Abstract
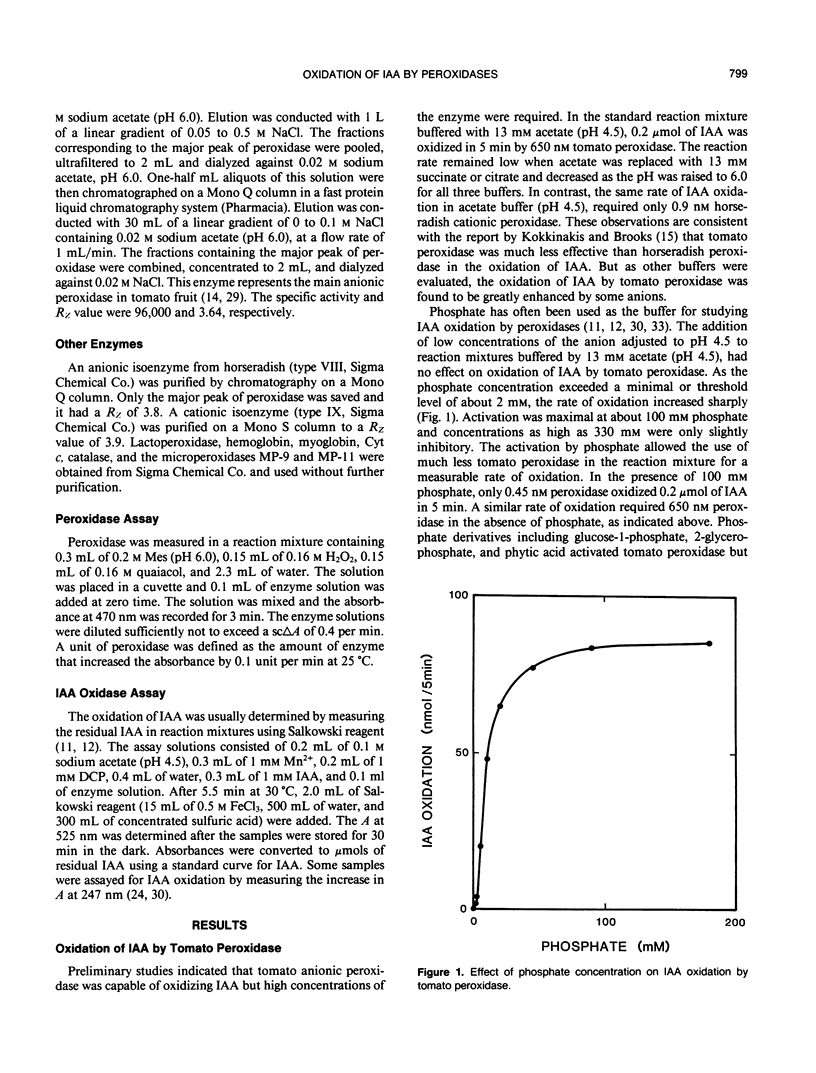
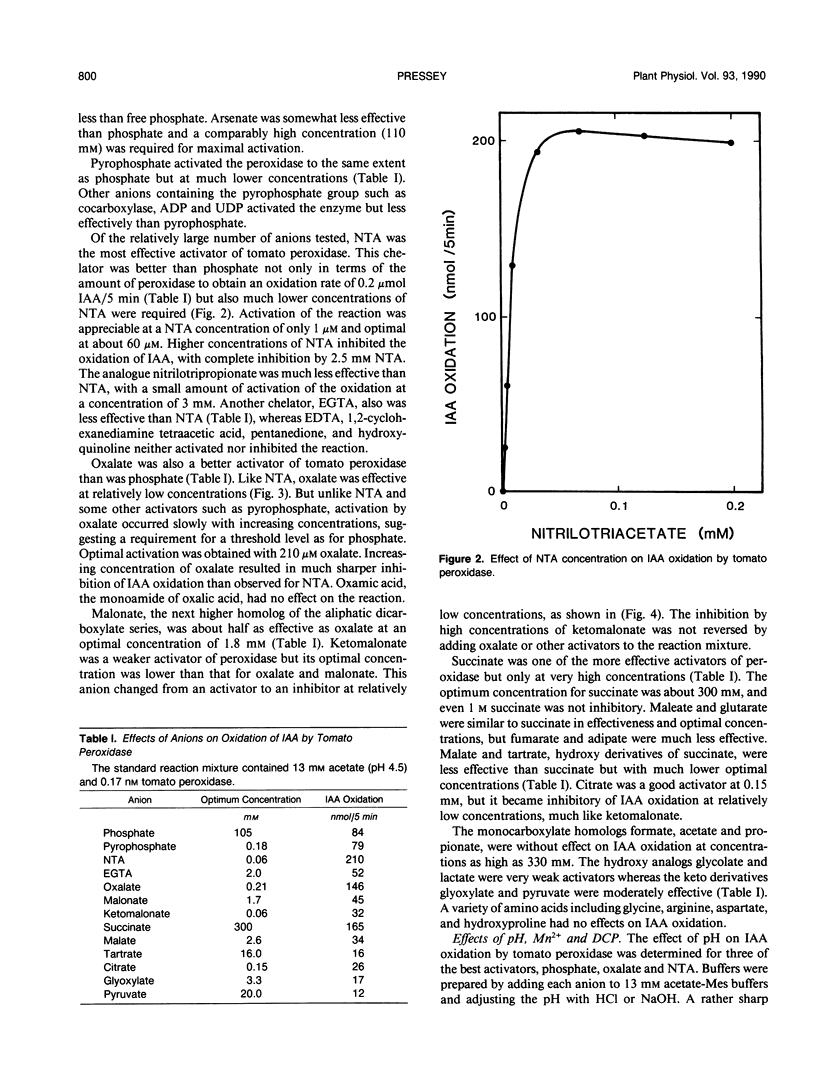
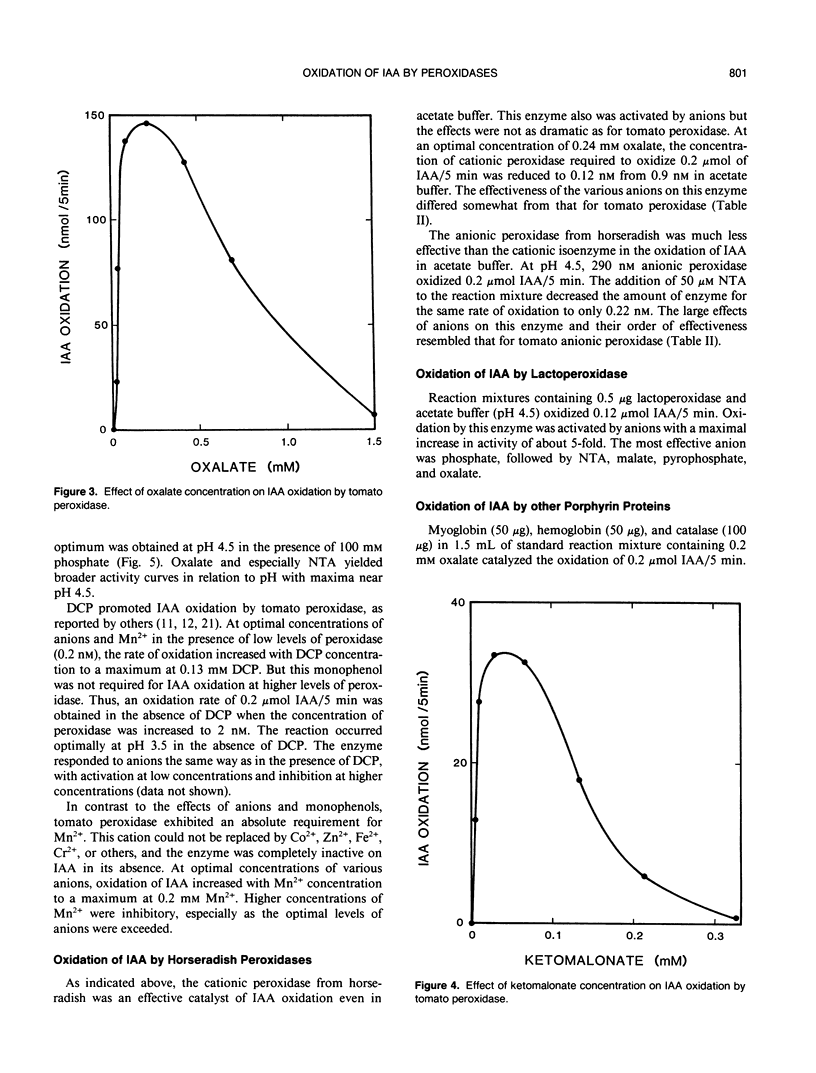
Anionic peroxidase from tomato (Lycopersicon esculentum) fruit oxidized indoleacetic acid (IAA) slowly in the presence of Mn2+ and dichlorophenol in acetate buffers. The addition of certain anions to the reaction mixture increased the rate of oxidation. Phosphate was one of the effective anions and exerted maximal activation at 0.1 molar. The most effective activator of tomato peroxidase was nitrilotriacetate (NTA) at an optimum concentration of 60 micromolar. Only 0.17 nanomolar peroxidase was needed to oxidize 0.1 micromole IAA/5 minutes in the presence of NTA compared to 650 nanomolar peroxidase for the same rate in the absence of NTA. Other effective anions were oxalate, pyrophosphate, malate, and citrate. Each activator exhibited an optimum concentration and higher concentrations were inhibitory. Anionic peroxidase from horseradish was activated by the same anions. A cationic peroxidase from horseradish and lactoperoxidase oxidized IAA in acetate buffer although anions activated these enzymes severalfold. Microperoxidase and other hematoporphrins also catalyzed IAA oxidation in the presence of anions. It is proposed that IAA oxidation by peroxidase may be important when vacuolar contents mix with peroxidase as during plant injury.
Full text
PDF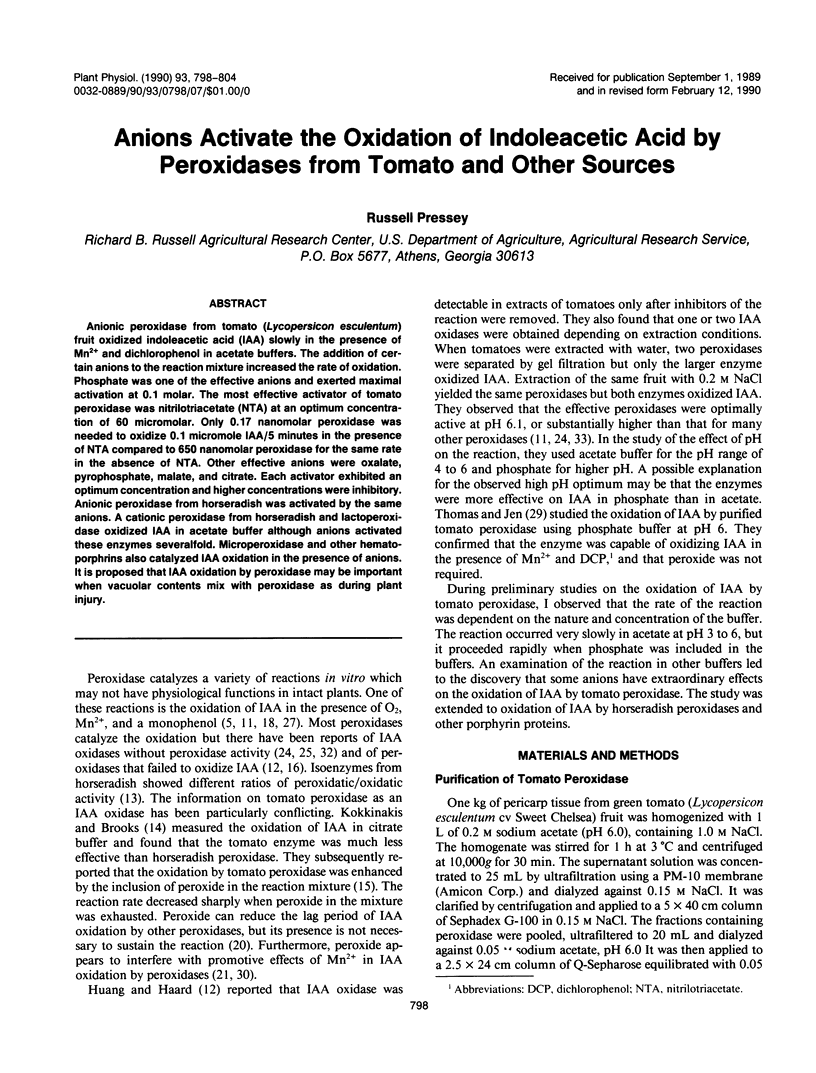

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin E. A., Pressey R. Pectic enzymes in pectolyase: separation, characterization, and induction of ethylene in fruits. Plant Physiol. 1989 May;90(1):191–196. doi: 10.1104/pp.90.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brecht J. K., Huber D. J. Products Released from Enzymically Active Cell Wall Stimulate Ethylene Production and Ripening in Preclimacteric Tomato (Lycopersicon esculentum Mill.) Fruit. Plant Physiol. 1988 Dec;88(4):1037–1041. doi: 10.1104/pp.88.4.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feder N. A heme-peptide as an ultrastructural tracer. J Histochem Cytochem. 1970 Dec;18(12):911–913. doi: 10.1177/18.12.911. [DOI] [PubMed] [Google Scholar]
- Felsenfeld G. DNA. Sci Am. 1985 Oct;253(4):58–67. doi: 10.1038/scientificamerican1085-58. [DOI] [PubMed] [Google Scholar]
- Frenkel C., Dyck R. Auxin inhibition of ripening in bartlett pears. Plant Physiol. 1973 Jan;51(1):6–9. doi: 10.1104/pp.51.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C. Involvement of Peroxidase and Indole-3-acetic Acid Oxidase Isozymes from Pear, Tomato, and Blueberry Fruit in Ripening. Plant Physiol. 1972 May;49(5):757–763. doi: 10.1104/pp.49.5.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel C. Oxidative turnover of auxins in relation to the onset of ripening in bartlett pear. Plant Physiol. 1975 Mar;55(3):480–484. doi: 10.1104/pp.55.3.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINMAN R. L., LANG J. PEROXIDASE-CATALYZED OXIDATION OF INDOLE-3-ACETIC ACID. Biochemistry. 1965 Jan;4:144–158. doi: 10.1021/bi00877a023. [DOI] [PubMed] [Google Scholar]
- Kay E., Shannon L. M., Lew J. Y. Peroxidase isozymes from horseradish roots. II. Catalytic properties. J Biol Chem. 1967 May 25;242(10):2470–2473. [PubMed] [Google Scholar]
- Kokkinakis D. M., Brooks J. L. Hydrogen Peroxide-mediated Oxidation of Indole-3-acetic Acid by Tomato Peroxidase and Molecular Oxygen. Plant Physiol. 1979 Aug;64(2):220–223. doi: 10.1104/pp.64.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkinakis D. M., Brooks J. L. Tomato peroxidase: purification, characterization, and catalytic properties. Plant Physiol. 1979 Jan;63(1):93–99. doi: 10.1104/pp.63.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart J. A. The Role of 2,4-Dichlorophenol in the Destruction of Indoleacetic Acid by Peroxidase. Plant Physiol. 1955 Jan;30(1):86–88. doi: 10.1104/pp.30.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAY P. M. Destruction of indoleacetic acid. IV. Kinetics of enzymic oxidation. Arch Biochem Biophys. 1962 Feb;96:199–209. doi: 10.1016/0003-9861(62)90399-5. [DOI] [PubMed] [Google Scholar]
- RAY P. M. The destruction of indoleacetic acid. III. Relationships between peroxidase action and indoleacetic acid oxidation. Arch Biochem Biophys. 1960 Mar;87:19–30. doi: 10.1016/0003-9861(60)90118-1. [DOI] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Metabolic conversion of 14C-indole-3-acetic acid to 14C-oxindole-3-acetic acid. Biochem Biophys Res Commun. 1981 Nov 30;103(2):429–433. doi: 10.1016/0006-291x(81)90470-8. [DOI] [PubMed] [Google Scholar]
- Reinecke D. M., Bandurski R. S. Oxindole-3-acetic Acid, an Indole-3-acetic Acid Catabolite in Zea mays. Plant Physiol. 1983 Jan;71(1):211–213. doi: 10.1104/pp.71.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequeira L., Mineo L. Partial purification and kinetics of indoleacetic Acid oxidase from tobacco roots. Plant Physiol. 1966 Sep;41(7):1200–1208. doi: 10.1104/pp.41.7.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel B. Z., Galston A. W. Indoleacetic acid oxidase activity of apoperoxidase. Science. 1967 Sep 29;157(3796):1557–1559. doi: 10.1126/science.157.3796.1557. [DOI] [PubMed] [Google Scholar]
- Thomas R. L., Jen J. J. Preparation of a homogeneous tomato fruit peroxidase. Prep Biochem. 1980;10(5):581–596. doi: 10.1080/00327488008061755. [DOI] [PubMed] [Google Scholar]
- Tong C. B., Labavitch J. M., Yang S. F. The induction of ethylene production from pear cell culture by cell wall fragments. Plant Physiol. 1986 Jul;81(3):929–930. doi: 10.1104/pp.81.3.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGENKNECHT A. C., BURRIS R. H. Indoleacetic acid inactivating enzymes from bean roots and pea seedlings. Arch Biochem. 1950 Jan;25(1):30–53. [PubMed] [Google Scholar]


