Abstract
Although there is ample evidence that the advanced glycation end-product (AGE) glucosepane contributes to age-related morbidities and diabetic complications, the impact of glucosepane modifications on proteins has not been extensively explored due to the lack of sufficient analytical tools. Here, we report the development of the first polyclonal anti-glucosepane antibodies using a synthetic immunogen that contains the core bicyclic ring structure of glucosepane. We investigate the recognition properties of these antibodies through ELISAs involving an array of synthetic AGE derivatives and determine them to be both high-affinity and selective in binding glucosepane. We then employ these antibodies to image glucosepane in aging mouse retinae via immunohistochemistry. Our studies demonstrate for the first time accumulation of glucosepane within the retinal pigment epithelium, Bruch’s membrane, and choroid: all regions of the eye impacted by age-related macular degeneration. Co-localization studies further suggest that glucosepane colocalizes with lipofuscin, which has previously been associated with lysosomal dysfunction and has been implicated in the development of age-related macular degeneration, among other diseases. We believe that the anti-glucosepane antibodies described in this study will prove highly useful for examining the role of glycation in human health and disease.
Graphical Abstract
INTRODUCTION
Advanced glycation end-products (AGEs) are a heterogeneous group of compounds formed as a result of non-enzymatic reactions between nucleophilic residues and sugars. AGEs have been shown to alter the structure of long-lived proteins, such as crystallin, albumin, and collagen,1 making them more resistant to degradation and promoting their accumulation in cells and tissues with age.2–5 There is increasing evidence that AGEs are involved in the pathogenesis of age-related and chronic diseases, including age-related macular degeneration (AMD) and diabetes.6–9
Glucosepane is among the most abundant AGEs found in human tissues. It is formed from lysine, arginine, and glucose, and it is over an order of magnitude more abundant than any other AGE cross-link in the extracellular matrix (ECM; Supplementary Scheme 1).10 Notably, glucosepane levels have been shown to correlate with various disease states, including diabetic retinopathy, microalbuminuria, and neuropathy.3,11–13 While the exact mechanisms behind glucosepane-mediated dysfunction remain unclear, it is believed to impair the functional and mechanical properties of proteins in the ECM14 and interfere with proteolytic degradation of collagen.15
To date, the primary method for identifying glucosepane in tissues has required exhaustive enzymatic degradation followed by high pressure liquid chromatography−mass spectrometry (LC/MS).13,16,17 Although these protocols have proven effective in quantifying glucosepane in bulk tissue extracts, they are labor-intensive, and the degradation process destroys the tissue architecture, making it difficult to examine the localization of glucosepane.
In recent years, anti-AGE antibodies have emerged as useful tools for studying AGEs and have the advantage of being compatible with the evaluation of intact tissues, enabling immunohistochemical staining and imaging procedures.18 nb Several anti-AGE antibodies have been produced by immunization of animals with AGEs generated either from total synthesis19 or through in vitro glycation methods. Such methods involve the incubation of an immunogenic carrier protein, such as BSA, with glucose or other reactive sugar metabolites.20–23 Reaction conditions that generate glucosepane24 are known also to generate a range of AGE byproducts, including carboxymethyllysine.25,26
These in vitro preparation methods are unlikely to produce antibodies that are specific for glucosepane, although no such studies have been reported. To avoid this expected complication, we decided to synthesize homogeneous, synthetic glucosepane immunogens.
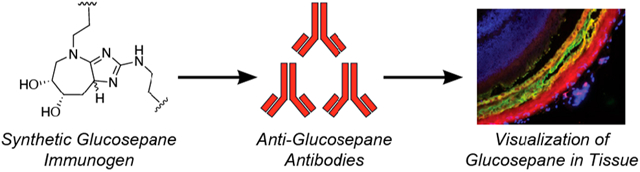
Herein, we describe the development and characterization of the first antibodies known to selectively recognize glucosepane. To this end, we have created a synthetic glucosepane immunogen that closely resembles glucosepane found in vivo and used it to generate a polyclonal antibody serum that recognizes glucosepane both in vitro and in ex vivo tissue samples. We have demonstrated that the antibodies can bind to glucosepane with high degrees of specificity and sensitivity through ELISA studies and have employed these antibodies in immunohistochemical experiments. Interestingly, these latter studies demonstrate that glucosepane accumulates within subcomponents of the retina, specifically the retinal pigment epithelium (RPE), Bruch’s membrane, and choroid, which are anatomic areas highly affected by AMD and diabetic retinopathy.27,28 Our results suggest that anti-glucosepane antibodies could be useful for uncovering mechanisms through which glucosepane contributes to aging and disease and could potentially serve as tools for the diagnosis of aging and diabetic complications.
RESULTS AND DISCUSSION
Immunogen Design and Synthesis.
In order to generate antibodies capable of binding glucosepane cross-links in proteins, we designed immunogen 1 (Scheme 1a), containing the glucosepane core incorporated into an immunogen peptide as our target epitope (highlighted in red, Scheme 1a). Surrounding the target epitope, we included polyethylene glycol and glycine residues. We hypothesized that these motifs would only contribute minimally to antibody binding affinity due to their flexibility and, as such, would allow us to obtain clones that interact preferentially with the target epitope. Cysteine was added to the C-terminus as a chemical handle to enable thiol-maleimide conjugation reactions to proteins and solid supports.
Scheme 1.
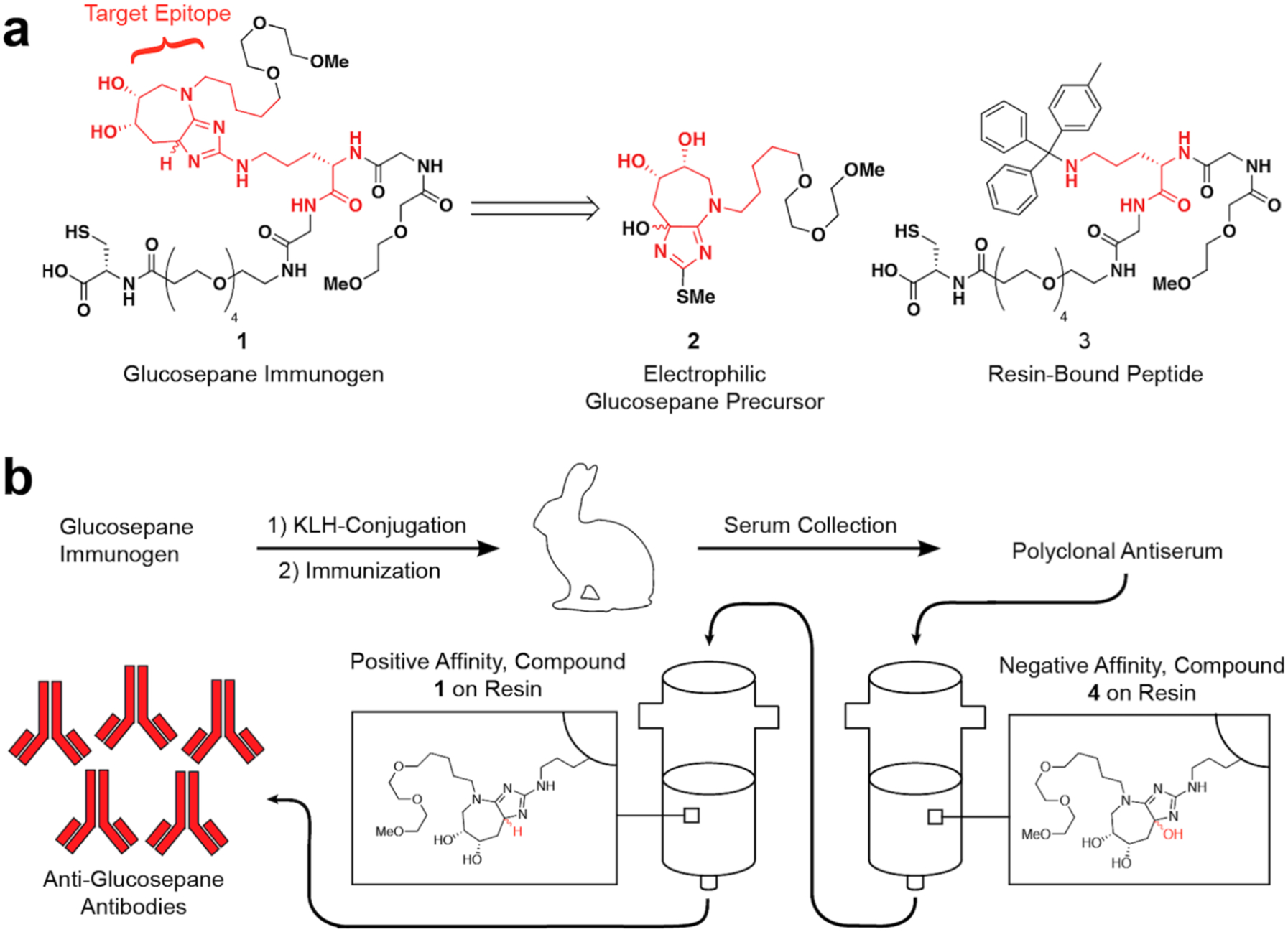
(a) Immunogen Retrosynthesisa and (b) Antibody Generation and Purification Processb
aImmunogen 1 was accessed from electrophilic glucosepane precursor 2 and resin-bound peptide 3. bThe immunogen was conjugated to keyhole limpet hemocyanin (KLH). Rabbits were immunized, and antiserum was collected. The antiserum was subjected to a series of affinity purifications, and the resulting antibodies were used for all subsequent experiments.
Immunogen 1 (Scheme 1a) was retrosynthetically disconnected in a manner to facilitate late-stage incorporation of advanced precursor 2 (Scheme 1a) into the peptide sequence. The key disconnection was made between the 2-amino group and the rest of the bicyclic glucosepane core. We hypothesized that we could construct this bond through a late-stage reaction of an unprotected ornithine side-chain with the electrophilic glucosepane precursor 2 using a strategy derived from our reported glucosepane total synthesis (Supplementary Figure 1).29 This, in turn, could be appended to resin-bound ornithine derivative 3, which could be readily prepared via solid-phase peptide synthesis (SPPS). We felt this late-stage installation of glucosepane precursor 2 would be more material-efficient than a traditional monomer-based approach with an Fmocprotected glucosepane amino acid monomer (Supplementary Figure 1).
For the synthesis of electrophilic glucosepane precursor 2, we followed a synthetic strategy similar to what we employed previously in the total synthesis of glucosepane (Supplementary Scheme 2).29 This 10-step procedure starts from readily available 1,5-dibromopentane and affords antigen precursor 2 with an overall yield of 3.6%. Compound 2 was then allowed to react with resin-bound ornithine derivative 3 to afford a 2,4-diamino-5-hydroxyimidazole species on-resin. After a sodium triacetoxyborohydride reduction and cleavage from the resin, we accessed the desired glucosepane immunogen 1 in 4.06% yield.
Antibody Generation and Characterization.
Upon completion of synthesis, peptide immunogen 1 was conjugated to keyhole limpet hemocyanin (KLH) via the pendant thiol (Scheme 1b). This conjugate was then used by New England Peptide, Inc. (Gardner, MA) to immunize New Zealand white rabbits. After an immunization period of three months, serum samples were collected from the rabbits. The polyclonal antiserum was then subjected to negative selection against oxidized glucosepane derivative 4 to remove antibodies specific for the peptide backbone, followed by positive selection against agarose beads coated with glucosepane immunogen 1. Following elution, we isolated anti-glucosepane antibodies.
The specificity of the purified anti-glucosepane antibodies was tested via ELISA against a panel of synthetic peptides (Figure 1a, 6−9) containing some of the most abundant AGEs, along with arginine-containing (Figure 1a, 5) and glucosepane-containing controls (Figure 1a, 10).30,31 Peptides were anchored to the wells of maleimide-coated 96-well plates via thiol-maleimide conjugation reactions. Binding of these synthetic peptides was then tested against the polyclonal serum. As hypothesized, peptides 5−9 exhibited no detectable binding to the antibodies, while the glucosepane peptide 10 bound tightly to the antibodies with an EC50 of 14.16 ± 0.20 nM (Figure 1b). To the best of our knowledge, this is the first report of the generation of glucosepane-specific antibodies.
Figure 1.
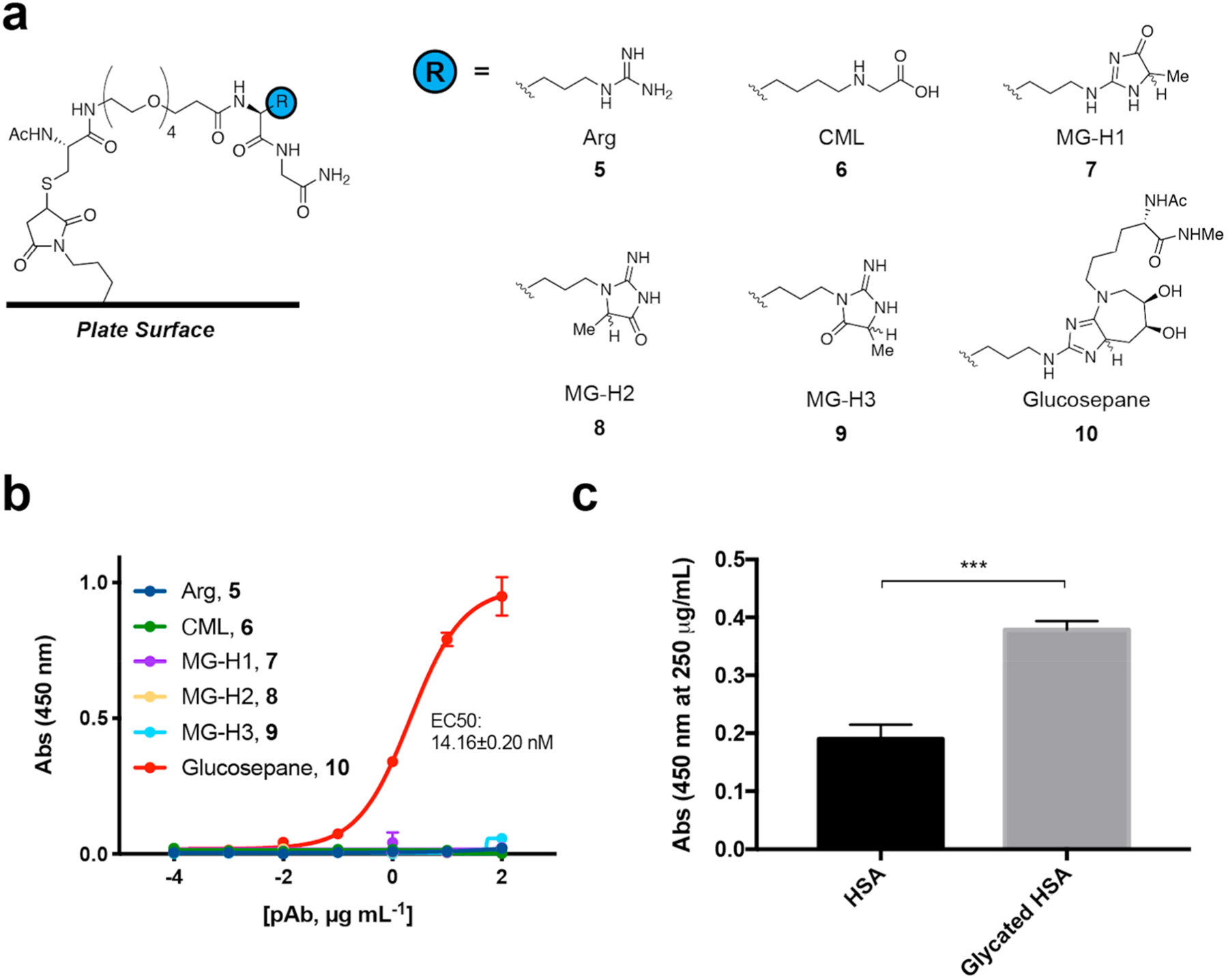
Chemical structures of synthetic ELISA substrates and the corresponding ELISA results used to demonstrate anti-glucosepane antibody selectivity. (a) Synthetic peptides containing arginine, other advanced glycation end-products, and glucosepane (CML = carboxymethyllysine; MG-H1 = methylglyoxal-derived hydroimidazolone 1; MG-H2 = methylglyoxal-derived hydroimidazolone 2; MG-H3 = methylglyoxal-derived hydroimidazolone 3). (b) ELISA data toward panel of synthetic peptides. Indirect ELISAs were run on maleimide-coated plates. Error bars represent standard deviation (n = 2). (c) Protein-based indirect ELISA on Nunc plates. Binding of anti-glucosepane antibodies was tested against commercially available HSA and glycated HSA (***P < 0.0005, two-tailed, unpaired t test).
To characterize the binding epitope of the antibodies further, additional ELISAs were performed with several abiotic glucosepane analogs. These experiments were designed to determine the importance of various structural elements of glucosepane toward the binding epitope of the antibodies. As expected, the glucosepane peptide most structurally similar to the immunogen (S24) exhibited the lowest EC50 for the antibodies at 5.6 ± 0.23 nM. Scaffolds that slightly perturbed the core immunogen scaffold demonstrated weaker affinities. For example, when the hydroxyl groups were not present (as in S22), the EC50 increased to 7.07 ± 0.13 nM, and when the core ring system was oxidized (as in S23), the EC50 increased to 11.86 ± 0.20 nM. Further differences, such as the absence of the polyethylene glycol motif in addition to the hydroxyl groups (as in S21), resulted in a more significant reduction in affinity to ~1 μM. Additional alterations, such as cleavage of the seven-membered ring (as in S20) caused the EC50 of the antibodies to rise to levels comparable to negative control compound 5 (Supplementary Figure 2).
In summary, the only analogs that showed significant affinity to the antibodies are abiotic. Two substrates in physiological systems that have the potential to cross-react with the antibodies generated herein are pentosinane and pentosidine, but to date there have been no reported synthetic routes to peptides containing these modifications.32 The abiotic analogs tested also offer insights into our antibody generation and purification process. The high binding affinity of oxidized substrate S23 highlights that negative affinity purification did not completely precipitate antibodies capable of binding to this substrate. Meanwhile, PEGylated S24 highlights that PEGylation of the immunogen results in a minor contribution to the binding epitope over AcHN-Lys-NHMe-modified 10 (5.6 ± 0.23 nM, S24; 14.16 ± 0.20 nM, 10).
An experiment was also performed to demonstrate the capacity of a soluble inhibitor S25 to inhibit binding of the antibodies to glucosepane substrate S24. When excess soluble glucosepane competitor S25 was present, the binding of the antibodies was reduced to background levels (Supplementary Figure 3).
Overall, these experiments demonstrate that antibody binding is highly specific for the molecular structure of glucosepane, and comparable binding properties are observed only for very closely related abiotic analogs.
Having demonstrated that the antibodies bound synthetic glucosepane specifically and with high affinity, we next sought to evaluate their utility in biological samples. To this end, we measured glucosepane levels in several commercial samples of human serum albumin (HSA) using an LC/MS method. Approximately 0.3% of the arginine residues in these HSA samples were found to be modified by glucosepane. We also employed in vitro glycation methods to enrich glucosepane content in HSA and found these samples to contain glucosepane levels of 1.3%.33
ELISAs were then performed, wherein our antibodies were exposed directly to low- and high-content HSA samples. As predicted, significantly greater binding was observed with glycated HSA compared to commercially available HSA (Figure 1c). Importantly, the glucosepane levels used in this experiment, 0.3% and 1.3%, align with the range of glucosepane levels that have been observed in human serum samples.16
Glucosepane in Aging Retinae.
Glucosepane has been found to accumulate in various tissues with age10,16 and has long been thought to play a role in promoting ocular diseases such as cataracts, diabetic retinopathy, and AMD.3,12,34 However, tissue localization studies of glucosepane have not been performed to our knowledge. Therefore, we next sought to apply the anti-glucosepane antibodies to study the localization of glucosepane in the retinae of aging mice via immunohistochemistry. In these studies, we sectioned, stained, and imaged retinae of young (2 month), middle-aged (7 month), and older (12 month) C57BL/6J mice (Figure 2a). These experiments revealed an increase in the area of glucosepane staining in retinal tissue samples with age. Further examination of the IHC images showed glucosepane accumulation in the retinal pigment epithelium (RPE), Bruch’s membrane, and choroid: regions associated with diseases such as AMD and retinopathy (Figure 2b).27,28 Weaker glucosepane staining was also observed in the photoreceptor inner and outer segments (Figure 2a). Additionally, in competition experiments, a reduced fluorescence signal was observed in the presence of peptidic competitor S25 (Supplementary Figure 3), suggesting that the antibodies were binding glucosepane cross-links.
Figure 2.
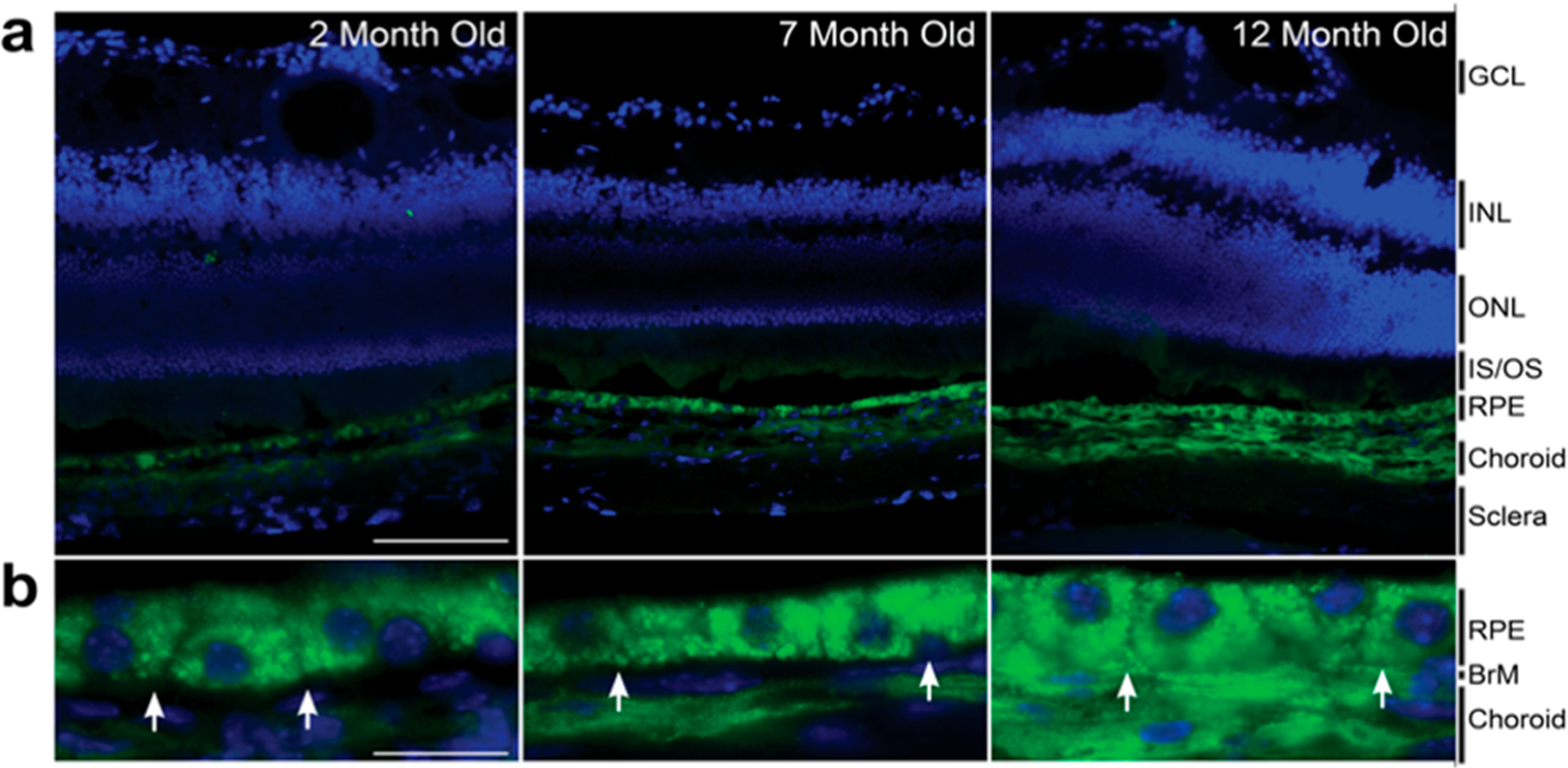
Immunohistochemical images of mouse retinae. Retinal samples were taken from 2, 7, and 12 month old mice. (a) Representative retinal sections labeled with anti-glucosepane antibody/anti-Rbt Alexa488 (green) and DAPI (blue). Scale bars are 100 μm (GCL = ganglion cell layer; INL = inner nuclear layer; ONL = outer nuclear layer; IS/OS = inner/outer segments; RPE = retinal pigmented epithelium). (b) Representative high-magnification images of the RPE, Bruch’s membrane, and choroid. White arrows highlight the position of Bruch’s membrane (BrM). Scale bars are 20 μm.
Notably, granular glucosepane staining was observed in the RPE at the subcellular level (Figure 2b). We, therefore, hypothesized that there could be colocalization between glucosepane and lipofuscin, a lysosomally derived, non-degradable, heterogeneous molecular aggregate that is partially characterized by its granular appearance. Previous reports have proposed that the accumulation of lipofuscin within the RPE is considered to be a risk factor for age-related diseases of the eye.35 Lipofuscin has been shown to induce lysosomal dysfunction and interfere with autophagic clearance, contributing to the development of various eye pathologies, including AMD.36–39 There is also evidence that AGEs promote formation of lipofuscin and that AGE-modified proteins may be components of lipofuscin granules.40,41
Lipofuscin granules are often identified by their characteristic autofluorescence, as well as their lysosomal origin, indicated by colocalization with lysosomal-associated membrane protein 1 (LAMP-1).42 After partial bleaching of retinal samples, colocalization between autofluorescent granules and glucosepane was observed in regions of the RPE (Figure 3a). Additional costaining experiments demonstrated colocalization between glucosepane and LAMP-1 (Figure 3b). Although other AGEs have been associated with lipofuscin formation,43,44 this is the first evidence for the colocalization of lipofuscin and glucosepane. Our findings therefore suggest that glucosepane-modified proteins may be constituents of lipofuscin and accumulate within the RPE with age. Given the known involvement of lipofuscin in disease, these findings warrant further investigations into the impact of glucosepane accumulation on lysosomal activity and disease onset. These data also suggest that glucosepane merits exploration as a biomarker for age-related eye disease.
Figure 3.
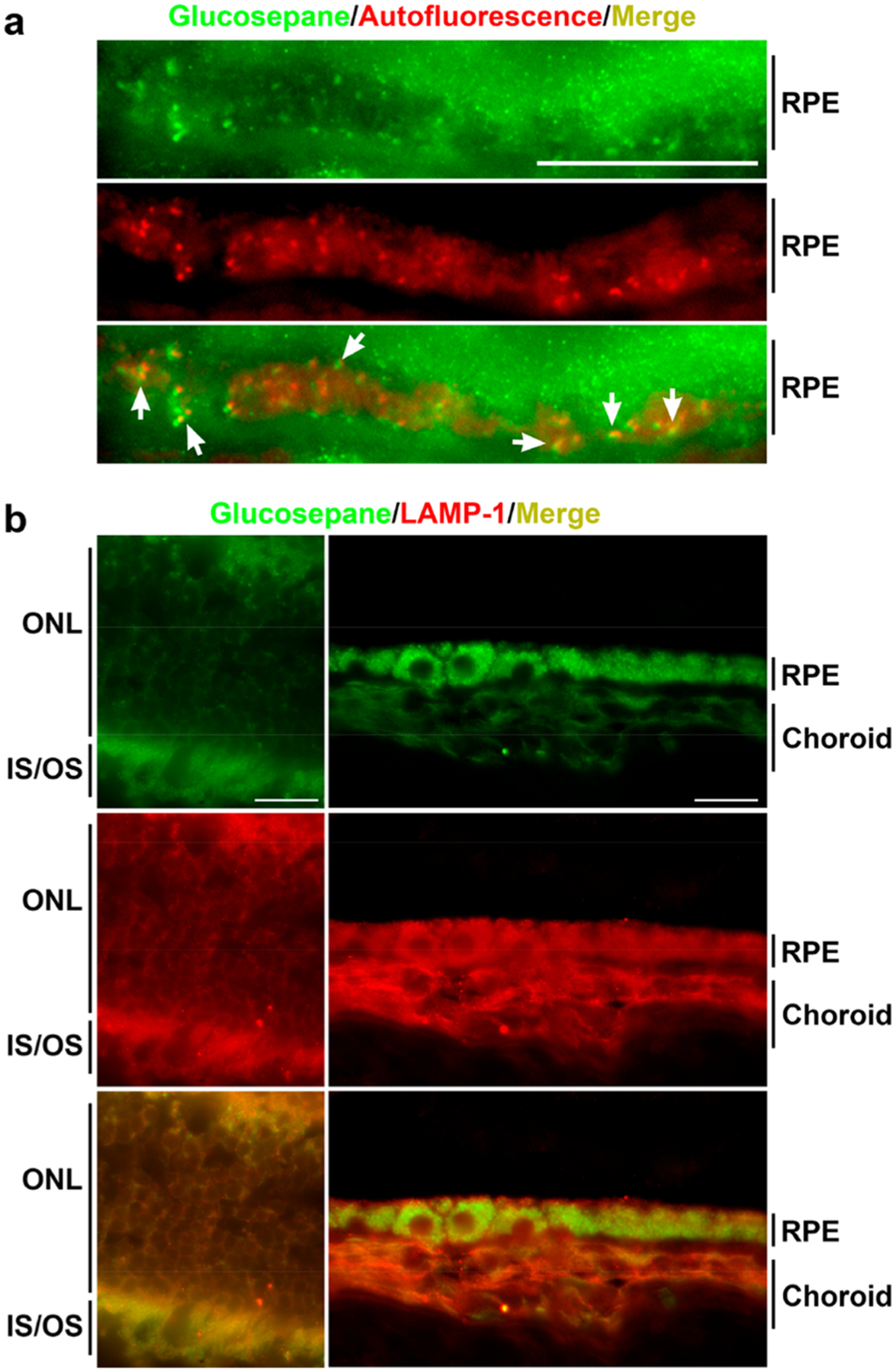
Immunohistochemical images of 12-month-old mouse retinae. (a) Localization of glucosepane (green) and autofluorescence (red) in partially bleached RPE. Yellow indicates colocalization. White arrows highlight areas of colocalization. (b) Localization of glucosepane (green) and LAMP1 (red) in unbleached retinal photoreceptors (left) and bleached RPE/choroid (right). Yellow indicates colocalization. Scale bars are 20 μm (ONL = outer nuclear layer; IS/OS = inner/outer segments; RPE = retinal pigmented epithelium).
CONCLUSIONS
Investigation into the role of AGEs in pathophysiology has been hindered by a lack of tools to study the distribution of individual AGEs in intact tissues. Through the preparation of a synthetic glucosepane immunogen, we have developed the first polyclonal anti-glucosepane antibodies, which recognize glucosepane with high specificity and sensitivity. These antibodies bind both synthetic glucosepane and glucosepane-like molecules, as well as glucosepane-modified HSA in a manner that correlates with traditional HPLC/MS protocols. Furthermore, we applied these antibodies to demonstrate, for the first time, that glucosepane accumulates in the RPE, Bruch’s membrane, and choroid in the retinae of aging mice. The accumulation of glucosepane in the RPE partially colocalizes with lipofuscin granules, suggesting a potential link to lipofuscin-related pathology. Taken together, these data demonstrate that anti-glucosepane antibodies are effective tools for examining glucosepane cross-links in tissue with subcellular resolution. In the future, these antibodies will serve as tools to provide insight into the role of glucosepane in human health and disease.
METHODS
A description of all chemicals, reagents, instrumentation, and procedures is available in the Supporting Information.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by funding from the American Diabetes Association Pathway to Stop Diabetes Grant 1-17-VSN-04 and the SENS Research Foundation. J.D.R. was funded by the National Institute of Health Yale Chemical Biology Training Grant (2T32GM06754 3-17). The authors would also like to thank S. Bhatt and D. McDonald for their help in preparing the manuscript and S. Lewis at New England Peptide for his helpful advice regarding immunization.
Footnotes
The authors declare the following competing financial interest(s): D.A.S. is co-founder and shareholder of Revel Pharmaceuticals, a company that develops AGE-related therapies.
Contributor Information
Matthew D. Streeter, Department of Chemistry, Yale University, New Haven, Connecticut 06511, United States
Sheldon Rowan, Tufts University, JM-USDA Human Nutrition Research Center on Aging, Boston, Massachusetts 02111, United States.
Jason Ray, Department of Chemistry, Yale University, New Haven, Connecticut 06511, United States.
David M. McDonald, Department of Chemistry, Yale University, New Haven, Connecticut 06511, United States
Jonathan Volkin, Tufts University, JM-USDA Human Nutrition Research Center on Aging, Boston, Massachusetts 02111, United States.
Jonathan Clark, Biological Chemistry Laboratory, Babraham Institute, Cambridge CB21 3AT, United Kingdom.
Allen Taylor, Tufts University, JM-USDA Human Nutrition Research Center on Aging, Boston, Massachusetts 02111, United States.
David A. Spiegel, Department of Chemistry, Yale University, New Haven, Connecticut 06511, United States
REFERENCES
- (1).Singh R, Barden A, Mori T, and Beilin L (2001) Advanced Glycation End-Products: A Review. Diabetologia 44, 129–146. [DOI] [PubMed] [Google Scholar]
- (2).Bartling B, Desole M, Rohrbach S, Silber R-E, and Simm A (2009) Age-associated changes of extracellular matrix collagen impair lung cancer cell migration. FASEB J. 23, 1510–1520. [DOI] [PubMed] [Google Scholar]
- (3).Monnier VM, Sun W, Sell DR, Fan X, Nemet I, and Genuth S (2014) Glucosepane: A Poorly Understood Advanced Glycation End-Product of Growing Importance for Diabetes and its Complications. Clin. Chem. Lab. Med 52, 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).DeGroot J, Verzijl N, Budde M, Bijlsma JWJ, Lafeber FPJG, and TeKoppele JM (2001) Accumulation of Advanced Glycation End Products Decreases Collagen Turnover by Bovine Chondrocytes. Exp. Cell Res 266, 303–310. [DOI] [PubMed] [Google Scholar]
- (5).Uchiki T, Weikel KA, Jiao W, Shang F, Caceres A, Pawlak D, Handa JT, Brownlee M, Nagaraj R, and Taylor A (2012) Glycation-altered Proteolysis as a Pathobiologic Mechanism that Links Dietary Glycemic index, Aging, and Age-related Disease (in Non-diabetics). Aging Cell 11, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Yamagishi S (2011) Role of advanced glycation endproducts (AGEs) and receptor for AGEs (RAGE) in vascular damage in diabetes. Exp. Gerontol 46, 217–224. [DOI] [PubMed] [Google Scholar]
- (7).Ishibashi T, Murata T, Hangai M, Nagai R, Horiuchi S, Lopez PF, Hinton DR, and Ryan SJ (1998) Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol 116, 1629–1632. [DOI] [PubMed] [Google Scholar]
- (8).Glenn JV, and Stitt AW (2009) The role of advanced glycation end products in retinal ageing and disease. Biochim. Biophys. Acta, Gen. Subj 1790, 1109–1116. [DOI] [PubMed] [Google Scholar]
- (9).Yan SF, Ramasamy R, and Schmidt AM (2008) Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Clin. Pract. Endocrinol. Metab 4, 285–293. [DOI] [PubMed] [Google Scholar]
- (10).Sell DR, Biemel KM, Reihl O, Lederer MO, Strauch CM, and Monnier VM (2005) Glucosepane is a major protein cross-link of the senescent human extracellular matrix. J. Biol. Chem 280, 12310–12315. [DOI] [PubMed] [Google Scholar]
- (11).Genuth S, Sun W, Cleary P, Gao X, Sell DR, Lachin J, and Monnier VM (2015) Skin advanced glycation end products glucosepane and methylglyoxal hydroimidazolone are independently associated with long-term microvascular complication progression of type 1 diabetes. Diabetes 64, 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Rowan S, Jiang S, Korem T, Szymanski J, Chang M-L, Szelog J, Cassalman C, Dasuri K, McGuire C, Nagai R, Du XL, Brownlee M, Rabbani N, Thornalley PJ, Baleja JD, Deik AA, Pierce KA, Scott JM, Clish CB, Smith DE, Weinberger A, Avnit-Sagi T, Lotan-Pompan M, Segal E, and Taylor A (2017) Involvement of a gut-retina axis in protection against dietary glycemia-induced age-related macular degeneration. Proc. Natl. Acad. Sci. U. S. A 114, E4472–E4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Chiu C-J, Rabbani N, Rowan S, Chang M-L, Sawyer S, Hu FB, Willett W, Thornalley PJ, Anwar A, Bar L, Kang JH, and Taylor A (2018) Studies of advanced glycation end products and oxidation biomarkers for type 2 diabetes. Biofactors 44, 281–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Collier TA, Nash A, Birch HL, and deLeeuw NH (2018) Effect on the mechanical properties of type I collagen of intramolecular lysine-arginine derived advanced glycation end-product cross-linking. J. Biomech 67, 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Collier TA, Nash A, Birch HL, and de Leeuw NH (2015) Preferential sites for intramolecular glucosepane cross-link formation in type I collagen: A thermodynamic study. Matrix Biol. 48, 78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Biemel KM, Friedl DA, and Lederer MO (2002) Identification and quantification of major maillard cross-links in human serum albumin and lens protein. J. Biol. Chem 277, 24907–24915. [DOI] [PubMed] [Google Scholar]
- (17).Rabbani N, and Thornalley PJ (2018) Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 93, 803–813. [DOI] [PubMed] [Google Scholar]
- (18).Nagai R, Shirakawa J, Ohno R, Hatano K, Sugawa H, Arakawa S, Ichimaru K, Kinoshita S, Sakata N, and Nagai M (2016) Antibody-based detection of advanced glycation end-products: promises vs. limitations. Glycoconjugate J. 33, 545–552. [DOI] [PubMed] [Google Scholar]
- (19).Wang T, Streeter MD, and Spiegel DA (2015) Generation and characterization of antibodies against arginine-derived advanced glycation endproducts. Bioorg. Med. Chem. Lett 25, 4881–4886. [DOI] [PubMed] [Google Scholar]
- (20).Finco AB, Machado-de-Avila RA, Maciel R, De Moura J, Billiald P, Stinghen AEM, and Alvarenga LM (2016) Generation and characterization of monoclonal antibody against advanced glycation endproducts in chronic kidney disease. Biochem. Biophys. Rep 6, 142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Nagai R, Fujiwara Y, Mera K, Yamagata K, Sakashita N, and Takeya M (2008) Immunochemical detection of Ne-(carboxyethyl)lysine using a specific antibody. J. Immunol. Methods 332, 112–120. [DOI] [PubMed] [Google Scholar]
- (22).Shamsi FA, Partal A, Sady C, Glomb MA, and Nagaraj RH (1998) Immunological evidence for methylglyoxal-derived modifications in vivo. J. Biol. Chem 273, 6928–6936. [DOI] [PubMed] [Google Scholar]
- (23).Morioka Y, Teshigawara K, Tomono Y, Wang D, Izushi Y, Wake H, Liu K, Takahashi HK, Mori S, and Nishibori M (2017) The specific localization of advanced glycation endproducts (AGEs) in rat pancreatic islets. J. Pharmacol. Sci 134, 218–224. [DOI] [PubMed] [Google Scholar]
- (24).Lederer MO, and Buhler HP (1999) Cross-linking of proteins by Maillard processes-Characterization and detection of a lysine-argining cross-link derived from D-glucose. Bioorg. Med. Chem 7, 1081–1088. [DOI] [PubMed] [Google Scholar]
- (25).Ahmad W, Li L, and Deng Y (2008) Identification of AGE-precursors and AGE formation in glycation-induced BSA peptides. BMB Rep. 41, 516–522. [DOI] [PubMed] [Google Scholar]
- (26).Zyzak DV, Richardson JM, Thorpe SR, and Baynes JW (1995) Formation of reactive intermediates from Amadori compounds under physiological conditions. Arch. Biochem. Biophys 316, 547–554. [DOI] [PubMed] [Google Scholar]
- (27).Strauss O (2005) The retinal pigment epithelium in visual function. Physiol. Rev 85, 845–881. [DOI] [PubMed] [Google Scholar]
- (28).Xu H-Z, Song Z, Fu S, Zhu M, and Le Y-Z (2011) RPE barrier breakdown in diabetic retinopathy: seeing is believing. J. Ocul. Biol. Dis. Inform 4, 83–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Draghici C, Wang T, and Spiegel DA (2015) Concise total synthesis of glucosepane. Science 350, 294–298. [DOI] [PubMed] [Google Scholar]
- (30).Wang T, Kartika R, and Spiegel DA (2012) Exploring post-translational arginine modification using chemically synthesized methylglyoxal hydroimidazolones. J. Am. Chem. Soc 134, 8958–8967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Woods TM, Kamalov M, Harris PWR, Cooper GJS, and Brimble M (2012) Synthesis of monolysyl advanced glycation endproducts and their incorporation into collage model peptides. Org. Lett 14, 5740–5743. [DOI] [PubMed] [Google Scholar]
- (32).Biemel KM, Reihl O, Conrad J, and Lederer MO (2001) Formation Pathways for Lysine-Arginine Cross-Links Derived from Hexoses and Pentoses by Maillard Processes. J. Biol. Chem 276, 23405–23412. [DOI] [PubMed] [Google Scholar]
- (33).Chesne S, Rondeau P, Armenta S, and Bourdon E (2006) Effects of oxidative modifications induced by the glycation of bovine serum albumin on its structure and on cultured adipose cells. Biochimie 88, 1467–1477. [DOI] [PubMed] [Google Scholar]
- (34).Tessier F, Obrenovich M, and Monnier VM (1999) Structure and Mechanism of Formation of Human Lens Fluorophore LM-1. Relationship to Vesperlysine A and the Advanced Maillard Reaction in Aging, Diabetes, and Cataractogenesis. J. Biol. Chem 274, 20796–20804. [DOI] [PubMed] [Google Scholar]
- (35).Nociari MM, Kiss S, and Rodriguez-Boulan E (2017) Lipofuscin Accumulation into and Clearance from Retinal Pigment Epithelium Lysosomes: Physiopathology and Emerging Therapeutics, In Lysosomes: Associated Diseases and Methods to Study Their Function (Dhiman P, Ed.), pp 1–29, IntechOpen. [Google Scholar]
- (36).Schutt F, Bergmann M, Holz FG, and Kopitz J (2003) Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Invest. Ophthalmol. Visual Sci 44, 3663. [DOI] [PubMed] [Google Scholar]
- (37).Wolf G (2003) Lipofuscin and macular degeneration. Nutr. Rev 61, 342–346. [DOI] [PubMed] [Google Scholar]
- (38).Mitter SK, Rao HV, Qi X, Cai J, Sugrue A, Dunn WA Jr., Grant MB, and Boulton ME (2012) Autophagy in the Retina: A Potential Role in Age-Related Macular Degeneration. Adv. Exp. Med. Biol 723, 83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Krohne TU, Kaemmerer E, Holz FG, and Kopitz J (2010) Lipid peroxidation products reduce lysosomal protease activities in human retinal pigment epithelial cells via two different mechanisms of action. Exp. Eye Res 90, 261–266. [DOI] [PubMed] [Google Scholar]
- (40).Horie K, Miyata T, Yasuda T, Takeda A, Yasuda Y, Maeda K, Sobue G, and Kurokawa K (1997) Immunohistochemical localization of advanced glycation endproducts, pentosidine, and carboxymethyllysine in lipofuscin pigments of Alzheimer’s disease and aged neurons. Biochem. Biophys. Res. Commun 236, 327–332. [DOI] [PubMed] [Google Scholar]
- (41).Glenn JV, Mahaffy H, Wu K, Smith G, Nagai R, Simpson DAC, Boulton ME, and Stitt AW (2009) Advanced glycation end products (AGE) accumulation on Bruch’s membrane: links to age-related RPE dysfunction. Invest. Ophthalmol. Visual Sci 50, 441–451. [DOI] [PubMed] [Google Scholar]
- (42).Finnemann SC, Leung LW, and Rodriguez-Boulan E (2002) The lipofuscin component A2E selectively inhibits phagolysosomal degradation of photoreceptor phospholipid by the retinal pigment epithelium. Proc. Natl. Acad. Sci. U. S. A 99, 3842–3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Ling X, Sakashita N, Takeya M, Nagai R, Horiuchi S, and Takahashi K (1998) Immunohistochemical distribution and subcellular localization of three distinct specific molecular structures of advanced glycation end products in human tissues. Lab. Invest 78, 1591–1606. [PubMed] [Google Scholar]
- (44).Glenn JV, Beattie JR, Barrett L, Frizzell N, Thorpe SR, Boulton ME, McGarvey JJ, and Stitt AW (2007) Confocal Raman microscopy can quantify advanced glycation end product (AGE) modifications in Bruch’s membrane leading to accurate, nondestructive prediction of ocular aging. FASEB J. 21, 3542–3552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


