Keywords: active breaks, physical activity, physiology, sedentary behavior, sitting
Abstract
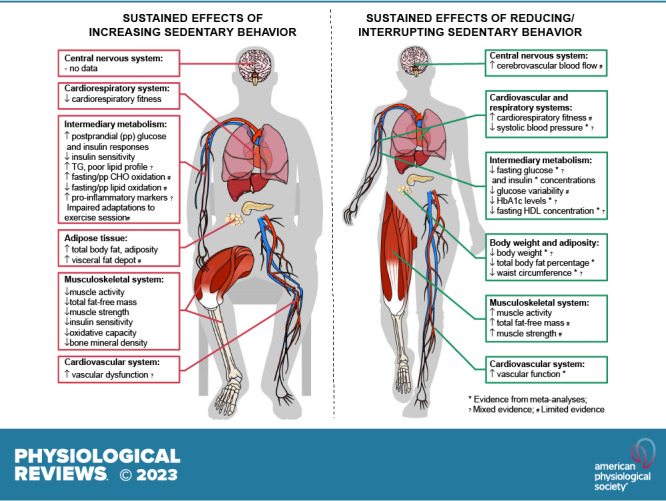
Sedentary behaviors (SB) are characterized by low energy expenditure while in a sitting or reclining posture. Evidence relevant to understanding the physiology of SB can be derived from studies employing several experimental models: bed rest, immobilization, reduced step count, and reducing/interrupting prolonged SB. We examine the relevant physiological evidence relating to body weight and energy balance, intermediary metabolism, cardiovascular and respiratory systems, the musculoskeletal system, the central nervous system, and immunity and inflammatory responses. Excessive and prolonged SB can lead to insulin resistance, vascular dysfunction, shift in substrate use toward carbohydrate oxidation, shift in muscle fiber from oxidative to glycolytic type, reduced cardiorespiratory fitness, loss of muscle mass and strength and bone mass, and increased total body fat mass and visceral fat depot, blood lipid concentrations, and inflammation. Despite marked differences across individual studies, longer term interventions aimed at reducing/interrupting SB have resulted in small, albeit marginally clinically meaningful, benefits on body weight, waist circumference, percent body fat, fasting glucose, insulin, HbA1c and HDL concentrations, systolic blood pressure, and vascular function in adults and older adults. There is more limited evidence for other health-related outcomes and physiological systems and for children and adolescents. Future research should focus on the investigation of molecular and cellular mechanisms underpinning adaptations to increasing and reducing/interrupting SB and the necessary changes in SB and physical activity to impact physiological systems and overall health in diverse population groups.
CLINICAL HIGHLIGHTS.
-
1)
Sedentary behavior (SB; time spent sitting) occupies a high proportion of adults’ waking hours, and its effects can be examined distinctly from lack of exercise or physical activity (PA).
-
2)
The average energy cost of common SBs ranges between 1.0 and 1.5 metabolic equivalent of task. Energy expenditure, heart rate, skeletal muscle blood flow, and contractile activity are higher during sitting than when reclining but lower than in a standing position and during PA of any intensity.
-
3)
Prolonged and uninterrupted SB leads to insulin resistance, vascular dysfunction, shift in substrate use toward carbohydrate oxidation, shift in muscle fiber from oxidative to glycolytic type, reduced cardiorespiratory fitness, loss of muscle mass and strength and bone mass, and increased total body fat mass and visceral fat depot, blood lipid concentrations, and inflammation.
-
4)
From a physiological perspective, there are impacts of SB on physiological responses that relate to those of physical inactivity, i.e., too little exercise. Even though such effects are similar, high volumes of SB can have adverse physiological impacts even in the presence of large volumes of aerobic and/or resistance exercise.
-
5)
Acutely, reducing/interrupting SB improves postprandial glucose and insulin responses, systolic blood pressure, mean arterial pressure, and lower limb vascular function. In the longer term, there are small improvements in body weight, waist circumference, percent body fat, fasting glucose, HbA1c and HDL concentrations, systolic blood pressure, and vascular function. Evidence is more limited for other health outcomes and physiological systems.
-
6)
Reducing/interrupting SB improves body composition, intermediary metabolism, and cardiovascular health outcomes, but the effects are small, albeit marginally clinically meaningful. Most studies have been conducted in healthy population groups (i.e., outcomes within normal ranges), and larger effects may be observed in unhealthy populations.
-
7)
The “sit less, move more and exercise” focus of contemporary public health guidelines is a consensus based primarily on epidemiological findings, and further experimental evidence is needed to elucidate the physiological effects of interventions combining exercise and reduction/interruptions to SB. Nevertheless, reducing/interrupting SB is a low-risk strategy of clinical and population health relevance and can serve as a stepping stone to regular participation in moderate-to-vigorous intensity PA.
1. INTRODUCTION
Sedentary behavior (SB) is defined as any waking behavior characterized by a low energy expenditure [≤1.5 metabolic equivalents of task (MET)] while sitting or lying down (1). Driven by environmental, economic, social, and technological changes, SB is now understood to be a major component of the human movement spectrum that can impact health adversely (2, 3). In adults and older adults, time spent sedentary can range from 5 to 11.5 h/day (4–7).
Particular attention has been given to SB only since the early 2000s when the term “inactivity physiology” [i.e., acute and chronic physiological effects of SB (nonexercise activity deficiency)] was put forward by Hamilton and colleagues (8–10) as a separate research field from exercise physiology. This shift was motivated by experimental findings demonstrating key differences in mechanisms driving skeletal muscle lipoprotein lipase (LPL) responses between physical inactivity and exercise compared to normal standing and ambulatory activity in rats (8, 9). The rapid accumulation of experimental evidence on SB over the past 20 years has built on these early insights, with greater attention being directed at understanding the health consequences of daily hours spent sedentary and the countermeasure strategies aimed at reducing/interrupting time spent sedentary.
This review provides a perspective on 1) how to characterize SB; 2) the pros and cons of the currently available experimental models employed in the investigation of the physiology of SB; 3) the physiological effects of variations in SB and potential underlying mechanisms; and 4) the gaps that currently exist in the scientific understanding of the physiology of SB. For a broad and practically informed perspective, we address the extent to which the physiological evidence base can help to further focus and sharpen public health and clinical practice guidelines, extending beyond the well-understood and accepted “exercise more” message, toward a more comprehensive “sit less, move more and exercise” message.
2. AN OPERATIONAL FRAMEWORK FOR SEDENTARY BEHAVIOR PHYSIOLOGY RESEARCH
The term SB, derived from the Latin word sedere (“to sit”), refers to any waking behavior posture (1) (see TABLE 1 for key terms in SB research). First, we discuss key features and themes of SB research. This operational framework provides guidance for more in-depth considerations on the physiology of SB later in the review.
Table 1.
Key terms and definitions in sedentary behavior and physical inactivity research
| Term | Definition | References |
|---|---|---|
| Continuum of human movement and nonmovement | Refers to all behaviors, including sleep, sedentary behavior, standing, and physical activity at any intensity, that occur in the 24-h interval. Behaviors comprised within the continuum differ in terms of type, posture, and physiological state—metabolic cost, oxygen consumption, heart rate, and skeletal muscle activity, and blood flow—which may underpin health effects associated with each behavior. | 1 |
| Nonmovement or stationary behaviors | Any walking behavior performed in a sitting, reclining, lying down, or standing position with no ambulation, irrespective of EE. | 1 |
| Sedentary behavior | Any waking behavior characterized by a low EE (≤1.5 METs) while in a sitting, reclined, or lying down posture. | 1, 11 |
| Sendentary behavior pattern | The manner in which sedentary behavior is accumulated, for example, timing of the day, duration, and frequency of bouts and breaks. | 1 |
| Standing | The act of one maintaining an upright position while supported by one’s feet. | 1 |
| Passive standing | Any waking activity characterized by an EE ≤2.0 METs while standing without ambulation. | 1 |
| Active standing | Any waking activity characterized by an EE >2.0 METs while standing without ambulation. | 1 |
| Movement or nonstationary behaviors | Any walking behavior performed in a standing position with ambulation, irrespective of energy expenditure. | 1 |
| Physical activity | Any bodily movement produced by the skeletal muscles that result in an increase in EE above resting levels. | 12 |
| Exercise | Refers to a physical activity that is planned, structured, repetitive, and purposeful (i.e., aimed to increase or maintain one or more components of physical fitness). It is considered as a subcategory of physical activity. | 12 |
| Light-intensity physical activity | Any waking behavior with an EE between 1.6 < 3.0 METs or the relative intensity is between 20 < 40% V̇o2max/%HRR and 40 < 55% HRmax. | 13 |
| Moderate physical activity | Any waking behavior with an EE between 3.0 < 6.0 METs or the relative intensity is between 40 < 60% V̇o2max/%HRR and 55 < 70% HRmax. | 13 |
| Vigorous physical activity | Any waking behavior with EE ≥6.0 METs or the relative intensity is between ≥60% V̇o2max/%HRR and ≥ 70% HRmax. | 13 |
| Physical inactivity | Insufficient level of moderate-to-vigorous physical activity to meet the current physical activity recommendations | 1, 11 |
| Physical activity recommendations | For adults and older adults, at least 150 min/wk of moderate-to-vigorous physical activity or 75 min/wk of vigorous physical activity. For children and adolescents, 60 min or more of moderate-to-vigorous physical activity daily. | 2, 14 |
| Sedentary behavior recommendations | For adults and older adults, limit the amount of time spent sedentary and replace sedentary time with more physical activity of any intensity. For children and adolescents, limit the amount of time spent sedentary particularly recreational screen time. | 2, 14 |
EE, energy expenditure; HR, heart rate; %HRR, percentage of heart rate reserve; METs, metabolic equivalent.
2.1. Characteristics of Sedentary Behavior
SBs are identified based on their physiological and postural characteristics. Physiologically, the average energy cost of common types of SB ranges between 1.0 and 1.5 METs in healthy adults (15) during fasting (16–18) and postprandial states (19, 20), as measured by indirect calorimetry (16–19) or by whole room calorimetry (20). Overall, energy expenditure during sitting is higher than reclining (17, 20) but lower than standing (16, 17) and lower than all intensities of physical activity (PA) (19). For heart rate (HR), similar responses are observed (16, 18).
In skeletal muscle, increased contractile activity is required to sustain standing and ambulatory activities (21); consequently, blood flow increases and the metabolic demands of the contracting muscles are accommodated (22). In contrast, muscle contractile activity during sitting postures [as measured by electromyographic (EMG) activity] is significantly lower than for standing and ambulatory activities (18, 21, 23). EMG activity in the quadriceps and hamstring muscle groups is ∼2.0–2.5, ∼7.0–10.5, and ∼18.0 times higher during standing, walking, and stair climbing in daily living settings, respectively, as compared to sitting (21, 23). To match the reduced metabolic demands of low muscle activity, skeletal muscle blood flow is also significantly lower and less variable during sitting as compared to standing and ambulatory activities (22, 24, 25).
Elements pertaining to SB that may explain the differential impacts of SB on health outcomes include frequency, intensity, time, and type, the so-called frequency, intensity, time, and type (FITT) principle (TABLE 2) (36). Engagement in SB can be accordingly described and monitored, which aligns with and builds on the FITT principle for exercise prescription. These key postural, physiological, and behavioral features are what define/characterize SB and should be considered explicitly in SB research.
Table 2.
Sedentary behavior frequency, intensity, time, and type principle
| Term | Definition | References |
|---|---|---|
| Frequency | Refers to the number of SB bouts over a given time frame. The most common SB bout lengths reported in the literature are ≥30 min, ≥60 min, and ≥120 min. Example: interruptions to sitting lasting 2–3 min every 20–30 min seems to yield greater benefits for glycemic control compared to prolonged, uninterrupted sitting. Study design, data analysis, and reporting: include experimental groups with a different frequency of interruptions to SB versus a more sedentary experimental group (control group). If possible, groups should be matched for duration and/or EE. Include detailed description about how participants were instructed about the frequency of interruptions and how adherence to prescribed frequency was assessed. Report changes in the number of daily interruptions to SB and number and duration of prolonged SB bouts. |
26–28 |
| Intensity | Refers to any waking behavior with an energy expenditure ≤1.5 MET, while sitting, reclining, or lying down. When focusing at reducing/ interrupting sedentary behavior, intensity refers to the physical activity used to replace sedentary behavior. Example: EE during sitting postures is lower than standing and all intensities of physical activity. Similar responses are observed for oxygen consumption and heart rate. Study design, data analysis, and reporting: include experimental groups with different intensity of interruptions to SB versus a more sedentary experimental group (control group). If possible, groups should be matched for EE. Include detailed description about how participants were instructed about the intensity of interruptions and how adherence to prescribed intensity was assessed. Report changes in objectively measured daily time spent in each physical activity intensity. |
5–20 |
| Time | Refers to the total duration of time spent in SB or time spent in bouts of uninterrupted, prolonged sitting (i.e., consecutive min accumulated in SB, usually reported as bouts of ≥30 min, ≥1 h, and ≥2 h of sitting). Example: increasing sedentary time results in maladaptations in physiological systems. In contrast, reducing/interrupting sedentary time results in small benefits. Study design, data analysis, and reporting: include detailed description about how participants were instructed to reduce/interrupt SB (as per other FITT elements) and how adherence to prescribed intervention was assessed. If possible, provide participants with specific and measurable goals, so adherence can be objectively assessed. Report changes in duration of objectively measured total daily SB and prolonged SB bouts. |
29–33 |
| Type | Refers to the main intention of the SB and the context in which it occurs. Example: SB associated with energy surplus is more detrimental than exposures to sedentary behavior in energy balance. Study design, data analysis, and reporting: measure participation in each type of SB using a validated questionnaire or diary. Similarly, record types of physical activity that can be used to reduce/interrupt SB. If appropriate, design the intervention to tackle specific type/context of SB (e.g., work-related, recreational screen time, etc.) and standardize the FITT of physical activities being used to reduce/interrupt SB. Report changes in duration of objectively measured total daily SB and prolonged SB bouts, and self-reported duration in each specific type/context of SB. |
34, 35 |
EE, energy expenditure; FITT, frequency, intensity, time, and type principle; MET, metabolic equivalent; SB, sedentary behavior.
2.2. Sedentary Behavior Versus Physical Inactivity
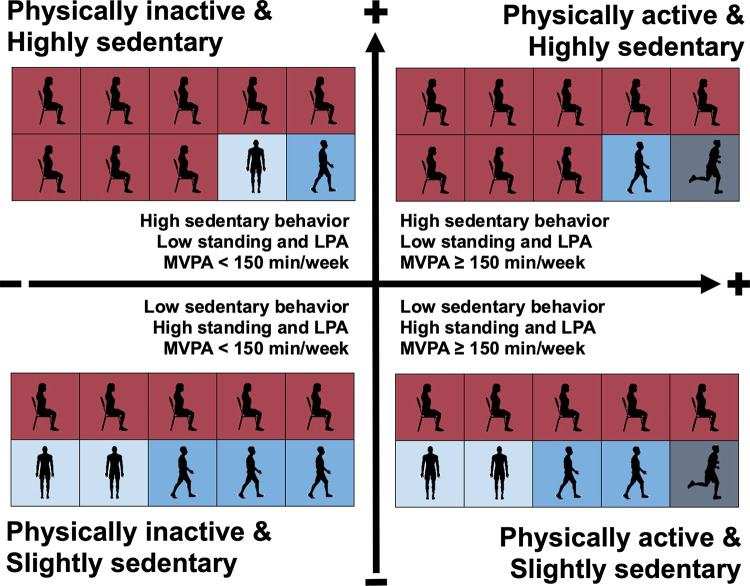
The term “sedentary” had previously been used interchangeably with physical inactivity to denote insufficient levels of moderate-to-vigorous intensity PA (MVPA), i.e., not achieving the current PA guidelines (1). However, SB and physical inactivity are now viewed as separate entities on a continuum of human movement and nonmovement behaviors (1). According to this definition, a person could be classified as being both highly sedentary and physically active. This points to four distinct classifications: being physically active and highly sedentary, physically active and slightly sedentary, physically inactive and highly sedentary, or physically inactive and slightly sedentary (FIGURE 1). The importance of addressing the behavioral phenotype of both excessive SB and physical inactivity is now embodied in contemporary public health PA guidelines (2, 14).
FIGURE 1.
Sedentary behavior and physical inactivity operationalized as distinct behaviors identify 4 key classifications. A person is classified as physically inactive if he/she engages in <150 min/wk of moderate-to-vigorous intensity physical activity or <75 min/wk of vigorous-intensity physical activity (1). Despite the absence of consensus on the cut point to classify “highly” sedentary; epidemiological evidence suggests a higher risk for premature mortality from ≥9.5 h/day for objectively measured sedentary time (4). Red boxes represent time spent in sedentary behavior, light blue represents time spent standing and in light-intensity physical activity, and dark blue represents time spent in moderate-to-vigorous intensity physical activity. The vertical arrow represents time spent in sedentary behavior; the horizontal arrow represents time spent in moderate-to-vigorous intensity physical activity. LPA, light-intensity physical activity; MVPA, moderate-to-vigorous intensity physical activity. Image was created with mindthegraph.com, with permission.
From a physiological perspective, differentiating between “SB” and “physical inactivity” may initially seem rather semantic. Indeed, reviews have already summarized the evidence to date on numerous physiological responses of increasing SB (37–42), and it is evident that many of these relate to the responses following imposed physical inactivity (43–46). Even though the effects of SB and physical inactivity are generally in a similar direction (deleterious), excessive participation in SB has been shown to result in adverse effects even in the presence of large volumes of aerobic and/or resistance exercise (a sedentary yet physically active condition) (29). Exercising (above current PA guidelines) during bed rest does not necessarily counteract, or only partially counteracts, some of the adverse effects of large volumes of SB in healthy adults (29). These findings point not only to likely independent adverse health effects of SB but also to the potential benefits of regular nonexercise activity and/or muscle contractions.
2.3. Sedentary Behavior Physiology Research: the Seminal Role of Inactivity Physiology and Animal Studies
The term inactivity physiology was first proposed in the early 2000s as a separate field and distinct from exercise physiology (8–10). The premise was that excessive SB was not the same as lack of exercise and that SBs have their own unique physiological consequences.
Research in the inactivity physiology context has been examined primarily using hindlimb unloading and wheel lock methodologies in rats. The key objective of these studies was to better understand how increasing SB and imposing physical inactivity (or rather hypodynamia and hypokinesia) may trigger maladaptations linked to chronic diseases. Here, the key findings pertinent to SB physiology are summarized.
Hindlimb unloading models involve suspending rodents by their tails, thereby preventing any weight-bearing activities of the lower limbs. Hindlimb unloading studies have reported rapid development of insulin resistance (increased glucose-insulin index) after 1 day of limb suspension (47). Notably, glucose transport activity and intramuscular triglycerides were significantly lower in the soleus muscle but not in the extensor digitorum longus after 1 day of limb suspension, which was driven by increases in p38 mitogen-activated protein kinase expression (MAPK), known to negatively interact with insulin signaling cascade (47). This suggests that those muscles that predominantly contain type 1 fibers are more susceptible to maladaptations related to increasing SB than muscles composed of type 2 fibers predominantly. This has also been shown in humans following periods of bed rest. For example, plantar flexor and monoarticular knee extensor muscles were found to be more affected than hip extensor/adductor muscles after head-down bed rest (HDBR) and horizontal bed rest (48–51). In contrast, the biarticular knee extensor and hip flexor rectus femoris, other anteromedial hip muscles, and the short head of biceps femoris were found to be comparatively less affected by horizontal bed rest (48). Notably, faster rates of muscle atrophy were observed in antigravity muscles and those that are more intensively required for standing and walking (48). Similarly, myosin heavy chain (MHC) distribution in the skeletal muscle shifted from slow-twitch (MHC I) toward hybrid (I/IIa and IIa/IIx) and fast-twitch (IIa and IIx) fiber types in the vastus lateralis following 35–84 days of HDBR and horizontal bed rest (52–54). Similar alterations in slow and hybrid fibers, but not fast fibers, were observed in the soleus muscle after 84 days of HDBR (53).
Bay and Hamilton (55) showed that distinctive physiological pathways are activated with hindlimb unloading, particularly LPL activity, which seems to remain largely unaffected by MVPA. Rat skeletal muscle triglyceride uptake was reduced by 75%, and LPL protein expression and enzymatic activity were rapidly suppressed during acute (1–18 h) and chronic (∼10 h/day over 11 days) periods of hindlimb unloading (55). Alterations in heparin-released and intracellular LPL activity decreased monoexponentially in both the soleus (type 1) and red quadriceps (predominantly type 2) muscles after 12 h of limb unloading. These alterations were rapidly reversed with light-intensity contractile activity in both soleus and quadriceps muscles (9, 55, 56). Interestingly, MVPA/exercise training did not enhance LPL regulation in type 1 muscles and type 2 muscles that were not recruited during running (8, 57). In type 1 muscles recruited during running, there was an increase in heparin-released LPL activity, LPL mRNA level, and LPL protein mass (8, 57). Additionally, heparin-released LPL activity was 10-fold less in the soleus and quadriceps muscles and 2-fold less in the rectus femoris muscle of rats subjected to 12-h limb unloading as compared with low-intensity ambulatory controls (55). The absence of changes in LPL activity in the heart and diaphragm, both muscles with high oxidative capacity, also suggested that loss of muscle LPL activity was constrained to unused muscles (55).
Despite changes in LPL activity, no changes were observed in skeletal muscle LPL gene expression following acute (1–18 h) and sustained (∼10 h/day over 11 days) periods of hindlimb unloading (56). However, Zderic and Hamilton (58) demonstrated that skeletal muscle differentially expresses at least 17 genes involved in homeostasis in humans and rats. Of particular interest, lipid phosphatase-1 (LPP1/PAP2A), a key gene for the degradation of prothrombotic and proinflammatory lysophospholipids, was suppressed locally in muscle tissue after 12 h of hindlimb unloading in rats and after 12 h of prolonged sitting in humans (58). Of note, exercise was ineffective at counteracting this decrease in both species (58).
Wheel lock models involve periods of habitual or voluntary activity (3–6 wk; typically, 5–10 km/day of running), which is suddenly restricted (i.e., running wheel locked) to permit only minimal movement within the cage for up to 7 days. While daily wheel running increased insulin-dependent glucose uptake in isolated skeletal muscle, a rapid decrease in insulin sensitivity was reported following only 2 days of wheel lock in rats (59). This reduction in insulin-dependent glucose transport was associated with reduced activation of the insulin-signaling pathway and glucose transporter 4 (GLUT-4) protein content. Pronounced gains in intra-abdominal fat mass (25 to 48%) were also reported following 1 wk of wheel lock (60, 61). Interestingly, lowering food intake during wheel lock did not significantly change fat mass increase compared to the rats that were fed ad libitum, indicating that fat storage was the result of SB and physical inactivity per se, rather than positive energy balance (60). Despite providing important initial insights for SB research, wheel lock models are considered to be extreme models of inactivity in which animals transition from very high daily amounts of exercise to sedentariness/inactivity. Therefore, it may be more a model of detraining from exercise rather than a model to study adaptations to increasing SB.
Evidence from inactivity physiology studies using hindlimb unloading and wheel lock methodologies has been instrumental in laying the foundation for experimental studies related to SB and physical inactivity physiology in humans by providing initial evidence on the potential adverse effects and underpinning mechanisms associated with these behaviors as compared to habitual activity and exercise.
2.4. Role of Sedentary Behavior in Health and Disease
Extensive epidemiological evidence has highlighted that excessive daily time in SB is associated with an increased risk of early mortality and chronic diseases, including obesity, type 2 diabetes, cardiovascular disease, metabolic syndrome, certain type of cancers, and others (4, 62–70). Yet, the mechanisms involved in this increased risk are poorly understood.
A systematic review synthesized current knowledge of the associations of SB with gene expression and epigenetic modifications in children and adolescents. Overall, the evidence is still limited, but some studies suggest candidate genes and noncoding ribonucleic acids (RNAs) that are linked to/regulated by SB, including higher miRNA-222 and miRNA-146a levels (related to angiogenesis and inflammation) and methylation at HSD11B2 promoter (related to stress/cortisol metabolism) (71). Additionally, screen time was a significant moderator in the association of the rs9939609 single nucleotide polymorphism (SNP) located on the fat mass and obesity-associated gene (FTO) with metabolic syndrome clustered cardiometabolic risk score in children and adolescents of low cardiorespiratory fitness (72). In adults, FTO SNP rs9939609 was significantly associated with self reported time spent in SB, and sedentary time partially mediated the association between FTO and body mass index (BMI) (73). In contrast, another study demonstrated that the association between objectively measured SB and FTO SNP rs17817449 was fully attenuated by BMI in adults, suggesting the association between SB and FTO was explained by adiposity (74). Although the mechanisms through which FTO increases BMI and adiposity have not been elucidated, knockout mouse models suggest that FTO may be involved in energy homeostasis via the regulation of energy expenditure (75).
Ascribing causality from observational evidence is difficult. To overcome this limitation some studies have used Mendelian randomization, which is a well-established tool that employs genetic variants as instrumental variables for exposures (e.g., SB and PA). Since the genetic variants are randomly assigned during meiosis, Mendelian randomization can minimize confounding and reverse causation, potentially providing stronger evidence for causal inference (76). Overall, findings from Mendelian randomization studies are aligned with those from observational studies.
Totals of 136, 43, and 5 genetic SNPs have been found to be associated with leisure-time TV watching, computer use, and driving, respectively. Genetically predicted duration of TV watching was positively associated with the risk of myocardial infarction, heart failure, and atrial fibrillation, which remained significant after adjustments for genetically predicted PA. Associations between computer time use and driving and cardiovascular diseases were inconsistent and nonsignificant (77). Similarly, another study identified a total of 89 genetic SNPs that were associated with TV watching. Genetically predicted duration of TV watching, but not computer use and driving, was positively associated with the risk of developing type 2 diabetes (78). A study using individual-level data from 130,957 females identified 6 SNPs as predictors of participation in SB. Females with genetic variants predisposing them to a higher time in SB had a higher risk of hormone-receptor-negative and in situ breast cancer. Subanalysis suggested that SB and PA independently influence the risk of breast cancer (79). Finally, a genome-wide association study of PA and SB provided some insights into underlying mechanisms and roles in disease prevention (80). Eighty-eight loci (89 independent SNPs) were associated with leisure screen time, eight loci for SB at work, and none for SB during commuting. The authors observed a significant genetic correlation between high leisure screen time and higher adiposity-related traits, particularly fat percentage; poor cardiometabolic status, including higher triglycerides, cholesterol, fasting glucose, and insulin concentrations; and odds of type 2 diabetes, coronary artery disease, cancer, worsened mental health outcomes, and decreased longevity. Additionally, Mendelian randomization has consistently shown that leisure screen time and BMI causally influence each other, with the causal role of leisure screen time in BMI being two-to-threefold larger than the effect of BMI on leisure screen time. In this same study, tissue and cell-type enrichment analysis has also suggested a role for visual information processing and the reward system in leisure screen time. Leisure screen time-associated loci were mildly enriched for genes whose expression in skeletal muscle is altered by resistance exercise training. Forty-six candidate genes pointed to pathways related to endocytosis, locomotion, and myopathy, but in vivo models are required to confirm or refuse a role in SB. Overall, such causal inferences can support the public health message that increasing MVPA and reducing SB mitigate the risk of multiple chronic diseases (80).
The summary of available literature highlights the small evidence base with respect to the mechanisms behind the relationship between SB, health, and disease. Very few genes and genetic regions have been studied in SB research. Despite the limited evidence, Mendelian randomization studies provide genetic support for a causal relationship between SB and the risk of chronic diseases. There is a need for larger cohort studies and randomized controlled trials using “omics” approaches (e.g., genomics, epigenomics, transcriptomics, proteomics, and metabolomics) to better understand the molecular mechanisms underlying the effects of SB on heath and disease.
2.5. The Influence of Sedentary Behaviors on the Relationship Between Exercise and Health Outcomes
Recent experimental evidence indicates excessive sedentary time also affects the relationship between participation in MVPA/exercise and health benefits (81–85).
Coyle and colleagues (83–85) have demonstrated the adverse effects of excessive participation in SB on postprandial metabolic responses and metabolic benefits of acute exercise. Two or four days of prolonged sitting (>14 h/day and ∼1,650 steps) resulted in increased postprandial plasma triglyceride responses regardless of energy intake, compared to 4 days of standing/walking (∼8.4 h/day of SB and ∼17,000 steps/day) in healthy, physically active males. Importantly, this altered response was not attenuated by a subsequent acute 1-h bout of MVPA (∼67% V̇o2max) performed at 1700 h of day 4 (83). Using a similar study design, Akins and colleagues (84) demonstrated that acute exposure to prolonged sitting (∼13.5 h/day of sitting and <4,000 steps/day) not only prevented the traditional exercise-related benefits in postprandial triglycerides responses but also improvements in postprandial plasma glucose and insulin responses in healthy adults. Furthermore, Burton and Coyle (85) compared postprandial plasma triglycerides responses to an acute exercise bout after 2 days of low (∼2,500 steps/day), limited (∼5,000 steps/day), or normal (∼8,500 steps/day) daily step count to determine the range of step counts that elicited this blunted postprandial metabolic response to acute exercise. Following low and limited step counts, postprandial triglyceride responses were elevated by 22–23%, and whole body fat oxidation was reduced by 14–19% as compared to normal step count in healthy adults (85). This finding indicates that altered metabolic responses to acute exercise can occur in those taking ∼5,000 steps/day or lower.
These studies provide initial insights into a unique perspective that excessive SB might be a health hazard, not only via the physiological maladaptations that occur during sitting but also by impacting the health benefits provided by MVPA/exercise. Collectively, these findings highlight the need for 1) addressing large amounts of time spent in SB to minimize/counteract its adverse effects; and 2) examining the physiological responses and adaptations within and across each of these distinct behavioral constructs, as there may be differential, additive and/or interacting physiological effects to consider.
These are research questions that arise from SB physiology that have not been pursued by exercise and physical inactivity physiology. A key feature in SB research has been to focus on shifting the balance of participation in SB toward LPA, rather than solely focusing on MVPA. This has also been included in the development of countermeasures to specifically address SB, with a growing body of experimental studies aiming to reduce and interrupt prolonged SB with various types of PA (30, 86–89). The understanding of the physiological impact of the interdependent relationships between SB, LPA, and MVPA is a more recent focus and available evidence has been limited to the abovementioned studies. In sect. 2.6, the pros and cons of the relevant experimental models will be discussed in the context of their potential implications for investigations of the physiology of SB.
2.6. Experimental Models with Relevance for Sedentary Behavior Research
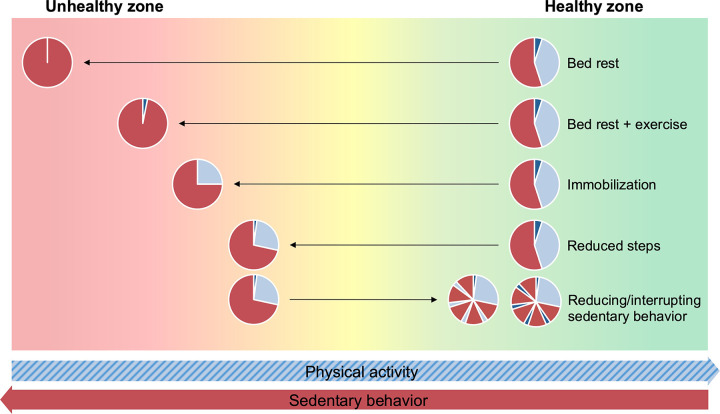
SB-induced physiological changes in humans can be inferred from a variety of experimental models, e.g., bed rest, limb immobilization/casting, reduced daily step count, reducing/interrupting prolonged sitting, and others (e.g., detraining, confinement, and natural experiments), that collectively induce variations in time spent in SB (FIGURE 2). Each of these approaches can provide complementary information related to the impacts of SB on health outcomes. Identifying and understanding the different goals, methodologies, and assumptions that can be made under these models are fundamental when attempting to generalize their findings to SB physiology research.
FIGURE 2.
Hypothetical representation of imposed changes in physical activity level by experimental models that can induce changes in time spent in sedentary behavior. Fluctuations in physical activity levels, such as those imposed by these experimental models, may result in increased or decreased risk of adverse health outcomes. For the pie charts, red represents sedentary behavior, light blue represents standing and light-intensity physical activity, and dark blue represents moderate-to-vigorous intensity physical activity. Horizontal arrows schematically represent the direction of changes in physical activity and sedentary behavior. Image was created with mindthegraph.com, with permission.
2.6.1. Bed rest.
Bed rest is a common practice within medical treatment for selected conditions. In research, bed rest was initially developed in the context of space exploration as a ground-based model used to mimic on Earth the physiological effects of microgravity. The bed rest model is characterized by a postural change [lying down or lying down combined with head tilt (i.e., HDBR)] and lack of muscle contraction for extended periods of time. In the quest of mitigating the adverse health effects of microgravity on the body, space agencies have developed and tested countermeasures during bed rest studies including different exercise training protocols (29, 43, 90–95).
Beyond the interest in space science, bed rest models have implications for SB and physical inactivity research. Bed rest has the advantage of taking place in a highly controlled environment, which allows accurate monitoring of the activity performed and of food intake. The degree of physical inactivity observed during bed rest may be seen as too extreme compared with that seen in the general population, who spend more time sitting with some level of body movement rather than strictly lying down. However, because of upper body movements and fidgeting movements in the bed, the PA level (i.e., the ratio between total and resting energy expenditures) measured during bed rest studies is similar to that of sedentary individuals (i.e., 1.4 to 1.5) (96, 97). In contrast, strict bed rest involves prolonged exposures to the lying down position, which distinctly affects organs and physiological systems as compared to the free-living context, where sitting is the most predominant type of SB. For example, lower limb muscles and weight-bearing muscles are more affected than upper limbs and nonweight-bearing muscles during bed rest (48, 98, 99). Shift of fluid from the lower to upper part of the body is a unique adaptation to the lying down position, particularly in HDBR (100), which may distinctly affect cardiovascular and central nervous systems, which is in contrast to how SB manifests in the free-living context.
2.6.2. Immobilization.
Limb immobilization/casting models are characterized by periods during which a limb is physically immobilized (101, 102). In the case of lower limb immobilization, participants are commonly supported by crutches and asked to refrain from weight-bearing activity on the immobilized leg. Consequently, there is an extensive restriction of motion for the target limb with a reduction in habitual ambulatory activity, which ultimately results in local muscle disuse (101). The main focus of contemporary studies has been to investigate mechanisms underlying muscle disuse atrophy (102, 103), particularly related to aging (104–107) and identifying potential countermeasures (107–109).
Lower limb immobilization models can be useful to investigate local muscle disuse since movements in the casted leg are tightly controlled during the protocol. Of importance for SB research, the lower limb immobilization protocol likely induces increases in sedentary time. This can be presumed from free-living studies showing a higher sedentary time and lower PA during casting due to lower or upper limb fractures compared to healthy peers (110) or previous PA levels (111). Similar to bed rest studies, immobilization protocols impose extreme changes in PA and SB levels. Additionally, the absence of control for PA level requires a cautious interpretation of findings on systemic alterations in organs and systems other than the impacted limb.
2.6.3. Reduced daily step count.
Reduced step count models focus on addressing how physical inactivity (reduced daily movement) is manifested in the daily lives of the majority of the population. For reference, the median daily steps count in adult and older adult populations is typically around 5,000 (112). However, it is common that people may intermittently transition to lower daily step counts. Reduced steps models aim to mimic such transitions by reducing participants’ daily ambulatory activity from normal to subnormal levels (113–116). Most protocols aim to reduce the total amount of daily steps to ∼1,500 steps/day (range: 750 to 5,000) (115, 117–123). While the subsequent reduction in PA does not constitute complete disuse, it is plausible to assume that even short-term exposure to such periods of physical inactivity may have profound physiological consequences (119).
The main strength of reduced steps models is that they induce changes that are more similar to typical reductions in ambulatory activity that can occur in daily living due to hospitalization (740 to 2,620 steps/day) (124, 125) as compared to strict bed rest, but they are less severe than those observed due to sickness (i.e., influenza-like symptoms; average of 924 steps/day) (126). The measure of PA level used in these studies has almost exclusively been step count. SB is not typically the focus, and only a few have accurately reported increases in sedentary time during the step reduction protocols (116, 122, 123, 127), with inferences made about changes in sedentary time as a consequence of reductions seen in time spent in ambulatory activities.
2.6.4. Reducing and interrupting prolonged sedentary behavior.
Experimental models aimed at reducing and/or interrupting sitting time in laboratory-based and free-living settings are a relatively new approach. A key distinction here from the experimental models described above is the “solution focused” treatment paradigm whereby typically physically inactive/sedentary individuals modify their typical low PA level to a higher PA level (128). Most reducing/interrupting prolonged SB models are acute in nature (most lasting >2 h but <24 h) (86–88, 129, 130). There is a growing number of multidays (≤4 days) (131–134) to longer term clinical trials (2 wk to 36 mo) (30, 89).
Acute and multiday protocols have utilized a control condition of imposed prolonged sitting and one or more experimental conditions involving, for example, a single continuous bout of activity and/or frequent, short bouts of activity (often referred to as “breaks”) (86–88). These experimental conditions may vary in terms of frequency, intensity, duration, and type of activity used to interrupt sitting (24, 86–88). Acute models targeting reducing/interrupting prolonged SB can provide insights into the physiological effects and underlying mechanisms of such strategies. These studies have been typically conducted in highly controlled research environments, which allows accurate monitoring of potential confounders, including PA level. Consequently, the control condition (prolonged sitting) and experimental conditions (activity protocols) are typically unrepresentative of daily living activity patterns (135). To date, it is still uncertain whether some of the acute adaptations observed within this experimental model can be sustained over time (30) and whether maladaptations to sitting are an impairment to, or a sign of plasticity of, the physiological systems, e.g., health being defined as the ability of our body to cope with daily-life challenges (i.e., phenotypic flexibility) (136).
Longer term clinical trials have generally incorporated a control condition, in which sedentary/physically inactive participants are instructed to maintain their lifestyle or have received usual care, and an experimental group, in which participants undergo an intervention to reduce/interrupt SB (30, 89). Longer term randomized clinical trials can provide useful information on the longer term dose-response effects of reducing/interrupting health outcomes. Due to the nature of these studies, the effectiveness of interventions at reducing/interrupting sedentary time, adherence to intervention, and duration, frequency, and intensity of interruptions to SB must be closely monitored, as these factors likely affect the effects of such interventions on health outcomes.
2.7. Semantic Considerations for Interpreting the Evidence That Can Be Pertinent to Sedentary Behavior Research
In this review, SB is defined based on both physiological and postural features (see sect. 2.1) and is considered a distinct behavior that coexists with physical inactivity in daily living. In studies where SB cannot be separated from physical inactivity due to methodological limitations, the evidence that we will now consider below will be discussed in light of study limitations and noted as a consequence of both behaviors. Given the interdependent nature of SB, LPA, and MVPA and the lack of studies focusing on this interrelationship, changes in SB and PA levels will be reported whenever original studies or meta-analyses reported such changes. This information will be particularly useful to triangulate available evidence to better understand potential differential, additive, and/or interacting effects of behaviors on physiological outcomes. The duration of SB interventions will also be reported as we address the relevant findings. The terms “acute” and “multidays” will be used for studies lasting hours to ≤14 days. The term “longer term” will be used for studies lasting >2 weeks to years.
Adaptations to physical inactivity are not the opposite of adaptations to exercise/PA (8–10, 137). Accordingly, evidence from studies using models for increasing SB will be discussed separately from those of models aimed at reducing/interrupting SB. The consequences of increasing SB will be discussed using evidence from bed rest, bed rest combined with exercise, immobilization/casting, reduced daily steps, and acute studies that included a condition imposing prolonged SB. In contrast, the effects of reducing/interrupting SB will be discussed using evidence from acute and longer term studies that include at least one condition aimed at reducing/interrupting SB with multiple active bouts. Because of the limitations associated with bed rest models, evidence from bed rest related to whole body outcomes will not be discussed herein. However, given the absence of evidence from other human models of increased SB, bed rest data will be used to inform the potential mechanistic underpinnings of excessive and prolonged SB. Evidence from detraining, confinement, and natural experiments has not been used either, as study findings are constrained by limitations related to study design, targeted population (e.g., athletes, those highly physically active), and the lack of control for sedentary time.
Finally, SB research studies to date have included a variety of population groups. Therefore, the use of some key terms has been standardized over the next sections to facilitate the discussion of available evidence. Adult and older adult groups have been defined according to age cut points used by the original studies (“adults”: 18–59 or 18–64 years old; “older adults”: ≥60 or ≥65 years old). The term “children” has been used for 5–12 years old and “adolescents” for 13–17 years old. With respect to health status, the term “healthy” will be used to refer to population groups without any existing medical condition. Otherwise, health status or condition (e.g., overweight, obesity, type 2 diabetes) will be reported along with study findings. The sex of participants will be reported for studies that included females or males only. Sex-neutral terms will be used in studies that included participants from both sexes.
The considerations outlined in sect. 2 provide perspectives and caveats of relevance to the evidence we address below for each of the relevant major bodily systems and processes. Accordingly, in sects. 3–8, we examine the relevant physiological evidence relating to body weight and energy balance, intermediary metabolism, cardiovascular and respiratory systems, the musculoskeletal system, the central nervous system, and immunity and inflammatory responses.
3. BODY WEIGHT AND ENERGY BALANCE
3.1. Body Mass and Composition
3.1.1. Increasing sedentary behavior.
3.1.1.1. evidence from longer term studies.
Fourteen days of reduced step count (from 10,501 to 1,344 steps/day) reduced leg lean mass (∼0.5 kg) and increased intra-abdominal fat mass (7%) but not total fat mass in healthy male adults (118, 119). Other studies (14 to 20 days; from ∼11,500 to 2,000 steps/day) have shown similar alterations in healthy male adults but also revealed increases in total and percent body fat (∼3 to 14%) (116, 117, 138). Interestingly, alterations in body composition observed after 14 days of reduced step count (81% reduction from baseline plus a 3.7 h/day increase in sedentary time) returned to baseline levels after resuming habitual PA for 14 days in healthy adults with or without a first-degree relative with type 2 diabetes (122). Although inconsistent across studies, most step-reduction protocols were detrimental for at least one body composition-related outcome in healthy older adults (115, 120, 139, 140). Fourteen days of reduced step count (from ∼9,000 to 3,000 steps/day) also resulted in intramuscular (nuclear/myofibrillar fraction) ceramide accumulation (∼20%) in healthy older adults (140).
3.1.1.2. clinical significance.
Increases in body fat mass induced by models involving increased SB are likely clinically relevant. Specifically, increases in body fat mass reported in reduced step studies (3 to 14%) are considerably more pronounced than longitudinal changes observed in the general population. A populational cohort study showed an ∼1% (0.7 kg) increase in body fat mass over the course of 12 years (141). Of concern, measures of adiposity (BMI, visceral fat mass or central adiposity, and body fat percentage) are positively associated with increased risk of all-cause cardiovascular disease and cancer mortality (142) and 21 major chronic diseases (143).
3.1.2. Reducing and interrupting sedentary behavior.
3.1.2.1. evidence from longer term studies.
A meta-analysis has analyzed data from longer term studies investigating the effects of SB interventions conducted in free-living settings on adiposity outcomes. Intervention duration ranged between 2 wk and 36 mo, and the average change in total sedentary time was −28.6 min/day (30). There were small significant reductions in body weight (−0.6 kg), waist circumference (−0.7 cm), and percent body fat (−0.3%) in adults and older adults, but no changes have been reported in BMI, total body fat, and total fat-free mass (30). Another meta-analysis investigating the effects of SB interventions (range: 6 to 24 wk) on body composition demonstrated significant decreases in total sedentary time (−64 min/day) and increases in walking time (27 min/day) but no significant changes in time spent standing and in MVPA (31). The authors noted small significant decreases in percent body fat (−0.7%) and waist circumference (−1.5 cm) following SB interventions but no changes in body weight and BMI in clinical population groups (those with overweight, obesity, type 2 diabetes, cardiovascular, neurological/cognitive, and musculoskeletal diseases) (31).
A meta-analysis of studies aiming to replace SB with standing time (mean follow-up: 3.8 mo) demonstrated a significant increase in total standing time (1.3 h/day) in adults. This was associated with a significant decrease in total body fat mass (−0.75 kg) but no changes in body weight and waist circumference (32). Finally, a systematic review of studies implementing workplace SB interventions in apparently healthy and overweight/obese desk-based office workers demonstrated that the effects of workplace interventions on body composition have been inconsistent across studies. Only 11 out of 29 studies reported improvements in measures of adiposity, with most studies showing no changes following SB interventions (144).
In children, an 8-mo, school-based intervention using height-adjustable desks in the classroom was ineffective at reducing classroom and total daily SB (145). Consequently, no significant changes were observed in BMI z-score and waist circumference (145). However, other longitudinal studies demonstrated that when coupled with increases in PA, reducing SB prevents unhealthy weight gain. The socioecological French ICAPS (Intervention Centered on Adolescents’ Physical Activity and Sedentary Behavior) study (146) concomitantly targeted PA and SB through a 4-yr multilevel intervention that focused on the school and family of the children and the children themselves. Compared to the controls (no intervention), pupils who received the intervention were more active, were less sedentary, and gained less weight throughout the 4-yr study (146). Importantly, these changes in physical behavior and the prevention of weight gain were maintained 2.6 years after the end of the intervention with the highest efficacy in the most sedentary adolescents (147).
In summary, it is evident that findings are inconsistent across studies and meta-analyses. It is not clear whether type, intensity, and frequency of interruptions to sitting differentially affect body composition outcomes nor if there are specific factors that mediate adaptations in body composition following SB interventions (e.g., age, sex, BMI, population group).
3.1.2.1.1. Reducing/interrupting SB versus continuous MVPA/exercise.
A small-scale, 12-wk, multifactorial, pilot study examined both the independent and the combined effects of exercise training (40–65% heart rate reserve, i.e., moderate to vigorous intensity) and reducing SB (replace SB with standing and LPA plus increasing daily step count by 5–10%) on body composition in adults with overweight/obesity (148). Compared to control, both exercise training and exercise training combined with reducing SB significantly changed SB and PA levels (no change in SB + 27 min/day increase in MVPA and −7.3% of daily hours in SB + 45 min/day increase in MVPA, respectively). This resulted in a significant decrease in BMI (−0.5 and −1.1 kg/m2, respectively), body weight (−2.3 and −3.4 kg, respectively), and total body fat (−1.0 and −1.4%, respectively). The addition of interruptions to SB did not result in greater improvements in body composition compared with exercise training only (148). No changes in body composition outcomes were observed in the group reducing SB only (148).
Another small-scale, 6-mo pilot study examined the effects of a stepping protocol during a TV commercial (brisk walking around the room during at least 90 min of TV programming at least 5 days/wk) versus brisk walking for 30 min/day (at least 5 days/wk) in adults with overweight/obesity (149). After 6 mo of intervention, both protocols increased the number of daily steps and decreased time watching TV (2,994 vs. 2,956 steps/day and −1.2 vs. −1.4 h/day, respectively) as well as TV-related energy intake (−282 vs. −517 kcal/day, respectively). Both interventions significantly reduced percent body fat (−1.0 vs. −0.9%, respectively), waist circumference (−2.5 vs. −1.6 cm, respectively), and hip circumference (−1.9 vs. −1.2 cm, respectively) at the 6-mo time point, but neither changed body weight and BMI (149). In contrast, 4 wk of interventions aimed at reducing SB (−53 min/day in SB with no change in MVPA) or increasing MVPA to at least 30 min day (+16 min/day of MVPA with no change in SB) did not lower BMI and waist circumference in physically inactive adults with obesity (150).
3.1.2.2. clinical significance.
There is some evidence of small to trivial improvements in body mass and composition [body weight (−0.6 kg), waist circumference (−0.7 to −1.5 cm), and percent body fat (−0.3 to −0.7%)] associated with reducing/interrupting SB. Mixed results have also been reported across original studies and meta-analyses. Despite intervention effects being small and likely not clinically relevant, it is important to highlight that mean baseline BMI ranged between 25 and 30 kg/m2 and study duration varied between 6 to 24 wk in available meta-analyses (30, 31). It has been suggested that the weight loss induced by 1-yr exercise programs is more pronounced in those with existing obesity as compared to individuals with overweight (1.1 to 1.5 kg less than individuals with obesity) (151). It is not clear whether baseline BMI, other measures of adiposity, and duration of intervention may have affected responses to reducing/interrupting SB interventions.
Reducing SB and increasing LPA without increasing engagement in MVPA/exercise do not seem to be an effective strategy to improve markers of adiposity as compared to traditional, continuous exercise (149, 150). These findings indicate that reducing/interrupting SB with PA in higher intensities might be required to improve markers of adiposity in those with overweight/obesity. As recently reviewed thoroughly (152), reducing/interrupting SB is likely not effective at inducing weight loss, like exercise but may prevent unhealthy weight gain. Future studies will also need to investigate whether changes in SB/LPA trigger spontaneous behavioral and physiological compensatory responses (e.g., decrease in activity and/or nonactivity energy expenditures, increase in appetite and food intake) like those observed following the initiation of exercise training and thought to minimize the effect of exercise on body weight (153, 154), as further discussed in the next topics.
3.2. Total Energy Expenditure
3.2.1. Increasing sedentary behavior.
3.2.1.1. evidence from multiday studies.
Participation in high levels of SB results in lower energy expenditure and PA levels. For example, 7 days of exposure to a highly sedentary condition in free-living (increased sedentary time and limited participation in PA of any intensity) significantly reduced energy expenditure (−15% in MET-hour/week estimated from a validated accelerometer) (155).
3.2.1.2. evidence from longer term studies.
In a clinical study requiring physically active, but not trained, lean male adults to refrain from PA for 1 mo, total daily energy expenditure decreased by 8% due to a drop in activity-related energy expenditure only (137).
3.2.2. Reducing and interrupting sedentary behavior.
3.2.2.1. evidence from acute studies.
Experimental studies have demonstrated that both standing and ambulatory interruptions to prolonged sitting time increased energy expenditure in adults, as a function of the duration, intensity, and modality used (156–158). Interestingly, the increased energy utilization in response to frequent 2-min moderate-intensity walking interruptions to sitting was maintained for ∼4 min after every walking bout in adults. When repeated throughout the day, performing 28 min of interruptions to sitting resulted in ∼70 min of elevated energy utilization over 7 h (158).
3.2.2.2. evidence from longer term studies.
It is still unclear whether SB reducing/interruption interventions may result in compensatory changes in total energy expenditure and/or energy balance over the longer term. A study demonstrating the differential impact of low- and moderate-intensity training provides some relevant insights (159). Moderate-intensity aerobic exercise training (60% V̇o2reserve) tended to increase total energy expenditure in female adults with overweight/obesity compared to low-intensity training (40% V̇o2reserve) matched for energy expenditure over 3 mo of intervention. Exercise energy expenditure was almost entirely compensated (96%). Interestingly, greater energy compensation was observed in the moderate-intensity group than in the low-intensity group (161% vs. 49%) (159). Participants in the low-intensity group spent more time walking and less time lying down compared to the moderate-intensity group. These behavioral and energetic differences translated into ∼1-kg weight gain in the moderate-intensity group versus 1-kg weight loss in the low-intensity group (159). The clinical significance of these findings is still unclear.
3.3. Energy Intake and Appetite
3.3.1. Increasing sedentary behavior.
3.3.1.1. evidence from acute studies.
Granados and colleagues (160) showed that 1 day of sitting decreased energy expenditure without a reduction in appetite in adults, suggesting this would favor a positive energy balance. This is consistent with findings that demonstrated no compensatory decline in ad libitum food intake in response to large reductions in energy expenditure (∼24%) in healthy male adults (161). Another study (162) also demonstrated that energy intake during 1 day of decreased energy expenditure (275 steps/day) was comparable to energy intake during higher PA levels (equivalent to ∼1.5 and 2.1 resting metabolic rate) in healthy male adults.
3.3.1.2. evidence from longer term studies.
While there is no evidence of the impact of a reduced step count protocol on appetite regulation, 14 days of reduced step count (81% reduction from baseline) did not significantly alter fasting adiponectin and leptin levels in health male adults (118).
Acute experimental findings indicate that increasing SB can result in positive energy balance, yet caution is warranted when interpreting the clinical relevance of these findings, as acute changes in appetite may not affect weight control in the longer term. Longer term investigation into the effects of increasing SB on energy balance is warranted.
3.3.1.2.1. Potential mechanisms.
Several hormones are involved in the regulation of appetite and feeding behavior. A study demonstrated a significant decrease in adiponectin levels (∼21%; a hormone associated with increased sensation of hunger) in healthy male adults following 16 days of horizontal bed rest but no changes in other appetite-regulating hormones [ghrelin, peptide YY (PYY), glucagon-like peptide 1 (GLP-1), and leptin] (163). In a 2-mo HDBR study in females, fasting leptin was negatively associated with the spontaneous decrease in energy intake, thus suggesting a relationship between PA, leptin, and food intake (96).
3.3.2. Reducing interrupting sedentary behavior.
3.3.2.1. evidence from acute studies.
Over a 12-h period, performing hourly 5-min vigorous-intensity walking bouts (60–65% V̇o2peak; total: 60 min) resulted in lower perceived hunger (∼23%) in the midafternoon hours as compared to prolonged sitting and an energy-matched moderate-intensity continuous walking bout (60–65% V̇o2peak, total: 60 min) in adults with obesity but did not affect PYY levels (164). Changes in perceived hunger were not observed for continuous exercise (60–65% V̇o2peak; total: 60 min) followed by prolonged sitting (164). Another study demonstrated that while frequent 5-min moderate-walking interruptions (perceived effort: 12–13 “somewhat hard”; total: 30 min) did not affect hunger and desire for food consumption, this strategy resulted in lower food cravings (∼6%) compared to prolonged sitting in healthy adults (165). This was not observed for continuous exercise (perceived effort: 12–13 somewhat hard; total: 30 min) followed by prolonged sitting (165). Furthermore, while interrupting sitting with 2-min light or moderate-intensity walking bouts every 20 min (perceived effort: 6–9 “very light” and 12–14 somewhat hard, respectively; total: 28 min) did not alter appetite, it resulted in reduced relative energy intake (39 and 120%, respectively) that was not compensated for in a subsequent meal in healthy adults, which could have important implications for weight management (166). In contrast, other studies showed no alterations in appetite measures, ad libitum intake, and circulating gut hormone concentrations following interruptions to sitting in healthy adults (166–168). It is not clear whether the type, intensity, and frequency of interruptions to sitting can differentially affect energy intake and appetite.
3.3.2.2. evidence from longer term studies.
Replacing prolonged sitting time with regular standing bouts at the workplace (a 21% reduction of workplace sedentary time) reduced dietary intake (∼10%) in sedentary, adult office workers after 4 wk of intervention (169). It remains unclear the extent to which this reduction in caloric intake impacted measures of adiposity and other cardiometabolic outcomes. Longer term investigation into the effects of reducing/interrupting SB on appetite and food intake is warranted.
4. INTERMEDIARY METABOLISM
4.1. Glucose Metabolism
4.1.1. Increasing sedentary behavior.
4.1.1.1. evidence from acute and multiday studies.
Experimental findings show that in healthy adults as little as 1 day of exposure to SB (∼17 h/day of SB) combined with energy surplus reduced whole body insulin sensitivity (−39%) but did not change fasting glucose and insulin concentrations, as compared to a minimal sitting condition (∼6 h of sitting). Importantly, reducing energy intake to match energy demand during prolonged sitting significantly attenuated, but did not fully mitigate, the decline in insulin action (−18%) (34). This finding indicates that excessive sitting might be detrimental to insulin sensitivity irrespective of energy balance. Seven days of exposure to a highly sedentary condition (increased sedentary time and limited participation in PA of any intensity) did not alter fasting glucose and insulin concentrations in healthy lean adults (155). Increasing SB significantly increased 2-h postload insulin concentration (38.8 uIU·mL−1) and reduced insulin sensitivity (−17.2%), as assessed by a composite insulin sensitivity index. Changes in time spent in prolonged sedentary bouts (>30 and 60 continuous min), but not in LPA and MVPA, were positively associated with 2-h postload insulin concentrations (155).
In a crossover randomized trial, healthy adults performed 10 days of reduced steps (from 12,154 to 4,275 steps/day, with a 10% increase in SB) while consuming a control diet (16% protein, 64% carbohydrate, 20% fat; 80% of daily energy need) or a high-protein diet (30% protein, 50% carbohydrate, 20% fat; 80% of daily energy need) (170). Independent of diet, there were no changes in fasting glucose and insulin concentrations and postload glucose and insulin responses in healthy adults following step reduction. However, in another study, 10 days of step reduction resulted in reduced insulin sensitivity along with increases in carbohydrate oxidation measured in response to an oral glucose tolerance text (84). Combining step reduction with overfeeding also increased fasting glucose and insulin concentrations, postload 2-h glucose concentrations, and postload glucose responses in healthy male adults (170), thus suggesting that energy surplus exacerbates the metabolic deteriorations triggered by exposures to both SB and physical inactivity.
4.1.1.2. evidence from longer term studies.
Fourteen days of reduced step count (from 10,501 to 1,344 steps/day) resulted in rapid decreases in whole body (∼58%) and peripheral insulin sensitivity (∼17%) in healthy male adults (118, 119), which was accompanied by a significant reduction in insulin-stimulated pAktthr308/total Akt protein expression (118). Similar alterations have been observed in healthy adults with/without a first-degree relative with type 2 diabetes (122) and older adults (140, 171). Reducing daily step count (3 days, from 12,956 to 4,319 steps/day) increased postprandial (30 to 90 min after a meal) glucose responses (6 to 9%) and glycemic variability (33 to 97%) in healthy adults as assessed by continuous glucose monitors, despite the absence of changes in postload glucose responses following an oral glucose tolerance test (127). Twenty days of reduced steps (from 14,000 to 3,000 steps/day) increased total glucose oxidation in healthy physically active male adults, which was associated with a significant decrease in nonprotein respiratory quotient during an oral glucose tolerance test (116), which indicates the development of metabolic inflexibility (i.e., the inability of the body to adjust substrate use to changes in substrate availability). While no changes have been reported for fasting glucose concentrations following step reduction protocols in healthy adults (116–118, 127), some studies demonstrated increases in fasting insulin concentrations (116, 117, 127). The absence of changes in glycemia may reflect compensatory increased insulin levels in response to reduced step count. Resuming habitual daily activities was sufficient to restore whole body insulin sensitivity to baseline levels in healthy adults and older adults (117, 122, 172).
4.1.1.2.1. Potential mechanisms.
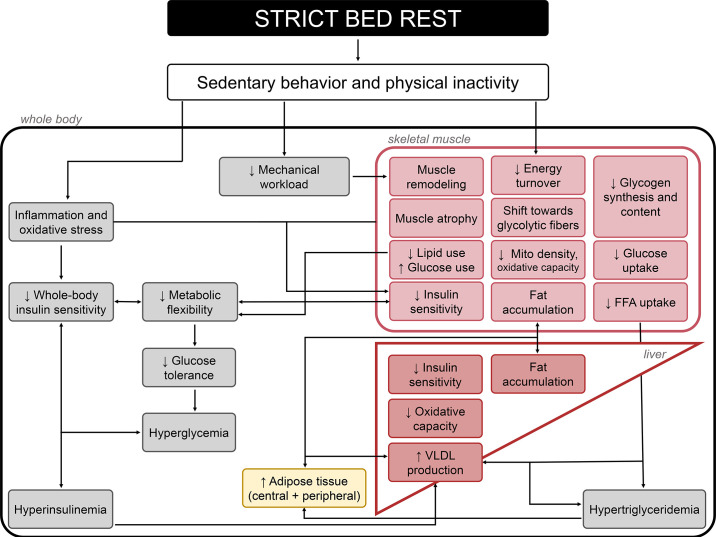
Short to longer term exposures (3 to 90 days) to horizontal bed rest and HDBR are associated with reduced whole body insulin sensitivity (173, 174), altered fuel selection toward the use of carbohydrates (173, 175), and metabolic inflexibility that precedes the development of glucose intolerance (175). Some mechanisms have been proposed including changes in body composition, body fat repartition, alterations in lipid content, oxidative stress, and capillary density (176). While some have been confirmed, controversial results have been obtained for others. For example, 60 days of HDBR induced fat accumulation in skeletal muscle (177) and with low-grade inflammation (178). However, Shur and colleagues (179) failed to demonstrate an increase in intramyocellular lipid content following 3 and 56 days of HDBR, which was not confounded by positive energy balance unlike previous studies (180, 181). Similarly, after 1 wk of bed rest, reduced insulin sensitivity was observed along with reduced oxidative capacity but not increases in muscle lipid level or degree of saturation, markers of oxidative stress, or reductions in capillary density, suggesting other mechanisms are likely at play (98). Pronounced transcriptomic changes of the skeletal muscle metabolic pathways have also been proposed. Shur and colleagues (179) reported extensive changes in mRNA abundance in gene targets controlling carbohydrate metabolism (40 transcripts) after only 3 days of HDBR. These changes preceded the modifications in whole body fuel selection and the reduction in muscle glycogen storage. Another study demonstrated similar alterations in the expression of genes associated with insulin resistance and fuel metabolism following 9 days of bed rest (182). Additionally, bed rest-induced reduction in insulin sensitivity has been accompanied by reduced skeletal muscle GLUT-4, hexokinase II, protein kinase B (Akt) 1, and Akt2 protein content and decreased insulin-stimulated glycogen synthase (GS) activity and Akt signaling (183). These findings suggest that both decreased glucose transport and decreased nonoxidative glucose metabolism in skeletal muscle contribute to changes in carbohydrate metabolism (FIGURE 3).
FIGURE 3.
Metabolic cascade induced by exposures to sustained periods of strict bed rest. This cascade ultimately results in a plethora of adverse events, including development of whole body insulin resistance, hyperglycemia, hyperinsulinemia, hypertriglyceridemia, metabolic inflexibility (i.e., the inability of the body to adjust substrate use to changes in substrate availability), and alterations on body composition (increased fat mass and muscle atrophy) (173). FFA, free-fatty acids; VLDL, very low-density lipoprotein. Image was created with mindthegraph.com, with permission.
4.1.1.3. clinical significance.
Excessive SB has been positively associated with increased 2-h postload glucose and fasting insulin concentrations in the general population, independent of participation in MVPA (184, 185). Experimental models imposing periods of increased sedentary time in both adults and older adults consistently induced alterations in whole body and peripheral insulin sensitivity (−17 to −58%) but not in fasting glucose concentrations. The clinical significance of these findings is unclear. However, the dramatic reduction in insulin action within days of increased prolonged SB is relevant given that SB is the most prevalent behavior (8.3 to 11.5 h/day) (4), and alterations in markers of glucose control are associated with greater risk for cardiovascular disease and events even in those without diabetes (186, 187).
4.1.2. Reducing and interrupting sedentary behavior.
4.1.2.1. evidence from acute and multiday studies.
Acutely, interrupting sitting with frequent, short bouts of LPA improved postprandial glucose responses by 17.5% and insulin responses by 25.1% compared to prolonged, uninterrupted sitting in both healthy and metabolically impaired adults and older adults, as evidenced by meta-analysis (86). These results have been corroborated by other meta-analyses that have included studies investigating other types (e.g., standing still, walking, and simple resistance activities) and intensities (e.g., light, moderate, and vigorous intensity) of interruptions to sitting (Cohen’s d for glucose and insulin: −0.26 to −0.83 favoring intervention) (87, 88, 188). However, it should be noted that some original studies have not demonstrated changes in postprandial glucose and/or insulin responses following at least one of the interruptions to SB protocols (standing, simple resistance activities, LPA to VPA walking) compared to prolonged sitting (25, 26, 133, 134, 189–202).
Reducing/interrupting sitting for 3 to 4 days reduced postprandial glucose (131, 203) and insulin responses (132–134, 203) in adults and older adults with overweight/obesity and type 2 diabetes (PA intensity and duration: 3.2 km/h, 2.8 METs, total: 34 min/day (131); perceived effort: 13 somewhat hard, total: 45 min/day (134); and 93–95 steps/min, total: 3.1–4.11 h/day of standing and 3.1–4.9 h/day of stepping (132, 133, 203). However, results have been more inconsistent for postprandial glucose responses following interruptions to sitting with standing/LPA (132, 133) and moderate PA (MPA) (134). No changes were observed in fasting glucose concentrations following 3 days of interrupting SB with standing or LPA (131–133, 203) and MPA (134), but reductions in fasting insulin concentration were reported in adults with overweight (−1.8 mIU/L) (132) and type 2 diabetes (−13 pmol/L) (203). Despite these inconsistencies, insulin sensitivity improved after 3 days of interrupting SB (12–29%) (132–134, 203). Interestingly, 3 days of interrupting prolonged sitting with regular 2-min bouts of light-intensity walking (pace: 3.2 km/h, 2.8 METs; perceived effort: 6–11 “fairly light”; total: 28 min) sustained, but did not further enhance, improvements in postprandial glucose (−4%; estimated average concentration: 103 mg/dL following interruptions vs. 107 mg/dL following prolonged sitting) and insulin responses (−12%; estimated average concentration: 262 pmol/L following interruptions vs. 297 pmol/L following prolonged sitting) observed on the first day of intervention (131).
Both light- and moderate-intensity bouts improved postprandial glucose and insulin responses, while standing interruptions did not significantly affect these responses (87, 188, 204, 205). A pooled analysis of three acute laboratory-based trials also showed that the estimated energy cost of interruptions to sitting was associated with lower postprandial glucose and insulin responses in a dose-dependent manner in sedentary adults with overweight or obesity. Specifically, light- and moderate-walking interruptions (3.2 and 5.8 km/h; 2.8 and ∼4.3 METs, respectively; 2-min bouts every 20 min, total: 28 min), but not standing still, significantly reduced postprandial responses of both markers compared to prolonged sitting (206). Yet, a meta-analyses (188) and a few original studies (207–209) suggested standing interruptions are effective at improving glucose responses.
As for frequency of the active interruptions, results have been inconsistent across studies. Interrupting prolonged sitting with 6-min bouts of simple resistance activities every 60 min (squatting, calf raises; total: 36 min) was more effective at decreasing postprandial glucose responses (−21%, estimated average concentration: 193 mg/dL following interruptions vs. 203 mg/dL following prolonged sitting) in adults and older adults with type 2 diabetes compared to a higher frequency of interruptions (i.e., 3-min bouts every 30 min, total: 39 min) and 8 h of prolonged sitting (193), for which no differences were observed. Additionally, less-frequent interruptions to sitting acutely improved glycemic control in the 4-h period following lunch, while more-frequent interruptions were likely more beneficial for nocturnal glucose control, as assessed by continuous glucose monitors over 22 h (27). No differences were noted for mean glucose and other markers of glucose variability between interruptions and prolonged sitting (27). Other studies also showed similar inconsistencies in glucose and insulin responses across different frequencies of interruptions to SB compared to prolonged sitting (26, 28, 195). In addition, Duran and colleagues (210) suggested higher frequency and higher duration of light-walking interruptions to sitting (3.2 km/h, 2.8 METs; 5 min every 30 min, total: 70 min) should be considered when targeting improvements in glycemic responses in healthy adults and older adults. This strategy significantly attenuated postprandial glucose responses as compared to sedentary control (total: 8 h) and conditions with lower frequency (every 60 min, total: 35 min) and lower duration (1 minute, total: ∼7 or 14 min) of interruptions to sitting, for which no changes were observed (210). To date, available evidence is not sufficient to draw clear-cut conclusions on the dose-response relationship between the frequency of the active bouts to interrupt prolonged sitting and postprandial glycemia and insulinemia, and further studies are needed.
Subgroup analyses in original studies showed that attenuations in the magnitude of reductions in postprandial glucose (132, 205, 211) and/or insulin (205) responses are more pronounced in females than in males following interruptions to sitting as compared to prolonged sitting, which was corroborated by meta-analytic evidence (88). Additionally, meta-regressions revealed that higher BMI is significantly associated with greater reductions in postprandial glucose and insulin responses following interruptions to sitting as compared to prolonged sitting (88). Pooled data from three randomized crossover trials showed that those with higher underlying levels of fasting insulin and insulin resistance may derive greater reductions in postprandial insulin responses from regularly interrupting prolonged sitting than their healthier counterparts. Similarly, those with poorer fasting glucose and β-cell function may derive greater reductions in postprandial glucose responses from performing walking interruptions to sitting (212). These findings have been corroborated by subgroup analyses in two meta-analyses indicating that improvements in glycemia were more prominent in metabolically impaired adults and older adults compared to healthy counterparts (86, 88). Moreover, McCarthy and colleagues (213) demonstrated that reductions in postprandial glucose responses were more pronounced in those with lower cardiorespiratory fitness (25th and 50th centiles) following light-intensity walking interruptions to sitting (3.0 km/h, 2.0 METs; 5-min bouts every 30 min, total: 1 h) (213). Altogether these findings show that females, those with higher BMI, higher insulin resistance, and lower cardiorespiratory fitness may derive greater benefit from interrupting sitting with respect to glucose metabolism.
Results have been mixed in children and adolescents. Interrupting sitting with 3-min bouts of moderate-intensity walking (heart rate at 80% of ventilatory threshold, total: 18 min) significantly reduced postprandial insulin responses (−21%; estimated average concentration: 92 mIU/dL following interruptions vs. 117 mIU/dL following prolonged sitting), but not glucose responses, in children with overweight/obesity compared to 3 h of prolonged sitting (214). Insulin sensitivity, as assessed by the Matsuda index, was also greater during the interruption protocol (17%) (214). In healthy-weight children, this same interruption protocol significantly reduced postprandial glucose (−7%; estimated average concentration: 105 mg/dL following interruptions vs. 112 mg/dL following prolonged sitting) and insulin (−32%; estimated average concentration: 30 uU/mL following interruptions vs. 45 uU/mL following prolonged sitting) responses compared to 3 h of prolonged sitting (215). Furthermore, in healthy children and adolescents, both light-intensity walking interruptions to sitting (30% V̇o2peak, 2-min every 20 min, total: 42 min) and interruptions combined with two 20-min bouts of moderate-intensity walking did not affect postprandial glucose and insulin responses compared to 8 h of prolonged sitting (216).
4.1.2.1.1. Reducing/interrupting SB versus continuous MVPA/exercise.
Acute (8 to 9 h) reductions in postprandial glucose responses were more pronounced following frequent light-walking interruptions to sitting (total: 30–42 min) compared to a continuous bout of activity (total: 30 min) in healthy and at-risk adults and older adults (217–219). However, other studies did not observe such differences between patterns of activity (220–223). Frequent moderate-intensity interruptions were more effective at improving postprandial glucose and insulin responses than a single, continuous bout of moderate-intensity activity when compared to prolonged sitting (Cohen’s d: −0.69 and −0.47 vs. −0.16 and −0.22, respectively), as evidenced by meta-analysis (204). Another meta-analysis demonstrated similar results for postprandial glucose responses, but no differences were observed for insulin responses (88). In multiday studies (2 to 4 days), reducing/interrupting SB was shown to be more effective at reducing fasting insulin, 24-h glucose responses, and duration of hyperglycemia episodes, but not other markers of glucose metabolism (e.g., fasting glucose), compared to continuous exercise in adults and older adults with type 2 diabetes (93 steps/min, total: 4.1 h/day of standing and 3.1 h/day of stepping vs. 50–60% Wmax, total: 1 h/day) (203). In healthy adults and adults with overweight/obesity, there were no differences between patterns of activity in markers of glucose metabolism (134, 224).
4.1.2.1.2. Potential mechanisms.
The effects of interrupting sedentary time on glucose control are likely related to a greater reliance upon carbohydrate oxidation as fuel. Acute studies (6 to 9 h) in healthy adults (218, 222) showed that the lower glucose response following light (25% V̇o2max, total: 3.5 h) and moderate (∼46% V̇o2max, total: 30 min) walking interruptions to sitting compared to prolonged sitting (218, 222) was associated greater total carbohydrate oxidation. De Jong and colleagues (134) also demonstrated in adults with overweight or obesity that hourly 5-min bouts of moderate-walking interruptions during sitting (perceived effort: 13 somewhat hard; total: 45 min/day) primarily rely on carbohydrate as fuel over 24 h after 4 days of intervention as compared to prolonged sitting and a time-matched continuous bout of brisk walking. This effect does not appear to be related to energy expenditure and balance but rather to increasing the frequency of muscle contractions spread across the day. However, these differences in substrate use were not accompanied by changes in fasting and postprandial glucose responses (134).
The skeletal muscle is the largest glucose-consuming organ of the body (225) and the largest lean tissue mass in adults without obesity (226). Lack of muscle contractions has been one of the proposed mechanisms for SB-related impairments in glucose metabolism. During periods of SB, skeletal muscle accounts for only 15% of whole body glucose (227), whereas it accounts for more than 80% of the insulin-stimulated glucose disposal and is quantitatively the most dominant tissue during exercise (225). Hamilton and colleagues (228) developed a physiological method of muscle contractile activity to magnify and sustain soleus oxidative metabolism (∼88% type I slow-twitch fibers) through performing “soleus push ups” (∼1.3 and 1.7 METs, 50 to 100 contractions/min). Sustained continuous soleus contractile activity improved systemic metabolic regulation, by reducing 3-h postload glucose and insulin responses (−39–52% and −41–60%, respectively) and 2-h postload glucose concentration (−29–46 mg/dL) in adults and older adults (BMI: 20–43 kg/m2). Sustained contractions also increased energy demand (91 kcal/h above sedentary control) and local carbohydrate oxidation (100–200 mg/min above sedentary control) (228). These findings indicate that increasing local contractile activity in small oxidative muscles can be a potent strategy for improving systemic metabolic regulation.
In the skeletal muscle, 5 h of frequent, walking interruptions during sitting (3.2 km/h, 2.8 METs; 2-min bouts every 20 min, total: 28 min) altered expression of 10 genes involved in carbohydrate metabolism, including increased gene expression of dynein light chain (DYNLL1), which may regulate translocation of the GLUT-4, and pyruvate dehydrogenase kinase 4 (PDK-4), which inhibits the pyruvate dehydrogenase complex and increases glucose utilization in adults with overweight/obesity (229). Five hours of interrupting prolonged sitting with light- and moderate-intensity walking (3.2 km/h, 2.8 METs and 5.8 km/h, ∼4.3 METs, respectively; 2-min bouts of walking every 20 min, total: 28 min) further resulted in an upregulation of the contraction-stimulated glucose uptake pathway [i.e., adenosine monophosphate-activated protein kinase (AMPK)-mediated], while 3 consecutive days of interrupting sitting resulted in a transition toward upregulation of the insulin-mediated glucose uptake pathway (i.e., Akt-mediated) along with greater capacity for glycogen synthesis (i.e., increase in total GSK3β protein expression) in the skeletal muscle of adults with overweight/obesity (230). In contrast, there were no differences in pAS160Thr642/AS160 ratio and GLUT4 protein expression in the skeletal muscle of postmenopausal females with rheumatoid arthritis following light-intensity walking interruptions to sitting (∼25% heart rate reserve, 3-min bouts every 30 min, total: 42 min) compared to 8 h of prolonged sitting (217). Finally, although acute exposure to moderate-intensity active interruptions in adults with overweight/obesity was associated with increased gene expression of complex V of the electron transport chain indicating a greater capacity for ATP production (230), 4 days of moderate-walking interruptions to sitting (perceived effort: 12–13 somewhat hard, 5-min bouts every hour, total: 45 min/day) did not elicit changes in mitochondrial respiration in presence of carbohydrates (231); of note this later measurement was performed in fasting state and >12 h after the last active interruption. Notably, pathways associated with muscle contraction transcription signaling, namely oxidative phosphorylation and sirtuin signaling expression, were enhanced in the skeletal muscle of these same participants when compared to a highly sedentary condition, as indicated by pathway enrichment analysis with RNA sequencing data (231).
In the subcutaneous abdominal adipose tissue, interrupting prolonged sitting with light-intensity walking (3.2 km/h, 2.8 METs; 2-min bouts of walking every 20 min, total: 28 min) led to a downregulation of pathways linked to carbohydrate oxidation and upregulation of pathways linked to lipid oxidation in adults with overweight/obesity as compared to prolonged sitting and moderate-intensity walking interruptions. In contrast, genes associated with glucose oxidation were upregulated in the moderate-intensity walking condition (5.8 km/h, ∼4.3 METs; 2-min bouts of every 20 min, total: 28 min) (232).
These studies provided the first insights into the muscle regulatory systems and molecular processes underlying the effects of interrupting prolonged sitting on glucose metabolism (FIGURE 4).
FIGURE 4.
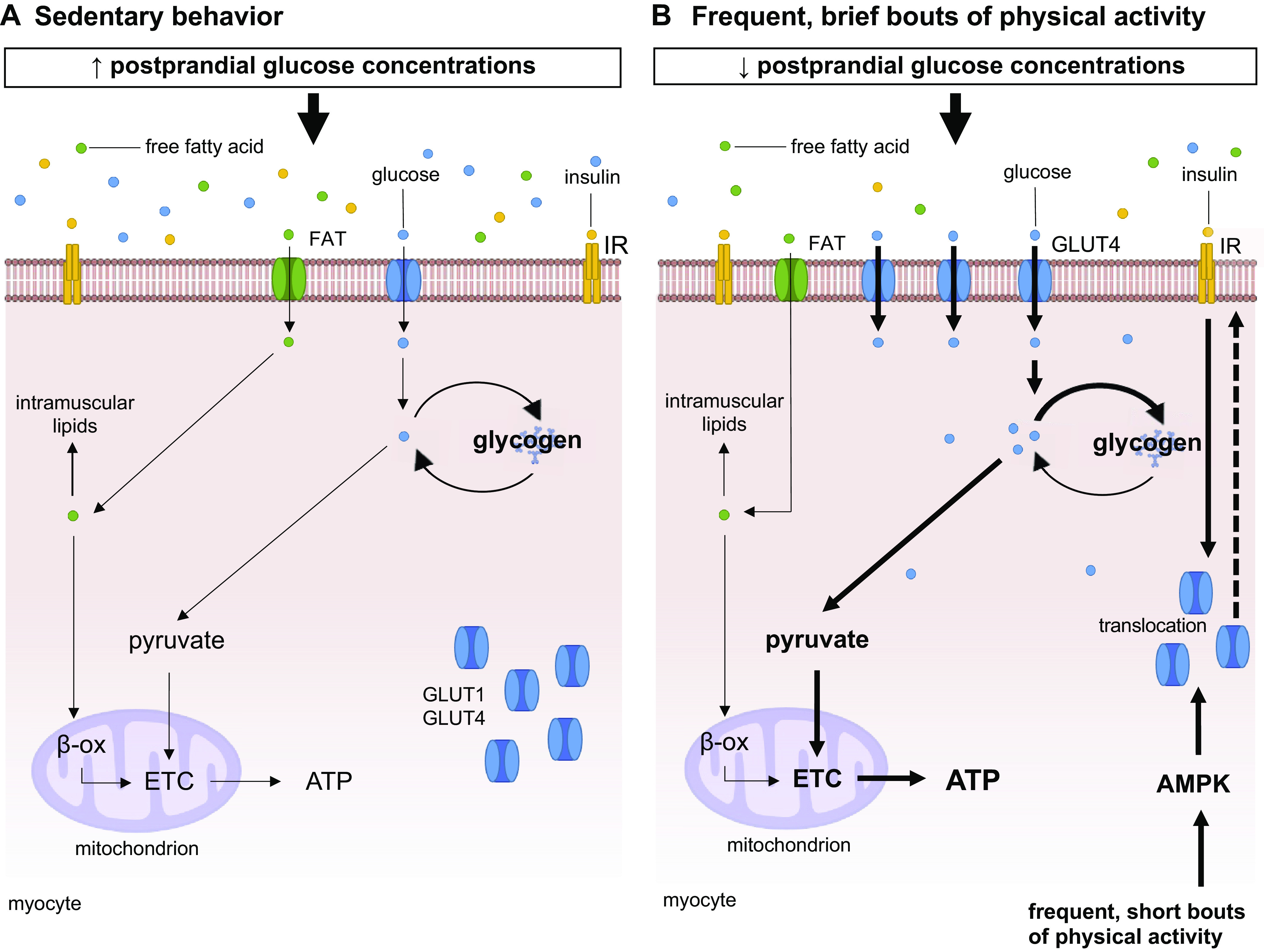
Mechanisms underlying sedentary behavior and interruptions to sitting-induced adaptations on carbohydrate metabolism in skeletal muscle. A: during rest (i.e., prolonged sitting), requirements for glucose and free fatty acids are minimal; therefore, delivery to skeletal muscle is low. Any glucose that does enter the myocyte is stored as glycogen or metabolized to ATP via glycolysis. Any free fatty acid is stored as intramuscular lipid or metabolized to ATP via β-oxidation. Acute exposures to prolonged sitting have been shown to subsequently increase postprandial glycemia (233). B: during short, interruptions to sitting with physical activity, muscle uses glycogen and the glucose available in the bloodstream as the main sources of glucose for the generation of ATP. Acutely, frequent muscle contractions increase AMPK levels in the myocytes, which results in the translocation of GLUT4 to the membrane facilitating glucose uptake. Interruptions to sitting also increase the capacity for ATP production and glycogen synthesis. With glucose constantly available and competing against lipids, free-fatty acid oxidation is not required to be increased for ATP production. Ultimately, interruptions over 3 days induce a transition to modulation of the insulin-dependent signaling pathway (230). Performing frequent, short bouts of physical activity has been shown to reduce postprandial glycemia (233). Differences in signaling pathways in B are relative to A, with bolded arrows representing pathways that are upregulated following interruptions to sitting. AMPK, adenosine monophosphate-activated protein kinase; ATP, adenosine triphosphate; β-oxi, β-oxidation; ETC, electron transport chain; FAT, fatty-acid transporter; GLUT1, glucose transporter type 1; GLUT4, glucose transporter type 4; IR, insulin receptor. Image was created with mindthegraph.com, with permission.
4.1.2.2. evidence from longer term studies.
A meta-analysis examined findings from longer term studies investigating the effects of SB interventions conducted in free-living settings on glucose/insulin outcomes. Intervention duration ranged between 2 wk and 36 mo, and the average change in total sedentary time was −28.6 min/day (30). There was a small significant decrease in fasting insulin concentration (−1.42 pM) but no changes in fasting glucose concentration and HbA1c (30). Another meta-analysis investigating the effects of SB interventions (range: 6 to 24 wk) on markers of glucose metabolism demonstrated significant decreases in total sedentary time (−64 min/day) and increases in walking time (27 min/day) but no significant changes in time spent standing and in MVPA (31). The authors noted a small significant decrease in HbA1c (−0.2%) but no change in fasting glucose concentration in individuals with overweight, obesity, type 2 diabetes, cardiovascular, neurological/cognitive, or musculoskeletal diseases (31). A meta-analysis of studies aiming to replace SB with standing time (mean follow-up: 3.8 mo) demonstrated a significant increase in total standing time (1.3 h/day) in adults along with significant decreases in fasting glucose (−2.53 mg/dL) and insulin (−2.30 mg/dL) concentrations (32). A systematic review of studies implementing workplace SB interventions in apparently healthy and overweight/obese desk-based office workers found that the effects of workplace interventions on markers of glucose metabolism have been inconsistent across studies. Only 6 out of 15 studies reported improvements in at least one glucose metabolism-related outcomes, while most studies showed no changes (144). Furthermore, 3 wk of frequent active interruptions to sitting (increase of 744 steps/day from baseline, with no changes reported for SB) reduced mean fasting glucose levels (−0.34 mmol/L) and glucose variability (−2%) in highly sedentary adults with obesity but did not improve glucose tolerance, insulin sensitivity, postload glucose responses, and average interstitial glucose concentrations (234).
Altogether, it becomes evident that results on the effects of reducing/interrupting SB on markers of glucose metabolism are mostly inconsistent across studies and meta-analyses. It is not clear whether type, intensity, and frequency of interruptions to sitting, characteristics of population groups, and/or duration of intervention affect responses to reducing/interrupting SB interventions.
4.1.2.2.1. Reducing/interrupting SB versus continuous MVPA/exercise.
A small-scale, 12-wk, multifactorial, pilot-study examined both the independent and combined effects of exercise training (40–65% hear rate reserve, i.e., moderate to vigorous intensity) and reducing SB (replace SB with standing and LPA plus increasing daily step count by 5–10%) on markers of insulin action in adults with overweight/obesity (148). Exercise training combined with reducing SB (−7.3% of daily hours in SB and +45 min/day increase in MVPA) was the most effective intervention to improve markers of insulin action. This approach improved insulin sensitivity (17.8%), as assessed by a composite insulin-sensitivity index, and decreased 2-h insulin concentration (−30.5 uIU·mL−1) and postload glucose responses (−19.4%). Reducing SB alone (−4.8% of daily hours in SB, +28 min/day in MVPA) reduced fasting insulin concentrations (−3.6 uIU·mL−1) but, surprisingly, concomitantly increased 2-h glucose (+26.5 mg/dL). In contrast, exercise training alone (no change in SB + 27 min/day increase in MVPA) did not improve any marker of insulin action. In addition, exercise and SB interventions, when performed independently, did not affect fasting and postload glycemia (148). Similarly, 4 wk of interventions aiming at reducing SB (−53 min/day in SB with no change in MVPA) or increasing MVPA to at least 30 min/day (+16 min/day of MVPA with no change in SB) did not change fasting glucose and HbA1c concentrations in physically inactive adults with obesity (150).
4.1.2.3. clinical significance.
There is some evidence of small improvements on markers of glucose control [fasting insulin (−1.42 pM), glucose (−2.53 mg/dL), and HbA1c (−0.2%) concentrations] associated with reducing/interrupting SB. However, mixed results have also been reported across original studies and meta-analyses. It is important to highlight that mean baseline levels of fasting glucose, insulin, and HbA1c were mostly within normal ranges in available meta-analyses (30–32), which may partly explain the small magnitude of reported changes following SB interventions. In fact, these small changes are comparable to the findings that have been observed with continuous exercise in people without glycemic dysfunction (235).
The most commonly used marker of glucose control in clinical practice is HbA1c. A reduction of 0.5 to 1.0% is generally used as a cut point for clinically meaningful change, which associates with significant reductions in risk of all-cause mortality, myocardial infarction, stroke, and heart failure in patients with type 2 diabetes (236). One meta-analysis of SB interventions demonstrated a 0.2% reduction in HbA1c (mean baseline level was 6.4%) after 6 to 24 wk of follow-up (31), which is not clinically meaningful. Interestingly, a 3-yr intervention (1 mo of exercise counseling every year; SB: −0.8 h/day, LPA: 0.7 h/day, and MVPA: 3 min/day) significantly reduced HbA1c values (−0.5%) in those with type 2 diabetes compared to standard care (i.e., general physician recommendations for increasing daily PA and decreasing SB) (237). The highest quartile for changes in SB (−1.53 h/day and +10.5 min/day of MVPA) significantly reduced HbA1c values (−0.85%) as compared to other quartiles, with SB being an independent predictor of changes in HbA1c (238). Another randomized controlled trial aiming at increasing participation in standing and LPA (SB: −39 min/day, standing: 39 min/day) performed a subgroup analysis in the participants who had high fasting glucose concentrations at baseline and found effects sizes to be larger for fasting glucose and HbA1c (−7.2 mg/dL and −0.3%, respectively) (239). Altogether, these findings indicate that reducing/interrupting SB has the potential to improve glucose control in those with dysglycemia and type 2 diabetes.
4.2. Lipid Metabolism
4.2.1. Increasing sedentary behavior.
4.2.1.1. evidence from acute and multiday studies.
Four days of exposure to a highly sedentary regime (14 h sitting/day) did not alter fasting blood lipid concentrations (i.e., total cholesterol, HDL, LDL, and triglycerides) in healthy sedentary adults (133). Similar results were observed in those with overweight and obesity (132) and type 2 diabetes (203). Seven days of exposure to a highly sedentary condition (increased sedentary time and limited participation in PA of any intensity) did not alter fasting blood lipids concentrations in healthy lean adults (155).
4.2.1.2. evidence from longer term studies.
Fourteen days of reduced step count (from 10,501 to 1,344 steps/day) led to increased triglyceride responses (∼21%) to an oral fat tolerance test in healthy male adults (119). A 14-day period of reduced steps (by 81%, and a 3.7 h/day increase in sedentary time) significantly increased fasting total cholesterol, LDL, and triglycerides (by 0.5, 0.3, and 0.5 mmol/L, respectively) in healthy adults with/without a first-degree relative diagnosed with type 2 diabetes. These changes returned to baseline levels after resuming habitual PA for 14 days (122). Twenty days of reduced steps (from 14,000 to 3,000 steps/day) not only worsened fasting HDL concentration but decreased total lipid oxidation and increased fasting triglycerides concentration (0.3 mmol/L) along with de novo lipogenesis in healthy physically active male adults (116). Periods of reduced step count (14 days; from 9,008 to 2,994 steps/day) also resulted in increased serum and intramuscular ceramides in healthy older adults (140). Other studies demonstrated deleterious effects of reduced steps on blood lipids in healthy male adults (138, 240); however, results have also been inconsistent, with some studies demonstrating no effects (117, 118, 241).
4.2.1.2.1. Potential mechanisms.
Results from bed rest studies have been inconsistent for blood lipids concentrations in healthy adults and older adults, with some reporting hypertriglyceridemia and hypercholesterolemia and others no effect (29, 173, 242). Bed rest also impairs lipid oxidation in both fasting and postprandial states, which was observed to be independent of detectable changes in energy balance (173) (FIGURE 3). This reduced fatty acid oxidation does not seem to be due to an impaired trafficking toward peripheral tissue but rather to structural, metabolic, and molecular changes in the skeletal muscle (177). For example, physical inactivity coupled with sedentariness has been shown to reduce the content of slow oxidative muscle fibers (243–245), reduce mitochondrial density and oxidative capacity (246), and decrease the expression of genes involved in mitochondrial function (182). Gene expression and activity of enzymes coupled with oxidative metabolism, such as skeletal muscle LPL, fatty acid transporter into the myocyte (CD36), and into the mitochondria [carnitine palmitoyltransferase I (CPT1b)] (137, 177), are also decreased (177, 182, 247). These changes are particularly relevant following meal ingestion since they lead to decreased clearance of dietary lipids, which can contribute to hyperlipidemia. In this line, decreases in the oxidation of dietary saturated, but not monounsaturated, fatty acids were observed (137, 177, 248). The changes in the oxidative rate of dietary saturated fatty acid have been associated with lower clearance and uptake by peripheral tissues and decrease in the gene expression of CD36, fatty acid binding protein (FABPpm), CPT1, and acyl-CoA synthetase long-chain family member 1 (ACSL1). Despite a reduction in adipose tissue lipolysis (249, 250), excess of plasma lipids has been shown to enhance fat accumulation in the visceral adipose depot (251) and ectopic fat storage in muscle, liver, and bone (177, 252–254). Fat accumulation is known to impair the function of the tissues. In line with this, a recent study in nonhuman primates (macaques) showed that 42 days of HDBR altered transcriptome signatures with upregulation of genes in lipid metabolisms in liver samples, revealing mildly disturbed fatty acid metabolism (255). In humans, indexes of fatty liver such as high levels of plasma transaminases (252) have been associated with increased de novo lipogenesis (116), an index of hepatic insulin resistance.
4.2.1.3. clinical significance.
Excessive SB has been positively associated with increased fasting triglyceride concentrations and decreased fasting HDL concentrations in the general population, independent of participation in MVPA (256–258). Some detrimental changes in blood lipids have been reported in reduced step count studies in both adults and older adults, but results have been inconsistent. The clinical significance of these findings is unclear.
4.2.2. Reducing and interrupting sedentary behavior.
4.2.2.1. evidence from acute and multiday studies.
Findings from single and multiday experimental studies examining the effects of interrupting prolonged sitting on fasting (132, 133, 155, 203) and postprandial lipid concentrations (86–88) have been less consistent than those observed for glucose and insulin responses. Some original studies failed to show improvements in triglycerides following at least one of the interruptions to SB compared to prolonged sitting (131, 134, 193, 195, 197, 198, 207, 211, 217, 218, 259). Two meta-analyses (86, 87) indicated no effect of standing and light- and moderate-intensity activity interruptions to sitting on postprandial triglycerides responses in healthy and metabolically impaired adults and older adults compared to prolonged, uninterrupted sitting. In contrast, one meta-analysis (88) indicated a small significant effect (Cohen’s d: −0.26 favoring intervention) of interruptions to sitting on postprandial triglycerides in healthy and metabolically impaired adults and older adults compared to prolonged, uninterrupted sitting. This was mainly driven by the evidence from multiday studies, which may suggest an additive effect of interruptions to sitting on blood lipids (88).
The magnitude of changes in triglyceride responses following interruptions to sitting has been reported to be independent of the intensity of PA interruptions (standing vs. light- vs. moderate-intensity interruptions), as indicated by subgroup analysis (87). For frequency of interruptions to sitting, there were no differences in postprandial triglycerides responses following light-intensity walking interruptions to sitting every 20, 60, and 120 min (30% V̇o2max, total: 48 min) in male adults with central obesity (195). Similarly, there were no differences between low- and high-frequency simple resistance activities interruptions to sitting (squatting, calf raises; total: 36 min; or moderate-intensity walking, i.e., 65% V̇o2peak; total: 30 min) on postprandial triglycerides responses in adults and older adults with type 2 diabetes (193) and in healthy female adults (26). It is unclear whether the type of interruptions to sitting differentially affect markers of lipid metabolism.
The magnitude of changes in triglyceride responses following interruptions to sitting was not affected by meal composition, BMI, and population characteristics, as indicated by subgroup analysis and meta-regressions (87, 88). Interestingly, fasting triglycerides had a negative quadratic association with postprandial triglyceride responses to a single, moderate-walking bout followed by intermittent light-walking interruptions compared to prolonged sitting in healthy older adults. This may imply that those with high triglyceride levels were more resistant to intervention-induced reductions in triglyceride responses (260).
In children with normal weight or overweight/obesity, interrupting sitting with 3-min bouts of moderate-intensity walking (heart rate at 80% of ventilatory threshold, total: 18 min) did not affect postprandial triglycerides and free fatty acids responses compared to 3 h of prolonged sitting (214, 215). Similarly, light-intensity walking interruptions to sitting (30% V̇o2peak, 2-min every 20 min, total: 42 min) did not affect postprandial triglyceride, HDL, and LDL responses in healthy children and adolescents compared to 8 h of prolonged sitting (216).
Regarding other markers of lipid metabolism, acute and multiday studies (6 h to up to 4 days) indicate no changes in postprandial concentrations of free fatty acids (134, 219, 221, 222, 224, 261), 3-hydroxybutyrate, apolipoproteins C-II and C-III (261), apolipoproteins B-48 and B-100, total ketone bodies, and acetoacetic acid (219) following interruptions to sitting (standing and moderate-intensity walking) in healthy adults, adults with overweight/obesity and postmenopausal females.
4.2.2.1.1. Reducing/interrupting SB versus continuous MVPA/exercise.
Acutely (7 to 9 h), a continuous bout of PA (moderate intensity, total: 30 min) in the morning was more effective at attenuating postprandial triglyceride responses than frequent standing (45-min interruptions every 15 min, total: 4.5 h) (261), moderate walking (46% V̇o2max, 1.6-min interruptions every 28 min, total: 30 min) (218), and vigorous cycling (70% V̇o2max, 6-min interruptions every 40 min, total: 30 min) (262) interruptions to sitting in healthy adults. In contrast, other studies did not report such differences between patterns of activity (217, 219, 220, 222) or showed a superiority of interruptions to sitting at decreasing postprandial triglyceride responses in healthy and at-risk adults and older adults (221, 223). A meta-analysis summarizing acute evidence demonstrated no differences between interruptions to sitting and a continuous bout of activity on postprandial triglycerides responses (88), but activity protocols were not always matched for duration and energy expenditure. In multiday studies (2 to 4 days), reducing/interrupting SB was shown to be as effective as continuous exercise in reducing fasting triglyceride concentrations in adults and older adults with type 2 diabetes (203). In healthy adults and adults with overweight and obesity, there were no differences between interruptions to sitting and continuous activity in postprandial triglycerides and free fatty acids responses (134, 224).
4.2.2.1.2. Potential mechanisms.
As expected, a single continuous bout of PA leads to increases in total fat oxidation compared to a sedentary control, while greater reliance on carbohydrates was shown following interruptions to sitting in healthy adults and adults with overweight/obesity (134, 218). Peddie and colleagues (218) observed a significant reduction in postprandial triglyceride responses following a single moderate-intensity walking bout (∼46% V̇o2max, total: 30 min) compared to prolonged sitting and interruptions to sitting (218, 222). Despite changes in substrate use, De Jong and colleagues (134) failed to detect reductions in fasting and postprandial triglycerides responses following a continuous bout of activity (perceived effort: 13 somewhat hard; total: 45 min/day). It is likely that glycogen storage was partly depleted when performing continuous PA, thus allowing lipids to be oxidized for energy expenditure and glycogen pools to be refilled, which likely did not occur during brief interruptions to sitting as glucose was constantly available and competing against lipids. Additionally, postprandial responses of markers for hepatic fatty acid oxidation (total ketone bodies and acetoacetic acid) were elevated following continuous exercise but not interruptions to sitting. This indicates increased hepatic fatty acid oxidation and reduced availability of triglycerides for incorporation into VLDL with exercise (219).
It is difficult to explain the inconsistencies in the acute effects of reducing/interrupting SB on postprandial triglyceride responses. Based on findings from animal models, it has been hypothesized that the lack of muscle contraction during exposures to increased SB reduces skeletal muscle LPL mass and activity and alters lipid metabolism (8, 55). Of note, these defects were reversible only with light-intensity contractile activity but not MVPA/exercise (9, 55). No change was observed in serum inactive monomeric LPL protein concentrations following standing interruptions to sitting in healthy males (261) and in plasma preheparin LPL concentrations following walking interruptions to sitting in postmenopausal females (219). Similarly, no changes in LPL gene expression were reported following frequent light- to moderate-intensity walking interruptions to sitting (over 5 to 8 h, 2–3-min bouts every 20–30 min) in the skeletal muscle of adults with overweight/obesity (229) and postmenopausal females with rheumatoid arthritis (217). One possibility is that the pattern and frequency of activity used in published studies were insufficient to elicit changes in LPL enzymatic activity.
4.2.2.2. evidence from longer term studies.
A meta-analysis examined data from longer term studies of the effects of SB interventions conducted in free-living settings on blood-lipid outcomes. Intervention duration ranged between 2 wk and 36 mo, and the average change in total sedentary time was −28.6 min/day (30). There was a small significant increase in HDL (0.04 mM) but no change in total cholesterol, LDL, and triglycerides in response to interrupting/reducing SB interventions (30). Another meta-analysis investigating the effects of SB interventions (range: 6 to 24 wk) showed that lipid profile was not ameliorated despite significant decreases in total sedentary time (−64 min/day) and increases in walking time (27 min/day) in individuals with overweight, obesity, type 2 diabetes, cardiovascular, neurological/cognitive, or musculoskeletal diseases (31). A meta-analysis of studies aiming at replacing SB with standing time (mean follow-up: 3.8 mo) showed that significant increases in total standing time (1.3 h/day) in adults had no effect on lipid profile (32). Another systematic review of studies also concluded that SB interventions did not improve blood lipids in most published studies (13 out of 18 studies) (144). Furthermore, skeletal muscle lipidome was largely unaffected after 3 wk of an intervention consisting of frequent active interruptions to sitting (increase of 744 steps/day from baseline, no change in SB) in adults with central obesity (234).
Taken these data together, it is evident that results on the effects of reducing/interrupting SB on markers of lipid metabolism are inconsistent across studies and meta-analysis. It is not clear whether type, intensity, and frequency of interruptions to sitting, characteristics of population groups, and/or duration of intervention influence the responses to reducing/interrupting SB interventions.
4.2.2.2.1. Reducing/interrupting SB versus continuous MVPA/exercise.
A small-scale, 12-wk, multifactorial, pilot-study examined both the independent and the combined effects of exercise training (40–65% hear rate reserve, i.e., moderate to vigorous intensity) and reducing SB (replace SB with standing and LPA plus increasing daily step count by 5–10%) on blood lipids in adults with overweight/obesity (148). Reducing SB with nonexercise PA (−4.8% of daily hours in SB, +28 min/day in MVPA) and exercise training combined with reducing SB (−7.3% of daily hours in SB + 45 min/day increase in MVPA) did not change fasting total cholesterol, HDL, and triglycerides concentrations. In contrast, exercise training only (no change in SB, + 27 min/day increase in MVPA) significantly reduced triglyceride concentrations (−0.4 mmol·L−1) (148). Four wk of intervention aiming at reducing SB (–53 min/day in SB with no change in MVPA) or increasing MVPA to at least 30 min/day (+16 min/day of MVPA with no change in SB) did not improve HDL, LDL, and triglyceride concentrations in physically inactive adults with obesity (150).
4.2.2.3. clinical significance.
There is some evidence of small improvements, likely not clinically meaningful, on fasting HDL (0.04 mM) concentrations associated with reducing/interrupting SB. However, no consistent improvements have been reported for other blood lipids. It is important to highlight that those studies within available meta-analyses reported mean baseline values for all fasting blood lipids within normal ranges (30, 31), which may contribute to the small magnitude of changes reported following SB interventions. Putting these results into perspective with the effects of continuous exercise, the small benefit for HDL is comparable to the increases in HDL associated with supervised aerobic exercise in adults and older adults with type 2 diabetes (263). A meta-analysis also demonstrated continuous aerobic exercise decreased fasting triglyceride concentrations (−6.8 mg/dL) but not total cholesterol, LDL, and HDL, in adults and older adults with overweight/obesity (264). The current evidence indicates that reducing/interrupting SB has marginal effects on fasting blood lipids. Longer term investigations into the effects of reducing/interrupting SB on blood lipids are warranted, particularly in individuals with dyslipidemia.
4.3. Protein Metabolism
4.3.1. Increasing sedentary behavior.
4.3.1.1. evidence from longer term studies.
Most evidence related to the effects of changing sedentary and physically active behaviors on protein metabolism has focused on skeletal muscle. To further understand how sedentary-induced hypokinesia may affect protein metabolism in skeletal muscle, it is important to acknowledge that muscle mass is regulated by an intricate and coordinated balance between daily fluctuations in muscle protein synthesis and breakdown (i.e., muscle protein balance) (265, 266). While breakdown is considered somewhat stable in nonpathological conditions, muscle protein synthesis is modulated by anabolic stimuli such as PA and nutrition (265, 266). This indicates that SB-related mechanical unloading of muscles may lead to muscle wasting (267, 268).
Step reduction (∼80% reduction from baseline) for 14 days resulted in a significant decrease in postprandial myofibrillar protein synthetic rate (−25 to −50%) in older adults (115, 120). This also seems to be the case in clinical populations, in which reducing step count (7,362 to 991 steps/day) significantly reduced measures of the 14-day integrated rates of muscle protein synthesis (approximately −12%) in older adults with overweight and prediabetes, which remained lower after resuming habitual activity for 14 days (171). Using a unilateral leg model, Devries and colleagues (115) demonstrated that the step reduction-induced decrease in postprandial myofibrillar protein synthetic rate can be fully mitigated in older adults by performing unilateral resistance exercise training (3 times/wk) while performing step reduction. A study employing 14 days of unilateral immobilization also showed reduced postabsorptive muscle protein synthesis (−27%) in healthy adults (103).
4.3.1.1.1. Potential mechanisms.
Bed rest studies indicate the loss of body protein with physical inactivity/SB is predominantly due to a decrease in muscle protein synthesis (269). This latter was associated with reductions in lean mass (−1.7%) following 14 days of HDBR (269).
4.3.1.2. clinical significance.
The pronounced reductions in muscle protein synthesis (−12 to 50%) following periods of increased SB are likely relevant. The physiological and clinical impact of increasing SB on skeletal muscle will be further discussed in sect. 6.1.
4.3.2. Reducing and interrupting sedentary behavior.
4.3.2.1. evidence from acute studies.
Acutely, myofibrillar protein synthesis was greater following light walking (1.9 km/h, <2.8 METs; 2 min of walking every 30 min, total: 30 min) and squatting interruptions to sitting (15 body-weight squats every 30 min, total: 225 repetitions) as compared to 7.5 h of prolonged sitting in healthy adults (∼47% and ∼20%, respectively) (270). The increases in myofibrillar protein synthesis are comparable to those observed following an acute bout of structured resistance exercise (∼40%) in healthy male adults (271). Additionally, rpS6Ser240/244 phosphorylation was greater in squatting interruptions compared to prolonged sitting, but no differences were observed for other anabolic signaling protein targets (4E-BP1Thr37/46, eEF2Thr56, mTORSer2448, and ERK1/2Thr202/Tyr204) (270). This could suggest that the squat interruptions resulted in the stimulation of translation initiation and myofibrillar protein synthesis. In contrast, walking had no effect on any anabolic signaling protein targets, which may be related to lower activation of the vastus lateralis during walking compared to squatting (272). It is not clear whether type, intensity, and frequency of interruptions to sitting differentially affect markers of protein metabolism.
As for whole body nutrient oxidation, De Jong and colleagues (134) showed that frequent 5-min bouts of brisk walking at every hour (total: 45 min/day) increased protein oxidation (11.4%) during the sleeping period (8 h) as compared to the sedentary condition (67% of waking h) in people with overweight/obesity. The authors suggest that the greater disappearance in protein may reflect the use of protein for gluconeogenesis to replenish muscle glycogen, as the short bouts of activity likely triggered the use and replenishment of glycogen stores, thus enhancing glycogen turnover (134).
It has been proposed that muscle hypertrophy following resistance exercise occurs as a result of summed periods of repeated acute exercise-induced positive protein balance where muscle protein synthesis exceeds muscle protein breakdown (273). As such, the practical relevance of acute increases in myofibrillar protein synthesis (20–47%) seen following aerobic and resistance activity interruptions to sitting and the extension of skeletal muscle remodeling should be addressed in future longer term studies.
5. CARDIOVASCULAR AND RESPIRATORY SYSTEMS
5.1. Hemodynamics
5.1.1. Increasing sedentary behavior.
5.1.1.1. evidence from acute studies.
A seated posture creates bends in major blood vessels, such as the femoral and popliteal arteries, which may result in turbulent blood flow patterns (274, 275). Also, such posture not only results in diminished skeletal muscle contractile activity that aids in venous return via the muscle pump but also detrimentally affects blood flow and vascular shear stress (physiological stressors that may underlie the health benefits of PA on the endothelium) (37). In healthy adults, the shear rate in the lower limbs, but not in the upper limbs, is reduced after only 30 min of uninterrupted sitting (24, 276). After ∼2 h, blood pools in the calf and whole blood leg viscosity are also reduced (277). After 3 h, blood flow in lower limbs is decreased in parallel with a further reduction in shear rate, as evidenced by meta-analysis (24). Although most of the evidence suggests that prolonged sitting detrimentally affects peripheral hemodynamics (278), some studies have demonstrated no alterations in blood flow (279) and retrograde shear rate (280, 281) in healthy males following 3–5 h of uninterrupted sitting.
A meta-analysis reported that exposures to prolonged, uninterrupted sitting resulted in significant increases in systolic blood pressure (3.2 mmHg) and mean arterial pressure (3.3 mmHg) among adults and older adults, but diastolic blood pressure was unaffected (129). In contrast, another meta-analysis demonstrated no change in mean arterial pressure following exposures to prolonged sitting >3 h (24). Some studies also failed to observe sitting-induced alterations in systolic (197, 282–285), diastolic pressure (201, 282, 286–288), and mean arterial pressure (285, 289–292). In addition, 7 days of reduced step count (∼9,000 to ∼6,000 steps/day) did not modify systolic and diastolic blood pressure and mean arterial pressure in healthy active adults (293).
Five days of reduced steps (from ∼12,000 to 4,000 steps/day) did not affect femoral or brachial artery blood flow responses (114) nor did it alter shear rate in healthy active male adults (113).
Changes in catecholamines have been inconsistent across studies; some indicated no changes and others small increases in plasma/serum concentrations in adults and older adults following 7–8 h of prolonged sitting (288, 294, 295). Increased lower leg and foot venous pressure/swelling was also observed after prolonged sitting, possibly impairing the regulation of capillary fluid filtration and edema formation in the feet (296).
5.1.1.1.1. Potential mechanisms.
Atherosclerotic plaques have been reported to commonly manifest near arterial bifurcations, which is at least partially due to an unfavorable local hemodynamic environment (297). The seated position may mimic a similar environment to arterial bifurcations due to the “bent artery” morphology created by 90° angles in the hip and knees. Acute evidence revealed that as little as 3 h of leg bending, similar to sitting, produces detrimental hemodynamic changes in the popliteal artery as compared with a straight limb (298). Therefore, the bent artery position induced by sitting may be a key contributor to changes in hemodynamics (278).
5.1.1.2. evidence from longer term studies.
Unilateral leg immobilization (12 days) did not induce changes in blood pressure, mean blood flow in carotid, femoral, and popliteal arteries in the immobilized leg versus the nonimmobilized leg in healthy adults (299). However, mean blood velocity (∼22%) and vessel shear rate (∼35%) in the femoral artery were increased in the immobilized leg (299). Hemodynamic adaptations to lower limb immobilization seem to be constrained to peripheral arteries, not altering large central arteries (299).
Fourteen days of step reduction (∼82% reduction from baseline combined or not with unilateral low-load resistance exercise training, 3 times/wk) did not alter superficial femoral artery blood flow during both fasted and fed state in healthy older males (115). Systolic blood pressure increased by 4 mmHg following a 14-day step reduction protocol (81% reduction from baseline; +3.7 h/day in sedentary time) in healthy adults with/without a first-degree relative with type 2 diabetes but returned to baseline after resuming habitual activity for 14 days (122). Diastolic blood pressure remained unchanged during step reduction and recovery periods (122).
5.1.1.3. clinical significance.
Acute exposures to prolonged sitting resulted in significant increases in systolic blood pressure (3.2 mmHg) and mean arterial pressure (3.3 mmHg) among adults and older adults (129). Similar increases (4 mmHg) were reported following 14 days of reduced step count in healthy adults with/without a first-degree relative with type 2 diabetes (122). While it is unclear whether these detrimental changes are sustained over time, the magnitude of these increases in blood pressure is likely to be clinically significant if maintained. For some perspective, at the population level, 1- to 10-mmHg increases in mean systolic blood pressure are associated with increases in cardiovascular disease incidence (300) and mortality (300, 301) and stroke mortality (302).
5.1.2. Reducing and interrupting sedentary behavior.
5.1.2.1. evidence from acute studies.
A meta-analysis of acute randomized crossover trials showed that interruptions to sitting (aerobic, simple resistance activities, and standing) reduced systolic blood pressure by 4.4 mmHg and diastolic blood pressure by 2.4 mmHg versus prolonged sitting (129). A meta-analysis demonstrated no change in systolic and diastolic blood pressure following standing interruptions to sitting compared to prolonged sitting in adults and older adults (188), suggesting interruptions at higher intensities may be necessary to affect blood pressure. However, mixed results have been reported by original studies. A systematic review summarized findings from acute randomized crossover studies investigating blood pressure responses to different intensities of interruptions to sitting in adults and older adults at risk for type 2 diabetes (303). Five out of ten studies found significant improvements in blood pressure responses following light-intensity interruptions, and three out of five studies demonstrated reductions following moderate-to-vigorous intensity interruptions (303). In contrast, only one out of six studies found reductions in blood pressure following standing interruptions to prolonged sitting (303), indicating that standing interruptions might be less effective at improving blood pressure responses.
Regarding the type of interruptions to sitting, the decreases in systolic blood pressure were mainly driven by studies implementing aerobic activity interruption strategies, as evidenced by a meta-analysis (129). In terms of frequency, interrupting prolonged sitting with 3-min or 6-min bouts of simple resistance activities every 30 or 60 min (squatting, calf raises; total: 36–39 min) did not elicit changes in blood pressure responses compared to 8 h of prolonged sitting in adults and older adults with type 2 diabetes (287). Similarly, there were no differences in blood pressure responses following high (2-min bouts every 20 min, total: 30 min) and low frequency (10-min bouts every 60 min, total: 50 min) of standing bouts compared to 6 h of prolonged sitting in older adults (201). In addition, Duran and colleagues (210) suggested light-walking interruptions to sitting (3.2 km/h, 2.8 METs) of high and low frequency (every 30 and 60 min, respectively) and high and low duration (5- and 1-min interruptions, respectively) can be considered when targeting improvements in blood pressure responses in adults and older adults. These strategies significantly attenuated systolic blood pressure responses (−3 to −5 mmHg) as compared to sedentary control (total: 8 h), and there were no differences between high/low frequencies and durations of interruptions to sitting protocols (210). Both high and low frequencies of single resistance activity interruptions (squatting, calf raises; every 30 vs. 60 min, total: 36–39 min) significantly increased blood flow (125 and 114%, respectively) and shear rate (87 and 98%, respectively) in adults and older adults with type 2 diabetes as compared to 8 h of prolonged sitting (287).
Reductions in systolic and diastolic blood pressure responses were more pronounced in older females than older males in response to a 30-min bout of exercise followed by frequent, brief light-intensity walking bouts as compared to 8 h of uninterrupted sitting (295). This finding suggests that females might benefit more from performing exercise plus interruption to sitting strategy.
In preadolescent children, interrupting sitting with light-, moderate- and high-intensity walking (25, 50, and 75% heart rate reserve, respectively, 2-min bouts every 20 min, total: 40 min) did not alter systolic and diastolic blood pressure responses compared to 8 h of prolonged sitting (304).
Simple resistance activity interruptions to sitting (squatting, calf raises; 3-min bouts every 30 min, total: 27 min) resulted in increased resting blood flow (∼43%) and shear rate (∼98%) in the femoral but not brachial artery, compared to 5 h uninterrupted sitting in adults with overweight and obesity (283). Interruptions to sitting with calisthenic exercises (squats, arm circles, calf raises; 2-min interruptions every 20 min, total: 6 min) increased shear rate in the brachial artery (∼16%) in healthy adults (291). Performing simple leg movements such as fidgeting was also sufficient to attenuate sitting-induced decreases in popliteal artery blood flow (−19% vs. −29%) and shear rate (−22% vs. −43%) in healthy adults (305). Finally, brief walking interruptions to sitting (3.2 and 5.8 km/h; 2.8 and ∼4.3 METs, respectively; 2-min bouts every 20 min, total: 28 min) may also play an important role in improving the concentration of hemostatic and/or procoagulant risk factors (e.g., fibrinogen, hematocrit, and hemoglobin) (306).
5.1.2.1.1. Reducing/interrupting SB versus continuous MVPA/exercise.
A 30-min bout of moderate-intensity walking (71% maximum heart rate), but not regular 2-min bouts of moderate- or vigorous-intensity walking (53 and 79% maximum heart rate, total: 42 and 16 min, respectively), significantly reduced ambulatory systolic blood pressure (−3 mmHg) in adults with overweight/obesity compared to 9 h of prolonged sitting (307). Similarly, a 30-min bout of moderate-intensity walking (55% heart rate reserve) reduced systolic blood pressure responses in the 4-h period after exercise in postmenopausal females with rheumatoid arthritis (47% participants had hypertension), which was not observed following 3-min boults of light-intensity walking every 30 min (24% heart rate reserve, total: 42 min) (217). Interestingly, resting systolic blood pressure was significantly lower in the morning after performing both a single bout and multiple 3-min bouts of brisk walking every 30 min (41–42% V̇o2max, total: 30 min) compared to a sedentary condition (−7 and −8 mmHg, respectively) in healthy male adults (308). Finally, a single 30-min bout of moderate-intensity walking (65–75% maximum heart rate) was effective at reducing systolic and diastolic blood pressure in older adults (−3 and −1 mmHg, respectively). Performing regular 3-min light-intensity walking interruptions every 30 min (3.2 km/h, 2.8 METs, total: 36 min) after exercising further reduced systolic blood pressure (−5 mmHg) but not diastolic blood pressure (295).
5.1.2.2. evidence from longer term studies.
A meta-analysis has summarized data from longer term studies investigating the effects of SB interventions conducted in free-living (−28.6 min/day of SB). Performing interruptions to sitting resulted in a small but significant reduction in systolic, but not diastolic, blood pressure (−1.1 mmHg) compared to control groups in both apparently healthy and clinical populations (30). Nonetheless, other meta-analyses of studies involving clinical population groups (31) and focused on reducing/interrupting SB with standing in adults and older adults (32) showed no change in blood pressure following intervention. A systematic review of workplace reducing/interrupting sitting interventions showed that only 5 out of 22 studies reported reductions in systolic blood pressure and 2 observed drops in diastolic blood pressure (144).
In children, an 8-mo, school-based intervention using height-adjustable desks in the classroom was ineffective at reducing classroom and total daily SB (145). Consequently, no significant changes were observed in systolic and diastolic blood pressure (145).
Regarding other hemodynamic parameters, the effect sizes for increases in shear rate in the femoral and brachial arteries following an 8-wk intervention to reduce/interrupt SB at work (−38 min/8-h workday of sedentary time and +35 min/8-h workday of standing time) were considered small (Cohen’s d: 0.31 and 0.23) (309). In a 16-wk nonrandomized trial, an intervention to reduce sitting (−60 min/day of sedentary, +36 min/day of standing, and +30 min/day of walking time) resulted in increased femoral artery antegrade shear rate (∼14%), but not in basal blood flow or retrograde shear rate, among those (≥55 years old) with increased cardiovascular risk (310).
In summary, the effects of reducing/interrupting SB on hemodynamics are inconsistent across studies and meta-analysis. It is not clear whether type, intensity, and frequency of interruptions to sitting, characteristics of population groups, and/or duration of intervention affect responses to reducing/interrupting SB interventions.
5.1.2.2.1. Reducing/interrupting SB versus continuous MVPA/exercise.
A small-scale, 12-wk, multifactorial, pilot-study examined both the independent and the combined effects of exercise training (40–65% heart rate reserve, i.e., moderate to vigorous intensity) and reducing SB (replace SB with standing and LPA plus increasing daily step count by 5–10%) on blood pressure in adults with overweight/obesity (148). Reducing SB with LPA (−4.8% of daily hours in SB, +28 min/day in MVPA) resulted in significant decreases in systolic and diastolic blood pressure (−4.7 and −4.0 mmHg, respectively). Interestingly, exercise training (no change in SB + 27 min/day increase in MVPA) and exercise training combined with reducing SB (−7.3% of daily hours in SB + 45 min/day increase in MVPA) significantly reduced systolic (−7.0 and –5.9 mmHg, respectively), but not diastolic, blood pressure. There were no between-group differences with respect to improvements in systolic blood pressure (148). In contrast, 4 wk of multiple interventions aimed at reducing SB (−53 min/day in SB with no change in MVPA) or increasing MVPA to at least 30 min/day (+16 min/day of MVPA with no change in SB) had no effect on blood pressure in physically inactive adults with obesity (150).
5.1.2.3. clinical significance.
Blood pressure is one of the most commonly used hemodynamic parameters in clinical practice. There is some evidence of small improvements in systolic blood pressure (−1.1 mmHg) associated with reducing/interrupting SB (30). Such benefits were not observed for diastolic blood pressure. It is important to highlight that the mean baseline blood pressure of the participants was typically within normal ranges in available meta-analyses (30, 31), which may reflect the small magnitude of reported changes following SB interventions. Importantly, these small changes in systolic blood pressure are comparable to those shown to be associated with continuous aerobic exercise and resistance exercise training in people with normal blood pressure (−0.6 to −0.8 mmHg) (311). In contrast, exercise training could be more effective at reducing diastolic blood pressure (−1.1 to −3.3 mmHg) (311). Despite the small effects of reducing/interrupting SB, even discrete reductions in blood pressure (e.g., 2 mmHg) are relevant at a population level, as they have been shown to be associated with significant reductions in the risk of all-cause coronary diseases and stroke mortality (12,000 lives saved per year) (312).
Evidence on the effects of reducing/interrupting SB on blood pressure in individuals with hypertension is still scarce. An analysis of pooled data from four acute randomized crossover trials indicated that blood pressure reductions were of greater magnitude in adults with overweight/obesity and hypertension (approximately −10 to −13 vs. −2 to −7 mmHg) following intermittent light-walking or simple resistance activity interruptions to sitting (3.2 km/h, 2.8 METs, or body-weight squatting and calf raises; 3-min bouts every 30 min, total: 36 min) compared to those without hypertension (37). Additionally, in a 12-mo randomized controlled trial, individuals who were randomized to the group STAND+ (sit-to-stand desk plus goal of ≥30 min of additional LPA) significantly reduced sitting time by −59.2 min/8-h workday compared to the group MOVE+ (goal of ≥30 min of additional LPA). These changes in physical behavior resulted in nonsignificant changes in cardiometabolic outcomes (239). A subgroup analysis in individuals with dysglycemia (baseline blood pressure: 135/83 mmHg) revealed larger reductions in systolic blood pressure (−6.6 mmHg) for participants randomized to STAND+ compared to MOVE+ (239). Altogether, these findings indicate that reducing/interrupting SB has the potential to improve blood pressure to a greater extent in those with hypertension.
5.2. Cardiovascular Function and Structure
5.2.1. Increasing sedentary behavior.
5.2.1.1. evidence from acute studies.
Prolonged sitting may induce endothelial dysfunction (i.e., the inability of the blood vessels to dilate appropriately) and oxidative stress (313, 314). Padilla and colleagues (276) observed that 3 h of uninterrupted sitting attenuated popliteal artery shear rate (∼75%) in healthy adults, but this reduction was not paralleled by a concomitant reduction in flow-mediated dilation (FMD). Conversely, Thosar and colleagues (280) reported a reduction in the superficial femoral artery FMD (∼2.5%FMD) following 3 h of uninterrupted sitting. Restaino and colleagues (315) also demonstrated that prolonged sitting for 6 h impairs lower limb FMD (∼5%FMD) but not upper limb. This is possibly because upper limb movement was not restricted in this study. These results are corroborated by meta-analytic findings showing that acute exposures to prolonged sitting result in reduced lower limb (∼2 to 5%FMD), but not upper limb, vascular function in healthy adults (24, 130). Notably, no significant reductions were observed for exposures that were shorter than 2 h of uninterrupted sitting (24). Additionally, some studies reported increases in artery stiffness, as measured by pulse wave velocity (PWV). Carotid-to-femoral PWV was increased in healthy adults following 3 h of prolonged sitting (292, 316), and carotid-to-ankle PWV has been also shown to increase in adults with overweight/obesity and elevated blood pressure following 10 h of prolonged sitting (317). However, these increases are rather marginal and likely not clinically relevant (318). Despite this evidence, some studies have not observed alterations in markers of macrovascular function [e.g., FMD (285, 287) and PWV (285, 317, 319)].
Some studies demonstrated that microvascular reactivity is blunted following 1.5- to 6-h exposures to prolonged sitting in both upper and lower extremities, as evidenced by reductions in peak blood flow and blood flow area under the curve (AUC) (286, 292, 315, 320–323). Studies also demonstrated impairments in tissue oxygenation index recovery rate during reactive hyperemia after ∼3 h of prolonged sitting (285, 319, 324). However, some studies demonstrated alteration in markers of microvascular function [e.g., shear rate (283) and blood flow AUC (323)].
Seven days of lower limb immobilization resulted in reductions in femoral artery base diameter (∼5%) and vascular conductance (∼23%) and increases in vascular resistance (∼35%) in healthy male adults, but all parameters returned to baseline levels after resuming habitual activity for 14 days (325).
Five days of reduced step count (∼12,000 to ∼3,500 steps/day) reduced popliteal artery (−3%FMD), but not brachial artery, FMD in healthy male adults (113). Conversely, base diameter was decreased in the brachial artery (∼5%, suggestive of inward vascular remodeling), but not in the popliteal artery (113). Additionally, CD31+/CD42b– endothelial microparticles concentration (a marker of endothelial apoptosis) increased significantly by ∼490% after 5 days of reduced activity (113).
5.2.1.1.1. Potential mechanisms.
The seated posture results in a greater amount of low and oscillatory shear rates in the lower extremities conduit arteries (276, 280, 281, 292, 315, 320). Both oscillatory and low shear stress can increase endothelial cell-derived reactive oxygen species (ROS) and downregulate endothelial nitric oxide synthase (eNOS) expression and production of nitric oxide (NO; a potent dilator released from endothelial cells) (326, 327). It has also been proposed that increased endothelin-1 concentrations (ET-1; a potent vasoconstrictor) may upregulate ROS and reduce total plasma nitrate and nitrite, markers of NO bioavailability (42). Increases in ET-1 concentrations, but not NO bioavailability, have been shown following exposures to prolonged sitting (283, 319). However, others did not report such increases in ET-1 concentrations (287, 316).
5.2.1.2. evidence from longer term studies.
Unilateral leg immobilization for 12 days reduced femoral and popliteal arteries’ mean diameter (∼7% and 14%, respectively) but did not change resting carotid artery diameter in healthy adults (299). Popliteal artery FMD increased in the immobilized leg (∼7%FMD) but not in the nonimmobilized leg (299).
5.2.1.3. clinical significance.
Acute exposures to prolonged sitting resulted in significant decreases in the lower limb (−2 to −5%FMD), but not the upper limb, in healthy adults (24, 130). Similar decreases in vascular function (−3%FMD) have been reported following 5 days of reduced step count in healthy male adults (113). FMD is a predictor of cardiovascular events in the general population (328). However, it is unclear whether these detrimental changes are likely to be sustained over time and whether upper limb FMD might also be impacted by SB.
5.2.2. Reducing and interrupting sedentary behavior.
5.2.2.1. evidence from acute studies.
Three meta-analyses have summarized the effects of interrupting sitting on FMD (24, 33, 130). Two of those indicated that vascular dysfunction can be mitigated by interrupting prolonged sitting with aerobic, standing, or simple resistance activities when compared to prolonged sitting (1.5 to 5 h) (33, 130). These short-term interventions improved FMD by 1.5 to 1.9%FMD (33, 130) and increased shear rate by 12.7 s−1 in adults and older adults (33). In contrast, the most recent meta-analysis demonstrated a small, nonsignificant effect of PA interruptions to sitting on FMD (24). Of note, some original studies failed to show improvements in lower and/or upper limb FMD following at least one of the interruptions to SB compared to prolonged sitting (25, 283, 284, 287, 291, 329, 330).
Meta-regressions revealed that higher BMI is significantly associated with greater reductions in FMD responses following interruptions to sitting as compared to prolonged sitting (33), indicating that those with higher BMI may benefit more from interrupting sitting. Subgroup analysis also indicated that aerobic and simple resistance activities may be more effective than standing interruptions at improving FMD, but this comparison did not reach statistical significance due to the low number of included studies (130). Regarding the frequency of interruptions to sitting, interrupting prolonged sitting with 3 min of simple resistance activities every 30 min (squat, calf raises; total: 39 min) was more effective at increasing FMD in the femoral artery in adults and older adults with type 2 diabetes compared to a lower frequency of interruptions (i.e., squatting, calf raises; 6-min bouts every 60 min, total: 36 min), for which FMD did not change (287). It is not clear whether the intensity of interruptions differentially affects FMD responses.
5.2.2.1.1. Reducing/interrupting SB versus continuous MVPA/exercise.
Continuous exercise (60 min/day of MVPA), but not substituting sitting with 5–6 h/day of LPA, over 4 days improved circulating markers of endothelial disfunction in adults with normal weight, overweight, and type 2 diabetes (331).
5.2.2.1.2. Potential mechanisms.
It has been suggested that changes in vascular function may be mediated by decreases in plasma ET-1 concentration (283, 319) and increases in plasma nitrate/nitrite concentration and NO bioavailability/endothelin-1 ratio (319). Changes in these biomarkers, along with increased skeletal muscle activity and other systemic changes (reduced postprandial glucose responses, blood lipids, inflammatory markers, and sympathetic nervous system activity), are thought to be the potential mechanisms underpinning the effects of reducing/interrupting SB on cardiovascular function (FIGURE 5) (39, 332).
FIGURE 5.
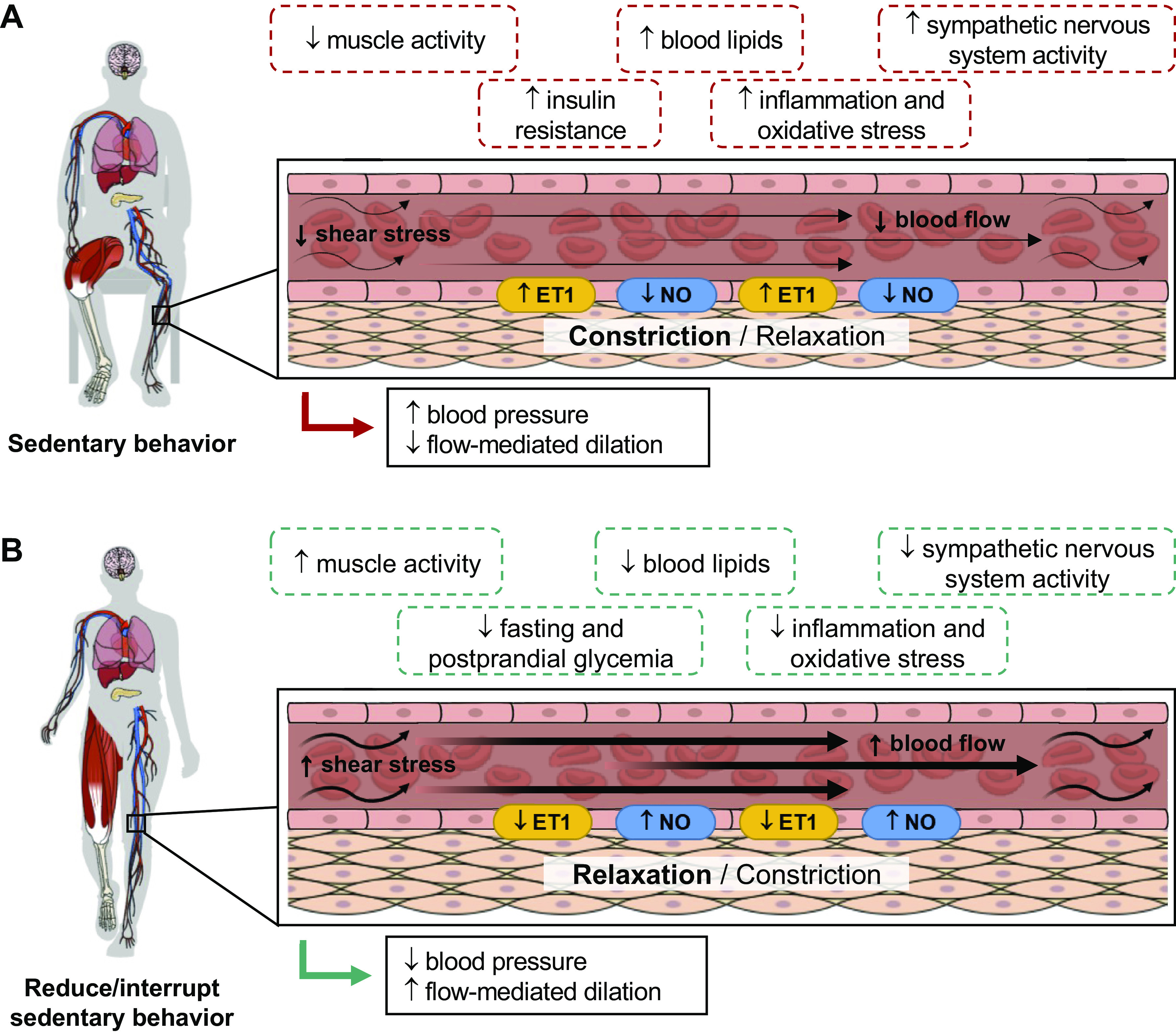
Vascular responses following exposures to prolonged sedentary behavior and to reducing/interrupting sedentary behavior. A: prolonged exposures to sedentary behavior reduce blood flow and shear stress, increasing endothelin-1 and attenuating nitric oxide, subsequently leading to reduced flow-mediated dilation, particularly in the lower limbs, and increased blood pressure. In addition, muscle inactivity, insulin resistance, hyperlipemia, inflammation, oxidative stress, and increased sympathetic nervous system activity may further augment the consequences of sedentary behavior on vascular health. B: reducing/interrupting sedentary behavior may be a potential strategy to improve cardiovascular health by attenuating sedentary behavior-induced maladaptations in cardiovascular and metabolic health (39, 332). ET1, endothelin-1; NO, nitric oxide. Image was created with mindthegraph.com, with permission.
5.2.2.2. evidence from longer term studies.
Only four longer term trials have investigated the effects of reducing/interrupting SB with standing, walking, and/or other types of PA on vascular function (33). A meta-analysis summarizing the available evidence showed a significant increase in FMD (0.93%FMD) in adults and older adults following 8 to 16 wk of intervention (33). However, this beneficial effect was mainly driven by one study (309), with other studies suggesting no change in lower and/or upper limb FMD following reducing/interrupting SB (309, 310, 333).
5.2.2.3. clinical significance.
There is some evidence of improvements in brachial and lower limb vascular function (0.93%FMD) associated with reducing/interrupting SB. This change is likely to be clinically meaningful, given that a 1% increase in FMD is significantly associated with a 17% decrease in future risk of cardiovascular events (334, 335). However, the effects of reducing/interrupting SB on vascular function appear to be less pronounced relative to those observed for aerobic, resistance, and combined exercise training (+2.1 to 2.8%FMD) in adults and older adults (336). Despite this initial evidence indicating a potential benefit of reducing/interrupting SB, the evidence base is still limited, and future studies are needed to elucidate the effects of reducing/interrupting SB on vascular function and the factors that mediate intervention effects.
5.3. Cardiorespiratory Fitness
5.3.1. Increasing sedentary behavior.
5.3.1.1. evidence from longer term studies.
Fourteen days of reduced step count (10,501 to 1,344 steps/day) resulted in a 6.6% decline in V̇o2max in healthy male adults, which was significantly correlated with reductions in daily steps (118). Other studies showed similar results in healthy adults (117, 122). Importantly, V̇o2max returned to baseline levels by simply resuming habitual activity for 14 days (117, 122).
5.3.1.2. clinical significance.
Increasing SB results in profound decreases in V̇o2max in healthy adults (e.g., −6.6% in 14 days). Changes observed over 14 days of increased SB are dramatically accelerated compared to age-related decline in V̇o2max per decade (∼10%, regardless of PA level) in females and males (337). Given low cardiorespiratory fitness is significantly associated with increased risk for all-cause mortality and cardiovascular events (338, 339), these findings hold important clinical implications for those who may undergo periods of reduced activity, bed rest, or immobilization. In contrast, it is important to highlight these alterations can be reverted after resuming habitual daily activities (117, 122).
5.3.2. Reducing and interrupting sedentary behavior.
5.3.2.1. evidence from acute studies.
Acutely, regular sit-to-stand transitions (stand up and return to a seated position every 1 minute for 10 min) significantly increased V̇o2max by ∼32% in healthy adults as compared to 10 min seated in a chair motionless (3.86 vs. 2.93 mL·kg−1·min−1) (16).
5.3.2.2. evidence from longer term studies.
A 3-yr randomized controlled trial involving physically inactive and sedentary individuals with type 2 diabetes compared 1 mo of exercise counseling every year to standard care (238). In an ancillary analysis, when participants were divided into quartiles based on changes in PA and SB irrespective of experimental arm, the highest quartile for changes in SB (−1.53 h/day) presented a significant increase in V̇o2max (4.49 mL·kg−1·min−1). Importantly, increases in time spent in SB were negatively associated with changes in V̇o2max, which was independent of time spent in MVPA (238). This finding indicates that reducing sedentary time, leading to substantial increases in total PA may be sufficient to improve cardiorespiratory fitness in a highly physically inactive and sedentary, at-risk population group.
5.3.2.2.1. Reducing/interrupting SB versus continuous MVPA/exercise.
In a small-scale, 12-wk, multifactorial, pilot study involving 57 sedentary, overweight/obese males and females, Kozey Keadle and colleagues (148) examined both the independent and the combined effects of exercise training (40–65% heart rate reserve, i.e., moderate to vigorous intensity) and reducing SB (replace SB with standing and LPA plus increasing daily step count by 5–10%) on V̇o2max. Compared to control, both exercise training (no change in SB, +27 min/day in MVPA) and exercise training combined with reducing SB (−7.3% of daily hours in SB, +45 min/day in MVPA) improved V̇o2max by ∼10% (2.5 mL·kg−1·min−1 and 2.9 mL·kg−1·min−1, respectively). Additionally, changes in V̇o2max resulting from reducing SB only (−4.8% of daily hours in SB, +28 min/day in MVPA) were not statistically significant (0.2 mL·kg−1·min−1) (148), indicating that improvements in V̇o2max may depend on the PA intensity. Four weeks of an intervention to reduce SB (−53 min/day in SB with no change in MVPA) resulted in significant increases in V̇o2max (1.9 mL·kg−1·min−1) in physically inactive adults with obesity (150). This improvement in V̇o2max was comparable to the effects of an intervention aiming to increase MVPA to at least 30 min day (2.2 mL·kg−1·min−1; +16 min/day of MVPA with no change in SB) (150).
5.3.2.3. clinical significance.
Significant increases in V̇o2max (1.9 to 4.4 mL·kg−1·min−1) have been reported following interventions to reduce/interrupt SB (−1 to 1.5 h/day) (150, 238). Despite these findings, evidence is still limited, and future studies are needed to elucidate the effects of reducing/interrupting SB on cardiorespiratory fitness, the clinical significance of these improvements, and the mediating factors (e.g., intensity of PA replacing SB).
6. MUSCULOSKELETAL SYSTEM
6.1. Skeletal Muscle
6.1.1. Increasing sedentary behavior.
6.1.1.1. evidence from longer term studies.
Skeletal muscle contractile activity during sitting is significantly lower than for standing and ambulatory activities (18, 21, 23). As discussed in sect. 4.3.1, existing studies are reasonably consistent in showing impaired anabolic responses after different periods and forms of SB. Significant losses in muscle mass have been consistently reported after only a few days of exposure to increased SB, regardless of study model.
Computed tomography scans revealed ∼2% decrements in quadriceps cross-sectional area (CSA) after only 5 days of a knee-immobilization protocol in healthy older males (340). After 14 days of full-leg casting, both young and older males had experienced significant decreases (∼5 to 9%) in quadriceps muscle volume as assessed by magnetic resonance imaging (MRI) (105). Longer time periods have also been studied (e.g., 3.5 mo), and muscle fiber CSA of the vastus lateralis was shown to be dramatically reduced (−24 to −51% depending on fiber type) in older males submitted to full-leg immobilization after total knee arthroplasty, when compared to control, physically inactive individuals (341). Notably, initial quadriceps CSA was positively associated with the magnitude of muscle atrophy following 14 days of lower limb immobilization in females but not in males (342). These findings highlight possible sex-based differences in skeletal muscle adaptations to immobilization, in which females with higher preimmobilization muscle mass are more likely to present with muscle loss.
Several studies have demonstrated that daily step reductions (∼67 to 87% reduction from baseline) resulted in lean mass decrements varying between 0.5 to 4.5% in healthy male adults (117–119, 240). Fourteen days of reduced daily steps (from 5,962 to 1,413 steps/day) elicited a 4% decrease in lower limb fat-free mass in healthy older adults (120). Furthermore, using a unilateral leg model, Devries and colleagues (115) demonstrated that 14 days of step reduction resulted in an ∼1.3% loss of leg fat-free mass in healthy older males. Performing concomitant unilateral resistance exercise counteracted maladaptations by increasing fat-free mass (∼1.4%) in the exercised leg. In addition, MHC distribution in the skeletal muscle shifted from slow-twitch (MHC I) toward hybrid and fast-twitch (IIa) fiber types in vastus lateralis following 20 days of reduced steps (from 14,000 to 3,000 steps/day) in healthy male adults (116), which has also been observed following periods of bed rest (52–54).
6.1.1.1.1. Potential mechanisms.
Bed rest studies showed that both decreased muscle protein synthesis and increased muscle protein breakdown contribute to skeletal muscle loss in healthy individuals and those with chronic disease conditions (343, 344). Skeletal muscle homeostasis is tightly controlled by numerous anabolic and catabolic pathways, although the precise interconnection and biological actions of these actors still need to be fully elucidated (345) (FIGURE 6). In muscle atrophy conditions, several anabolic signaling pathways may be suppressed, including phosphoinositide 3-kinase (PI3K)-Akt-mechanistic target of rapamycin 1 (mTORC1), β2-adrenergic, wingless/int1-frizzled (WNT/FZD), calcineurin, hippo, and/or bone morphogenetic protein (BMP). In contrast, several catabolic signaling pathways may be overactivated, including transforming growth factor-β (TGF-β), AMPK, nuclear factor-κ light chain enhancer of activated β-cells (NF-κβ), glucocorticoid receptors, angiotensin, IL-6-Janus kinases/signal transducers and activators of transcription (JAK/STAT), kinin, sphingolipids, notch, and/or activating transcription factor 4 (ATF4)-endoplasmic reticulum stress (345–348). Finally, recent a study showed that diminished mitochondrial energetics and lipid remodeling and increased H2O2 emission in hindlimb muscles of mice were early features preceding loss of muscle function (349). In addition, the shift in MHC distribution from slow-twitch toward hybrid and fast-twitch following bed rest (52–54) may contribute to muscle fatigue, dependence on muscle glycogen as fuel, and reduced capacity for fat oxidation (350).
FIGURE 6.
Key anabolic and catabolic pathways in muscle atrophy conditions. Several anabolic pathways signaling might be suppressed in muscle atrophy conditions, including PI3K-Akt-mTORC1, β2-adrenergic, WNT/FZD, calcineurin, hippo, and/or BMP. In contrast, several catabolic pathways signaling might be overactivated, including TGF-β, AMPK, NF-κβ, glucocorticoid receptors, angiotensin, IL-6-JAK/STAT, kinin, sphingolipids, notch, and/or ATF4-ER stress. Physical (in)activity and sedentary behavior also influence muscle protein synthesis and breakdown (345–348). Akt, protein kinase B; AMPK, adenosine monophosphate-activated protein kinase; ATF4, activating transcription factor 4; BMP, bone morphogenetic protein; ER, endoplasmic reticulum; IL-6, interleukin 6; JAK, janus kinases; mTORC1, mechanistic target of rapamycin 1; NF-κβ nuclear factor-κ light chain enhancer of activated β-cells; PI3K, phosphoinositide 3-kinase; STAT, signal transducers and activators of transcription; TGF- β, transforming growth factor-β; WNT/FZD, wingless-int1-frizzled. Image was created with mindthegraph.com, with permission.
6.1.1.2. clinical significance.
Increasing SB results in profound decreases (1.3 to 9%) in skeletal muscle or fat-free mass in adults and older adults. Changes observed over a few days of increased SB are comparable to muscle mass decreases (∼3 to 8%) over a decade after the age of 30 (351, 352). Given that low muscle mass is significantly associated with an increased risk for all-cause mortality (353), these findings could hold important clinical implications for those who may undergo periods of reduced activity, bed rest, or immobilization and for older adults who present higher rates of muscle mass loss compared to younger individuals (351).
6.1.2. Reducing and interrupting sedentary behavior.
6.1.2.1. evidence from longer term studies.
Five studies investigated the longer term effects (4 to 16 wk) of reducing/interrupting SB on fat-free mass. Four of them showed no effect in adult office workers and adults and older adults with type 2 diabetes (354–357). In contrast, a 3-mo cluster randomized trial conducted in 161 adult office workers significantly reduced sedentary time by 35 min/8-h workday compared to control individuals (n = 131), which was primarily driven by an increase in standing time. After intervention, there was an increase in total fat-free mass (0.5 kg) in the intervention group compared to the control group (358).
Overall, longer term findings related to the effects of reducing/interrupting prolonged sitting on skeletal muscle mass have been inconsistent. The extent to which reducing/interrupting SB increases muscle mass remains unclear. Future experimental studies are warranted to investigate whether reducing/interrupting SB and which type and intensity of PA can meaningfully impact skeletal muscle mass.
6.2. Muscle Strength and Functioning
6.2.1. Increasing sedentary behavior.
6.2.1.1. evidence from longer term studies.
Two weeks of unilateral whole leg casting significantly impaired muscle function by ∼16–31% in both adults and older male adults, as measured by maximal voluntary contraction, peak torque, specific force, and rate of force development (105). Interestingly, 4 wk of rehabilitation (unilateral resistance training, 3 times/wk) after a 2-wk immobilization restored muscle function in both age groups (105). Another study found that as little as 5 days of knee immobilization resulted in an 8–9% reduction in muscle strength in healthy older males (340). Four weeks of unilateral lower limb suspension (99% reduction in daily step count) reduced isometric force during plantar flexion and knee extension tasks by ∼15–25% and increased isometric fluctuations for both tasks by ∼12–22%. In contrast, no change was observed in EMG activity for soleus and gastrocnemius muscles (359).
Reduction in daily steps (75% reduction from baseline) for 14 days impaired muscle strength by ∼8% in healthy older adults. In contrast, a rehabilitation program of the same length (i.e., 14 days) was shown to be ineffective in restoring physical function (140), indicating that this population may be at higher risk for accelerated age-related loss in muscle mass/function after transitions to reduced activity levels. Nonetheless, the literature is inconsistent, with other studies having failed to show muscle strength decrements in response to step reduction in older adults (115, 120, 171).
6.2.1.2. clinical significance.
Increasing SB results in significant decreases in skeletal muscle strength (8 to 25%) in both adults and older adults. Changes observed with increased SB are comparable to annual decreases in grip and knee extension/flexion (2.2 to 3.1% and 3.6 to 5.0%, respectively) observed in older adults (360, 361). Given that low muscle strength is independently associated with increased risk for all-cause mortality regardless of muscle mass and participation in SB and leisure time PA (353), these findings could hold important clinical implications for those who may be exposed to periods of reduced activity, bed rest, or immobilization and for older adults who may be more susceptible than younger adults to muscle loss after increased SB (362).
6.2.2. Reducing and interrupting sedentary behavior.
6.2.2.1. evidence from longer term studies.
A previously described, a 3-yr randomized controlled trial involving physically inactive and sedentary individuals with type 2 diabetes compared 1 mo of exercise counseling every year to standard care. Participants in the intervention group experienced a significant reduction in sedentary time (−0.9 h/day) and increase in LPA (0.8 h/day) and MVPA (8 min/day), which resulted in improved lower body strength (∼19%) as compared to the control group (237). Additionally, participants were divided into quartiles based on changes in PA and SB irrespective of experimental arm. The highe st quartile for changes in SB (−1.53 h/day and +10.5 min/day of MVPA) did not have different isometric muscle strength in the upper body (shoulder press) but did have significantly greater lower body strength (leg extension) as compared to other quartiles, which may have been driven by the higher participation in PA of any intensity observed in this quartile (238). Interestingly, age was negatively and positively associated with intervention-induced changes in upper and lower body strength, respectively (238).
In relation to physical functioning, a Cochrane systematic review and meta-analysis of interventions aiming at reducing SB (1 wk to 1 yr) in community-dwelling older adults showed low-certainty evidence related to reductions in sedentary time (−45 min/day vs. control group, which was not significant) and improvements in physical function following intervention (2 studies; changes in gait speed and physical function as assessed by a physical performance battery were not significant) (363). Another meta-analysis, including interventions aimed at increasing participation in PA (52 min/week) and reducing SB (−58 min/day) among older adults showed a small, significant increase in physical functioning (standard mean difference: 0.21) compared to the control group (364). In patients with rheumatoid arthritis, a 4-mo motivational intervention aimed at reducing SB (−1.6 h/day of sedentary, 1.3 h/day of standing, and +0.5 h/day of stepping time) resulted in increased physical functioning (31%) as assessed by a disease-specific questionnaire (365). Notably, this effect was sustained up to 18 mo after the end of the intervention (366).
Only a few studies have investigated the effects of reducing/interrupting prolonged sitting on muscle function. Given the limited number of studies, the extent to which reducing/interrupting SB increases muscle strength and functioning and the associated clinical significance remain unclear.
6.3. Bone
6.3.1. Increasing sedentary behavior.
6.3.1.1. evidence from longer term studies.
Increased resorption and decreased formation are considered to be the primary drivers of immobilization-induced bone loss in weight-bearing bones (367). Experimental studies have consistently shown significant alterations in bone parameters when exposed to periods of increased SB. Lower limb suspension has been used as a model for such investigations, and 24 days of unilateral lower limb suspension induced losses in bone (tibia: 0.3 to 0.9%) of healthy male adults comparable to those seen after bed rest (368).
6.3.1.1.1. Potential mechanisms.
Markers of bone resorption (urine: hydroxyproline, deoxypyridinoline, and N-telopeptide of type I collagen; serum: type I collagen carboxytelopeptide) were significantly increased during bed rest, returning to baseline levels after resuming ambulation (369).
The effects of bone loss and recovery during/following disuse are unclear in older adults and individuals with osteopenia/osteoporosis. This should be addressed by future studies, particularly given the known negative effect of aging on cellular and molecular processes throughout the different stages of bone fracture healing (370).
6.3.1.2. clinical significance.
Limb immobilization results in profound decreases in bone mineral density (∼1%), primarily in weight-bearing bones in adults. These alterations are likely to be clinically significant given that the changes observed over 24 days of immobilization are comparable to annual bone loss (∼1%) in older adults (360). These findings hold important clinical implications for those who may undergo bed rest and immobilization periods due to injury and for older adults, particularly postmenopausal females who present higher rates of bone loss compared to males of similar age (360).
6.3.2. Reducing and interrupting sedentary behavior.
To our knowledge, no experimental study has investigated the effects of reducing/interrupting SB on bone metabolism, which is a promising area to be addressed in future studies.
7. CENTRAL NERVOUS SYSTEM
7.1. Central and Peripheral Neural Effects
7.1.1. Increasing sedentary behavior.
7.1.1.1. evidence from acute studies.
Four to six hours of exposure to prolonged, uninterrupted sitting decreased middle cerebral artery blood flow velocity and cerebrovascular conductance (∼3 to 6%) in healthy adults with desk-based jobs as compared to baseline levels, indicating impaired dynamic cerebral autoregulation (371, 372). Three hours of exposure to prolonged, uninterrupted sitting resulted in reductions in cerebrovascular conductance index (∼8%) in individuals (≥55 years old) with increased cardiovascular risk (310). In contrast, 3 h of uninterrupted sitting (with low or high mental activity) did not change cerebral blood flow in older adults but increased blood pressure (mean arterial pressure: 8.6 mmHg) and cerebrovascular resistance (∼13%), which are known to negatively impact brain health in the long term (35). Additionally, 3 h of prolonged, uninterrupted sitting did not affect corticospinal excitability in adult office workers (373) nor cerebral vasomotor reactivity in individuals with increased cardiovascular risk (310).
Exposure to prolonged sitting did not change plasma concentrations of brain-derived neurotrophic factor (BDNF), catecholamines, and related precursors or metabolites [norepinephrine, epinephrine, dopamine, DOPA, and dihydroxyphenylglycol (DHPG)] (288). Interestingly, changes in total fatigue levels were significantly associated with increased DHPG and decreased DOPA concentrations over 4 h of prolonged sitting, which may reflect alterations in the sympathetic nervous system in response to prolonged exposures to sitting (288).
7.1.1.1.1. Potential mechanisms.
It has been proposed that there may be involvement of altered cerebral glucose utilization due to increased postprandial glucose responses (41, 86–88); altered cortical perfusion and oxygen delivery due to alterations in cerebral (310, 371, 372) and peripheral vascular function (24, 33, 130) and in the supply of BDNF (374); and increased levels of inflammatory markers and reactive oxygen species (314, 375–377). Importantly, all these factors are recognized contributors to cognitive decline and dementia (41, 378, 379) (FIGURE 7). With respect to glucose utilization, altered cerebral glucose utilization seems to be a response to increased circulating glucose concentration. Acute hyperglycemia leads to a reduction in regional cerebral blood flow and a spike in insulin levels to facilitate glucose clearance. Together, these two factors favor a glucose nadir. The glucose nadir can impair endocrine counterregulation to a subsequent dip in glucose, thus exaggerating the hypoglycemic episode (41).
FIGURE 7.
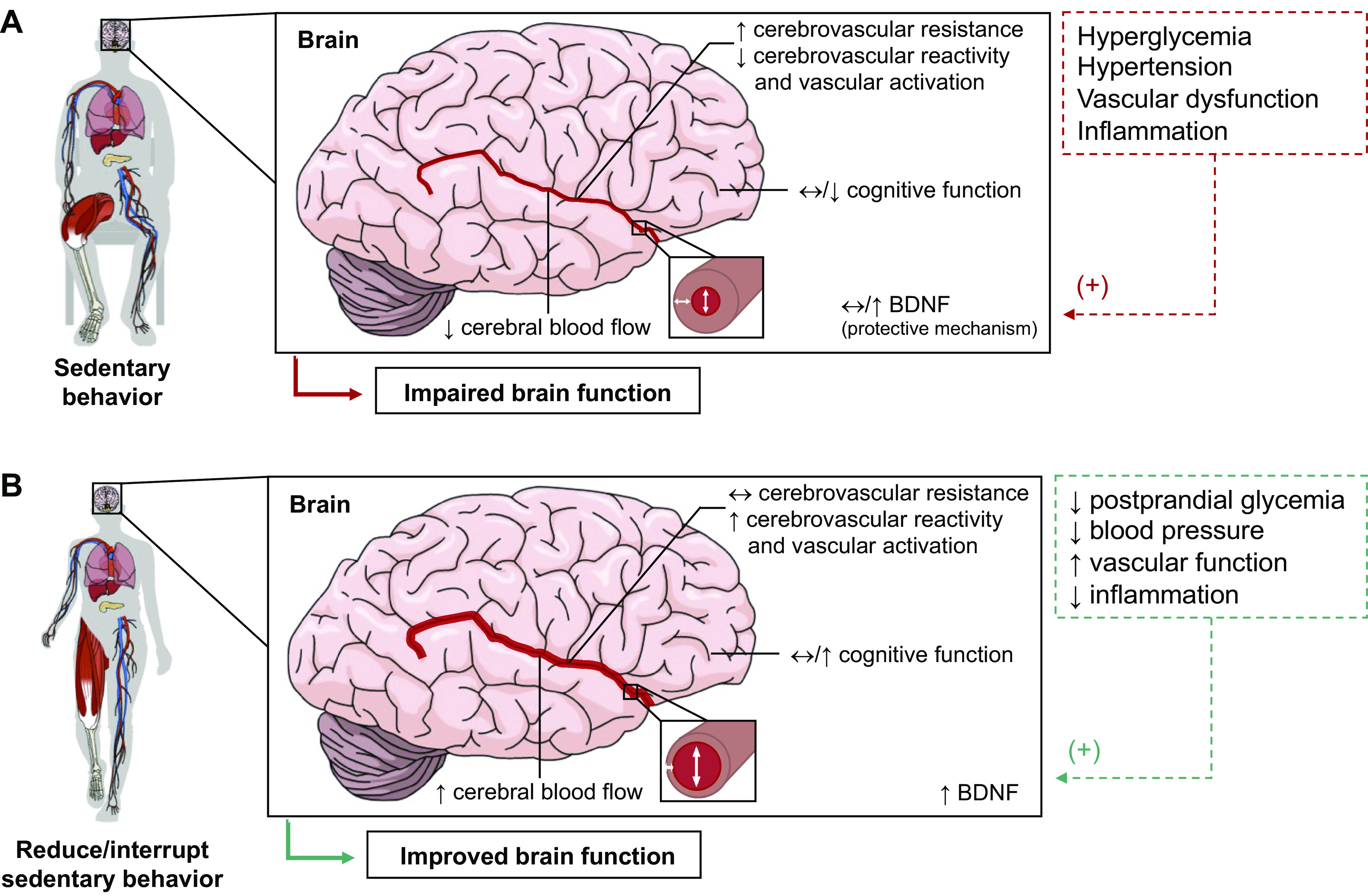
Cerebrovascular responses following exposures to prolonged sedentary behavior and to reducing/interrupting sedentary behavior. A: exposures to prolonged sedentary behavior may result in decreased cerebral blood flow velocity, cerebrovascular reactivity, and vascular activation and increased cerebrovascular resistance. Additionally, poor glucose control, vascular dysfunction, low-grade inflammation, and altered levels of neurotrophic biomarkers and their precursors/metabolites may further impair brain and central nervous system function. B: reducing/interrupting sedentary behaviors may be a potential strategy to attenuate sedentary behavior-induced maladaptations in the central nervous system function (41). BDNF, brain-derived neurotrophic factor. Image was created with mindthegraph.com, with permission.
SB and physical inactivity have also been shown to alter hippocampal mitochondrial and synaptic function in rats. Using selective breeding for physical inactivity, male and female sedentary low-voluntary runners, wild-type, and high-voluntary runner rats underwent cognitive behavioral testing, analysis of hippocampal neurogenesis and mitochondrial respiration, and molecular analysis of the dentate gyrus. Preference for physical inactivity (i.e., low voluntary runners) resulted in major detriments to cognition (spatial learning and memory), brain mitochondrial respiration (coupled and uncoupled respiration), and neurogenesis (reduced AMPA receptor subunit GluA1 protein levels, which is suggested to be an initiator of Alzheimer’s disease pathogenesis) compared to wild-type and high voluntary runner rats. Of note, a significant sex-effect was noted: these differences were essentially noted in females with males being more comparable to the wild-type rats (260). These findings provide evidence that selective breeding for physical inactivity has a heritable and detrimental effect on brain health and females appear to be more susceptible.
Future studies are required to better understand longer term adaptations associated with increased SB on cerebrovascular function.
7.1.2. Reducing and interrupting sedentary behavior.
7.1.2.1. evidence from acute studies.
Mixed findings have been reported related to alterations in cerebral blood flow responses when interrupting prolonged sitting. Carter and colleagues (371) showed that 2-min walking interruptions every 30 min during sitting, but not 8-min interruptions every 120 min (3.6 km/h, ∼2.8–3.0 METs, total: 16 min), increased middle cerebral artery blood flow velocity in healthy sedentary adults when compared to prolonged sitting over 4 h (0.6 vs. −1.2 vs. −3.2 cm/s, respectively) (371). In contrast, while a single 30-min exercise bout (65–75% maximum heart rate) increased middle cerebral artery blood flow velocity (2 cm/s) in healthy older adults compared to prolonged sitting, adding frequent light-intensity walking interruptions (3.2 km/h, 2.8 METs, 3-min bouts every 30 min, total: 36 min) following the exercise bout did not counteract the sitting-induced decrease in mean middle cerebral artery blood flow velocity over the 6.5-h period following exercise (380). Three hours of frequent walking interruptions (self-selected pace, 2-min bouts every 30 min, total: 12 min) during sitting with low or high mental activity (watch TV vs. cognitive puzzles) did not change cerebral blood flow in older adults compared to prolonged, uninterrupted sitting (35). It also did not counteract the sitting-induced increase in cerebrovascular resistance. Frequent, 2-min light-intensity walking interruptions every 30 min of sitting (self-selected pace, total: 10 min) prevented sitting-induced reductions in cerebral blood flow velocity and cerebrovascular conductance index in individuals (≥55 years old) with increased cardiovascular risk as compared to 3 h of prolonged sitting (310). Other markers of cerebrovascular flow and function were not affected by interruptions to sitting (310).
Bojsen-Møller and colleagues (373) showed that 3-min simple resistance activities interruptions every 30 min of sitting (body-weight squatting, calf raises; total: ∼15 min) increased corticospinal excitability in adult office workers compared to 3 h of prolonged sitting, suggesting that interruptions to sitting may promote corticospinal neuroplasticity. In contrast, short-interval intracortical inhibition was unchanged following interruptions to sitting (373).
Interrupting prolonged sitting with light-intensity walking (3.2 km/h, 2.8 METs; 3-min bouts every 30 min, total: 30 min) did not change plasma concentration of BDNF and, catecholamines and its precursors or metabolites as compared to 7 h of prolonged, uninterrupted sitting in overweight and obese adults (288). In contrast, performing a single 30-min exercise bout (65–75% maximum heart rate) with or without subsequent light-walking interruptions to sitting (3.2 km/h, 2.8 METs; 3-min bouts every 30 min, total: 36 min) increased serum BDNF responses (by 160 and 175%, respectively) over 8 h in older adults, relative to prolonged sitting (374). In contrast, no differences were observed for salivary cortisol levels between sitting interrupted with a 6-min single bout of high-intensity interval training (∼84% maximum heart rate) and uninterrupted sitting in healthy male adults (381). Similarly, no differences were reported for urinary catecholamines and cortisol concentrations between six hourly 5-min bouts of moderate-walking interruptions to sitting (perceived effort: 12–13 somewhat hard; total: 30 min) and uninterrupted sitting in healthy sedentary adults (165). It is not clear whether type, intensity, and frequency of interruptions to sitting differentially affect central and peripheral neural effects.
7.1.2.2. evidence from longer term studies.
The effect size for decreasing sedentary time can be considered large following an 8-wk e-health intervention to reduce/interrupt SB at the workplace versus a control condition (−38 min/8-h workday of sedentary time and +35 min/8-h workday of standing time) (309). As for changes in cerebrovascular function, large effects were observed for the change in gain (Cohen’s d: 1.25) and normalized gain (Cohen’s d: 0.91). Effect sizes were considered small to moderate for all other outcomes (Cohen’s d: 0.00 to 0.74), indicating little to no improvements in cerebrovascular function following intervention among healthy adult office workers (309). In a 16-wk nonrandomized trial, an intervention to reduce sitting (−60 min/day of sedentary, +36 min/day of standing, and +30 min/day of walking time) resulted in increased resting cerebral blood flow velocity (∼6%) and cerebrovascular conductance index (4%) among individuals (≥55 years old) with increased cardiovascular risk (310); no alterations in cerebral autoregulation, cerebral vasomotor reactivity, and cardiac baroreflex sensitivity were observed. These findings indicate an overall increase in cerebral perfusion after SB reduction intervention.
7.1.2.3. clinical significance.
Small increases in cerebral blood flow, but not cerebrovascular function, have been reported following 16 wk of reducing/interrupting SB (310). It is important to highlight that these improvements are in line with those observed following exercise programs in older adults (382, 383). It should be noted that even conditions such as Alzheimer’s disease do not markedly affect cerebrovascular function (384). This highlights the robustness of the cerebrovascular system to regulate fluctuations in cerebral blood flow and may justify the small magnitude of changes observed following PA/SB interventions. Given the limited number of studies, the extent to which reducing/interrupting SB alters cerebrovascular flow and function, as well as the clinical significance of these findings, remains imprecise. Future studies are required to better understand adaptations associated with reducing/interrupting SB in the central nervous system.
7.2. Cognitive Performance
7.2.1. Increasing sedentary behavior.
7.2.1.1. evidence from acute studies.
Eight hours of prolonged, uninterrupted sitting decreased working memory, executive function, and visual learning in healthy older adults as compared to exercise followed by prolonged sitting and exercise combined with frequent light-walking interruptions to sitting (374). In contrast, 6 h of exposure to prolonged, uninterrupted sitting did not affect cognitive function in healthy, sedentary adults (372). Other acute studies also indicate that acute exposures to prolonged, uninterrupted sitting (3 to 7 h) do not affect cognitive function in adults and older adults (288, 381, 385, 386). For the acute context, while prolonged sitting decreases cerebral blood flow (371, 372), prefrontal cortex perfusion and oxygen delivery to specific brain regions are maintained (387), which may preserve cognitive performance. Future studies are required to better understand longer term adaptations associated with increased SB on cognitive performance.
7.2.2. Reducing and interrupting sedentary behavior.
7.2.2.1. evidence from acute studies.
Findings on the effects of interrupting prolonged sitting on cognitive performance have been highly inconsistent. Interrupting sitting with bouts of standing, light- or moderate-intensity walking improved some cognitive performance components in three studies, namely, attention (388, 389), executive function (388), working memory (374, 389), and psychomotor function (389) in adults and older adults. In contrast, six hourly 5-min bouts of moderate-intensity walking (perceived effort: 12–13 somewhat hard; total: 30 min) did not affect cognitive function in healthy sedentary adults compared to prolonged sitting and a time-matched continuous bout of moderate-intensity walking (perceived effort: 12–13 somewhat hard) (165). No changes in cognitive performance following interruptions to sitting have been reported in other studies (165, 210, 288, 372, 381, 385, 386, 390, 391). Nonetheless, none of these studies showed detrimental effects of interruptions to sitting on cognitive performance. It is not clear whether type, intensity, and frequency of interruptions to sitting differentially affect cognitive performance.
7.2.2.2. evidence from longer term studies.
An 8-wk e-health intervention to reduce/interrupt SB within the workplace resulted in large reductions in sedentary time (−38 min/8-h workday of sedentary time and +35 min/8-h workday of standing time). Effect sizes were considered small for changes in work productivity (Cohen’s d: 0.47) and concentration/focus (Cohen’s d: 0.00), indicating little to no improvements following intervention among healthy adult office workers (309). In adults (≥50 years old) with knee osteoarthritis, an intervention aimed at increasing MVPA and reducing SB did not significantly increase working memory and episodic memory (392). Changes in MVPA and SB were not associated with changes in cognitive function during the study protocol (392).
7.2.2.3. clinical significance.
Acute and longer term findings related to the effects of interrupting prolonged sitting on cognitive performance have been highly inconsistent. It remains unclear the extent to which reducing/interrupting SB alters cognitive function, but studies indicate that this strategy does not negatively affect cognition. Longer term investigation into the effects of reducing/interrupting SB on cognitive performance is warranted.
8. IMMUNE SYSTEM
8.1. Immunity and Inflammatory Responses
8.1.1. Increasing sedentary behavior.
8.1.1.1. evidence from acute and multiday studies.
Two experimental studies reported that an acute 3- to 5-h exposure to prolonged sitting increased plasma IL-6 (by ∼38 to 50%) concentrations in healthy adults (393) and adults with central obesity (394); IL-6 is a pleiotropic cytokine with a broad range of inflammatory, immune, and hematopoietic effects. However, three other studies showed no change in this proinflammatory marker after 7–8 h of uninterrupted sitting in healthy adults (372), adults with overweight or obesity (288), and postmenopausal females with rheumatoid arthritis (217). Another study reported significant decreases in high-sensitivity C-reactive protein (∼91%) concentration following 6 h of prolonged, uninterrupted sitting (372). In the adipose tissue, uninterrupted sitting increased gene expression of IL-6 and monocyte chemoattractant protein-1 (MCP1) but not of tumor necrosis factor-α (TNF-α) and IL-18 (394).
In a 2-day crossover trial, healthy male adults performed prolonged sitting (for 7 h), standing (6 times, for a 45-min period each time, for 7 h), or moderate-intensity exercise (60% maximum heart rate, total: 30 min) on day 1 and remained seated for 6 h on day 2 (310). On day 1, plasma superoxide dismutase concentration increased by ∼13% during 7 h of prolonged sitting as compared to standing and exercise conditions, but no difference was observed for biological antioxidant potential and catalase concentrations. Postprandial concentrations of serum derivatives of reactive oxygen metabolites tended to be higher than in fasting state following 6 h of prolonged sitting on day 2 (314).
8.1.1.2. evidence from longer term studies.
Fourteen days of step count reduction (81% reduction from baseline) did not alter fasting plasma TNF, IL-6, IL-15, and adiponectin concentration in healthy male adults (118). In healthy older adults, a 14-day step reduction protocol (∼70 to 76% reduction from baseline) resulted in increases in plasma C-reactive protein (120, 171), IL-6 (171), and TNF-α concentrations (120, 171). Surprisingly, cytokine levels remained elevated throughout a 14-day recovery period (171). In the skeletal muscle, 14 days of step count reduction (from ∼9,000 to 3,000 steps/day) increased expression of key proteins in inflammatory signaling pathways [i.e., c-Jun NH2 terminal kinase (JNK), NF-kβ inhibitor-α (Ikβα), and Toll-like receptor 4 (TLR4)] (140) and macrophage infiltration in healthy older adults, likely due to an increase in transient muscle edema and/or minor myofiber damage (172).
8.1.1.3. clinical significance.
Exposures to prolonged SB increased some inflammatory markers, including plasma C-reactive protein, IL-6, and TNF-α concentrations, in both adults and older adults. It is unclear whether these detrimental changes are clinically meaningful and sustained over time. Given that low-grade inflammation is important in the pathogenesis of cardiovascular and other chronic diseases (395, 396), longer term studies should further investigate whether the altered inflammatory responses following increased SB are associated with alterations in other physiological systems.
8.1.2. Reducing and interrupting sedentary behavior.
8.1.2.1. evidence from acute and multiday studies.
Little is known about the effects of reducing/interrupting SB on inflammatory markers and the immune system. For instance, plasma/serum IL-6 responses were not affected by frequent, short bouts of light- and moderate-intensity walking interruptions to sitting as compared to 5.5 to 8 h of prolonged, uninterrupted sitting in adults and older adults (217, 288, 394). Regarding the other cytokines, frequent, 3-min light-intensity walking interruptions to sitting (24% heart rate reserve, total: 42 min) decreased plasma IL-1β (∼21%) and IL-10 (∼17%) and increased IL-1ra (∼25%) concentrations but did not change IL-4, IL-6, IL-8, IL-17, and IFN-γ, compared to 8 h of prolonged sitting in postmenopausal females with rheumatoid arthritis (217). These acute responses were not observed with the traditional single 30-min bout of moderate-intensity walking (55% heart rate reserve) performed early in the morning (217). In a crossover trial, performing multiple standing bouts (6 times, total: 4.5 h) or a moderate-intensity exercise bout (60% maximum heart rate, total: 30 min) protected against the increase in plasma superoxide dismutase observed following 7 h of prolonged sitting in healthy male adults, with no differences between standing interruptions and continuous exercise trials (314). It is not clear whether type, intensity, and frequency of interruptions to sitting differentially affect markers of inflammation and oxidative stress.
Regular light-intensity and simple resistance activity interruptions to sitting over 7 h (3.2 km/h, 2.8 METs or body-weight squatting, calf raises; 3-min bouts every 30 min, total: 36 min) increased plasma lysoalkylphosphatidylcholine (associated with anti-inflammatory pathways) and alkenylphosphatidylcholine (associated with antioxidant capacity) and decreased diacylglycerols and triacylglycerols (associated with proinflammatory pathways) concentrations, but did not affect other lipid species and subspecies, in adults and older adults with overweight/obesity and type 2 diabetes (397). In contrast, a 30-min bout of moderate-intensity walking (55% heart rate reserve), but not light-intensity walking interruptions to sitting (24% heart rate reserve; 3-min bouts every 30 min, total: 42 min), modified serum concentration of 6 lipid classes and subclasses in a direction that indicates reduction in inflammation and platelet activation and increase in antioxidant capacity in postmenopausal females with rheumatoid arthritis (217).
Moderate-intensity walking interruptions to sitting over 5 h (5.8 km/h, ∼4.3 METs; 2-min bouts every 20 min, total: 28 min) resulted in increased nicotamide N-methyltransferase (NNMT; modulates anti-inflammatory and antioxidative pathways) gene expression in the skeletal muscle as compared to prolonged, uninterrupted sitting in adults with overweight/obesity (229). In subcutaneous abdominal adipose tissue, interrupting sitting with light-intensity walking hours (3.2 km/h, 2.8 METs; 2-min bouts every 20 min, total: 28 min) resulted in upregulation of immune function and downregulation of inflammatory pathways (total of 8 pathways) as compared to uninterrupted sitting in adults with overweight/obesity (232).
8.1.2.2. clinical significance.
Currently, evidence related to the effects of reducing/interrupting sitting is restricted to acute exposures. Although small improvements were shown in some inflammatory markers, it remains unclear whether these changes are clinically meaningful and sustained over time. Longer term investigation into the effects of reducing/interrupting SB on inflammatory responses is warranted, particularly in population groups characterized by a low-grade inflammatory profile and/or high-grade systemic inflammation.
9. SUMMARY OF PHYSIOLOGICAL IMPACTS AND FUTURE DIRECTIONS
The rapid accumulation of epidemiological and experimental evidence on SB over the past 20 years has provided a foundation for understanding the physiology of SB. To date, evidence on the physiological effects of exposures to increased SB and the potential impact of reducing and interrupting SB raise several pertinent questions, research needs and opportunities. These include 1) how evidence on physiological consequences of SB relates to the already vast knowledge base on physical inactivity (lack of sufficient exercise); 2) what are the effects of reducing/interrupting SB on acute/chronic physiological processes or health outcomes and the specific mechanisms involved; and 3) how the evolving knowledge about reducing/interrupting SB can provide rational mechanistic bases for interventions and future clinical and public health recommendations. Hereafter, we provide a summary of available evidence and a perspective on some of the priority areas for future work in SB physiology.
9.1. What Are the Effects of Sedentary Behaviors on Physiological Systems?
From a physiological perspective, the evidence to date indicates the numerous physiological responses resulting from increasing SB (TABLE 3). To summarize, excessive and prolonged SB leads to insulin resistance, vascular dysfunction, shift in substrate use toward carbohydrate oxidation, shift in muscle fiber from oxidative to glycolytic type, reduced cardiorespiratory fitness, loss of muscle mass and strength and bone mass, and increased total body fat mass and visceral fat depot, blood lipid concentrations, and inflammation. These adaptations relate to those reported for physical inactivity (43–46, 398, 399).
Table 3.
Summary of acute (hours to <4 days) and longer term (>2 wk) effects (experimental/intervention) of increasing sedentary behavior in adults and older adults
| Outcomes | Acute Effects |
Longer Term Effects |
||
|---|---|---|---|---|
| Mean Change | Referencesa | Mean Change | Referencesa | |
| Body mass and composition | ||||
| Body weight, kg | NA | −2% | 118 | |
| Total and percent body fat, % | NA | 3 to 14% | 116, 117, 138 | |
| Intra-abdominal fat mass, % | NA | 7% | 118, 119 | |
| Glucose metabolism | ||||
| Fasting glucose, mg/dL | NA | NS | 118, 155 | |
| 2-h postload glucose, mg/dL | ? | NS | 155 | |
| Postprandial glucose, %b | 17.5 (−26.2 to −8.7) | 86* | 6 to 9% | 127 |
| Glycemic variability | NA | 33 to 97% | 127 | |
| Whole body insulin sensitivity, % | −39 to −18% | 34 | −17% | 155 |
| Fasting insulin, μU/mL | NA | NS | 118, 155 | |
| 2-h postload insulin, μU/mL | ? | 38.8 μU/mL | 155 | |
| Postprandial insulin, %b | 25.1 (−31.8 to −18.3) | 86* | 32% | 155 |
| Lipid metabolism | ||||
| Fasting triglycerides, mg/dL | NA | 0.3 mmol/L | 116, 122 | |
| Postprandial triglycerides, %b | 27% | 83 | 21% | 119 |
| Fasting total cholesterol, mmol/L | NA | 0.5 mmol/L | 122 | |
| Fasting LDL, mmol/L | NA | 0.3 mmol/L | 122 | |
| Fasting HDL, mmol/L | NA | −0.1 mmol/L | 116 | |
| Hemodynamics | ||||
| Systolic blood pressure, mmHg | 3.2 (0.6 to 5.8) | 129* | 4 mmHg | 122 |
| Diastolic blood pressure, mmHg | NS | 129* | NS | 122 |
| Mean arterial pressure, mmHg | 3.3 (2.2 to 4.4) | 129* | ? | |
| Blood flow, mL/min | −1.0 (−1.6 to −0.4) | 24* | NS | 115 |
| Shear rate, SMD | −0.8 (−1.0 to −0.5) | 24* | ? | |
| Cardiovascular function | ||||
| Flow-mediated dilation, %FMD | −1.2 (−1.7 to −0.7) | 24 | −3 %FMD | 113 |
| Base diameter, % | NS | 283 | −5% | 113, 299 |
| Cardiorespiratory fitness | ||||
| V̇o2max, ml·kg−1·min−1 or % | NA | −6.6% | 117, 118, 122 | |
| Musculoskeletal system | ||||
| Lean mass, % | NA | −9 to −0.5% | 105, 117–119, 340 | |
| Muscle strength, % | NA | −31 to −8% | 105, 140, 340, 359 | |
| Bone mineral density, % | NA | −0.3 to −1% | 368 | |
| Central nervous system | ||||
| Cerebral artery blood flow, % | −6 to −3% | 371, 372 | ? | |
| Cerebrovascular conductance, % | −8% | 310 | ? | |
| Cerebrovascular resistance, % | 13% | 35 | ? | |
| Inflammatory responses | ||||
| C-reactive protein, % | −91% | 372 | 25 to 45% | 120, 171 |
| Interleukin 6, % | 38 to 50% | 393, 394 | 30% | 171 |
| Tumor necrosis factor-α, % | ? | 12 to 31% | 120, 171 | |
For meta-analyses, data are presented as mean absolute or percent change (95% confidence interval) from baseline to postexposure to increased sedentary behavior. For original studies, data are presented as mean change or the range of mean change from baseline to postexposure to increased sedentary behavior. *Evidence from meta-analyses; awhen meta-analyses are not available, key references are provided: refer to main document for detailed description and detailed information on study models; bas measured by area under the curve. ?, No data available; NA, not applicable; NS, nonsignificant; FMD, flow-mediated dilation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SMD, standardized mean difference.
A standing question is whether SB (too much sitting) and physical inactivity (too little exercise) lead to similar or distinct adaptations in relation to the number of physiological systems being impaired, magnitude of changes, and mechanisms. This question is challenging to address with the current available study models. However, bed rest studies that tested the effects of exercise training to prevent the physiological adaptations to bed rest can provide some insights. While individuals in strict bed rest are deficient in both LPA and MVPA, those who are concomitantly subjected to bed rest and exercise training are only deficient in LPA. Therefore, bed rest combined with exercise represents a model to study the adaptations and potential mechanisms of exposures to a highly sedentary yet physically active condition. Findings from these studies suggest excessive participation in SB results in adverse effects, at least for some outcomes, even in the presence of large volumes of aerobic and/or resistance exercise, i.e., above current guidelines (29) (TABLE 4). This supports that SB and physical inactivity likely coexist in a continuum related to energy demand and muscle contraction and that the magnitude of maladaptations following physical inactivity seems to be more pronounced than those of SB in most physiological systems (29), but it is not clear whether these behaviors differentially affect physiological systems or present distinct underpinning mechanisms. It also highlights the potential benefits of regular nonexercise activity and/or muscle contractions.
Table 4.
Summary of effects of increasing sedentary behavior through strict bed rest with or without exercise training in healthy adults
| Outcomes | Strict Bed Rest | Bed Rest with Exercise* | References |
|---|---|---|---|
| Fat mass and repartition | |||
| Intra-abdominal fat mass | Increases | Counteracted | 29, 251 |
| Liver fat accumulation | Increases | Attenuated | 29 |
| Glucose metabolism | |||
| Whole body insulin sensitivity | Decreases | Attenuated to counteracted | 29, 177, 248 |
| Fasting insulin | Increases | No effect to counteracted | 29, 177, 248 |
| Fasting carbohydrate oxidation | Increases | No effect to attenuated | 29 |
| Postprandial carbohydrate oxidation | Increases | No effect to counteracted | 29, 248 |
| Lipid metabolism | |||
| Fasting triglycerides | Increases | No effect | 29 |
| Fasting HDL | Decreases | No effect | 29 |
| Fasting lipid oxidation | Decreases | No effect to attenuated | 29, 177, 248 |
| Postprandial lipid oxidation | Decreases | No effect to counteracted | 29, 248 |
| Cardiorespiratory fitness | |||
| V̇o2max | Decreases | Attenuated to counteracted | 29, 400 |
| Musculoskeletal system | |||
| Muscle mass | Decreases | Attenuated to counteracted | 29 |
| Muscle fiber type toward glycolytic | Increases | Attenuated | 29 |
| Mitochondrial oxidative capacity | Decreases | Attenuated | 29 |
| Muscle strength | Decreases | Counteracted | 53, 54, 243 |
| Muscle fat storage | Increases | No effect | 177 |
| Bone mineral density | Decreases | Attenuated or counteracted | 401, 402 |
| Bone fat storage | Increases | No effect to counteracted | 29 |
| Inflammatory responses | |||
| Proinflammatory markers | Increases | Counteracted | 29, 252, 403 |
*Protocols involved resistance exercise alone or resistance and aerobic exercise training. In general, combined exercise training was more effective at attenuating or counteracting adverse effects related to bed rest than resistance exercise training alone.
Our premise in this review is that the adaptations to increasing SB are not the opposite of adaptations to reducing/interrupting SB (i.e., increasing PA). Accordingly, evidence from each paradigm was addressed in separate sections. Notably, a few studies in rodents and humans have shed some light on potential distinct adaptations to increasing SB versus increasing nonexercise activity (e.g., LPA) versus MVPA/exercise training. For example, alterations in heparin-released and intracellular LPL activity decreased monoexponentially in both type 1 and predominantly type 2 muscles after 12 h of limb unloading. These alterations were rapidly reversed with light-intensity contractile activity in both soleus and quadriceps muscles (9, 55, 56), but MVPA/exercise training did not enhance LPL regulation in type 1 muscles and type 2 muscles that were not recruited during running (8, 57). In addition, LPP1/PAP2A has been proposed as a potential gene that modulates maladaptation related to increased SB/inactivity in both humans and rats, but exercise has been found to be ineffective at counteracting alterations in LPP1/PAP2A in both species (51). Finally, a clinical study compared the effects of 2 mo of exercise training in sedentary male adults with the effects of 1 mo of reducing nonexercise PA (i.e., increased SB) in active male adults. Notably, the deleterious effect of increased SB was more marked than the beneficial effects of exercise training following current PA guidelines for dietary fat oxidation, fasting and postprandial insulin concentration/response, postprandial triglycerides response, and fat-free and fat mass (137). In contrast, the magnitude of changes in V̇o2max was more pronounced following exercise training than increased SB (137). Altogether, these findings suggest distinct mechanisms may underpin adaptations to increased SB and exercise training. Further investigations are however clearly required.
It is critical that well-powered, rigorous studies are conducted to examine the acute and chronic physiological adaptations to imposed SB. Studies aiming to investigate the physiological consequences of imposed SB (“problem-focused” approach) should focus on recruiting individuals with low sedentary time who will experimentally modify their usual activity (low SB and high LPA) to more sedentary states (higher SB and lower PA of any intensity). This concept is analogous to the paradigm put forward initially by Booth and Lees (404) stating that appropriate models of physical inactivity should utilize an approach whereby physically active individuals become physically inactive (405). To effectively isolate the effects of physical inactivity from those of SB, studies should focus on recruiting individuals who do not meet current PA guidelines (150 min/wk of MVPA) or perform regular exercise training. Although bed rest can be a well-controlled and useful model to study SB physiology, studies should also focus on increased SB with behaviors more commonly seen in free-living (i.e., sitting not strict lying down).
Future studies should i) investigate whether differential changes in muscle mass and fiber type are associated with SB-related metabolic adaptations; 2) determine the molecular and cellular mechanisms underpinning adaptations to increasing SB, e.g., using omics approaches (genomics, epigenomics, transcriptomics, proteomics, metabolomics, etc.); 3) implement different volumes and types of PA/exercise to determine how much PA and/or exercise is needed to offset the adverse effects of increased SB; and 4) include rigorous measurements and control PA level at baseline and during the study protocol, preferably via device-based measures, which is a major limitation of free-living studies to increase SB (i.e., reduced step count and immobilization). Given that most of the available evidence is limited to healthy male adults, future studies should focus on investigating the effects of increasing SB in females, children, adolescents, older adults, more-diverse population groups, and individuals at risk for or with chronic conditions. This will assist in providing the strong rational biological bases that are much needed for improving our understanding of the physiology of SB and its multiple health consequences.
9.2. What Are the Beneficial Effects of Reducing/Interrupting Sedentary Behavior?
Acutely, reducing/interrupting SB improves postprandial glucose and insulin responses, systolic and diastolic blood pressure, and lower limb vascular function in adults and older adults (TABLE 5) (33, 86–88, 129, 130). In the longer term, reducing/interrupting SB interventions results in small improvements in body weight (−0.6 kg), waist circumference (−0.7 to −1.5 cm), percent body fat (−0.3 to −0.7%), fasting glucose (−2.5 mg/dL), insulin (1.4 pM), HbA1c (−0.2%) and HDL (0.04 mM) concentrations, systolic blood pressure (−1.1 mmHg), and brachial and lower limb vascular function (0.93%FMD) in adults and older adults (TABLE 5) (30–33). There is more limited evidence for other health outcomes and physiological systems. Despite this initial evidence, findings from acute and longer term studies aimed at reducing/interrupting SB are inconsistent with other findings of no benefit for some outcomes. Overall, the effects of reducing/interrupting SB are small and likely not to be clinically and physiologically meaningful in healthy population groups, but plausibly, the effects are likely to be larger in less-healthy populations. Future studies need to investigate whether these small improvements in health outcomes observed with reducing/interrupting sedentary behavior are associated with reduced risk of chronic diseases and early mortality.
Table 5.
Summary of acute (<24 h) and longer term (>2 wk) beneficial effects (experimental/intervention) of reducing/interrupting sedentary behavior in adults and older adults, as evidenced by meta-analyses
| Outcomes | Acute Effects |
Longer Term Effects |
||
|---|---|---|---|---|
| Mean Difference | References | Mean Difference | References | |
| Body mass and composition | ||||
| Body weight, kg | NA | −0.6 (−0.9 to −0.2) | 30 | |
| NS | 31, 32 | |||
| Body mass index, kg/m2 | NA | NS | 30, 31 | |
| Waist circumference, cm | NA | −0.7 (−1.2 to −0.2) | 30 | |
| −1.5 (−2.8 to −0.2) | 31 | |||
| NS | 32 | |||
| Body fat percentage, % | NA | −0.3 (−0.5 to −0.0) | 30 | |
| −0.7 (−1.3 to −0.1) | 31 | |||
| Fat mass, kg | NA | −0.8 (−0.9 to −0.6) | 32 | |
| NS | 30 | |||
| Fat-free mass, kg | NA | NS | 30 | |
| Glucose metabolism | ||||
| Fasting glucose, mg/dL | NA | −2.5 (−4.3 to −0.8) | 32 | |
| NS | 30, 31 | |||
| Postprandial glucose, % or SMDa | −17.5 (−26.2 to −8.7) | 86 | ? | |
| −0.4 (−0.5 to −0.2) | 87 | |||
| −0.5 (−0.7 to −0.4) | 88 | |||
| −0.3 (−0.6 to −0.03) | 188 | |||
| −0.7 (−1.0 to −0.4) | 188 | |||
| Fasting insulin, pM, | NA | −1.4 (−2.8 to −0.0) | 30 | |
| −2.3 (−4.4 to −0.3) | 32 | |||
| Postprandial insulin, % or SMDa | −25.1 (−31.8 to −18.3) | 86 | ? | |
| −0.4 (−0.5 to −0.2) | 87 | |||
| −0.6 (−0.7 to −0.4) | 88 | |||
| −0.8 (−1.2 to −0.5) | 188 | |||
| NS | 188 | |||
| HbA1c, % | NA | −0.2 (−0.3 to −0.04) | 31 | |
| NS | 30 | |||
| Lipid metabolism | ||||
| Fasting triglycerides, mg/dL | NA | NS | 30, 31, 32 | |
| Postprandial triglycerides, SMDa | −0.3 (−0.4 to −0.1) | 88 | ? | |
| NS | 86, 87 | |||
| Fasting total cholesterol, mg/dL | NA | NS | 30, 31, 32 | |
| Fasting LDL, mg/dL | NA | NS | 30, 31, 32 | |
| Fasting HDL, mM | NA | 0.04 (0.02 to 0.07) | 30 | |
| NS | 31, 32 | |||
| Cardiorespiratory system | ||||
| Systolic blood pressure, mmHg | −4.4 (−7.4 to −1.5) | 129 | −1.1 (−2.1 to −0.0) | 30 |
| NS | 188 | NS | 32 | |
| Diastolic blood pressure, mmHg | −2.4 (−4.5 to −0.3) | 129 | NS | 30, 31, 32 |
| Flow-mediated dilation, %FMD | 1.5 (1.0 to 2.0) | 33 | 0.9 (0.3 to 1.6) | 33 |
| 1.9 (0.4 to 3.4) | 130 | |||
| NS | 24 | |||
| Pulse wave velocity, m/s | 0.02 (−0.27 to 0.32) | 33 | 0.27 (−0.32 to 0.87) | 33 |
| Shear rate, s−1 | 12.7 (7.9 to 17.5) | 33 | ? | |
Data are presented as mean difference (95% confidence interval) between reducing/interrupting sedentary behavior and prolonged sedentary behavior/control group. aAs measured by area under the curve. ?, No data available; NA, not applicable; NS, nonsignificant; FMD, flow-mediated dilation; HDL, high-density lipoprotein; LDL, low-density lipoprotein; SMD, standard mean difference.
For studies focused on the consequences of reducing/interrupting SB (solution focused treatment paradigm), the emphasis should be directed toward recruiting physically inactive-sedentary individuals with modification of their typical low PA level (high SB and low PA) to higher PA levels (low SB and high PA of any intensity). This includes conducting randomized controlled trials (with appropriate control groups) of longer duration (>12 wk); familiarizing/habituating study participants to interventions and study procedures; continuously monitoring the adherence to interventions; controlling for potential confounders during the design, conduct, and analysis of the trial; and analyzing experimental data considering both the intention to treat principle and adherence to the interventions.
Studies should include rigorous measurements of primary, secondary, and exploratory outcomes and report all possible outcomes and indexes being measured (e.g., acute studies measuring glucose concentrations should report not only AUC but also mean glucose concentration during the protocol). The monitoring of PA level at baseline, preferably via device-based measures, to access eligibility and during study follow-up can enhance scientific rigor. Longer term, randomized controlled trials should also combine device-based measures with self-reported (e.g., questionnaires and diaries) to gather information on activity type and context of SB and PA (303).
Future studies should focus on reporting data by key factors, including, but not limited to, age groups (preferably by smaller age groups, such as 5-yr increments), BMI, sex/gender, race/ethnicity, genetic profiles, menopausal and pregnancy status, medications, dietary habits, cardiorespiratory fitness, baseline SB/PA/exercise levels, sleep duration and quality, and populations at increased risk of or with chronic diseases, as indicated by the 2020 World Health Organization Guidelines Development Group (406, 407). Identifying whether such factors hold significant importance that will help identify more “at-risk” population groups and those who may derive more benefits from reducing/interrupting SB. Harmonized analysis using individual participant data and dose-escalation trials could also be implemented to evaluate the effect of different “doses” of SB reductions on physiological outcomes. More robust evidence on both the direct and indirect underlying cellular and molecular mechanisms associated with reducing/interrupting SB is also needed. This may be garnered through the collection of tissue samples (e.g., muscle, bone, adipose tissue), including more direct and integrated physiological measurements, rather than surrogate markers. This will assist in providing the strong rational biological bases that are much needed for improving our understanding of the physiology of reducing/interrupting SB and refining intervention strategies and guidelines to address SB as a clinical and public health problem.
9.3. What is the “Optimum” Frequency, Intensity, Time, and Type of Activities When Reducing/Interrupting Sedentary Behavior?
Elements pertaining to SB FITT may influence the effects of reducing/interrupting SB on glucose responses, which is the most-studied outcome. To summarize, it seems that more frequent (2–6-min bouts every 20–60 min) and higher intensity (light- to moderate-intensity) interruptions yield more pronounced improvements in relation to postprandial glucose responses than less frequent (every 120 min or more) and lower intensity (i.e., standing still) interruptions to sitting (86–88, 188, 204, 210). Despite being beneficial, it is still unclear whether there are differences between higher frequencies of interruptions (e.g., every 20 min vs. 30 min) or between intensities (i.e., light vs. moderate vs. vigorous). There are also some inconsistencies across original studies and meta-analysis on what is the ideal SB FITT. There is more limited evidence on the influence of FITT elements of an intervention on changes in health outcomes other than glycemia.
To summarize, current evidence does not allow us to conclude what is the desirable FITT to reducing/interrupting SB and significantly affect physiological systems in healthy adults and older adults and those at risk or with chronic diseases. The “ideal” or “optimal” FITT of SB and PA elements is likely to be based on the requirements, context, and activity/health status of the subpopulation, rather than a “one size fits all” approach. However, in terms of potential countermeasures applicable to the population, it may be that certain minimal combinations or criteria of mode or posture (e.g., active sitting, fidgeting, acute or extended postural changes, standing, activities involving resistance, and/or sit-to-stand transitions), volume or intensity (e.g., LPA or MVPA), or patterning (e.g., activity bout, active around meals, or standing length/accumulation) of physical movement are all that is required to derive physiological benefit.
Another pertinent question is whether regular interruptions to sitting would be more beneficial than performing the traditional continuous bout of activity. Acutely, reductions in postprandial glucose responses are more pronounced following frequent interruptions to sitting compared to a time-matched continuous bout of activity (88, 203, 204, 217). In contrast, a continuous bout of PA in the morning is more effective at attenuating postprandial triglycerides responses and systolic blood pressure than frequent interruptions to sitting (217, 218, 261, 262, 307). Nonetheless, evidence on the differential effects of frequent interruptions to sitting versus a continuous bout of PA/exercise is still limited to acute settings, a select number of health outcomes, and generally healthy population groups. Evidence on the combined effects of exercise and reducing/interrupting SB is also very limited. This is a critical gap in the literature, given the combination of both strategies, is what has been currently recommended in public health guidelines for all age and population groups.
Future studies should address the evidence gap on the optimum FITT of activities when reducing/interrupting SB, particularly in the longer term. To do so, studies should include experimental groups with different FITT of interruptions to SB versus a sedentary (control) group. If possible, groups should be matched for energy expenditure and FITT elements other than the one being tested should remain unchanged. For example, when testing the influence of frequency, total duration, intensity, and type of activity should be the same across experimental groups. Studies should also 1) include a detailed description of how participants were instructed to reduce/interrupt SB (as per all FITT elements); 2) provide participants with specific and measurable goals, so that adherence to FITT prescription can be objectively assessed; 3) describe how adherence was assessed and check adherence to the prescribed intervention throughout the study protocol; 4) report behavioral outcomes that reflect changes in the FITT element being tested, e.g., report changes in the number of daily interruptions to SB and number/duration of prolonged SB bouts when testing the effects of different frequencies of interruptions to SB; and 5) report adherence to the prescribed intervention (as per FITT elements being measured/controlled) using objective assessment (e.g., accelerometers, HR monitors, wearables, etc.) (TABLE 2). This will improve the study design, data analysis, reporting/data harmonization in future studies, and hence the robustness of the findings. It will ultimately assist with refining preventative strategies and guidelines to combat excessive SB.
10. CONCLUSIONS
SB is highly prevalent in daily living, and most of the population is exposed, to a greater or lesser extent, to the health risks of too much sitting. Excessive SB negatively impacts a multitude of physiological systems, leading to insulin resistance, vascular dysfunction, shift in substrate use toward carbohydrate oxidation, shift in muscle fiber from oxidative to glycolytic type, reduced cardiorespiratory fitness, loss of muscle mass and strength and bone mass, and increased total body fat mass and visceral fat depot, blood lipid concentrations, and inflammation. From a physiological perspective, exposures to increased SB result in maladaptations that are similar to those that have been reported for physical inactivity but generally lower in terms of magnitude.
Longer term interventions aimed at reducing/interrupting SB have only resulted in small improvements in body weight, waist circumference, percent body fat, fasting glucose, insulin, HbA1c and HDL concentrations, systolic blood pressure, and vascular function in adults and older adults. Because of inconsistencies in the reported effects of reducing/interrupting SB, the clinical significance of these findings still remains somewhat unclear. Although the sit less, move more and exercise message currently promoted by contemporary public health guidelines has received a clear general consensus based on a growing body of epidemiological findings, further experimental studies are needed to elucidate the physiological effects of interventions combining exercise and reduction/interruptions to sitting. Nonetheless, reducing/interrupting SB is a low-risk strategy and is likely relevant from a population point of view, particularly given that it can serve as a stepping stone to increase participation in PA/exercise for those who do not, or have significant challenges to, achieve the minimum guidelines on MVPA.
DATA AVAILABILITY
Data will be made available upon reasonable request.
GRANTS
A.J.P. and B.G. were supported by Fundação de Amparo à Pesquisa do Estado de São Paulo Grants 2015/26937-4 and 2018/19418-9 and 2017/13552-2. A.J.P. received funding from the Ludeman Family Center for Women’s Health Research at the University of Colorado Anschutz Medical Campus. A.B. was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK123334. D.W.D., P.C.D., and N.O. were supported by the National Health and Medical Research Council of Australia (NHMRC) Center of Research Excellence Grants 1057608 and 1142685), the Victorian state Government Operational Infrastructure Support scheme, and the NHMRC Fellowships Scheme.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
A.J.P., A.B., P.C.D., H.R., N.O., B.G., and D.W.D. conceived and designed research; A.J.P., A.B., P.C.D., N.O., and D.W.D. prepared figures; A.J.P., A.B., P.C.D., H.R., N.O., B.G., and D.W.D. drafted manuscript; A.J.P., A.B., P.C.D., H.R., N.O., B.G., and D.W.D. edited and revised manuscript; A.J.P., A.B., P.C.D., H.R., N.O., B.G., and D.W.D. approved final version of manuscript.
ACKNOWLEDGMENTS
We are extremely grateful for the resourcefulness and hard work of our broader network of collaborators and the research fellows, PhD students, and support staff who have worked with us in our programs of sedentary behavior research. The figures were created with Mind the Graph (mindthegraph.com).
REFERENCES
- 1. Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SF, Altenburg TM, Chinapaw MJ, SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN) – terminology consensus project process and outcome. Int J Behav Nutr Phys Act 14: 75, 2017. doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bull FC, Al-Ansari SS, Biddle S, Borodulin K, Buman MP, Cardon G, Carty C, Chaput JP, Chastin S, Chou R, Dempsey PC, DiPietro L, Ekelund U, Firth J, Friedenreich CM, Garcia L, Gichu M, Jago R, Katzmarzyk PT, Lambert E, Leitzmann M, Milton K, Ortega FB, Ranasinghe C, Stamatakis E, Tiedemann A, Troiano RP, van der Ploeg HP, Wari V, Willumsen JF. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med 54: 1451–1462, 2020. doi: 10.1136/bjsports-2020-102955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Katzmarzyk PT, Powell KE, Jakicic JM, Troiano RP, Piercy K, Tennant B, Physical Activity Guidelines Advisory Committee. Sedentary behavior and health: update from the 2018 Physical Activity Guidelines Advisory Committee. Med Sci Sports Exerc 51: 1227–1241, 2019. doi: 10.1249/MSS.0000000000001935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ekelund U, Tarp J, Steene-Johannessen J, Hansen BH, Jefferis B, Fagerland MW, Whincup P, Diaz KM, Hooker SP, Chernofsky A, Larson MG, Spartano N, Vasan RS, Dohrn IM, Hagstromer M, Edwardson C, Yates T, Shiroma E, Anderssen SA, Lee IM. Dose-response associations between accelerometry measured physical activity and sedentary time and all cause mortality: systematic review and harmonised meta-analysis. BMJ 366: 14570, 2019. doi: 10.1136/bmj.l4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bauman A, Ainsworth BE, Sallis JF, Hagstromer M, Craig CL, Bull FC, Pratt M, Venugopal K, Chau J, Sjostrom M, Group IPS. The descriptive epidemiology of sitting. A 20-country comparison using the International Physical Activity Questionnaire (IPAQ). Am J Prev Med 41: 228–235, 2011. doi: 10.1016/j.amepre.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 6. Bennie JA, Chau JY, van der Ploeg HP, Stamatakis E, Do A, Bauman A. The prevalence and correlates of sitting in european adults – a comparison of 32 eurobarometer-participating countries. Int J Behav Nutr Phys Act 10: 107, 2013. doi: 10.1186/1479-5868-10-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Luis de Moraes Ferrari G, Kovalskys I, Fisberg M, Gomez G, Rigotti A, Sanabria LY, Garcia MC, Torres RG, Herrera-Cuenca M, Zimberg IZ, Guajardo V, Pratt M, King AC, Sole D, ELANS Study Group. Original research socio-demographic patterning of self-reported physical activity and sitting time in Latin American countries: findings from ELANS. BMC Public Health 19: 1723, 2019. doi: 10.1186/s12889-019-8048-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamilton MT, Hamilton DG, Zderic TW. Exercise physiology versus inactivity physiology: an essential concept for understanding lipoprotein lipase regulation. Exerc Sport Sci Rev 32: 161–166, 2004. doi: 10.1097/00003677-200410000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamilton MT, Hamilton DG, Zderic TW. Role of low energy expenditure and sitting in obesity, metabolic syndrome, type 2 diabetes, and cardiovascular disease. Diabetes 56: 2655–2667, 2007. doi: 10.2337/db07-0882. [DOI] [PubMed] [Google Scholar]
- 10. Hamilton MT, Healy GN, Dunstan DW, Zderic TW, Owen N. Too little exercise and too much sitting: inactivity physiology and the need for new recommendations on sedentary behavior. Curr Cardiovasc Risk Rep 2: 292–298, 2008. doi: 10.1007/s12170-008-0054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedentary Behaviour Research Network. Letter to the editor: standardized use of the terms “sedentary” and “sedentary behaviours”. Appl Physiol Nutr Metab 37: 540–542, 2012. doi: 10.1139/h2012-024. [DOI] [PubMed] [Google Scholar]
- 12. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep 100: 126–131, 1985. [PMC free article] [PubMed] [Google Scholar]
- 13. Norton K, Norton L, Sadgrove D. Position statement on physical activity and exercise intensity terminology. J Sci Med Sport 13: 496–502, 2010. doi: 10.1016/j.jsams.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 14. Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA 320: 2020–2028, 2018. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 43: 1575–1581, 2011. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 16. Judice PB, Hamilton MT, Sardinha LB, Zderic TW, Silva AM. What is the metabolic and energy cost of sitting, standing and sit/stand transitions? Eur J Appl Physiol 116: 263–273, 2016. doi: 10.1007/s00421-015-3279-5. [DOI] [PubMed] [Google Scholar]
- 17. Betts JA, Smith HA, Johnson-Bonson DA, Ellis TI, Dagnall J, Hengist A, Carroll H, Thompson D, Gonzalez JT, Afman GH. The energy cost of sitting versus standing naturally in man. Med Sci Sports Exerc 51: 726–733, 2019. doi: 10.1249/MSS.0000000000001841. [DOI] [PubMed] [Google Scholar]
- 18. Gao Y, Silvennoinen M, Pesola AJ, Kainulainen H, Cronin NJ, Finni T. Acute metabolic response, energy expenditure, and EMG activity in sitting and standing. Med Sci Sports Exerc 49: 1927–1934, 2017. doi: 10.1249/MSS.0000000000001305. [DOI] [PubMed] [Google Scholar]
- 19. Mansoubi M, Pearson N, Clemes SA, Biddle SJ, Bodicoat DH, Tolfrey K, Edwardson CL, Yates T. Energy expenditure during common sitting and standing tasks: examining the 1.5 MET definition of sedentary behaviour. BMC Public Health 15: 516, 2015. doi: 10.1186/s12889-015-1851-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Newton RL Jr, Han H, Zderic T, Hamilton MT. The energy expenditure of sedentary behavior: a whole room calorimeter study. PLoS One 8: e63171, 2013. doi: 10.1371/journal.pone.0063171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pesola AJ, Laukkanen A, Tikkanen O, Sipila S, Kainulainen H, Finni T. Muscle inactivity is adversely associated with biomarkers in physically active adults. Med Sci Sports Exerc 47: 1188–1196, 2015. doi: 10.1249/MSS.0000000000000527. [DOI] [PubMed] [Google Scholar]
- 22. Joyner MJ, Casey DP. Regulation of increased blood flow (hyperemia) to muscles during exercise: a hierarchy of competing physiological needs. Physiol Rev 95: 549–601, 2015. doi: 10.1152/physrev.00035.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tikkanen O, Haakana P, Pesola AJ, Hakkinen K, Rantalainen T, Havu M, Pullinen T, Finni T. Muscle activity and inactivity periods during normal daily life. PLoS One 8: e52228, 2013. doi: 10.1371/journal.pone.0052228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Taylor FC, Pinto AJ, Maniar N, Dunstan DW, Green DJ. The acute effects of prolonged uninterrupted sitting on vascular function: a systematic review and meta-analysis. Med Sci Sports Exerc 54: 67–76, 2022. doi: 10.1249/MSS.0000000000002763. [DOI] [PubMed] [Google Scholar]
- 25. Peddie MC, Kessell C, Bergen T, Gibbons TD, Campbell HA, Cotter JD, Rehrer NJ, Thomas KN. The effects of prolonged sitting, prolonged standing, and activity breaks on vascular function, and postprandial glucose and insulin responses: a randomised crossover trial. PLoS One 16: e0244841, 2021. doi: 10.1371/journal.pone.0244841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Maylor BD, Zakrzewski-Fruer JK, Stensel DJ, Orton CJ, Bailey DP. Effects of frequency and duration of interrupting sitting on cardiometabolic risk markers. Int J Sports Med 40: 818–824, 2019. doi: 10.1055/a-0997-6650. [DOI] [PubMed] [Google Scholar]
- 27. Homer AR, Taylor FC, Dempsey PC, Wheeler MJ, Sethi P, Grace MS, Green DJ, Cohen ND, Larsen RN, Kingwell BA, Owen N, Dunstan DW. Different frequencies of active interruptions to sitting have distinct effects on 22 h glycemic control in type 2 diabetes. Nutr Metab Cardiovasc Dis 31: 2969–2978, 2021. doi: 10.1016/j.numecd.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 28. Paing AC, McMillan KA, Kirk AF, Collier A, Hewitt A, Chastin SF. Dose-response between frequency of breaks in sedentary time and glucose control in type 2 diabetes: a proof of concept study. J Sci Med Sport 22: 808–813, 2019. doi: 10.1016/j.jsams.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 29. Le Roux E, De Jong NP, Blanc S, Simon C, Bessesen DH, Bergouignan A. Physiology of physical inactivity, sedentary behaviours and non-exercise activity: insights from the space bedrest model. J Physiol 600: 1037–1051, 2022. doi: 10.1113/JP281064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hadgraft NT, Winkler E, Climie RE, Grace MS, Romero L, Owen N, Dunstan D, Healy G, Dempsey PC. Effects of sedentary behaviour interventions on biomarkers of cardiometabolic risk in adults: systematic review with meta-analyses. Br J Sports Med 55: 144–154, 2021. doi: 10.1136/bjsports-2019-101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nieste I, Franssen WM, Spaas J, Bruckers L, Savelberg H, Eijnde BO. Lifestyle interventions to reduce sedentary behaviour in clinical populations: a systematic review and meta-analysis of different strategies and effects on cardiometabolic health. Prev Med 148: 106593, 2021. doi: 10.1016/j.ypmed.2021.106593. [DOI] [PubMed] [Google Scholar]
- 32. Saeidifard F, Medina-Inojosa JR, Supervia M, Olson TP, Somers VK, Prokop LJ, Stokin GB, Lopez-Jimenez F. The effect of replacing sitting with standing on cardiovascular risk factors: a systematic review and meta-analysis. Mayo Clin Proc Innov Qual Outcomes 4: 611–626, 2020. doi: 10.1016/j.mayocpiqo.2020.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng C, Zhang X, Sheridan S, Ho RS, Sit CH, Huang Y, Wong SH. Effect of sedentary behavior interventions on vascular function in adults: a systematic review and meta-analysis. Scand J Med Sci Sports 31: 1395–1410, 2021. doi: 10.1111/sms.13947. [DOI] [PubMed] [Google Scholar]
- 34. Stephens BR, Granados K, Zderic TW, Hamilton MT, Braun B. Effects of 1 day of inactivity on insulin action in healthy men and women: interaction with energy intake. Metabolism 60: 941–949, 2011. doi: 10.1016/j.metabol.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 35. Maasakkers CM, Melis RJ, Kessels RP, Gardiner PA, Olde Rikkert MG, Thijssen DH, Claassen J. The short-term effects of sedentary behaviour on cerebral hemodynamics and cognitive performance in older adults: a cross-over design on the potential impact of mental and/or physical activity. Alzheimers Res Ther 12: 76, 2020. doi: 10.1186/s13195-020-00644-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab 35: 725–740, 2010. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- 37. Dempsey PC, Larsen RN, Dunstan DW, Owen N, Kingwell BA. Sitting less and moving more: implications for hypertension. Hypertension 72: 1037–1046, 2018. doi: 10.1161/HYPERTENSIONAHA.118.11190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dempsey PC, Owen N, Yates TE, Kingwell BA, Dunstan DW. Sitting less and moving more: improved glycaemic control for type 2 diabetes prevention and management. Curr Diab Rep 16: 114, 2016. doi: 10.1007/s11892-016-0797-4. [DOI] [PubMed] [Google Scholar]
- 39. Dunstan DW, Dogra S, Carter SE, Owen N. Sit less and move more for cardiovascular health: emerging insights and opportunities. Nat Rev Cardiol 18: 637–648, 2021. doi: 10.1038/s41569-021-00547-y. [DOI] [PubMed] [Google Scholar]
- 40. Homer AR, Owen N, Dunstan DW. Too much sitting and dysglycemia: mechanistic links and implications for obesity. Curr Opin Endocr Metab Res 4: 42–49, 2019. doi: 10.1016/j.coemr.2018.09.003. [DOI] [Google Scholar]
- 41. Wheeler MJ, Dempsey PC, Grace MS, Ellis KA, Gardiner PA, Green DJ, Dunstan DW. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimers Dement (N Y) 3: 291–300, 2017. doi: 10.1016/j.trci.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Padilla J, Fadel PJ. Prolonged sitting leg vasculopathy: contributing factors and clinical implications. Am J Physiol Heart Circ Physiol 313: H722–H728, 2017. doi: 10.1152/ajpheart.00326.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. J Appl Physiol (1985) 111: 1201–1210, 2011. doi: 10.1152/japplphysiol.00698.2011. [DOI] [PubMed] [Google Scholar]
- 44. Thyfault JP, Krogh-Madsen R. Metabolic disruptions induced by reduced ambulatory activity in free-living humans. J Appl Physiol (1985) 111: 1218–1224, 2011. doi: 10.1152/japplphysiol.00478.2011. [DOI] [PubMed] [Google Scholar]
- 45. Booth FW, Roberts CK, Thyfault JP, Ruegsegger GN, Toedebusch RG. Role of inactivity in chronic diseases: evolutionary insight and pathophysiological mechanisms. Physiol Rev 97: 1351–1402, 2017. doi: 10.1152/physrev.00019.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol 2: 1143–1211, 2012. doi: 10.1002/cphy.c110025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. O'Keefe MP, Perez FR, Kinnick TR, Tischler ME, Henriksen EJ. Development of whole-body and skeletal muscle insulin resistance after one day of hindlimb suspension. Metabolism 53: 1215–1222, 2004. doi: 10.1016/j.metabol.2004.02.025. [DOI] [PubMed] [Google Scholar]
- 48. Belavy DL, Miokovic T, Armbrecht G, Richardson CA, Rittweger J, Felsenberg D. Differential atrophy of the lower-limb musculature during prolonged bed-rest. Eur J Appl Physiol 107: 489–499, 2009. doi: 10.1007/s00421-009-1136-0. [DOI] [PubMed] [Google Scholar]
- 49. Berg HE, Eiken O, Miklavcic L, Mekjavic IB. Hip, thigh and calf muscle atrophy and bone loss after 5-week bedrest inactivity. Eur J Appl Physiol 99: 283–289, 2007. doi: 10.1007/s00421-006-0346-y. [DOI] [PubMed] [Google Scholar]
- 50. Dilani Mendis M, Hides JA, Wilson SJ, Grimaldi A, Belavy DL, Stanton W, Felsenberg D, Rittweger J, Richardson C. Effect of prolonged bed rest on the anterior hip muscles. Gait Posture 30: 533–537, 2009. doi: 10.1016/j.gaitpost.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 51. Miokovic T, Armbrecht G, Felsenberg D, Belavy DL. Differential atrophy of the postero-lateral hip musculature during prolonged bedrest and the influence of exercise countermeasures. J Appl Physiol (1985) 110: 926–934, 2011. doi: 10.1152/japplphysiol.01105.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Borina E, Pellegrino MA, D'Antona G, Bottinelli R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand J Med Sci Sports 20: 65–73, 2010. doi: 10.1111/j.1600-0838.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 53. Gallagher P, Trappe S, Harber M, Creer A, Mazzetti S, Trappe T, Alkner B, Tesch P. Effects of 84-days of bedrest and resistance training on single muscle fibre myosin heavy chain distribution in human vastus lateralis and soleus muscles. Acta Physiol Scand 185: 61–69, 2005. doi: 10.1111/j.1365-201X.2005.01457.x. [DOI] [PubMed] [Google Scholar]
- 54. Trappe S, Trappe T, Gallagher P, Harber M, Alkner B, Tesch P. Human single muscle fibre function with 84 day bed-rest and resistance exercise. J Physiol 557: 501–513, 2004. doi: 10.1113/jphysiol.2004.062166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical activity: a molecular reason to maintain daily low-intensity activity. J Physiol 551: 673–682, 2003. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bey L, Akunuri N, Zhao P, Hoffman EP, Hamilton DG, Hamilton MT. Patterns of global gene expression in rat skeletal muscle during unloading and low-intensity ambulatory activity. Physiol Genomics 13: 157–167, 2003. doi: 10.1152/physiolgenomics.00001.2002. [DOI] [PubMed] [Google Scholar]
- 57. Hamilton MT, Etienne J, McClure WC, Pavey BS, Holloway AK. Role of local contractile activity and muscle fiber type on LPL regulation during exercise. Am J Physiol Endocrinol Metab 275: E1016–E1022, 1998. doi: 10.1152/ajpendo.1998.275.6.E1016. [DOI] [PubMed] [Google Scholar]
- 58. Zderic TW, Hamilton MT. Identification of hemostatic genes expressed in human and rat leg muscles and a novel gene (LPP1/PAP2A) suppressed during prolonged physical inactivity (sitting). Lipids Health Dis 11: 137, 2012. doi: 10.1186/1476-511X-11-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kump DS, Booth FW. Alterations in insulin receptor signalling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol 562: 829–838, 2005. doi: 10.1113/jphysiol.2004.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Laye MJ, Thyfault JP, Stump CS, Booth FW. Inactivity induces increases in abdominal fat. J Appl Physiol (1985) 102: 1341–1347, 2007. doi: 10.1152/japplphysiol.01018.2006. [DOI] [PubMed] [Google Scholar]
- 61. Kump DS, Booth FW. Sustained rise in triacylglycerol synthesis and increased epididymal fat mass when rats cease voluntary wheel running. J Physiol 565: 911–925, 2005. doi: 10.1113/jphysiol.2005.084525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Diaz KM, Howard VJ, Hutto B, Colabianchi N, Vena JE, Safford MM, Blair SN, Hooker SP. Patterns of sedentary behavior and mortality in U.S. middle-aged and older adults: a national cohort study. Ann Intern Med 167: 465–475, 2017. doi: 10.7326/M17-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nicholas JA, Lo Siou G, Lynch BM, Robson PJ, Friedenreich CM, Csizmadi I. Leisure-time physical activity does not attenuate the association between occupational sedentary behavior and obesity: results from Alberta’s tomorrow project. J Phys Act Health 12: 1589–1600, 2015. doi: 10.1123/jpah.2014-0370. [DOI] [PubMed] [Google Scholar]
- 64. van der Berg JD, Stehouwer CD, Bosma H, van der Velde JH, Willems PJ, Savelberg HH, Schram MT, Sep SJ, van der Kallen CJ, Henry RM, Dagnelie PC, Schaper NC, Koster A. Associations of total amount and patterns of sedentary behaviour with type 2 diabetes and the metabolic syndrome: the Maastricht Study. Diabetologia 59: 709–718, 2016. doi: 10.1007/s00125-015-3861-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wilmot EG, Edwardson CL, Achana FA, Davies MJ, Gorely T, Gray LJ, Khunti K, Yates T, Biddle SJ. Sedentary time in adults and the association with diabetes, cardiovascular disease and death: systematic review and meta-analysis. Diabetologia 55: 2895–2905, 2012. doi: 10.1007/s00125-012-2677-z. [DOI] [PubMed] [Google Scholar]
- 66. Patterson R, McNamara E, Tainio M, de Sa TH, Smith AD, Sharp SJ, Edwards P, Woodcock J, Brage S, Wijndaele K. Sedentary behaviour and risk of all-cause, cardiovascular and cancer mortality, and incident type 2 diabetes: a systematic review and dose response meta-analysis. Eur J Epidemiol 33: 811–829, 2018. doi: 10.1007/s10654-018-0380-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Engelen L, Gale J, Chau JY, Hardy LL, Mackey M, Johnson N, Shirley D, Bauman A. Who is at risk of chronic disease? Associations between risk profiles of physical activity, sitting and cardio-metabolic disease in Australian adults. Aust N Z J Public Health 41: 178–183, 2017. doi: 10.1111/1753-6405.12627. [DOI] [PubMed] [Google Scholar]
- 68. Lynch BM. Sedentary behavior and cancer: a systematic review of the literature and proposed biological mechanisms. Cancer Epidemiol Biomarkers Prev 19: 2691–2709, 2010. doi: 10.1158/1055-9965.EPI-10-0815. [DOI] [PubMed] [Google Scholar]
- 69. Matthews CE, George SM, Moore SC, Bowles HR, Blair A, Park Y, Troiano RP, Hollenbeck A, Schatzkin A. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am J Clin Nutr 95: 437–445, 2012. doi: 10.3945/ajcn.111.019620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Falck RS, Davis JC, Liu-Ambrose T. What is the association between sedentary behaviour and cognitive function? A systematic review. Br J Sports Med 51: 800–811, 2016. doi: 10.1136/bjsports-2015-095551. [DOI] [PubMed] [Google Scholar]
- 71. Plaza-Florido A, Perez-Prieto I, Molina-Garcia P, Radom-Aizik S, Ortega FB, Altmae S. Transcriptional and epigenetic response to sedentary behavior and physical activity in children and adolescents: a systematic review. Front Pediatr 10: 917152, 2022. doi: 10.3389/fped.2022.917152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sehn AP, Brand C, de Castro Silveira JF, Andersen LB, Gaya AR, Todendi PF, de Moura Valim AR, Reuter CP. What is the role of cardiorespiratory fitness and sedentary behavior in relationship between the genetic predisposition to obesity and cardiometabolic risk score? BMC Cardiovasc Disord 22: 92, 2022. doi: 10.1186/s12872-022-02537-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Klimentidis YC, Arora A, Chougule A, Zhou J, Raichlen DA. FTO association and interaction with time spent sitting. Int J Obes (Lond) 40: 411–416, 2016. doi: 10.1038/ijo.2015.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Martorell M, Mardones L, Petermann-Rocha F, Martinez-Sanguinetti MA, Leiva-Ordonez AM, Troncoso-Pantoja C, Flores F, Cigarroa I, Perez-Bravo F, Ulloa N, Mondaca-Rojas D, Diaz-Martinez X, Celis-Morales C, Villagran M, Epidemiology of Lifestyle and Health Outcomes in Chile Consortium. The FTO RS17817449 polymorphism is not associated with sedentary time, physical activity, or cardiorespiratory fitness: findings from the GENADIO Cross-Sectional Study. J Phys Act Health 18: 1352–1357, 2021. doi: 10.1123/jpah.2021-0076. [DOI] [PubMed] [Google Scholar]
- 75. Fischer J, Koch L, Emmerling C, Vierkotten J, Peters T, Bruning JC, Ruther U. Inactivation of the FTO gene protects from obesity. Nature 458: 894–898, 2009. doi: 10.1038/nature07848. [DOI] [PubMed] [Google Scholar]
- 76. Davey Smith G, Hemani G. Mendelian randomization: genetic anchors for causal inference in epidemiological studies. Hum Mol Genet 23: R89–R98, 2014. doi: 10.1093/hmg/ddu328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yang F, Huangfu N, Chen S, Hu T, Qu Z, Wang K, Cui H, Xie X. Genetic liability to sedentary behavior in relation to myocardial infarction and heart failure: a Mendelian randomization study. Nutr Metab Cardiovasc Dis 32: 2621–2629, 2022. doi: 10.1016/j.numecd.2022.07.005. [DOI] [PubMed] [Google Scholar]
- 78. Deng MG, Cui HT, Lan YB, Nie JQ, Liang YH, Chai C. Physical activity, sedentary behavior, and the risk of type 2 diabetes: a two-sample Mendelian randomization analysis in the European population. Front Endocrinol (Lausanne) 13: 964132, 2022. doi: 10.3389/fendo.2022.964132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Dixon-Suen SC, Lewis SJ, Martin RM, English DR, Boyle T, Giles GG, et al. Physical activity, sedentary time and breast cancer risk: a Mendelian randomisation study. Br J Sports Med 56: 1157–1170, 2022. doi: 10.1136/bjsports-2021-105132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang Z, Emmerich A, Pillon NJ, Moore T, Hemerich D, Cornelis MC, et al. Genome-wide association analyses of physical activity and sedentary behavior provide insights into underlying mechanisms and roles in disease prevention. Nat Genet 54: 1332–1344, 2022. doi: 10.1038/s41588-022-01165-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Chastin SF, McGregor DE, Biddle SJ, Cardon G, Chaput JP, Dall PM, Dempsey PC, DiPietro L, Ekelund U, Katzmarzyk PT, Leitzmann M, Stamatakis E, Van der Ploeg HP. Striking the right balance: evidence to inform combined physical activity and sedentary behavior recommendations. J Phys Act Health 18: 631–637, 2021. doi: 10.1123/jpah.2020-0635. [DOI] [PubMed] [Google Scholar]
- 82. Ekelund U, Tarp J, Fagerland MW, Johannessen JS, Hansen BH, Jefferis BJ, Whincup PH, Diaz KM, Hooker S, Howard VJ, Chernofsky A, Larson MG, Spartano N, Vasan RS, Dohrn IM, Hagstromer M, Edwardson C, Yates T, Shiroma EJ, Dempsey P, Wijndaele K, Anderssen SA, Lee IM. Joint associations of accelerometer measured physical activity and sedentary time with all-cause mortality: a harmonised meta-analysis in more than 44 000 middle-aged and older individuals. Br J Sports Med 54: 1499–1506, 2020. doi: 10.1136/bjsports-2020-103270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kim IY, Park S, Chou TH, Trombold JR, Coyle EF. Prolonged sitting negatively affects the postprandial plasma triglyceride-lowering effect of acute exercise. Am J Physiol Endocrinol Metab 311: E891–E898, 2016. doi: 10.1152/ajpendo.00287.2016. [DOI] [PubMed] [Google Scholar]
- 84. Akins JD, Crawford CK, Burton HM, Wolfe AS, Vardarli E, Coyle EF. Inactivity induces resistance to the metabolic benefits following acute exercise. J Appl Physiol (1985) 126: 1088–1094, 2019. doi: 10.1152/japplphysiol.00968.2018. [DOI] [PubMed] [Google Scholar]
- 85. Burton HM, Coyle EF. Daily step count and postprandial fat metabolism. Med Sci Sports Exerc 53: 333–340, 2021. doi: 10.1249/MSS.0000000000002486. [DOI] [PubMed] [Google Scholar]
- 86. Chastin SF, De Craemer M, De Cocker K, Powell L, Van Cauwenberg J, Dall P, Hamer M, Stamatakis E. How does light-intensity physical activity associate with adult cardiometabolic health and mortality? Systematic review with meta-analysis of experimental and observational studies. Br J Sports Med 53: 370–376, 2019. doi: 10.1136/bjsports-2017-097563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Saunders TJ, Atkinson HF, Burr J, MacEwen B, Skeaff CM, Peddie MC. The acute metabolic and vascular impact of interrupting prolonged sitting: a systematic review and meta-analysis. Sports Med 48: 2347–2366, 2018. doi: 10.1007/s40279-018-0963-8. [DOI] [PubMed] [Google Scholar]
- 88. Loh R, Stamatakis E, Folkerts D, Allgrove JE, Moir HJ. Effects of interrupting prolonged sitting with physical activity breaks on blood glucose, insulin and triacylglycerol measures: a systematic review and meta-analysis. Sports Med 50: 295–330, 2020. doi: 10.1007/s40279-019-01183-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Shrestha N, Grgic J, Wiesner G, Parker A, Podnar H, Bennie JA, Biddle SJ, Pedisic Z. Effectiveness of interventions for reducing non-occupational sedentary behaviour in adults and older adults: a systematic review and meta-analysis. Br J Sports Med 53: 1206–1213, 2019. doi: 10.1136/bjsports-2017-098270. [DOI] [PubMed] [Google Scholar]
- 90. Rynders CA, Blanc S, DeJong N, Bessesen DH, Bergouignan A. Sedentary behaviour is a key determinant of metabolic inflexibility. J Physiol 596: 1319–1330, 2018. doi: 10.1113/JP273282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Hodgson H, Wilkinson M, Bowen S, Giannoudis P, Howard A. Older adults are not more susceptible to acute muscle atrophy after immobilisation compared to younger adults: a systematic review. Eur J Trauma Emerg Surg 48: 1167–1176, 2022. doi: 10.1007/s00068-021-01694-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sonne MP, Hojbjerre L, Alibegovic AC, Nielsen LB, Stallknecht B, Vaag AA, Dela F. Endothelial function after 10 days of bed rest in individuals at risk for type 2 diabetes and cardiovascular disease. Exp Physiol 96: 1000–1009, 2011. doi: 10.1113/expphysiol.2011.058511. [DOI] [PubMed] [Google Scholar]
- 93. Sonne MP, Alibegovic AC, Hojbjerre L, Vaag A, Stallknecht B, Dela F. Effect of 10 days of bedrest on metabolic and vascular insulin action: a study in individuals at risk for type 2 diabetes. J Appl Physiol (1985) 108: 830–837, 2010. doi: 10.1152/japplphysiol.00545.2009. [DOI] [PubMed] [Google Scholar]
- 94. Friedrichsen M, Ribel-Madsen R, Mortensen B, Hansen CN, Alibegovic AC, Hojbjerre L, Sonne MP, Wojtaszewski JF, Stallknecht B, Dela F, Vaag A. Muscle inflammatory signaling in response to 9 days of physical inactivity in young men with low compared with normal birth weight. Eur J Endocrinol 167: 829–838, 2012. doi: 10.1530/EJE-12-0498. [DOI] [PubMed] [Google Scholar]
- 95. Mortensen B, Friedrichsen M, Andersen NR, Alibegovic AC, Hojbjerre L, Sonne MP, Stallknecht B, Dela F, Wojtaszewski JF, Vaag A. Physical inactivity affects skeletal muscle insulin signaling in a birth weight-dependent manner. J Diabetes Complications 28: 71–78, 2014. doi: 10.1016/j.jdiacomp.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 96. Bergouignan A, Momken I, Schoeller DA, Normand S, Zahariev A, Lescure B, Simon C, Blanc S. Regulation of energy balance during long-term physical inactivity induced by bed rest with and without exercise training. J Clin Endocrinol Metab 95: 1045–1053, 2010. doi: 10.1210/jc.2009-1005. [DOI] [PubMed] [Google Scholar]
- 97. Blanc S, Normand S, Ritz P, Pachiaudi C, Vico L, Gharib C, Gauquelin-Koch G. Energy and water metabolism, body composition, and hormonal changes induced by 42 days of enforced inactivity and simulated weightlessness. J Clin Endocrinol Metab 83: 4289–4297, 1998. doi: 10.1210/jcem.83.12.5340. [DOI] [PubMed] [Google Scholar]
- 98. Dirks ML, Wall BT, van de Valk B, Holloway TM, Holloway GP, Chabowski A, Goossens GH, van Loon LJ. One week of bed rest leads to substantial muscle atrophy and induces whole-body insulin resistance in the absence of skeletal muscle lipid accumulation. Diabetes 65: 2862–2875, 2016. doi: 10.2337/db15-1661. [DOI] [PubMed] [Google Scholar]
- 99. LeBlanc AD, Schneider VS, Evans HJ, Pientok C, Rowe R, Spector E. Regional changes in muscle mass following 17 weeks of bed rest. J Appl Physiol (1985) 73: 2172–2178, 1992. doi: 10.1152/jappl.1992.73.5.2172. [DOI] [PubMed] [Google Scholar]
- 100. Pavy-Le Traon A, Heer M, Narici MV, Rittweger J, Vernikos J. From space to earth: advances in human physiology from 20 years of bed rest studies (1986–2006). Eur J Appl Physiol 101: 143–194, 2007. doi: 10.1007/s00421-007-0474-z. [DOI] [PubMed] [Google Scholar]
- 101. Adams GR, Caiozzo VJ, Baldwin KM. Skeletal muscle unweighting: spaceflight and ground-based models. J Appl Physiol (1985) 95: 2185–2201, 2003. doi: 10.1152/japplphysiol.00346.2003. [DOI] [PubMed] [Google Scholar]
- 102. Yasuda N, Glover EI, Phillips SM, Isfort RJ, Tarnopolsky MA. Sex-based differences in skeletal muscle function and morphology with short-term limb immobilization. J Appl Physiol (1985) 99: 1085–1092, 2005. doi: 10.1152/japplphysiol.00247.2005. [DOI] [PubMed] [Google Scholar]
- 103. Glover EI, Phillips SM, Oates BR, Tang JE, Tarnopolsky MA, Selby A, Smith K, Rennie MJ. Immobilization induces anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol 586: 6049–6061, 2008. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hvid LG, Ortenblad N, Aagaard P, Kjaer M, Suetta C. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol 589: 4745–4757, 2011. doi: 10.1113/jphysiol.2011.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Suetta C, Hvid LG, Justesen L, Christensen U, Neergaard K, Simonsen L, Ortenblad N, Magnusson SP, Kjaer M, Aagaard P. Effects of aging on human skeletal muscle after immobilization and retraining. J Appl Physiol (1985) 107: 1172–1180, 2009. doi: 10.1152/japplphysiol.00290.2009. [DOI] [PubMed] [Google Scholar]
- 106. Perkin O, McGuigan P, Thompson D, Stokes K. A reduced activity model: a relevant tool for the study of ageing muscle. Biogerontology 17: 435–447, 2016. doi: 10.1007/s10522-015-9613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Vigelso A, Gram M, Wiuff C, Hansen CN, Prats C, Dela F, Helge JW. Effects of immobilization and aerobic training on proteins related to intramuscular substrate storage and metabolism in young and older men. Eur J Appl Physiol 116: 481–494, 2016. doi: 10.1007/s00421-015-3302-x. [DOI] [PubMed] [Google Scholar]
- 108. Mitchell CJ, D'Souza RF, Mitchell SM, Figueiredo VC, Miller BF, Hamilton KL, Peelor FF 3rd, Coronet M, Pileggi CA, Durainayagam B, Fanning AC, Poppitt SD, Cameron-Smith D. Impact of dairy protein during limb immobilization and recovery on muscle size and protein synthesis; a randomized controlled trial. J Appl Physiol (1985) 124: 717–728, 2018. doi: 10.1152/japplphysiol.00803.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Edwards SJ, Smeuninx B, McKendry J, Nishimura Y, Luo D, Marshall RN, Perkins M, Ramsay J, Joanisse S, Philp A, Breen L. High-dose leucine supplementation does not prevent muscle atrophy or strength loss over 7 days of immobilization in healthy young males. Am J Clin Nutr 112: 1368–1381, 2020. doi: 10.1093/ajcn/nqaa229. [DOI] [PubMed] [Google Scholar]
- 110. Ceroni D, Martin X, Delhumeau C, Farpour-Lambert N. Decrease of physical activity level in adolescents with limb fractures: an accelerometry-based activity monitor study. BMC Musculoskelet Disord 12: 87, 2011. doi: 10.1186/1471-2474-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Rickert C, Grabowski M, Gosheger G, Schorn D, Schneider KN, Klingebiel S, Liem D. How shoulder immobilization influences daily physical activity – an accelerometer based preliminary study. BMC Musculoskelet Disord 21: 126, 2020. doi: 10.1186/s12891-020-3133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Kraus WE, Janz KF, Powell KE, Campbell WW, Jakicic JM, Troiano RP, Sprow K, Torres A, Piercy KL, Physical Activity Guidelines Advisory Committee. Daily step counts for measuring physical activity exposure and its relation to health. Med Sci Sports Exerc 51: 1206–1212, 2019. doi: 10.1249/MSS.0000000000001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Boyle LJ, Credeur DP, Jenkins NT, Padilla J, Leidy HJ, Thyfault JP, Fadel PJ. Impact of reduced daily physical activity on conduit artery flow-mediated dilation and circulating endothelial microparticles. J Appl Physiol (1985) 115: 1519–1525, 2013. doi: 10.1152/japplphysiol.00837.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Reynolds LJ, Credeur DP, Holwerda SW, Leidy HJ, Fadel PJ, Thyfault JP. Acute inactivity impairs glycemic control but not blood flow to glucose ingestion. Med Sci Sports Exerc 47: 1087–1094, 2015. doi: 10.1249/MSS.0000000000000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Devries MC, Breen L, Von Allmen M, MacDonald MJ, Moore DR, Offord EA, Horcajada MN, Breuille D, Phillips SM. Low-load resistance training during step-reduction attenuates declines in muscle mass and strength and enhances anabolic sensitivity in older men. Physiol Rep 3: e12493, 2015. doi: 10.14814/phy2.12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Damiot A, Demangel R, Noone J, Chery I, Zahariev A, Normand S, Brioche T, Crampes F, de Glisezinski I, Lefai E, Bareille MP, Chopard A, Drai J, Collin-Chavagnac D, Heer M, Gauquelin-Koch G, Prost M, Simon P, Py G, Blanc S, Simon C, Bergouignan A, O'Gorman DJ. A nutrient cocktail prevents lipid metabolism alterations induced by 20 days of daily steps reduction and fructose overfeeding: result from a randomized study. J Appl Physiol (1985) 126: 88–101, 2019. doi: 10.1152/japplphysiol.00018.2018. [DOI] [PubMed] [Google Scholar]
- 117. Knudsen SH, Hansen LS, Pedersen M, Dejgaard T, Hansen J, Hall GV, Thomsen C, Solomon TP, Pedersen BK, Krogh-Madsen R. Changes in insulin sensitivity precede changes in body composition during 14 days of step reduction combined with overfeeding in healthy young men. J Appl Physiol (1985) 113: 7–15, 2012. doi: 10.1152/japplphysiol.00189.2011. [DOI] [PubMed] [Google Scholar]
- 118. Krogh-Madsen R, Thyfault JP, Broholm C, Mortensen OH, Olsen RH, Mounier R, Plomgaard P, van Hall G, Booth FW, Pedersen BK. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J Appl Physiol (1985) 108: 1034–1040, 2010. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- 119. Olsen RH, Krogh-Madsen R, Thomsen C, Booth FW, Pedersen BK. Metabolic responses to reduced daily steps in healthy nonexercising men. JAMA 299: 1261–1263, 2008. doi: 10.1001/jama.299.11.1259. [DOI] [PubMed] [Google Scholar]
- 120. Breen L, Stokes KA, Churchward-Venne TA, Moore DR, Baker SK, Smith K, Atherton PJ, Phillips SM. Two weeks of reduced activity decreases leg lean mass and induces “anabolic resistance” of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab 98: 2604–2612, 2013. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 121. Moore DR, Kelly RP, Devries MC, Churchward-Venne TA, Phillips SM, Parise G, Johnston AP. Low-load resistance exercise during inactivity is associated with greater fibre area and satellite cell expression in older skeletal muscle. J Cachexia Sarcopenia Muscle 9: 747–754, 2018. doi: 10.1002/jcsm.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Bowden Davies KA, Sprung VS, Norman JA, Thompson A, Mitchell KL, Halford JC, Harrold JA, Wilding JP, Kemp GJ, Cuthbertson DJ. Short-term decreased physical activity with increased sedentary behaviour causes metabolic derangements and altered body composition: effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia 61: 1282–1294, 2018. doi: 10.1007/s00125-018-4603-5. [DOI] [PubMed] [Google Scholar]
- 123. Shad BJ, Thompson JL, Holwerda AM, Stocks B, Elhassan YS, Philp A, Vanl LJ, Wallis GA. One week of step reduction lowers myofibrillar protein synthesis rates in young men. Med Sci Sports Exerc 51: 2125–2134, 2019. doi: 10.1249/MSS.0000000000002034. [DOI] [PubMed] [Google Scholar]
- 124. Fisher SR, Goodwin JS, Protas EJ, Kuo YF, Graham JE, Ottenbacher KJ, Ostir GV. Ambulatory activity of older adults hospitalized with acute medical illness. J Am Geriatr Soc 59: 91–95, 2011. doi: 10.1111/j.1532-5415.2010.03202.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Takahashi T, Kumamaru M, Jenkins S, Saitoh M, Morisawa T, Matsuda H. In-patient step count predicts re-hospitalization after cardiac surgery. J Cardiol 66: 286–291, 2015. doi: 10.1016/j.jjcc.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 126. Mezlini A, Shapiro A, Daza EJ, Caddigan E, Ramirez E, Althoff T, Foschini L. Estimating the burden of influenza-like illness on daily activity at the population scale using commercial wearable sensors. JAMA Netw Open 5: e2211958, 2022. doi: 10.1001/jamanetworkopen.2022.11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mikus CR, Oberlin DJ, Libla JL, Taylor AM, Booth FW, Thyfault JP. Lowering physical activity impairs glycemic control in healthy volunteers. Med Sci Sports Exerc 44: 225–231, 2012. doi: 10.1249/MSS.0b013e31822ac0c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dunstan D, Howard B, Bergouignan A, Kingwell B, Owen N. Chapter 3: physiological effects of reducing and breaking up sitting time. In: Sedentary Behavior and Health: Concepts, Assessments, and Interventions edited by Zhu Q, Owen N. Urbana, IL: Human Kinetics, 2017. [Google Scholar]
- 129. Paterson C, Fryer S, Stone K, Zieff G, Turner L, Stoner L. The effects of acute exposure to prolonged sitting, with and without interruption, on peripheral blood pressure among adults: a systematic review and meta-analysis. Sports Med 52: 1369–1383, 2022. doi: 10.1007/s40279-021-01614-7. [DOI] [PubMed] [Google Scholar]
- 130. Paterson C, Fryer S, Zieff G, Stone K, Credeur DP, Barone Gibbs B, Padilla J, Parker JK, Stoner L. The effects of acute exposure to prolonged sitting, with and without interruption, on vascular function among adults: a meta-analysis. Sports Med 50: 1929–1942, 2020. doi: 10.1007/s40279-020-01325-5. [DOI] [PubMed] [Google Scholar]
- 131. Larsen RN, Kingwell BA, Robinson C, Hammond L, Cerin E, Shaw JE, Healy GN, Hamilton MT, Owen N, Dunstan DW. Breaking up of prolonged sitting over three days sustains, but does not enhance, lowering of postprandial plasma glucose and insulin in overweight and obese adults. Clin Sci (Lond) 129: 117–127, 2015. doi: 10.1042/CS20140790. [DOI] [PubMed] [Google Scholar]
- 132. Duvivier B, Schaper NC, Koster A, van Kan L, Peters HP, Adam JJ, Giesbrecht T, Kornips E, Hulsbosch M, Willems P, Hesselink MK, Schrauwen P, Savelberg H. Benefits of substituting sitting with standing and walking in free-living conditions for cardiometabolic risk markers, cognition and mood in overweight adults. Front Physiol 8: 353, 2017. doi: 10.3389/fphys.2017.00353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Duvivier BM, Schaper NC, Bremers MA, van Crombrugge G, Menheere PP, Kars M, Savelberg HH. Minimal intensity physical activity (standing and walking) of longer duration improves insulin action and plasma lipids more than shorter periods of moderate to vigorous exercise (cycling) in sedentary subjects when energy expenditure is comparable. PLoS One 8: e55542, 2013. doi: 10.1371/journal.pone.0055542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. De Jong NP, Rynders CA, Goldstrohm DA, Pan Z, Lange AH, Mendez C, Melanson EL, Bessesen DH, Bergouignan A. Effect of frequent interruptions of sedentary time on nutrient metabolism in sedentary overweight male and female adults. J Appl Physiol (1985) 126: 984–992, 2019. doi: 10.1152/japplphysiol.00632.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Blankenship JM, Winkler EA, Healy GN, Dempsey PC, Bellettiere J, Owen N, Dunstan DW. Descriptive epidemiology of interruptions to free-living sitting time in middle-age and older adults. Med Sci Sports Exerc 53: 2503–2511, 2021. doi: 10.1249/MSS.0000000000002750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stroeve JH, van Wietmarschen H, Kremer BH, van Ommen B, Wopereis S. Phenotypic flexibility as a measure of health: the optimal nutritional stress response test. Genes Nutr 10: 13, 2015. doi: 10.1007/s12263-015-0459-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Bergouignan A, Momken I, Lefai E, Antoun E, Schoeller DA, Platat C, Chery I, Zahariev A, Vidal H, Gabert L, Normand S, Freyssenet D, Laville M, Simon C, Blanc S. Activity energy expenditure is a major determinant of dietary fat oxidation and trafficking, but the deleterious effect of detraining is more marked than the beneficial effect of training at current recommendations. Am J Clin Nutr 98: 648–658, 2013. doi: 10.3945/ajcn.112.057075. [DOI] [PubMed] [Google Scholar]
- 138. Krogh-Madsen R, Pedersen M, Solomon TP, Knudsen SH, Hansen LS, Karstoft K, Lehrskov-Schmidt L, Pedersen KK, Thomsen C, Holst JJ, Pedersen BK. Normal physical activity obliterates the deleterious effects of a high-caloric intake. J Appl Physiol (1985) 116: 231–239, 2014. doi: 10.1152/japplphysiol.00155.2013. [DOI] [PubMed] [Google Scholar]
- 139. Oikawa SY, McGlory C, D'Souza LK, Morgan AK, Saddler NI, Baker SK, Parise G, Phillips SM. A randomized controlled trial of the impact of protein supplementation on leg lean mass and integrated muscle protein synthesis during inactivity and energy restriction in older persons. Am J Clin Nutr 108: 1060–1068, 2018. doi: 10.1093/ajcn/nqy193. [DOI] [PubMed] [Google Scholar]
- 140. Reidy PT, McKenzie AI, Mahmassani Z, Morrow VR, Yonemura NM, Hopkins PN, Marcus RL, Rondina MT, Lin YK, Drummond MJ. Skeletal muscle ceramides and relationship with insulin sensitivity after 2 weeks of simulated sedentary behaviour and recovery in healthy older adults. J Physiol 596: 5217–5236, 2018. doi: 10.1113/JP276798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Kim BS, Lee Y, Kim HJ, Shin JH, Park JK, Park HC, Lim YH, Shin J. Influence of changes in body fat on clinical outcomes in a general population: a 12-year follow-up report on the Ansan-Ansung cohort in the Korean Genome Environment Study. Ann Med 53: 1646–1658, 2021. doi: 10.1080/07853890.2021.1976416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Sanchez-Lastra MA, Ding D, Dalene KE, Ekelund U, Tarp J. Physical activity and mortality across levels of adiposity: a prospective cohort study from the UK biobank. Mayo Clin Proc 96: 105–119, 2021. doi: 10.1016/j.mayocp.2020.06.049. [DOI] [PubMed] [Google Scholar]
- 143. Kivimäki M, Strandberg T, Pentti J, Nyberg ST, Frank P, Jokela M, Ervasti J, Suominen SB, Vahtera J, Sipilä PN, Lindbohm JV, Ferrie JE. Body-mass index and risk of obesity-related complex multimorbidity: an observational multicohort study. Lancet Diabetes Endocrinol 10: 253–263, 2022. doi: 10.1016/S2213-8587(22)00033-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Brierley ML, Chater AM, Smith LR, Bailey DP. The effectiveness of sedentary behaviour reduction workplace interventions on cardiometabolic risk markers: a systematic review. Sports Med 49: 1739–1767, 2019. doi: 10.1007/s40279-019-01168-9. [DOI] [PubMed] [Google Scholar]
- 145. Contardo Ayala AM, Salmon J, Timperio A, Sudholz B, Ridgers ND, Sethi P, Dunstan DW. Impact of an 8-month trial using height-adjustable desks on children’s classroom sitting patterns and markers of cardio-metabolic and musculoskeletal health. Int J Environ Res Public Health 13: 1227, 2016. doi: 10.3390/ijerph13121227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Simon C, Wagner A, Platat C, Arveiler D, Schweitzer B, Schlienger JL, Triby E. ICAPS: a multilevel program to improve physical activity in adolescents. Diabetes Metab 32: 41–49, 2006. doi: 10.1016/s1262-3636(07)70245-8. [DOI] [PubMed] [Google Scholar]
- 147. Simon C, Kellou N, Dugas J, Platat C, Copin N, Schweitzer B, Hausser F, Bergouignan A, Lefai E, Blanc S. A socio-ecological approach promoting physical activity and limiting sedentary behavior in adolescence showed weight benefits maintained 2.5 years after intervention cessation. Int J Obes (Lond) 38: 936–943, 2014. doi: 10.1038/ijo.2014.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Kozey Keadle S, Lyden K, Staudenmayer J, Hickey A, Viskochil R, Braun B, Freedson PS. The independent and combined effects of exercise training and reducing sedentary behavior on cardiometabolic risk factors. Appl Physiol Nutr Metab 39: 770–780, 2014. doi: 10.1139/apnm-2013-0379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Steeves JA, Bassett DR, Fitzhugh EC, Raynor HA, Thompson DL. Can sedentary behavior be made more active? A randomized pilot study of TV commercial stepping versus walking. Int J Behav Nutr Phys Act 9: 95, 2012. doi: 10.1186/1479-5868-9-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Overgaard K, Nannerup K, Lunen MK, Maindal HT, Larsen RG. Exercise more or sit less? a randomized trial assessing the feasibility of two advice-based interventions in obese inactive adults. J Sci Med Sport 21: 708–713, 2018. doi: 10.1016/j.jsams.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 151. Barte JC, Veldwijk J, Teixeira PJ, Sacks FM, Bemelmans WJ. Differences in weight loss across different bmi classes: a meta-analysis of the effects of interventions with diet and exercise. Int J Behav Med 21: 784–793, 2014. doi: 10.1007/s12529-013-9355-5. [DOI] [PubMed] [Google Scholar]
- 152. Bourdier P, Simon C, Bessesen DH, Blanc S, Bergouignan A. The role of physical activity in the regulation of body weight: the overlooked contribution of light physical activity and sedentary behaviors. Obes Rev 24: e13528, 2023. doi: 10.1111/obr.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Careau V, Halsey LG, Pontzer H, Ainslie PN, Andersen LF, Anderson LJ, et al. Energy compensation and adiposity in humans. Curr Biol 31: 4659–4666.e4652, 2021. doi: 10.1016/j.cub.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Pontzer H, Durazo-Arvizu R, Dugas LR, Plange-Rhule J, Bovet P, Forrester TE, Lambert EV, Cooper RS, Schoeller DA, Luke A. Constrained total energy expenditure and metabolic adaptation to physical activity in adult humans. Curr Biol 26: 410–417, 2016. doi: 10.1016/j.cub.2015.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Lyden K, Keadle SK, Staudenmayer J, Braun B, Freedson PS. Discrete features of sedentary behavior impact cardiometabolic risk factors. Med Sci Sports Exerc 47: 1079–1086, 2015. doi: 10.1249/MSS.0000000000000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Carter SE, Jones M, Gladwell VF. Energy expenditure and heart rate response to breaking up sedentary time with three different physical activity interventions. Nutr Metab Cardiovasc Dis 25: 503–509, 2015. doi: 10.1016/j.numecd.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 157. Swartz AM, Squires L, Strath SJ. Energy expenditure of interruptions to sedentary behavior. Int J Behav Nutr Phys Act 8: 69, 2011. doi: 10.1186/1479-5868-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Fenemor SP, Homer AR, Perry TL, Skeaff CM, Peddie MC, Rehrer NJ. Energy utilization associated with regular activity breaks and continuous physical activity: a randomized crossover trial. Nutr Metab Cardiovasc Dis 28: 557–564, 2018. doi: 10.1016/j.numecd.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 159. Riou ME, Jomphe-Tremblay S, Lamothe G, Finlayson GS, Blundell JE, Decarie-Spain L, Gagnon JC, Doucet E. Energy compensation following a supervised exercise intervention in women living with overweight/obesity is accompanied by an early and sustained decrease in non-structured physical activity. Front Physiol 10: 1048, 2019. doi: 10.3389/fphys.2019.01048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Granados K, Stephens BR, Malin SK, Zderic TW, Hamilton MT, Braun B. Appetite regulation in response to sitting and energy imbalance. Appl Physiol Nutr Metab 37: 323–333, 2012. doi: 10.1139/h2012-002. [DOI] [PubMed] [Google Scholar]
- 161. Stubbs RJ, Hughes DA, Johnstone AM, Horgan GW, King N, Blundell JE. A decrease in physical activity affects appetite, energy, and nutrient balance in lean men feeding ad libitum. Am J Clin Nutr 79: 62–69, 2004. doi: 10.1093/ajcn/79.1.62. [DOI] [PubMed] [Google Scholar]
- 162. Hatamoto Y, Takae R, Goya R, Yoshimura E, Higaki Y, Tanaka H. Effects of different physical activity levels during a single day on energy intake, appetite, and energy balance: a preliminary study. Nutrients 11: 690, 2019. doi: 10.3390/nu11030690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Debevec T, Simpson EJ, Mekjavic IB, Eiken O, Macdonald IA. Effects of prolonged hypoxia and bed rest on appetite and appetite-related hormones. Appetite 107: 28–37, 2016. doi: 10.1016/j.appet.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 164. Holmstrup ME, Fairchild TJ, Keslacy S, Weinstock RS, Kanaley JA. Satiety, but not total PYY, is increased with continuous and intermittent exercise. Obesity (Silver Spring) 21: 2014–2020, 2013. doi: 10.1002/oby.20335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Bergouignan A, Legget KT, De Jong N, Kealey E, Nikolovski J, Groppel JL, Jordan C, O'Day R, Hill JO, Bessesen DH. Effect of frequent interruptions of prolonged sitting on self-perceived levels of energy, mood, food cravings and cognitive function. Int J Behav Nutr Phys Act 13: 113, 2016. doi: 10.1186/s12966-016-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Bailey DP, Broom DR, Chrismas BC, Taylor L, Flynn E, Hough J. Breaking up prolonged sitting time with walking does not affect appetite or gut hormone concentrations but does induce an energy deficit and suppresses postprandial glycaemia in sedentary adults. Appl Physiol Nutr Metab 41: 324–331, 2016. doi: 10.1139/apnm-2015-0462. [DOI] [PubMed] [Google Scholar]
- 167. Mete EM, Perry TL, Haszard JJ, Homer AR, Fenemor SP, Rehrer NJ, Skeaff CM, Peddie MC. Interrupting prolonged sitting with regular activity breaks does not acutely influence appetite: a randomised controlled trial. Nutrients 10: 125, 2018. doi: 10.3390/nu10020125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Maylor BD, Zakrzewski-Fruer JK, Stensel DJ, Orton CJ, Bailey DP. Breaking up sitting with short frequent or long infrequent physical activity breaks does not lead to compensatory changes in appetite, appetite-regulating hormones or energy intake. Appetite 182: 106445, 2023. doi: 10.1016/j.appet.2022.106445. [DOI] [PubMed] [Google Scholar]
- 169. Dutta N, Koepp GA, Stovitz SD, Levine JA, Pereira MA. Using sit-stand workstations to decrease sedentary time in office workers: a randomized crossover trial. Int J Environ Res Public Health 11: 6653–6665, 2014. doi: 10.3390/ijerph110706653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Winn NC, Pettit-Mee R, Walsh LK, Restaino RM, Ready ST, Padilla J, Kanaley JA. Metabolic implications of diet and energy intake during physical inactivity. Med Sci Sports Exerc 51: 995–1005, 2019. doi: 10.1249/MSS.0000000000001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. McGlory C, von Allmen MT, Stokes T, Morton RW, Hector AJ, Lago BA, Raphenya AR, Smith BK, McArthur AG, Steinberg GR, Baker SK, Phillips SM. Failed recovery of glycemic control and myofibrillar protein synthesis with 2 wk of physical inactivity in overweight, prediabetic older adults. J Gerontol A Biol Sci Med Sci 73: 1070–1077, 2018. doi: 10.1093/gerona/glx203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Reidy PT, Yonemura NM, Madsen JH, McKenzie AI, Mahmassani ZS, Rondina MT, Lin YK, Kaput K, Drummond MJ. An accumulation of muscle macrophages is accompanied by altered insulin sensitivity after reduced activity and recovery. Acta Physiol (Oxf) 226: e13251, 2019. doi: 10.1111/apha.13251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Bergouignan A, Rudwill F, Simon C, Blanc S. Physical inactivity as the culprit of metabolic inflexibility: evidence from bed-rest studies. J Appl Physiol (1985) 111: 1201–1210, 2011. doi: 10.1152/japplphysiol.00698.2011. [DOI] [PubMed] [Google Scholar]
- 174. Yanagibori R, Suzuki Y, Kawakubo K, Makita Y, Gunji A. Carbohydrate and lipid metabolism after 20 days of bed rest. Acta Physiol Scand Suppl 616: 51–57, 1994. [PubMed] [Google Scholar]
- 175. Rudwill F, O'Gorman D, Lefai E, Chery I, Zahariev A, Normand S, Pagano AF, Chopard A, Damiot A, Laurens C, Hodson L, Canet-Soulas E, Heer M, Meuthen PF, Buehlmeier J, Baecker N, Meiller L, Gauquelin-Koch G, Blanc S, Simon C, Bergouignan A. Metabolic inflexibility is an early marker of bed-rest-induced glucose intolerance even when fat mass is stable. J Clin Endocrinol Metab 103: 1910–1920, 2018. doi: 10.1210/jc.2017-02267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Gemmink A, Goodpaster BH, Schrauwen P, Hesselink MK. Intramyocellular lipid droplets and insulin sensitivity, the human perspective. Biochim Biophys Acta Mol Cell Biol Lipids 1862: 1242–1249, 2017. doi: 10.1016/j.bbalip.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 177. Bergouignan A, Trudel G, Simon C, Chopard A, Schoeller DA, Momken I, Votruba SB, Desage M, Burdge GC, Gauquelin-Koch G, Normand S, Blanc S. Physical inactivity differentially alters dietary oleate and palmitate trafficking. Diabetes 58: 367–376, 2009. doi: 10.2337/db08-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Rudwill F, Blanc S, Gauquelin-Koch G, Chouker A, Heer M, Simon C, Bergouignan A. Effects of different levels of physical inactivity on plasma visfatin in healthy normal-weight men. Appl Physiol Nutr Metab 38: 689–693, 2013. doi: 10.1139/apnm-2012-0434. [DOI] [PubMed] [Google Scholar]
- 179. Shur NF, Simpson EJ, Crossland H, Chivaka PK, Constantin D, Cordon SM, Constantin-Teodosiu D, Stephens FB, Lobo DN, Szewczyk N, Narici M, Prats C, Macdonald IA, Greenhaff PL. Human adaptation to immobilization: novel insights of impacts on glucose disposal and fuel utilization. J Cachexia Sarcopenia Muscle 13: 2999–3013, 2022. doi: 10.1002/jcsm.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Manini TM, Clark BC, Nalls MA, Goodpaster BH, Ploutz-Snyder LL, Harris TB. Reduced physical activity increases intermuscular adipose tissue in healthy young adults. Am J Clin Nutr 85: 377–384, 2007. doi: 10.1093/ajcn/85.2.377. [DOI] [PubMed] [Google Scholar]
- 181. Cree MG, Paddon-Jones D, Newcomer BR, Ronsen O, Aarsland A, Wolfe RR, Ferrando A. Twenty-eight-day bed rest with hypercortisolemia induces peripheral insulin resistance and increases intramuscular triglycerides. Metabolism 59: 703–710, 2010. doi: 10.1016/j.metabol.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Alibegovic AC, Sonne MP, Hojbjerre L, Bork-Jensen J, Jacobsen S, Nilsson E, Faerch K, Hiscock N, Mortensen B, Friedrichsen M, Stallknecht B, Dela F, Vaag A. Insulin resistance induced by physical inactivity is associated with multiple transcriptional changes in skeletal muscle in young men. Am J Physiol Endocrinol Metab 299: E752–E763, 2010. doi: 10.1152/ajpendo.00590.2009. [DOI] [PubMed] [Google Scholar]
- 183. Bienso RS, Ringholm S, Kiilerich K, Aachmann-Andersen NJ, Krogh-Madsen R, Guerra B, Plomgaard P, van Hall G, Treebak JT, Saltin B, Lundby C, Calbet JA, Pilegaard H, Wojtaszewski JF. GLUT4 and glycogen synthase are key players in bed rest-induced insulin resistance. Diabetes 61: 1090–1099, 2012. doi: 10.2337/db11-0884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Healy GN, Dunstan DW, Salmon J, Cerin E, Shaw JE, Zimmet PZ, Owen N. Objectively measured light-intensity physical activity is independently associated with 2-h plasma glucose. Diabetes Care 30: 1384–1389, 2007. doi: 10.2337/dc07-0114. [DOI] [PubMed] [Google Scholar]
- 185. Helmerhorst HJ, Wijndaele K, Brage S, Wareham NJ, Ekelund U. Objectively measured sedentary time may predict insulin resistance independent of moderate- and vigorous-intensity physical activity. Diabetes 58: 1776–1779, 2009. doi: 10.2337/db08-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4 years. Diabetes Care 22: 233–240, 1999. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 187. Levitan EB, Song Y, Ford ES, Liu S. Is nondiabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med 164: 2147–2155, 2004. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 188. Buffey AJ, Herring MP, Langley CK, Donnelly AE, Carson BP. The acute effects of interrupting prolonged sitting time in adults with standing and light-intensity walking on biomarkers of cardiometabolic health in adults: a systematic review and meta-analysis. Sports Med 52: 1765–1787, 2022. doi: 10.1007/s40279-022-01649-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Bailey DP, Stringer CA, Maylor BD, Zakrzewski-Fruer JK. Lower amounts of daily and prolonged sitting do not lower free-living continuously monitored glucose concentrations in overweight and obese adults: a randomised crossover study. Nutrients 14: 605, 2022. doi: 10.3390/nu14030605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Correia IR, Magalhaes JP, Judice PB, Hetherington-Rauth M, Freitas SP, Lopes JM, Gama FF, Sardinha LB. Breaking-up sedentary behavior and detraining effects on glycemic control: a randomized crossover trial in trained older adults. J Aging Phys Act 31: 391–399, 2022. doi: 10.1123/japa.2022-0124. [DOI] [PubMed] [Google Scholar]
- 191. Engeroff T, Fuzeki E, Vogt L, Banzer W. The acute effects of single or repeated bouts of vigorous-intensity exercise on insulin and glucose metabolism during postprandial sedentary behavior. Int J Environ Res Public Health 19: 4422, 2022. doi: 10.3390/ijerph19084422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Nieste I, Franssen WM, Duvivier B, Spaas J, Savelberg H, Eijnde BO. Replacing sitting with light-intensity physical activity throughout the day versus 1 bout of vigorous-intensity exercise: similar cardiometabolic health effects in multiple sclerosis. A randomised cross-over study. Disabil Rehabil. In press. doi: 10.1080/09638288.2022.2122601. [DOI] [PubMed] [Google Scholar]
- 193. Homer AR, Taylor FC, Dempsey PC, Wheeler MJ, Sethi P, Townsend MK, Grace MS, Green DJ, Cohen ND, Larsen RN, Kingwell BA, Owen N, Dunstan DW. Frequency of interruptions to sitting time: benefits for postprandial metabolism in type 2 diabetes. Diabetes Care 44: 1254–1263, 2021. doi: 10.2337/dc20-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Larsen R, Ali H, Dempsey PC, Grace M, Dillon F, Kingwell BA, Cohen N, Owen N, Green DJ, Dunstan DW. Interrupting sitting time with simple resistance activities lowers postprandial insulinemia in adults with overweight or obesity. Obesity (Silver Spring) 27: 1428–1433, 2019. doi: 10.1002/oby.22554. [DOI] [PubMed] [Google Scholar]
- 195. Thorsen IK, Johansen MY, Pilmark NS, Jespersen NZ, Brinklov CF, Benatti FB, Dunstan DW, Karstoft K, Pedersen BK, Ried-Larsen M. The effect of frequency of activity interruptions in prolonged sitting on postprandial glucose metabolism: a randomized crossover trial. Metabolism 96: 1–7, 2019. doi: 10.1016/j.metabol.2019.04.003. [DOI] [PubMed] [Google Scholar]
- 196. Wheeler MJ, Green DJ, Cerin E, Ellis KA, Heinonen I, Lewis J, Naylor LH, Cohen N, Larsen R, Dempsey PC, Kingwell BA, Owen N, Dunstan DW. Combined effects of continuous exercise and intermittent active interruptions to prolonged sitting on postprandial glucose, insulin, and triglycerides in adults with obesity: a randomized crossover trial. Int J Behav Nutr Phys Act 17: 152, 2020. doi: 10.1186/s12966-020-01057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yates T, Edwardson CL, Celis-Morales C, Biddle SJ, Bodicoat D, Davies MJ, Esliger D, Henson J, Kazi A, Khunti K, Sattar N, Sinclair AJ, Rowlands A, Velayudhan L, Zaccardi F, Gill JM. Metabolic effects of breaking prolonged sitting with standing or light walking in older South Asians and white Europeans: a randomized acute study. J Gerontol A Biol Sci Med Sci 75: 139–146, 2020. doi: 10.1093/gerona/gly252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Hawari NS, Wilson J, Gill JM. Effects of breaking up sedentary time with “chair squats” on postprandial metabolism. J Sports Sci 37: 331–338, 2019. doi: 10.1080/02640414.2018.1500856. [DOI] [PubMed] [Google Scholar]
- 199. Blankenship JM, Chipkin SR, Freedson PS, Staudenmayer J, Lyden K, Braun B. Managing free-living hyperglycemia with exercise or interrupted sitting in type 2 diabetes. J Appl Physiol (1985) 126: 616–625, 2019. doi: 10.1152/japplphysiol.00389.2018. [DOI] [PubMed] [Google Scholar]
- 200. Blankenship JM, Granados K, Braun B. Effects of subtracting sitting versus adding exercise on glycemic control and variability in sedentary office workers. Appl Physiol Nutr Metab 39: 1286–1293, 2014. doi: 10.1139/apnm-2014-0157. [DOI] [PubMed] [Google Scholar]
- 201. Kerr J, Crist K, Vital DG, Dillon L, Aden SA, Trivedi M, Castellanos LR, Godbole S, Li H, Allison MA, Khemlina GL, Takemoto ML, Schenk S, Sallis JF, Grace M, Dunstan DW, Natarajan L, LaCroix AZ, Sears DD. Acute glucoregulatory and vascular outcomes of three strategies for interrupting prolonged sitting time in postmenopausal women: a pilot, laboratory-based, randomized, controlled, 4-condition, 4-period crossover trial. PLoS One 12: e0188544, 2017. doi: 10.1371/journal.pone.0188544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Holmstrup M, Fairchild T, Keslacy S, Weinstock R, Kanaley J. Multiple short bouts of exercise over 12-h period reduce glucose excursions more than an energy-matched single bout of exercise. Metabolism 63: 510–519, 2014. doi: 10.1016/j.metabol.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Duvivier BM, Schaper NC, Hesselink MK, van Kan L, Stienen N, Winkens B, Koster A, Savelberg HH. Breaking sitting with light activities vs structured exercise: a randomised crossover study demonstrating benefits for glycaemic control and insulin sensitivity in type 2 diabetes. Diabetologia 60: 490–498, 2017. doi: 10.1007/s00125-016-4161-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Quan M, Xun P, Wu H, Wang J, Cheng W, Cao M, Zhou T, Huang T, Gao Z, Chen P. Effects of interrupting prolonged sitting on postprandial glycemia and insulin responses: a network meta-analysis. J Sport Health Sci 10: 419–429, 2021. doi: 10.1016/j.jshs.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Henson J, Edwardson CL, Celis-Morales CA, Davies MJ, Dunstan DW, Esliger DW, Gill JM, Kazi A, Khunti K, King J, McCarthy M, Sattar N, Stensel DJ, Velayudhan L, Zaccardi F, Yates T. Predictors of the acute postprandial response to breaking up prolonged sitting. Med Sci Sports Exerc 52: 1385–1393, 2020. doi: 10.1249/MSS.0000000000002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Larsen RN, Dempsey PC, Dillon F, Grace M, Kingwell BA, Owen N, Dunstan DW. Does the type of activity “break” from prolonged sitting differentially impact on postprandial blood glucose reductions? An exploratory analysis. Appl Physiol Nutr Metab 42: 897–900, 2017. doi: 10.1139/apnm-2016-0642. [DOI] [PubMed] [Google Scholar]
- 207. Henson J, Davies MJ, Bodicoat DH, Edwardson CL, Gill JM, Stensel DJ, Tolfrey K, Dunstan DW, Khunti K, Yates T. Breaking up prolonged sitting with standing or walking attenuates the postprandial metabolic response in postmenopausal women: a randomized acute study. Diabetes Care 39: 130–138, 2016. doi: 10.2337/dc15-1240. [DOI] [PubMed] [Google Scholar]
- 208. Toledo MJ, Ainsworth BE, Gaesser GA, Hooker SP, Pereira MA, Buman MP. Does frequency or duration of standing breaks drive changes in glycemic response? A randomized crossover trial. Scand J Med Sci Sports 33: 1135–1145, 2023. doi: 10.1111/sms.14344. [DOI] [PubMed] [Google Scholar]
- 209. Benatti FB, Larsen SA, Kofoed K, Nielsen ST, Harder-Lauridsen NM, Lyngbaek MP, Eriksen D, Karstoft K, Krogh-Madsen R, Pedersen BK, Ried-Larsen M. Intermittent standing but not a moderate exercise bout reduces postprandial glycemia. Med Sci Sports Exerc 49: 2305–2314, 2017. doi: 10.1249/MSS.0000000000001354. [DOI] [PubMed] [Google Scholar]
- 210. Duran AT, Friel CP, Serafini MA, Ensari I, Cheung YK, Diaz KM. Breaking up prolonged sitting to improve cardiometabolic risk: dose-response analysis of a randomized crossover trial. Med Sci Sports Exerc 55: 847–855, 2023. doi: 10.1249/MSS.0000000000003109. [DOI] [PubMed] [Google Scholar]
- 211. Dempsey PC, Larsen RN, Sethi P, Sacre JW, Straznicky NE, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA, Dunstan DW. Benefits for type 2 diabetes of interrupting prolonged sitting with brief bouts of light walking or simple resistance activities. Diabetes Care 39: 964–972, 2016. doi: 10.2337/dc15-2336. [DOI] [PubMed] [Google Scholar]
- 212. Dempsey PC, Larsen RN, Winkler EA, Owen N, Kingwell BA, Dunstan DW. Prolonged uninterrupted sitting elevates postprandial hyperglycaemia proportional to degree of insulin resistance. Diabetes Obes Metab 20: 1526–1530, 2018. doi: 10.1111/dom.13254. [DOI] [PubMed] [Google Scholar]
- 213. McCarthy M, Edwardson CL, Davies MJ, Henson J, Bodicoat DH, Khunti K, Dunstan DW, King JA, Yates T. Fitness moderates glycemic responses to sitting and light activity breaks. Med Sci Sports Exerc 49: 2216–2222, 2017. doi: 10.1249/MSS.0000000000001338. [DOI] [PubMed] [Google Scholar]
- 214. Broadney MM, Belcher BR, Berrigan DA, Brychta RJ, Tigner IL Jr, Shareef F, Papachristopoulou A, Hattenbach JD, Davis EK, Brady SM, Bernstein SB, Courville AB, Drinkard BE, Smith KP, Rosing DR, Wolters PL, Chen KY, Yanovski JA. Effects of interrupting sedentary behavior with short bouts of moderate physical activity on glucose tolerance in children with overweight and obesity: a randomized crossover trial. Diabetes Care 41: 2220–2228, 2018. doi: 10.2337/dc18-0774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Belcher BR, Berrigan D, Papachristopoulou A, Brady SM, Bernstein SB, Brychta RJ, Hattenbach JD, Tigner IL Jr, Courville AB, Drinkard BE, Smith KP, Rosing DR, Wolters PL, Chen KY, Yanovski JA. Effects of interrupting children's sedentary behaviors with activity on metabolic function: a randomized trial. J Clin Endocrinol Metab 100: 3735–3743, 2015. doi: 10.1210/jc.2015-2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Saunders TJ, Chaput JP, Goldfield GS, Colley RC, Kenny GP, Doucet E, Tremblay MS. Prolonged sitting and markers of cardiometabolic disease risk in children and youth: a randomized crossover study. Metabolism 62: 1423–1428, 2013. doi: 10.1016/j.metabol.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 217. Pinto AJ, Meireles K, Pecanha T, Mazzolani BC, Smaira FI, Rezende D, Benatti FB, Ribeiro AC, Pinto AL, Lima FR, Shinjo SK, Dantas WS, Mellett NA, Meikle PJ, Owen N, Dunstan DW, Roschel H, Gualano B. Acute cardiometabolic effects of brief active breaks in sitting for patients with rheumatoid arthritis. Am J Physiol Endocrinol Metab 321: E782–E794, 2021. doi: 10.1152/ajpendo.00259.2021. [DOI] [PubMed] [Google Scholar]
- 218. Peddie MC, Bone JL, Rehrer NJ, Skeaff CM, Gray AR, Perry TL. Breaking prolonged sitting reduces postprandial glycemia in healthy, normal-weight adults: a randomized crossover trial. Am J Clin Nutr 98: 358–366, 2013. doi: 10.3945/ajcn.112.051763. [DOI] [PubMed] [Google Scholar]
- 219. Kashiwabara K, Kidokoro T, Yanaoka T, Burns SF, Stensel DJ, Miyashita M. Different patterns of walking and postprandial triglycerides in older women. Med Sci Sports Exerc 50: 79–87, 2018. doi: 10.1249/MSS.0000000000001413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Miyashita M. Effects of continuous versus accumulated activity patterns on postprandial triacylglycerol concentrations in obese men. Int J Obes (Lond) 32: 1271–1278, 2008. doi: 10.1038/ijo.2008.73. [DOI] [PubMed] [Google Scholar]
- 221. Miyashita M, Edamoto K, Kidokoro T, Yanaoka T, Kashiwabara K, Takahashi M, Burns S. Interrupting sitting time with regular walks attenuates postprandial triglycerides. Int J Sports Med 37: 97–103, 2016. doi: 10.1055/s-0035-1559791. [DOI] [PubMed] [Google Scholar]
- 222. Kim IY, Park S, Trombold JR, Coyle EF. Effects of moderate- and intermittent low-intensity exercise on postprandial lipemia. Med Sci Sports Exerc 46: 1882–1890, 2014. doi: 10.1249/MSS.0000000000000324. [DOI] [PubMed] [Google Scholar]
- 223. Maylor BD, Zakrzewski-Fruer JK, Orton CJ, Bailey DP. Beneficial postprandial lipaemic effects of interrupting sedentary time with high-intensity physical activity versus a continuous moderate-intensity physical activity bout: a randomised crossover trial. J Sci Med Sport 21: 1250–1255, 2018. doi: 10.1016/j.jsams.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 224. Homer AR, Fenemor SP, Perry TL, Rehrer NJ, Cameron CM, Skeaff CM, Peddie MC. Regular activity breaks combined with physical activity improve postprandial plasma triglyceride, nonesterified fatty acid, and insulin responses in healthy, normal weight adults: a randomized crossover trial. J Clin Lipidol 11: 1268–1279, 2017. doi: 10.1016/j.jacl.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 225. Egan B, Zierath JR. Exercise metabolism and the molecular regulation of skeletal muscle adaptation. Cell Metab 17: 162–184, 2013. doi: 10.1016/j.cmet.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 226. Heymsfield SB, Smith B, Chung EA, Watts KL, Gonzalez MC, Yang S, Heo M, Thomas DM, Turner D, Bosy-Westphal A, Muller MJ. Phenotypic differences between people varying in muscularity. J Cachexia Sarcopenia Muscle 13: 1100–1112, 2022. doi: 10.1002/jcsm.12959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Kelley D, Mokan M, Veneman T. Impaired postprandial glucose utilization in non-insulin-dependent diabetes mellitus. Metabolism 43: 1549–1557, 1994. doi: 10.1016/0026-0495(94)90015-9. [DOI] [PubMed] [Google Scholar]
- 228. Hamilton MT, Hamilton DG, Zderic TW. A potent physiological method to magnify and sustain soleus oxidative metabolism improves glucose and lipid regulation. iScience 25: 104869, 2022. doi: 10.1016/j.isci.2022.104869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Latouche C, Jowett JB, Carey AL, Bertovic DA, Owen N, Dunstan DW, Kingwell BA. Effects of breaking up prolonged sitting on skeletal muscle gene expression. J Appl Physiol (1985) 114: 453–460, 2013. doi: 10.1152/japplphysiol.00978.2012. [DOI] [PubMed] [Google Scholar]
- 230. Bergouignan A, Latouche C, Heywood S, Grace MS, Reddy-Luthmoodoo M, Natoli AK, Owen N, Dunstan DW, Kingwell BA. Frequent interruptions of sedentary time modulates contraction- and insulin-stimulated glucose uptake pathways in muscle: ancillary analysis from randomized clinical trials. Sci Rep 6: 32044, 2016. doi: 10.1038/srep32044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. De Jong NP, Rudolph MC, Jackman MR, Sharp RR, Jones K, Houck J, Pan Z, Reusch JE, MacLean PS, Bessesen DH, Bergouignan A. Short-term adaptations in skeletal muscle mitochondrial oxidative capacity and metabolic pathways to breaking up sedentary behaviors in overweight or obese adults. Nutrients 14: 454, 2022. doi: 10.3390/nu14030454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Grace MS, Formosa MF, Bozaoglu K, Bergouignan A, Brozynska M, Carey AL, Veiga CB, Sethi P, Dillon F, Bertovic DA, Inouye M, Owen N, Dunstan DW, Kingwell BA. Acute effects of active breaks during prolonged sitting on subcutaneous adipose tissue gene expression: an ancillary analysis of a randomised controlled trial. Sci Rep 9: 3847, 2019. doi: 10.1038/s41598-019-40490-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Dunstan DW, Kingwell BA, Larsen R, Healy GN, Cerin E, Hamilton MT, Shaw JE, Bertovic DA, Zimmet PZ, Salmon J, Owen N. Breaking up prolonged sitting reduces postprandial glucose and insulin responses. Diabetes Care 35: 976–983, 2012. doi: 10.2337/dc11-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Smith JA, Savikj M, Sethi P, Platt S, Gabriel BM, Hawley JA, Dunstan D, Krook A, Zierath JR, Naslund E. Three weeks of interrupting sitting lowers fasting glucose and glycemic variability, but not glucose tolerance, in free-living women and men with obesity. Am J Physiol Endocrinol Metab 321: E203–E216, 2021. doi: 10.1152/ajpendo.00599.2020. [DOI] [PubMed] [Google Scholar]
- 235. Shambrook P, Kingsley M, Taylor N, Gordon B. Accumulated or continuous exercise for glycaemic regulation and control: a systematic review with meta-analysis. BMJ Open Sport Exerc Med 4: e000470, 2018. doi: 10.1136/bmjsem-2018-000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Stratton IM, Adler AI, Neil HA, Matthews DR, Manley SE, Cull CA, Hadden D, Turner RC, Holman RR. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ 321: 405–412, 2000. doi: 10.1136/bmj.321.7258.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Balducci S, D'Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, Vitale M, Bollanti L, Conti F, Zanuso S, Lucisano G, Nicolucci A, Pugliese G, Italian Diabetes and Exercise Study 2 (IDES_2) Investigators. Effect of a behavioral intervention strategy on sustained change in physical activity and sedentary behavior in patients with type 2 diabetes: the IDES_2 Randomized Clinical Trial. JAMA 321: 880–890, 2019. doi: 10.1001/jama.2019.0922. [DOI] [PubMed] [Google Scholar]
- 238. Balducci S, Haxhi J, Sacchetti M, Orlando G, Cardelli P, Vitale M, et al. Relationships of changes in physical activity and sedentary behavior with changes in physical fitness and cardiometabolic risk profile in individuals with type 2 diabetes: the Italian Diabetes and Exercise Study 2 (IDES_2). Diabetes Care 45: 213–221, 2022. doi: 10.2337/dc21-1505. [DOI] [PubMed] [Google Scholar]
- 239. Pereira MA, Mullane SL, Toledo MJ, Larouche ML, Rydell SA, Vuong B, Feltes LH, Mitchell NR, de Brito JN, Hasanaj K, Carlson NG, Gaesser GA, Crespo NC, Oakes JM, Buman MP. Efficacy of the ‘stand and move at work’ multicomponent workplace intervention to reduce sedentary time and improve cardiometabolic risk: a group randomized clinical trial. Int J Behav Nutr Phys Act 17: 133, 2020. doi: 10.1186/s12966-020-01033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Walhin JP, Richardson JD, Betts JA, Thompson D. Exercise counteracts the effects of short-term overfeeding and reduced physical activity independent of energy imbalance in healthy young men. J Physiol 591: 6231–6243, 2013. doi: 10.1113/jphysiol.2013.262709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Dixon NC, Hurst TL, Talbot DC, Tyrrell RM, Thompson D. Effect of short-term reduced physical activity on cardiovascular risk factors in active lean and overweight middle-aged men. Metabolism 62: 361–368, 2013. doi: 10.1016/j.metabol.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 242. Kehler DS, Theou O, Rockwood K. Bed rest and accelerated aging in relation to the musculoskeletal and cardiovascular systems and frailty biomarkers: a review. Exp Gerontol 124: 110643, 2019. doi: 10.1016/j.exger.2019.110643. [DOI] [PubMed] [Google Scholar]
- 243. Trappe S, Creer A, Slivka D, Minchev K, Trappe T. Single muscle fiber function with concurrent exercise or nutrition countermeasures during 60 days of bed rest in women. J Appl Physiol (1985) 103: 1242–1250, 2007. doi: 10.1152/japplphysiol.00560.2007. [DOI] [PubMed] [Google Scholar]
- 244. Trappe S, Creer A, Minchev K, Slivka D, Louis E, Luden N, Trappe T. Human soleus single muscle fiber function with exercise or nutrition countermeasures during 60 days of bed rest. Am J Physiol Regul Integr Comp Physiol 294: R939–R947, 2008. doi: 10.1152/ajpregu.00761.2007. [DOI] [PubMed] [Google Scholar]
- 245. Salanova M, Schiffl G, Püttmann B, Schoser BG, Blottner D. Molecular biomarkers monitoring human skeletal muscle fibres and microvasculature following long-term bed rest with and without countermeasures. J Anat 212: 306–318, 2008. doi: 10.1111/j.1469-7580.2008.00854.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Kenny HC, Rudwill F, Breen L, Salanova M, Blottner D, Heise T, Heer M, Blanc S, O'Gorman DJ. Bed rest and resistive vibration exercise unveil novel links between skeletal muscle mitochondrial function and insulin resistance. Diabetologia 60: 1491–1501, 2017. doi: 10.1007/s00125-017-4298-z. [DOI] [PubMed] [Google Scholar]
- 247. Fernandez-Gonzalo R, Tesch PA, Lundberg TR, Alkner BA, Rullman E, Gustafsson T. Three months of bed rest induce a residual transcriptomic signature resilient to resistance exercise countermeasures. FASEB J 34: 7958–7969, 2020. doi: 10.1096/fj.201902976R. [DOI] [PubMed] [Google Scholar]
- 248. Bergouignan A, Schoeller DA, Normand S, Gauquelin-Koch G, Laville M, Shriver T, Desage M, Le Maho Y, Ohshima H, Gharib C, Blanc S. Effect of physical inactivity on the oxidation of saturated and monounsaturated dietary fatty acids: results of a randomized trial. PLoS Clin Trials 1: e27, 2006. doi: 10.1371/journal.pctr.0010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Alibegovic AC, Højbjerre L, Sonne MP, van Hall G, Alsted TJ, Kiens B, Stallknecht B, Dela F, Vaag A. Increased rate of whole body lipolysis before and after 9 days of bed rest in healthy young men born with low birth weight. Am J Physiol Endocrinol Metab 298: E555–E564, 2010. doi: 10.1152/ajpendo.00223.2009. [DOI] [PubMed] [Google Scholar]
- 250. Alibegovic AC, Højbjerre L, Sonne MP, van Hall G, Stallknecht B, Dela F, Vaag A. Impact of 9 days of bed rest on hepatic and peripheral insulin action, insulin secretion, and whole-body lipolysis in healthy young male offspring of patients with type 2 diabetes. Diabetes 58: 2749–2756, 2009. doi: 10.2337/db09-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Belavy DL, Mohlig M, Pfeiffer AF, Felsenberg D, Armbrecht G. Preferential deposition of visceral adipose tissue occurs due to physical inactivity. Int J Obes (Lond) 38: 1478–1480, 2014. doi: 10.1038/ijo.2014.26. [DOI] [PubMed] [Google Scholar]
- 252. Rudwill F, Bergouignan A, Gastebois C, Gauquelin-Koch G, Lefai E, Blanc S, Simon C. Effect of enforced physical inactivity induced by 60-day of bed rest on hepatic markers of NAFLD in healthy normal-weight women. Liver Int 35: 1700–1706, 2015. doi: 10.1111/liv.12743. [DOI] [PubMed] [Google Scholar]
- 253. Trudel G, Coletta E, Cameron I, Belavy DL, Lecompte M, Armbrecht G, Felsenberg D, Uhthoff HK. Resistive exercises, with or without whole body vibration, prevent vertebral marrow fat accumulation during 60 days of head-down tilt bed rest in men. J Appl Physiol (1985) 112: 1824–1831, 2012. doi: 10.1152/japplphysiol.00029.2012. [DOI] [PubMed] [Google Scholar]
- 254. Trudel G, Payne M, Mädler B, Ramachandran N, Lecompte M, Wade C, Biolo G, Blanc S, Hughson R, Bear L, Uhthoff HK. Bone marrow fat accumulation after 60 days of bed rest persisted 1 year after activities were resumed along with hemopoietic stimulation: the Women International Space Simulation for Exploration Study. J Appl Physiol (1985) 107: 540–548, 2009. doi: 10.1152/japplphysiol.91530.2008. [DOI] [PubMed] [Google Scholar]
- 255. Zong B, Wang Y, Wang J, Zhang P, Kan G, Li M, Feng J, Wang Y, Chen X, Jin R, Ge Q. Effects of long-term simulated microgravity on liver metabolism in rhesus macaques. FASEB J 36: e22536, 2022. doi: 10.1096/fj.202200544RR. [DOI] [PubMed] [Google Scholar]
- 256. Healy GN, Matthews CE, Dunstan DW, Winkler EA, Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: NHANES 2003-06. Eur Heart J 32: 590–597, 2011. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Healy GN, Wijndaele K, Dunstan DW, Shaw JE, Salmon J, Zimmet PZ, Owen N. Objectively measured sedentary time, physical activity, and metabolic risk: the Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 31: 369–371, 2008. doi: 10.2337/dc07-1795. [DOI] [PubMed] [Google Scholar]
- 258. Edwardson CL, Henson J, Biddle SJ, Davies MJ, Khunti K, Maylor B, Yates T. Activpal and actigraph assessed sedentary behavior and cardiometabolic health markers. Med Sci Sports Exerc 52: 391–397, 2020. doi: 10.1249/MSS.0000000000002138. [DOI] [PubMed] [Google Scholar]
- 259. Bailey DP, Locke CD. Breaking up prolonged sitting with light-intensity walking improves postprandial glycemia, but breaking up sitting with standing does not. J Sci Med Sport 18: 294–298, 2015. doi: 10.1016/j.jsams.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 260. Kerr NR, Kelty TJ, Mao X, Childs TE, Kline DD, Rector RS, Booth FW. Selective breeding for physical inactivity produces cognitive deficits via altered hippocampal mitochondrial and synaptic function. Front Aging Neurosci 15: 1147420, 2023. doi: 10.3389/fnagi.2023.1147420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261. Miyashita M, Park JH, Takahashi M, Suzuki K, Stensel D, Nakamura Y. Postprandial lipaemia: effects of sitting, standing and walking in healthy normolipidaemic humans. Int J Sports Med 34: 21–27, 2013. doi: 10.1055/s-0032-1321897. [DOI] [PubMed] [Google Scholar]
- 262. Engeroff T, Fuzeki E, Vogt L, Banzer W. Breaking up sedentary time, physical activity and lipoprotein metabolism. J Sci Med Sport 20: 678–683, 2017. doi: 10.1016/j.jsams.2016.11.018. [DOI] [PubMed] [Google Scholar]
- 263. Hayashino Y, Jackson JL, Fukumori N, Nakamura F, Fukuhara S. Effects of supervised exercise on lipid profiles and blood pressure control in people with type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Diabetes Res Clin Pract 98: 349–360, 2012. doi: 10.1016/j.diabres.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 264. Cai M, Zou Z. Effect of aerobic exercise on blood lipid and glucose in obese or overweight adults: a meta-analysis of randomised controlled trials. Obes Res Clin Pract 10: 589–602, 2016. doi: 10.1016/j.orcp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 265. McGlory C, Devries MC, Phillips SM. Skeletal muscle and resistance exercise training; the role of protein synthesis in recovery and remodeling. J Appl Physiol (1985) 122: 541–548, 2017. doi: 10.1152/japplphysiol.00613.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. McGlory C, van Vliet S, Stokes T, Mittendorfer B, Phillips SM. The impact of exercise and nutrition on the regulation of skeletal muscle mass. J Physiol 597: 1251–1258, 2019. doi: 10.1113/JP275443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Bell KE, von Allmen MT, Devries MC, Phillips SM. Muscle disuse as a pivotal problem in sarcopenia-related muscle loss and dysfunction. J Frailty Aging 5: 33–41, 2016. doi: 10.14283/jfa.2016.78. [DOI] [PubMed] [Google Scholar]
- 268. Hartley P, Costello P, Fenner R, Gibbins N, Quinn E, Kuhn I, Keevil VL, Romero-Ortuno R. Change in skeletal muscle associated with unplanned hospital admissions in adult patients: a systematic review and meta-analysis. PLoS One 14: e0210186, 2019. doi: 10.1371/journal.pone.0210186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Ferrando AA, Lane HW, Stuart CA, Davis-Street J, Wolfe RR. Prolonged bed rest decreases skeletal muscle and whole body protein synthesis. Am J Physiol Endocrinol Metab 270: E627–E633, 1996. doi: 10.1152/ajpendo.1996.270.4.E627. [DOI] [PubMed] [Google Scholar]
- 270. Moore DR, Williamson EP, Hodson N, Estafanos S, Mazzulla M, Kumbhare D, Gillen JB. Walking or body weight squat ‘activity snacks’ increase dietary amino acid utilization for myofibrillar protein synthesis during prolonged sitting. J Appl Physiol (1985) 133: 777–785, 2022. doi: 10.1152/japplphysiol.00106.2022. [DOI] [PubMed] [Google Scholar]
- 271. Wall BT, Burd NA, Franssen R, Gorissen SH, Snijders T, Senden JM, Gijsen AP, van Loon LJ. Presleep protein ingestion does not compromise the muscle protein synthetic response to protein ingested the following morning. Am J Physiol Endocrinol Metab 311: E964–E973, 2016. doi: 10.1152/ajpendo.00325.2016. [DOI] [PubMed] [Google Scholar]
- 272. Lee N. Does the relative muscle activation of the vastus medialis, rectus femoris, and vastus lateralis, during the various activities, change in relation to the quadriceps angle? J Phys Ther Sci 30: 540–543, 2018. doi: 10.1589/jpts.30.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Phillips SM. A brief review of critical processes in exercise-induced muscular hypertrophy. Sports Med 44, Suppl 1: S71–S77, 2014. doi: 10.1007/s40279-014-0152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Ku DN, Giddens DP, Zarins CK, Glagov S. Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis 5: 293–302, 1985. doi: 10.1161/01.atv.5.3.293. [DOI] [PubMed] [Google Scholar]
- 275. Liepsch D. An introduction to biofluid mechanics–basic models and applications. J Biomech 35: 415–435, 2002. doi: 10.1016/s0021-9290(01)00185-3. [DOI] [PubMed] [Google Scholar]
- 276. Padilla J, Sheldon RD, Sitar DM, Newcomer SC. Impact of acute exposure to increased hydrostatic pressure and reduced shear rate on conduit artery endothelial function: a limb-specific response. Am J Physiol Heart Circ Physiol 297: H1103–H1108, 2009. doi: 10.1152/ajpheart.00167.2009. [DOI] [PubMed] [Google Scholar]
- 277. Hitosugi M, Niwa M, Takatsu A. Rheologic changes in venous blood during prolonged sitting. Thromb Res 100: 409–412, 2000. doi: 10.1016/s0049-3848(00)00348-0. [DOI] [PubMed] [Google Scholar]
- 278. Pekas EJ, Allen MF, Park SY. Prolonged sitting and peripheral vascular function: potential mechanisms and methodological considerations. J Appl Physiol (1985) 134: 810–822, 2023. doi: 10.1152/japplphysiol.00730.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279. Shvartz E, Gaume JG, White RT, Reibold RC. Hemodynamic responses during prolonged sitting. J Appl Physiol Respir Environ Exerc Physiol 54: 1673–1680, 1983. doi: 10.1152/jappl.1983.54.6.1673. [DOI] [PubMed] [Google Scholar]
- 280. Thosar SS, Bielko SL, Mather KJ, Johnston JD, Wallace JP. Effect of prolonged sitting and breaks in sitting time on endothelial function. Med Sci Sports Exerc 47: 843–849, 2015. doi: 10.1249/MSS.0000000000000479. [DOI] [PubMed] [Google Scholar]
- 281. Thosar SS, Bielko SL, Wiggins CC, Wallace JP. Differences in brachial and femoral artery responses to prolonged sitting. Cardiovasc Ultrasound 12: 50, 2014. doi: 10.1186/1476-7120-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Dogra S, Wolf M, Jeffrey MP, Foley RC, Logan-Sprenger H, Jones-Taggart H, Green-Johnson JM. Disrupting prolonged sitting reduces il-8 and lower leg swell in active young adults. BMC Sports Sci Med Rehabil 11: 23, 2019. doi: 10.1186/s13102-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Climie RE, Wheeler MJ, Grace M, Lambert E, Cohen N, Owen N, Kingwell B, Dunstan DW, Green DJ. Simple intermittent resistance activity mitigates the detrimental effect of prolonged unbroken sitting on arterial function in overweight and obese adults. J Appl Physiol (1985) 125: 1787–1794, 2018. doi: 10.1152/japplphysiol.00544.2018. [DOI] [PubMed] [Google Scholar]
- 284. Kruse NT, Hughes WE, Benzo RM, Carr LJ, Casey DP. Workplace strategies to prevent sitting-induced endothelial dysfunction. Med Sci Sports Exerc 50: 801–808, 2018. doi: 10.1249/MSS.0000000000001484. [DOI] [PubMed] [Google Scholar]
- 285. Headid RJ 3rd, Pekas EJ, Wooden TK, Son WM, Layec G, Shin J, Park SY. Impacts of prolonged sitting with mild hypercapnia on vascular and autonomic function in healthy recreationally active adults. Am J Physiol Heart Circ Physiol 319: H468–H480, 2020. doi: 10.1152/ajpheart.00354.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. O'Brien MW, Johns JA, Williams TD, Kimmerly DS. Sex does not influence impairments in popliteal endothelial-dependent vasodilator or vasoconstrictor responses following prolonged sitting. J Appl Physiol (1985) 127: 679–687, 2019. doi: 10.1152/japplphysiol.00887.2018. [DOI] [PubMed] [Google Scholar]
- 287. Taylor FC, Dunstan DW, Homer AR, Dempsey PC, Kingwell BA, Climie RE, Owen N, Cohen ND, Larsen RN, Grace M, Eikelis N, Wheeler MJ, Townsend MK, Maniar N, Green DJ. Acute effects of interrupting prolonged sitting on vascular function in type 2 diabetes. Am J Physiol Heart Circ Physiol 320: H393–H403, 2021. doi: 10.1152/ajpheart.00422.2020. [DOI] [PubMed] [Google Scholar]
- 288. Wennberg P, Boraxbekk CJ, Wheeler M, Howard B, Dempsey PC, Lambert G, Eikelis N, Larsen R, Sethi P, Occleston J, Hernestal-Boman J, Ellis KA, Owen N, Dunstan DW. Acute effects of breaking up prolonged sitting on fatigue and cognition: a pilot study. BMJ Open 6: e009630, 2016. doi: 10.1136/bmjopen-2015-009630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Ballard KD, Duguid RM, Berry CW, Dey P, Bruno RS, Ward RM, Timmerman KL. Effects of prior aerobic exercise on sitting-induced vascular dysfunction in healthy men. Eur J Appl Physiol 117: 2509–2518, 2017. doi: 10.1007/s00421-017-3738-2. [DOI] [PubMed] [Google Scholar]
- 290. Larsen RN, Kingwell BA, Sethi P, Cerin E, Owen N, Dunstan DW. Breaking up prolonged sitting reduces resting blood pressure in overweight/obese adults. Nutr Metab Cardiovasc Dis 24: 976–982, 2014. doi: 10.1016/j.numecd.2014.04.011. [DOI] [PubMed] [Google Scholar]
- 291. Carter SE, Gladwell VF. Effect of breaking up sedentary time with callisthenics on endothelial function. J Sports Sci 35: 1508–1514, 2017. doi: 10.1080/02640414.2016.1223331. [DOI] [PubMed] [Google Scholar]
- 292. Credeur DP, Miller SM, Jones R, Stoner L, Dolbow DR, Fryer SM, Stone K, McCoy SM. Impact of prolonged sitting on peripheral and central vascular health. Am J Cardiol 123: 260–266, 2019. doi: 10.1016/j.amjcard.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 293. Blough J, Loprinzi PD. Randomized controlled trial investigating the experimental effects of reduced habitual physical activity on cardiometabolic profile. Physiol Behav 194: 48–55, 2018. doi: 10.1016/j.physbeh.2018.04.036. [DOI] [PubMed] [Google Scholar]
- 294. Dempsey PC, Sacre JW, Larsen RN, Straznicky NE, Sethi P, Cohen ND, Cerin E, Lambert GW, Owen N, Kingwell BA, Dunstan DW. Interrupting prolonged sitting with brief bouts of light walking or simple resistance activities reduces resting blood pressure and plasma noradrenaline in type 2 diabetes. J Hypertens 34: 2376–2382, 2016. doi: 10.1097/HJH.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 295. Wheeler MJ, Dunstan DW, Ellis KA, Cerin E, Phillips S, Lambert G, Naylor LH, Dempsey PC, Kingwell BA, Green DJ. Effect of morning exercise with or without breaks in prolonged sitting on blood pressure in older overweight/obese adults. Hypertension 73: 859–867, 2019. doi: 10.1161/HYPERTENSIONAHA.118.12373. [DOI] [PubMed] [Google Scholar]
- 296. Noddeland H, Ingemansen R, Reed RK, Aukland K. A telemetric technique for studies of venous pressure in the human leg during different positions and activities. Clin Physiol 3: 573–576, 1983. doi: 10.1111/j.1475-097x.1983.tb00866.x. [DOI] [PubMed] [Google Scholar]
- 297. Morbiducci U, Kok AM, Kwak BR, Stone PH, Steinman DA, Wentzel JJ. Atherosclerosis at arterial bifurcations: evidence for the role of haemodynamics and geometry. Thromb Haemost 115: 484–492, 2016. doi: 10.1160/TH15-07-0597. [DOI] [PubMed] [Google Scholar]
- 298. Walsh LK, Restaino RM, Martinez-Lemus LA, Padilla J. Prolonged leg bending impairs endothelial function in the popliteal artery. Physiol Rep 5: e13478, 2017. doi: 10.14814/phy2.13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Rakobowchuk M, Crozier J, Glover EI, Yasuda N, Phillips SM, Tarnopolsky MA, MacDonald MJ. Short-term unilateral leg immobilization alters peripheral but not central arterial structure and function in healthy young humans. Eur J Appl Physiol 111: 203–210, 2011. doi: 10.1007/s00421-010-1636-y. [DOI] [PubMed] [Google Scholar]
- 300. Wei YC, George NI, Chang CW, Hicks KA. Assessing sex differences in the risk of cardiovascular disease and mortality per increment in systolic blood pressure: a systematic review and meta-analysis of follow-up studies in the United States. PLoS One 12: e0170218, 2017. doi: 10.1371/journal.pone.0170218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301. van den Hoogen PC, Feskens EJ, Nagelkerke NJ, Menotti A, Nissinen A, Kromhout D. The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven Countries Study Research Group. N Engl J Med 342: 1–8, 2000. doi: 10.1056/NEJM200001063420101. [DOI] [PubMed] [Google Scholar]
- 302. Palmer AJ, Bulpitt CJ, Fletcher AE, Beevers DG, Coles EC, Ledingham JG, O'Riordan PW, Petrie JC, Rajagopalan BE, Webster J. Relation between blood pressure and stroke mortality. Hypertension 20: 601–605, 1992. doi: 10.1161/01.hyp.20.5.601. [DOI] [PubMed] [Google Scholar]
- 303. Whipple MO, Masters KS, Huebschmann AG, Scalzo RL, Reusch JE, Bergouignan A, Regensteiner JG. Acute effects of sedentary breaks on vascular health in adults at risk for type 2 diabetes: a systematic review. Vasc Med 26: 448–458, 2021. doi: 10.1177/1358863X211009307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304. Weston E, Nagy M, Ajibewa TA, O'Sullivan M, Block S, Hasson RE. Acute effects of interrupting prolonged sitting with intermittent physical activity on blood pressure in preadolescent children. Pediatr Exerc Sci 31: 408–415, 2019. doi: 10.1123/pes.2018-0224. [DOI] [PubMed] [Google Scholar]
- 305. Morishima T, Restaino RM, Walsh LK, Kanaley JA, Fadel PJ, Padilla J. Prolonged sitting-induced leg endothelial dysfunction is prevented by fidgeting. Am J Physiol Heart Circ Physiol 311: H177–H182, 2016. doi: 10.1152/ajpheart.00297.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Howard BJ, Fraser SF, Sethi P, Cerin E, Hamilton MT, Owen N, Dunstan DW, Kingwell BA. Impact on hemostatic parameters of interrupting sitting with intermittent activity. Med Sci Sports Exerc 45: 1285–1291, 2013. doi: 10.1249/MSS.0b013e318285f57e. [DOI] [PubMed] [Google Scholar]
- 307. Bhammar DM, Sawyer BJ, Tucker WJ, Gaesser GA. Breaks in sitting time: effects on continuously monitored glucose and blood pressure. Med Sci Sports Exerc 49: 2119–2130, 2017. doi: 10.1249/MSS.0000000000001315. [DOI] [PubMed] [Google Scholar]
- 308. Miyashita M, Burns SF, Stensel DJ. Accumulating short bouts of brisk walking reduces postprandial plasma triacylglycerol concentrations and resting blood pressure in healthy young men. Am J Clin Nutr 88: 1225–1231, 2008. doi: 10.3945/ajcn.2008.26493. [DOI] [PubMed] [Google Scholar]
- 309. Carter SE, Draijer R, Maxwell JD, Morris AS, Pedersen SJ, Graves LE, Thijssen DH, Hopkins ND. Using an e-health intervention to reduce prolonged sitting in UK office workers: a randomised acceptability and feasibility study. Int J Environ Res Public Health 17: 8942, 2020. doi: 10.3390/ijerph17238942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310. Hartman YA, Tillmans LC, Benschop DL, Hermans AN, Nijssen KM, Eijsvogels TM, Willems P, Tack CJ, Hopman MT, Claassen J, Thijssen DH. Long-term and acute benefits of reduced sitting on vascular flow and function. Med Sci Sports Exerc 53: 341–350, 2021. doi: 10.1249/MSS.0000000000002462. [DOI] [PubMed] [Google Scholar]
- 311. Cornelissen VA, Smart NA. Exercise training for blood pressure: a systematic review and meta-analysis. J Am Heart Assoc 2: e004473, 2013. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312. Stamler R. Implications of the INTERSALT Study. Hypertension 17: I16–I20, 1991. doi: 10.1161/01.hyp.17.1_suppl.i16. [DOI] [PubMed] [Google Scholar]
- 313. Thosar SS, Johnson BD, Johnston JD, Wallace JP. Sitting and endothelial dysfunction: the role of shear stress. Med Sci Monit 18: RA173–180, 2012. doi: 10.12659/msm.883589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Takahashi M, Miyashita M, Park JH, Sakamoto S, Suzuki K. Effects of breaking sitting by standing and acute exercise on postprandial oxidative stress. Asian J Sports Med 6: e24902, 2015. doi: 10.5812/asjsm.24902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Restaino RM, Holwerda SW, Credeur DP, Fadel PJ, Padilla J. Impact of prolonged sitting on lower and upper limb micro- and macrovascular dilator function. Exp Physiol 100: 829–838, 2015. doi: 10.1113/EP085238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Evans WS, Stoner L, Willey Q, Kelsch E, Credeur DP, Hanson ED. Local exercise does not prevent the aortic stiffening response to acute prolonged sitting: a randomized crossover trial. J Appl Physiol (1985) 127: 781–787, 2019. doi: 10.1152/japplphysiol.00318.2019. [DOI] [PubMed] [Google Scholar]
- 317. Barone Gibbs B, Kowalsky RJ, Perdomo SJ, Taormina JM, Balzer JR, Jakicic JM. Effect of alternating standing and sitting on blood pressure and pulse wave velocity during a simulated workday in adults with overweight/obesity. J Hypertens 35: 2411–2418, 2017. doi: 10.1097/HJH.0000000000001463. [DOI] [PubMed] [Google Scholar]
- 318. Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol 55: 1318–1327, 2010. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 319. Park SY, Wooden TK, Pekas EJ, Anderson CP, Yadav SK, Slivka DR, Layec G. Effects of passive and active leg movements to interrupt sitting in mild hypercapnia on cardiovascular function in healthy adults. j Appl Physiol (1985) 132: 874–887, 2022. doi: 10.1152/japplphysiol.00799.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Restaino RM, Walsh LK, Morishima T, Vranish JR, Martinez-Lemus LA, Fadel PJ, Padilla J. Endothelial dysfunction following prolonged sitting is mediated by a reduction in shear stress. Am J Physiol Heart Circ Physiol 310: H648–H653, 2016. doi: 10.1152/ajpheart.00943.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Vranish JR, Young BE, Kaur J, Patik JC, Padilla J, Fadel PJ. Influence of sex on microvascular and macrovascular responses to prolonged sitting. Am J Physiol Heart Circ Physiol 312: H800–H805, 2017. doi: 10.1152/ajpheart.00823.2016. [DOI] [PubMed] [Google Scholar]
- 322. Vranish JR, Young BE, Stephens BY, Kaur J, Padilla J, Fadel PJ. Brief periods of inactivity reduce leg microvascular, but not macrovascular, function in healthy young men. Exp Physiol 103: 1425–1434, 2018. doi: 10.1113/EP086918. [DOI] [PubMed] [Google Scholar]
- 323. Decker KP, Feliciano PG, Kimmel MT, Hogwood AC, Weggen JB, Darling AM, Richardson JW, Garten RS. Examining sex differences in sitting-induced microvascular dysfunction: insight from acute vitamin c supplementation. Microvasc Res 135: 104147, 2021. doi: 10.1016/j.mvr.2021.104147. [DOI] [PubMed] [Google Scholar]
- 324. Horiuchi M, Stoner L. Macrovascular and microvascular responses to prolonged sitting with and without bodyweight exercise interruptions: a randomized cross-over trial. Vasc Med 27: 127–135, 2022. doi: 10.1177/1358863X211053381. [DOI] [PubMed] [Google Scholar]
- 325. Sugawara J, Hayashi K, Kaneko F, Yamada H, Kizuka T, Tanaka H. Reductions in basal limb blood flow and lumen diameter after short-term leg casting. Med Sci Sports Exerc 36: 1689–1694, 2004. doi: 10.1249/01.mss.0000142410.45142.28. [DOI] [PubMed] [Google Scholar]
- 326. Chao Y, Ye P, Zhu L, Kong X, Qu X, Zhang J, Luo J, Yang H, Chen S. Low shear stress induces endothelial reactive oxygen species via the AT1R/eNOS/NO pathway. J Cell Physiol 233: 1384–1395, 2018. doi: 10.1002/jcp.26016. [DOI] [PubMed] [Google Scholar]
- 327. Hosoya T, Maruyama A, Kang MI, Kawatani Y, Shibata T, Uchida K, Warabi E, Noguchi N, Itoh K, Yamamoto M. Differential responses of the Nrf2-Keap1 system to laminar and oscillatory shear stresses in endothelial cells. J Biol Chem 280: 27244–27250, 2005. doi: 10.1074/jbc.M502551200. [DOI] [PubMed] [Google Scholar]
- 328. Yeboah J, Folsom AR, Burke GL, Johnson C, Polak JF, Post W, Lima JA, Crouse JR, Herrington DM. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation 120: 502–509, 2009. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Taylor FC, Dunstan DW, Fletcher E, Townsend MK, Larsen RN, Rickards K, Maniar N, Buman M, Dempsey PC, Joham AE, Cohen N, Owen N, Moran LJ, Green DJ. Interrupting prolonged sitting and endothelial function in polycystic ovary syndrome. Med Sci Sports Exerc 53: 479–486, 2021. doi: 10.1249/MSS.0000000000002513. [DOI] [PubMed] [Google Scholar]
- 330. Carter SE, Draijer R, Holder SM, Brown L, Thijssen DH, Hopkins ND. Effect of different walking break strategies on superficial femoral artery endothelial function. Physiol Rep 7: e14190, 2019. doi: 10.14814/phy2.14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331. Duvivier B, Bolijn JE, Koster A, Schalkwijk CG, Savelberg H, Schaper NC. Reducing sitting time versus adding exercise: differential effects on biomarkers of endothelial dysfunction and metabolic risk. Sci Rep 8: 8657, 2018. doi: 10.1038/s41598-018-26616-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Carter S, Hartman Y, Holder S, Thijssen DH, Hopkins ND. Sedentary behavior and cardiovascular disease risk: mediating mechanisms. Exerc Sport Sci Rev 45: 80–86, 2017. doi: 10.1249/JES.0000000000000106. [DOI] [PubMed] [Google Scholar]
- 333. Graves LE, Murphy RC, Shepherd SO, Cabot J, Hopkins ND. Evaluation of sit-stand workstations in an office setting: a randomised controlled trial. BMC Public Health 15: 1145, 2015. doi: 10.1186/s12889-015-2469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Green DJ, Jones H, Thijssen D, Cable NT, Atkinson G. Flow-mediated dilation and cardiovascular event prediction: does nitric oxide matter? Hypertension 57: 363–369, 2011. doi: 10.1161/HYPERTENSIONAHA.110.167015. [DOI] [PubMed] [Google Scholar]
- 335. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow-mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta-analysis. J Am Heart Assoc 4: e002270, 2015. doi: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Ashor AW, Lara J, Siervo M, Celis-Morales C, Oggioni C, Jakovljevic DG, Mathers JC. Exercise modalities and endothelial function: a systematic review and dose-response meta-analysis of randomized controlled trials. Sports Med 45: 279–296, 2015. doi: 10.1007/s40279-014-0272-9. [DOI] [PubMed] [Google Scholar]
- 337. Hawkins S, Wiswell R. Rate and mechanism of maximal oxygen consumption decline with aging: implications for exercise training. Sports Med 33: 877–888, 2003. doi: 10.2165/00007256-200333120-00002. [DOI] [PubMed] [Google Scholar]
- 338. Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, Sugawara A, Totsuka K, Shimano H, Ohashi Y, Yamada N, Sone H. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA 301: 2024–2035, 2009. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 339. Blair SN, Kohl HW 3rd, Paffenbarger RS Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395–2401, 1989. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 340. Dirks ML, Wall BT, Nilwik R, Weerts DH, Verdijk LB, van Loon LJ. Skeletal muscle disuse atrophy is not attenuated by dietary protein supplementation in healthy older men. J Nutr 144: 1196–1203, 2014. doi: 10.3945/jn.114.194217. [DOI] [PubMed] [Google Scholar]
- 341. D'Antona G, Pellegrino MA, Adami R, Rossi R, Carlizzi CN, Canepari M, Saltin B, Bottinelli R. The effect of ageing and immobilization on structure and function of human skeletal muscle fibres. J Physiol 552: 499–511, 2003. doi: 10.1113/jphysiol.2003.046276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Coffey VG, McGlory C, Phillips SM, Doering TM. Does initial skeletal muscle size or sex affect the magnitude of muscle loss in response to 14 days immobilization? Appl Physiol Nutr Metab 48: 411–416, 2023. doi: 10.1139/apnm-2022-0458. [DOI] [PubMed] [Google Scholar]
- 343. Arnold J, Campbell IT, Samuels TA, Devlin JC, Green CJ, Hipkin LJ, MacDonald IA, Scrimgeour CM, Smith K, Rennie MJ. Increased whole body protein breakdown predominates over increased whole body protein synthesis in multiple organ failure. Clin Sci (Lond) 84: 655–661, 1993. doi: 10.1042/cs0840655. [DOI] [PubMed] [Google Scholar]
- 344. Rennie MJ, Edwards RH, Emery PW, Halliday D, Lundholm K, Millward DJ. Depressed protein synthesis is the dominant characteristic of muscle wasting and cachexia. Clin Physiol 3: 387–398, 1983. doi: 10.1111/j.1475-097x.1983.tb00847.x. [DOI] [PubMed] [Google Scholar]
- 345. Peris-Moreno D, Cussonneau L, Combaret L, Polge C, Taillandier D. Ubiquitin ligases at the heart of skeletal muscle atrophy control. Molecules 26: 407, 2021. doi: 10.3390/molecules26020407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Yin L, Li N, Jia W, Wang N, Liang M, Yang X, Du G. Skeletal muscle atrophy: from mechanisms to treatments. Pharmacol Res 172: 105807, 2021. doi: 10.1016/j.phrs.2021.105807. [DOI] [PubMed] [Google Scholar]
- 347. Vainshtein A, Sandri M. Signaling pathways that control muscle mass. Int J Mol Sci 21: 4759, 2020. doi: 10.3390/ijms21134759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348. Zumbaugh MD, Johnson SE, Shi TH, Gerrard DE. Molecular and biochemical regulation of skeletal muscle metabolism. J Anim Sci 100: skac035, 2022. doi: 10.1093/jas/skac035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349. Trevino MB, Zhang X, Standley RA, Wang M, Han X, Reis FC, Periasamy M, Yu G, Kelly DP, Goodpaster BH, Vega RB, Coen PM. Loss of mitochondrial energetics is associated with poor recovery of muscle function but not mass following disuse atrophy. Am J Physiol Endocrinol Metab 317: E899–E910, 2019. doi: 10.1152/ajpendo.00161.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Fitts RH, Riley DR, Widrick JJ. Physiology of a microgravity environment invited review: microgravity and skeletal muscle. J Appl Physiol (1985) 89: 823–839, 2000. doi: 10.1152/jappl.2000.89.2.823. [DOI] [PubMed] [Google Scholar]
- 351. Volpi E, Nazemi R, Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care 7: 405–410, 2004. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Melton LJ 3rd, Khosla S, Crowson CS, O'Connor MK, O'Fallon WM, Riggs BL. Epidemiology of sarcopenia. J Am Geriatr Soc 48: 625–630, 2000. doi: 10.1111/j.1532-5415.2000.tb04719.x. [DOI] [PubMed] [Google Scholar]
- 353. Li R, Xia J, Zhang XI, Gathirua-Mwangi WG, Guo J, Li Y, McKenzie S, Song Y. Associations of muscle mass and strength with all-cause mortality among US older adults. Med Sci Sports Exerc 50: 458–467, 2018. doi: 10.1249/MSS.0000000000001448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354. Alkhajah TA, Reeves MM, Eakin EG, Winkler EA, Owen N, Healy GN. Sit-stand workstations: a pilot intervention to reduce office sitting time. Am J Prev Med 43: 298–303, 2012. doi: 10.1016/j.amepre.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 355. Balducci S, D'Errico V, Haxhi J, Sacchetti M, Orlando G, Cardelli P, Vitale M, Bollanti L, Conti F, Zanuso S, Nicolucci A, Pugliese G, Italian Diabetes and Exercise Study 2 (IDES-_2) Investigators. Effect of a behavioral intervention strategy for adoption and maintenance of a physically active lifestyle: the Italian Diabetes and Exercise Study 2 (IDES_2): a randomized controlled trial. Diabetes Care 40: 1444–1452, 2017. doi: 10.2337/dc17-0594. [DOI] [PubMed] [Google Scholar]
- 356. Healy GN, Eakin EG, Lamontagne AD, Owen N, Winkler EA, Wiesner G, Gunning L, Neuhaus M, Lawler S, Fjeldsoe BS, Dunstan DW. Reducing sitting time in office workers: short-term efficacy of a multicomponent intervention. Prev Med 57: 43–48, 2013. doi: 10.1016/j.ypmed.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 357. Peterman JE, Morris KL, Kram R, Byrnes WC. Cardiometabolic effects of a workplace cycling intervention. J Phys Act Health 16: 547–555, 2019. doi: 10.1123/jpah.2018-0062. [DOI] [PubMed] [Google Scholar]
- 358. Danquah IH, Kloster S, Holtermann A, Aadahl M, Bauman A, Ersboll AK, Tolstrup JS. Take a stand!–a multi-component intervention aimed at reducing sitting time among office workers–a cluster randomized trial. Int J Epidemiol 46: 128–140, 2017. doi: 10.1093/ije/dyw009. [DOI] [PubMed] [Google Scholar]
- 359. Clark BC, Pierce JR, Manini TM, Ploutz-Snyder LL. Effect of prolonged unweighting of human skeletal muscle on neuromotor force control. Eur J Appl Physiol 100: 53–62, 2007. doi: 10.1007/s00421-007-0399-6. [DOI] [PubMed] [Google Scholar]
- 360. Daly RM, Rosengren BE, Alwis G, Ahlborg HG, Sernbo I, Karlsson MK. gender specific age-related changes in bone density, muscle strength and functional performance in the elderly: a-10 year prospective population-based study. BMC Geriatr 13: 71, 2013. doi: 10.1186/1471-2318-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Marcell TJ, Hawkins SA, Wiswell RA. Leg strength declines with advancing age despite habitual endurance exercise in active older adults. J Strength Cond Res 28: 504–513, 2014. doi: 10.1519/JSC.0b013e3182a952cc. [DOI] [PubMed] [Google Scholar]
- 362. Tanner RE, Brunker LB, Agergaard J, Barrows KM, Briggs RA, Kwon OS, Young LM, Hopkins PN, Volpi E, Marcus RL, LaStayo PC, Drummond MJ. Age-related differences in lean mass, protein synthesis and skeletal muscle markers of proteolysis after bed rest and exercise rehabilitation. J Physiol 593: 4259–4273, 2015. doi: 10.1113/JP270699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363. Chastin S, Gardiner PA, Harvey JA, Leask CF, Jerez-Roig J, Rosenberg D, Ashe MC, Helbostad JL, Skelton DA. Interventions for reducing sedentary behaviour in community-dwelling older adults. Cochrane Database Syst Rev 6: CD012784, 2021. doi: 10.1002/14651858.CD012784.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364. Stockwell S, Schofield P, Fisher A, Firth J, Jackson SE, Stubbs B, Smith L. Digital behavior change interventions to promote physical activity and/or reduce sedentary behavior in older adults: a systematic review and meta-analysis. Exp Gerontol 120: 68–87, 2019. doi: 10.1016/j.exger.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 365. Thomsen T, Aadahl M, Beyer N, Hetland ML, Loppenthin K, Midtgaard J, Christensen R, Ostergaard M, Jennum PJ, Esbensen BA. The efficacy of motivational counselling and sms reminders on daily sitting time in patients with rheumatoid arthritis: a randomised controlled trial. Ann Rheum Dis 76: 1603–1606, 2017. doi: 10.1136/annrheumdis-2016-210953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Thomsen T, Aadahl M, Beyer N, Hetland ML, Loppenthin KB, Midtgaard J, Christensen R, Nielsen SM, Ostergaard M, Jennum P, Esbensen BA. Sustained long-term efficacy of motivational counseling and text message reminders on daily sitting time in patients with rheumatoid arthritis: long-term follow-up of a randomized, parallel-group trial. Arthritis Care Res (Hoboken) 72: 1560–1570, 2020. doi: 10.1002/acr.24060. [DOI] [PubMed] [Google Scholar]
- 367. Alexandre C, Vico L. Pathophysiology of bone loss in disuse osteoporosis. Joint Bone Spine 78: 572–576, 2011. doi: 10.1016/j.jbspin.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 368. Rittweger J, Winwood K, Seynnes O, de Boer M, Wilks D, Lea R, Rennie M, Narici M. Bone loss from the human distal tibia epiphysis during 24 days of unilateral lower limb suspension. J Physiol 577: 331–337, 2006. doi: 10.1113/jphysiol.2006.115782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369. Zerwekh JE, Ruml LA, Gottschalk F, Pak CY. The effects of twelve weeks of bed rest on bone histology, biochemical markers of bone turnover, and calcium homeostasis in eleven normal subjects. J Bone Miner Res 13: 1594–1601, 1998. doi: 10.1359/jbmr.1998.13.10.1594. [DOI] [PubMed] [Google Scholar]
- 370. Clark D, Nakamura M, Miclau T, Marcucio R. Effects of aging on fracture healing. Curr Osteoporos Rep 15: 601–608, 2017. doi: 10.1007/s11914-017-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Carter SE, Draijer R, Holder SM, Brown L, Thijssen DH, Hopkins ND. Regular walking breaks prevent the decline in cerebral blood flow associated with prolonged sitting. J Appl Physiol (1985) 125: 790–798, 2018. doi: 10.1152/japplphysiol.00310.2018. [DOI] [PubMed] [Google Scholar]
- 372. Carter SE, Draijer R, Stewart CE, Moss AD, Thijssen DH, Hopkins ND. Are acute sitting-induced changes in inflammation and cerebrovascular function related to impaired mood and cognition? Sport Sci Health 17: 753–762, 2021. doi: 10.1007/s11332-021-00753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Bojsen-Moller E, Ekblom MM, Tarassova O, Dunstan DW, Ekblom O. The effect of breaking up prolonged sitting on paired associative stimulation-induced plasticity. Exp Brain Res 238: 2497–2506, 2020. doi: 10.1007/s00221-020-05866-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Wheeler MJ, Green DJ, Ellis KA, Cerin E, Heinonen I, Naylor LH, Larsen R, Wennberg P, Boraxbekk CJ, Lewis J, Eikelis N, Lautenschlager NT, Kingwell BA, Lambert G, Owen N, Dunstan DW. Distinct effects of acute exercise and breaks in sitting on working memory and executive function in older adults: a three-arm, randomised cross-over trial to evaluate the effects of exercise with and without breaks in sitting on cognition. Br J Sports Med 54: 776–781, 2020. doi: 10.1136/bjsports-2018-100168. [DOI] [PubMed] [Google Scholar]
- 375. Howard BJ, Balkau B, Thorp AA, Magliano DJ, Shaw JE, Owen N, Dunstan DW. Associations of overall sitting time and TV viewing time with fibrinogen and c reactive protein: the AusDiab Study. Br J Sports Med 49: 255–258, 2015. doi: 10.1136/bjsports-2013-093014. [DOI] [PubMed] [Google Scholar]
- 376. Henson J, Edwardson CL, Bodicoat DH, Bakrania K, Davies MJ, Khunti K, Talbot DC, Yates T. Reallocating sitting time to standing or stepping through isotemporal analysis: associations with markers of chronic low-grade inflammation. J Sports Sci 36: 1586–1593, 2018. doi: 10.1080/02640414.2017.1405709. [DOI] [PubMed] [Google Scholar]
- 377. Vandercappellen EJ, Koster A, Savelberg H, Eussen S, Dagnelie PC, Schaper NC, Schram MT, van der Kallen CJ, van Greevenbroek MM, Wesselius A, Schalkwijk CG, Kroon AA, Henry RM, Stehouwer CD. Sedentary behaviour and physical activity are associated with biomarkers of endothelial dysfunction and low-grade inflammation-relevance for (pre)diabetes: the Maastricht Study. Diabetologia 65: 777–789, 2022. doi: 10.1007/s00125-022-05651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Panza F, D'Introno A, Colacicco AM, Capurso C, Pichichero G, Capurso SA, Capurso A, Solfrizzi V. Lipid metabolism in cognitive decline and dementia. Brain Res Rev 51: 275–292, 2006. doi: 10.1016/j.brainresrev.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 379. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, Brayne C, Burns A, Cohen-Mansfield J, Cooper C, Costafreda SG, Dias A, Fox N, Gitlin LN, Howard R, Kales HC, Kivimäki M, Larson EB, Ogunniyi A, Orgeta V, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 396: 413–446, 2020. doi: 10.1016/s0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380. Wheeler MJ, Dunstan DW, Smith B, Smith KJ, Scheer A, Lewis J, Naylor LH, Heinonen I, Ellis KA, Cerin E, Ainslie PN, Green DJ. Morning exercise mitigates the impact of prolonged sitting on cerebral blood flow in older adults. J Appl Physiol (1985) 126: 1049–1055, 2019. doi: 10.1152/japplphysiol.00001.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381. Sperlich B, De Clerck I, Zinner C, Holmberg HC, Wallmann-Sperlich B. prolonged sitting interrupted by 6-min of high-intensity exercise: circulatory, metabolic, hormonal, thermal, cognitive, and perceptual responses. Front Physiol 9: 1279, 2018. doi: 10.3389/fphys.2018.01279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382. Aengevaeren VL, Claassen JA, Levine BD, Zhang R. Cardiac baroreflex function and dynamic cerebral autoregulation in elderly masters athletes. J Appl Physiol (1985) 114: 195–202, 2013. doi: 10.1152/japplphysiol.00402.2012. [DOI] [PubMed] [Google Scholar]
- 383. Burdette JH, Laurienti PJ, Espeland MA, Morgan A, Telesford Q, Vechlekar CD, Hayasaka S, Jennings JM, Katula JA, Kraft RA, Rejeski WJ. Using network science to evaluate exercise-associated brain changes in older adults. Front Aging Neurosci 2: 23, 2010. doi: 10.3389/fnagi.2010.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384. de Heus RA, de Jong DL, Sanders ML, van Spijker GJ, Oudegeest-Sander MH, Hopman MT, Lawlor BA, Olde Rikkert MG, Claassen J. Dynamic regulation of cerebral blood flow in patients with Alzheimer Disease. Hypertension 72: 139–150, 2018. doi: 10.1161/HYPERTENSIONAHA.118.10900. [DOI] [PubMed] [Google Scholar]
- 385. Charlett OP, Morari V, Bailey DP. Impaired postprandial glucose and no improvement in other cardiometabolic responses or cognitive function by breaking up sitting with bodyweight resistance exercises: a randomised crossover trial. J Sports Sci 39: 792–800, 2021. doi: 10.1080/02640414.2020.1847478. [DOI] [PubMed] [Google Scholar]
- 386. Wanders L, Cuijpers I, Kessels RP, van de Rest O, Hopman MT, Thijssen DH. Impact of prolonged sitting and physical activity breaks on cognitive performance, perceivable benefits, and cardiometabolic health in overweight/obese adults: the role of meal composition. Clin Nutr 40: 2259–2269, 2021. doi: 10.1016/j.clnu.2020.10.006. [DOI] [PubMed] [Google Scholar]
- 387. Stoner L, Willey Q, Evans WS, Burnet K, Credeur DP, Fryer S, Hanson ED. Effects of acute prolonged sitting on cerebral perfusion and executive function in young adults: a randomized cross-over trial. Psychophysiology 56: e13457, 2019. doi: 10.1111/psyp.13457. [DOI] [PubMed] [Google Scholar]
- 388. Chrismas BC, Taylor L, Cherif A, Sayegh S, Bailey DP. Breaking up prolonged sitting with moderate-intensity walking improves attention and executive function in qatari females. PLoS One 14: e0219565, 2019. doi: 10.1371/journal.pone.0219565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389. Mullane SL, Buman MP, Zeigler ZS, Crespo NC, Gaesser GA. Acute effects on cognitive performance following bouts of standing and light-intensity physical activity in a simulated workplace environment. J Sci Med Sport 20: 489–493, 2017. doi: 10.1016/j.jsams.2016.09.015. [DOI] [PubMed] [Google Scholar]
- 390. Schwartz B, Kapellusch JM, Schrempf A, Probst K, Haller M, Baca A. Effect of alternating postures on cognitive performance for healthy people performing sedentary work. Ergonomics 61: 778–795, 2018. doi: 10.1080/00140139.2017.1417642. [DOI] [PubMed] [Google Scholar]
- 391. Vincent GE, Jay SM, Sargent C, Kovac K, Vandelanotte C, Ridgers ND, Ferguson SA. The impact of breaking up prolonged sitting on glucose metabolism and cognitive function when sleep is restricted. Neurobiol Sleep Circadian Rhythms 4: 17–23, 2018. doi: 10.1016/j.nbscr.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Falck RS, Best JR, Li LC, Chan PC, Feehan LM, Liu-Ambrose T. Can we improve cognitive function among adults with osteoarthritis by increasing moderate-to-vigorous physical activity and reducing sedentary behaviour? Secondary analysis of the Monitor-OA Study. BMC Musculoskelet Disord 19: 447, 2018. doi: 10.1186/s12891-018-2369-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 393. Nelson MC, Casanova MP, Ball JR, Midence RD, Johnson TR, Martin B, Fehrenkamp BD, Baker RT, Drum SN, Vella CA. Effects of uninterrupted sitting: are there differences across sex in vascular and inflammatory biomarkers? Med Sci Sports Exerc 53: 81–81, 2021. doi: 10.1249/01.mss.0000760060.63336.ad. [DOI] [Google Scholar]
- 394. Chen YC, Betts JA, Walhin JP, Thompson D. Adipose tissue responses to breaking sitting in men and women with central adiposity. Med Sci Sports Exerc 50: 2049–2057, 2018. doi: 10.1249/MSS.0000000000001654. [DOI] [PubMed] [Google Scholar]
- 395. Jager A, van Hinsbergh VW, Kostense PJ, Emeis JJ, Yudkin JS, Nijpels G, Dekker JM, Heine RJ, Bouter LM, Stehouwer CD. Von Willebrand factor, c-reactive protein, and 5-year mortality in diabetic and nondiabetic subjects: the Hoorn Study. Arterioscler Thromb Vasc Biol 19: 3071–3078, 1999. doi: 10.1161/01.atv.19.12.3071. [DOI] [PubMed] [Google Scholar]
- 396. Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342: 836–843, 2000. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 397. Grace MS, Dempsey PC, Sethi P, Mundra PA, Mellett NA, Weir JM, Owen N, Dunstan DW, Meikle PJ, Kingwell BA. Breaking up prolonged sitting alters the postprandial plasma lipidomic profile of adults with type 2 diabetes. J Clin Endocrinol Metab 102: 1991–1999, 2017. doi: 10.1210/jc.2016-3926. [DOI] [PubMed] [Google Scholar]
- 398. Kerr NR, Booth FW. Contributions of physical inactivity and sedentary behavior to metabolic and endocrine diseases. Trends Endocrinol Metab 33: 817–827, 2022. doi: 10.1016/j.tem.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 399. Booth FW, Laye MJ. Lack of adequate appreciation of physical exercise's complexities can pre-empt appropriate design and interpretation in scientific discovery. J Physiol 587: 5527–5539, 2009. doi: 10.1113/jphysiol.2009.179507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400. Lee SM, Moore AD, Everett ME, Stenger MB, Platts SH. Aerobic exercise deconditioning and countermeasures during bed rest. Aviat Space Environ Med 81: 52–63, 2010. doi: 10.3357/asem.2474.2010. [DOI] [PubMed] [Google Scholar]
- 401. Shackelford LC, LeBlanc AD, Driscoll TB, Evans HJ, Rianon NJ, Smith SM, Spector E, Feeback DL, Lai D. Resistance exercise as a countermeasure to disuse-induced bone loss. J Appl Physiol (1985) 97: 119–129, 2004. doi: 10.1152/japplphysiol.00741.2003. [DOI] [PubMed] [Google Scholar]
- 402. Belavy DL, Beller G, Ritter Z, Felsenberg D. Bone structure and density Via Hr-Pqct in 60d bed-rest, 2-years recovery with and without countermeasures. J Musculoskelet Neuronal Interact 11: 215–226, 2011. [PubMed] [Google Scholar]
- 403. Mutin-Carnino M, Carnino A, Roffino S, Chopard A. Effect of muscle unloading, reloading and exercise on inflammation during a head-down bed rest. Int J Sports Med 35: 28–34, 2014. doi: 10.1055/s-0033-1343407. [DOI] [PubMed] [Google Scholar]
- 404. Booth FW, Lees SJ. Fundamental questions about genes, inactivity, and chronic diseases. Physiol Genomics 28: 146–157, 2007. doi: 10.1152/physiolgenomics.00174.2006. [DOI] [PubMed] [Google Scholar]
- 405. Booth FW, Lees SJ. Physically active subjects should be the control group. Med Sci Sports Exerc 38: 405–406, 2006. doi: 10.1249/01.mss.0000205117.11882.65. [DOI] [PubMed] [Google Scholar]
- 406. Dempsey PC, Biddle SJ, Buman MP, Chastin S, Ekelund U, Friedenreich CM, Katzmarzyk PT, Leitzmann MF, Stamatakis E, van der Ploeg HP, Willumsen J, Bull F. New global guidelines on sedentary behaviour and health for adults: broadening the behavioural targets. Int J Behav Nutr Phys Act 17: 151, 2020. doi: 10.1186/s12966-020-01044-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407. Dempsey PC, Friedenreich CM, Leitzmann MF, Buman MP, Lambert E, Willumsen J, Bull F. Global public health guidelines on physical activity and sedentary behavior for people living with chronic conditions: a call to action. J Phys Act Health 18: 76–85, 2021. doi: 10.1123/jpah.2020-0525. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available upon reasonable request.