Abstract
Bean (Phaseolus vulgaris L.) seeds contain a putative plant defense protein that inhibits insect and mammalian but not plant α-amylases. We recently (J Moreno, MJ Chrispeels [1989] Proc Natl Acad Sci USA 86:7885-7889) presented strong circumstantial evidence that this α-amylase inhibitor (αAI) is encoded by an already-identified lectin gene whose product is referred to as lectin-like-protein (LLP). We have now made a chimeric gene consisting of the coding sequence of the lectin gene that encodes LLP and the 5′ and 3′ flanking sequences of the lectin gene that encodes phytohemagglutinin-L. When this chimeric gene was expressed in transgenic tobacco (Nicotiana tabacum), we observed in the seeds a series of polypeptides (Mr 10,000-18,000) that cross-react with antibodies to the bean α-amylase inhibitor. Most of these polypeptides bind to a pig pancreas α-amylase affinity column. An extract of the seeds of the transformed tobacco plants inhibits pig pancreas α-amylase activity as well as the α-amylase present in the midgut of Tenebrio molitor. We suggest that introduction of this lectin gene (to be called αai) into other leguminous plants may be a strategy to protect the seeds from the seed-eating larvae of Coleoptera.
Full text
PDF

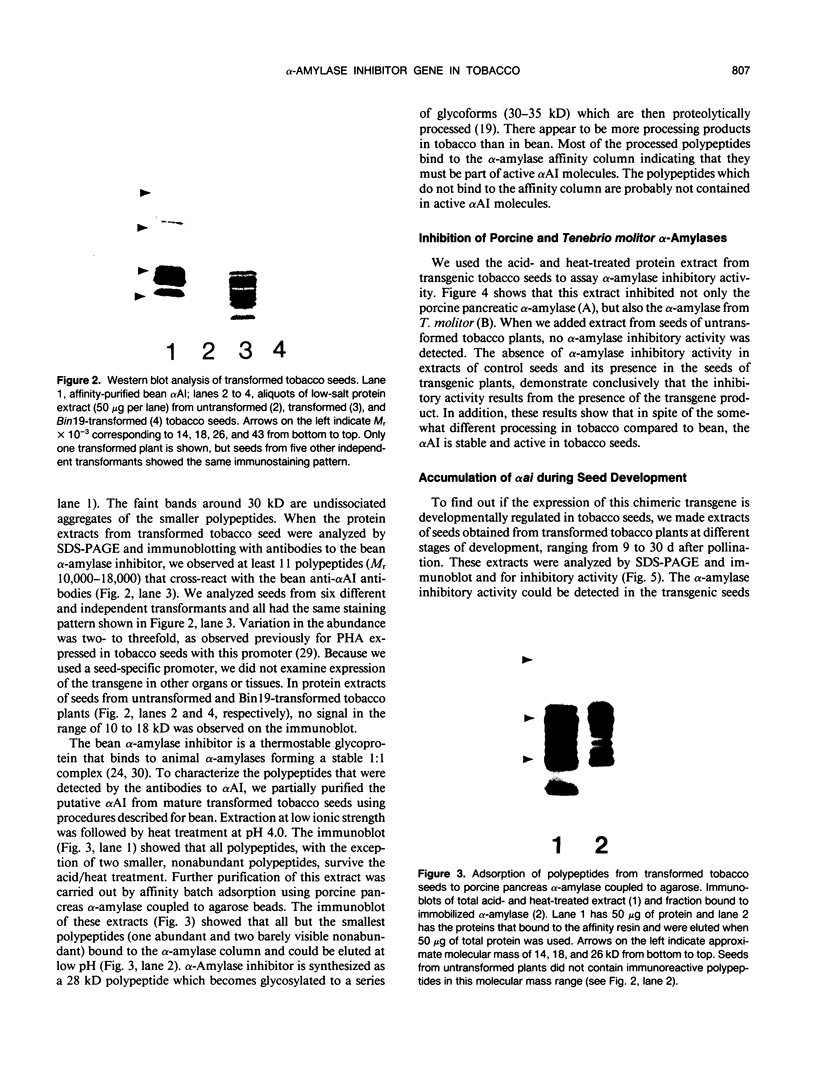


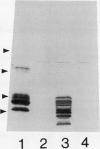
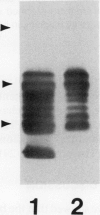
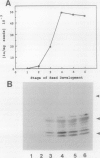
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beachy R. N., Chen Z. L., Horsch R. B., Rogers S. G., Hoffmann N. J., Fraley R. T. Accumulation and assembly of soybean beta-conglycinin in seeds of transformed petunia plants. EMBO J. 1985 Dec 1;4(12):3047–3053. doi: 10.1002/j.1460-2075.1985.tb04044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979 Jun;63(6):1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris N., Chrispeels M. J. Histochemical and biochemical observations on storage protein metabolism and protein body autolysis in cotyledons of germinating mung beans. Plant Physiol. 1975 Aug;56(2):292–299. doi: 10.1104/pp.56.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M., Donaldson D. D. Characterization of two Phaseolus vulgaris phytohemagglutinin genes closely linked on the chromosome. EMBO J. 1985 Apr;4(4):883–889. doi: 10.1002/j.1460-2075.1985.tb03714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M., Ma Y., Barker R. F. Molecular cloning of Phaseolus vulgaris lectin mRNA and use of cDNA as a probe to estimate lectin transcript levels in various tissues. Nucleic Acids Res. 1982 Dec 11;10(23):7819–7828. doi: 10.1093/nar/10.23.7819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman L. M. Structure of a chromosomal Phaseolus vulgaris lectin gene and its transcript. J Mol Appl Genet. 1984;2(5):447–453. [PubMed] [Google Scholar]
- Janzen D. H., Juster H. B., Liener I. E. Insecticidal action of the phytohemagglutinin in black beans on a bruchid beetle. Science. 1976 May 21;192(4241):795–796. doi: 10.1126/science.1265481. [DOI] [PubMed] [Google Scholar]
- Johnson R., Narvaez J., An G., Ryan C. Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9871–9875. doi: 10.1073/pnas.86.24.9871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Moreno J., Chrispeels M. J. A lectin gene encodes the alpha-amylase inhibitor of the common bean. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7885–7889. doi: 10.1073/pnas.86.20.7885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamuro J. K., Jofuku K. D., Goldberg R. B. Soybean seed lectin gene and flanking nonseed protein genes are developmentally regulated in transformed tobacco plants. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8240–8244. doi: 10.1073/pnas.83.21.8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborni T. C., Alexander D. C., Sun S. S., Cardona C., Bliss F. A. Insecticidal activity and lectin homology of arcelin seed protein. Science. 1988 Apr 8;240(4849):207–210. doi: 10.1126/science.240.4849.207. [DOI] [PubMed] [Google Scholar]
- Pick K. H., Wöber G. Proteinaceous alpha-amylase inhibitor from beans (Phaseolus vulgaris). Purification and partial characterization. Hoppe Seylers Z Physiol Chem. 1978 Oct;359(10):1371–1377. doi: 10.1515/bchm2.1978.359.2.1371. [DOI] [PubMed] [Google Scholar]
- Sengupta-Gopalan C., Reichert N. A., Barker R. F., Hall T. C., Kemp J. D. Developmentally regulated expression of the bean beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985 May;82(10):3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varner J. E., Mense R. M. Characteristics of the process of enzyme release from secretory plant cells. Plant Physiol. 1972 Feb;49(2):187–189. doi: 10.1104/pp.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker T. A., Herman E. M., Chrispeels M. J. In vitro mutated phytohemagglutinin genes expressed in tobacco seeds: role of glycans in protein targeting and stability. Plant Cell. 1989 Jan;1(1):95–104. doi: 10.1105/tpc.1.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker T., Sturm A., Chrispeels M. J. Differences in expression between two seed lectin alleles obtained from normal and lectin-deficient beans are maintained in transgenic tobacco. EMBO J. 1987 Dec 1;6(12):3571–3577. doi: 10.1002/j.1460-2075.1987.tb02687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]