas S. Olfactory receptor genes make the case for inter-chromosomal interactions. Curr Opin Genet Dev 55: 106–113, 2019. doi: 10.1016/j.gde.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kraus A, Huertas M, Ellis L, Boudinot P, Levraud JP, Salinas I. Intranasal delivery of SARS-CoV-2 spike protein is sufficient to cause olfactory damage, inflammation and olfactory dysfunction in zebrafish. Brain Behav Immun 102: 341–359, 2022. doi: 10.1016/j.bbi.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bourgon C, Albin AS, Ando-Grard O, Da Costa B, Domain R, Korkmaz B, Klonjkowski B, Le Poder S, Meunier N. Neutrophils play a major role in the destruction of the olfactory epithelium during SARS-CoV-2 infection in hamsters. Cell Mol Life Sci 79: 616, 2022. doi: 10.1007/s00018-022-04643-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Finlay JB, Brann DH, Abi Hachem R, Jang DW, Oliva AD, Ko T, Gupta R, Wellford SA, Moseman EA, Jang SS, Yan CH, Matsunami H, Tsukahara T, Datta SR, Goldstein BJ. Persistent post-COVID-19 smell loss is associated with immune cell infiltration and altered gene expression in olfactory epithelium. Sci Transl Med 14: eadd0484, 2022. doi: 10.1126/scitranslmed.add0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. LaFever BJ, Imamura F. Effects of nasal inflammation on the olfactory bulb. J Neuroinflammation 19: 294, 2022. doi: 10.1186/s12974-022-02657-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell 183: 16–27.e1, 2020. doi: 10.1016/j.cell.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bocci M, Oudenaarden C, Sàenz-Sardà X, Simrén J, Edén A, Sjölund J, Möller C, Gisslén M, Zetterberg H, Englund E, Pietras K. Infection of brain pericytes underlying neuropathology of COVID-19 patients. Int J Mol Sci 22: 11622, 2021. doi: 10.3390/ijms222111622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Khan M, Clijsters M, Choi S, Backaert W, Claerhout M, Couvreur F, , et al. Anatomical barriers against SARS-CoV-2 neuroinvasion at vulnerable interfaces visualized in deceased COVID-19 patients. Neuron 110: 3919–3935.e6, 2022. doi: 10.1016/j.neuron.2022.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Frere JJ, Serafini RA, Pryce KD, Zazhytska M, Oishi K, Golynker I, Panis M, Zimering J, Horiuchi S, Hoagland DA, Møller R, Ruiz A, Kodra A, Overdevest JB, Canoll PD, Borczuk AC, Chandar V, Bram Y, Schwartz R, Lomvardas S, Zachariou V, tenOever BR. SARS-CoV-2 infection in hamsters and humans results in lasting and unique systemic perturbations after recovery. Sci Transl Med 14: eabq3059, 2022. doi: 10.1126/scitranslmed.abq3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kishimoto-Urata M, Urata S, Kagoya R, Imamura F, Nagayama S, Reyna RA, Maruyama J, Yamasoba T, Kondo K, Hasegawa-Ishii S, Paessler S. Prolonged and extended impacts of SARS-CoV-2 on the olfactory neurocircuit. Sci Rep 12: 5728, 2022. doi: 10.1038/s41598-022-09731-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Käufer C, Schreiber CS, Hartke AS, Denden I, Stanelle-Bertram S, Beck S, Kouassi NM, Beythien G, Becker K, Schreiner T, Schaumburg B, Beineke A, Baumgärtner W, Gabriel G, Richter F. Microgliosis and neuronal proteinopathy in brain persist beyond viral clearance in SARS-CoV-2 hamster model. eBioMedicine 79: 103999, 2022. doi: 10.1016/j.ebiom.2022.103999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Yang AC, Kern F, Losada PM, Agam MR, Maat CA, Schmartz GP, Fehlmann T, Stein JA, Schaum N, Lee DP, Calcuttawala K, Vest RT, Berdnik D, Lu N, Hahn O, Gate D, McNerney MW, Channappa D, Cobos I, Ludwig N, Schulz-Schaeffer WJ, Keller A, Wyss-Coray T. Dysregulation of brain and choroid plexus cell types in severe COVID-19. Nature 595: 565–571, 2021. doi: 10.1038/s41586-021-03710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JL, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE, Smith SM. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature 604: 697–707, 2022. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ellul MA, Benjamin L, Singh B, Lant S, Michael BD, Easton A, Kneen R, Defres S, Sejvar J, Solomon T. Neurological associations of COVID-19. Lancet Neurol 19: 767–783, 2020. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Monje M, Iwasaki A. The neurobiology of long COVID. Neuron 110: 3484–3496, 2022. doi: 10.1016/j.neuron.2022.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Xu E, Xie Y, Al-Aly Z. Long-term neurologic outcomes of COVID-19. Nat Med 28: 2406–2415, 2022. doi: 10.1038/s41591-022-02001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Albers MW, Tabert MH, Devanand DP. Olfactory dysfunction as a predictor of neurodegenerative disease. Curr Neurol Neurosci Rep 6: 379–386, 2006. doi: 10.1007/s11910-996-0018-7. [DOI] [PubMed] [Google Scholar]