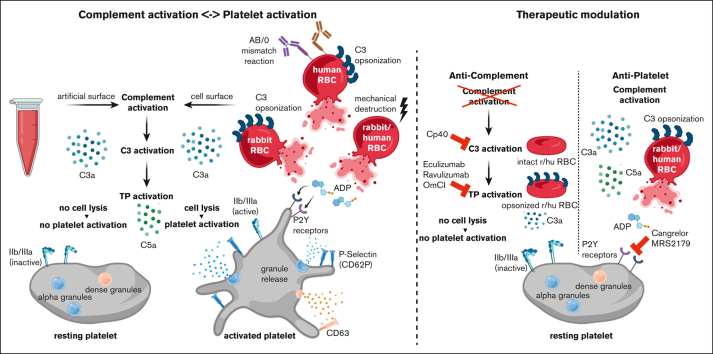
Key Points
-
•
Proximal and terminal complement activation products fail to directly induce prothrombotic platelet activation.
-
•
MAC-mediated release of intracellular ADP is sufficient and necessary for complement-mediated platelet activation.
Visual Abstract
Abstract
Complement activation in the diseases paroxysmal nocturnal hemoglobinuria (PNH) and atypical hemolytic uremic syndrome (aHUS) results in cytolysis and fatal thrombotic events, which are largely refractory to anticoagulation and/or antiplatelet therapy. Anticomplement therapy, however, efficiently prevents thrombotic events in PNH and aHUS, but the underlying mechanisms remain unresolved. We show that complement-mediated hemolysis in whole blood induces platelet activation similarly to activation by adenosine 5′-diphosphate (ADP). Blockage of C3 or C5 abolished platelet activation. We found that human platelets failed to respond functionally to the anaphylatoxins C3a and C5a. Instead, complement activation did lead to prothrombotic cell activation in the whole blood when membrane attack complex (MAC)-mediated cytolysis occurred. Consequently, we demonstrate that ADP receptor antagonists efficiently inhibited platelet activation, although full complement activation, which causes hemolysis, occurred. By using an established model of mismatched erythrocyte transfusions in rats, we crossvalidated these findings in vivo using the complement inhibitor OmCI and cobra venom factor. Consumptive complement activation in this animal model only led to a thrombotic phenotype when MAC-mediated cytolysis occurred. In conclusion, complement activation only induces substantial prothrombotic cell activation if terminal pathway activation culminates in MAC-mediated release of intracellular ADP. These results explain why anticomplement therapy efficiently prevents thromboembolisms without interfering negatively with hemostasis.
Introduction
Not all thrombotic conditions respond substantially to treatment with anticoagulation and/or antiplatelet therapy. A key example is the complement model disease, paroxysmal nocturnal hemoglobinuria (PNH), which has been described as the “most vicious acquired thrombophilic state known in medicine.”1 Clinically, classical PNH is characterized by anemia due to complement-mediated hemolysis, smooth muscle dystonia, and arterial and/or venous thrombosis, among other symptoms (reviewed previously2, 3, 4). To prevent or treat thromboembolic complications, patients with PNH underwent therapeutic anticoagulation, whereas antiplatelet therapy was usually not used.4,5 However, thrombotic complications in PNH proved largely resistant to anticoagulation, with thrombi (re)occurring often despite anticoagulation therapy,4 including in patients receiving combined anticoagulation and antiplatelet therapy.5,6 In contrast, a therapeutic block of the terminal and lytic complement pathway reduced thromboembolic events in patients with PNH, for example, from a rate of 10.6 events per 100 patient-years on antithrombotic therapy to 0.6 events per 100 patient-years on treatment with the C5-inhibiting monoclonal antibody eculizumab.7 Eculizumab also reliably reduced thrombotic microangiopathy in the complement-mediated disease atypical hemolytic uremic syndrome (aHUS), with 81% of patients reaching a complete thrombotic microangiopathy–free status during treatment.8,9
Several other disease entities exhibit a substantial complement activation signature, which leads to thrombosis despite application of established antithrombotic drugs (reviewed in10,11). This includes antiphospholipid syndrome (APS) or catastrophic APS (CAPS)12, 13, 14, 15 and autoimmune hemolytic anemia.16, 17, 18, 19, 20 Yet, not every disease entity with aberrant and strong complement activation presents with thromboembolic events, suggesting that complement activation products alone are not sufficient to induce a prothrombotic state of clinical manifestation. This becomes obvious in a comparison among the 3 complement-driven diseases PNH, aHUS, and C3 glomerulonephritis (C3G).10 Common among them is the dysregulation of the complement alternative pathway (AP), leading to C5 activation. However, thrombotic events are only typical for PNH and aHUS. A unifying feature of PNH and aHUS is the occurrence of complement-mediated cytolysis affecting erythrocytes and endothelial cells, respectively, whereas membrane attack complex (MAC)-mediated cytolysis does not occur in C3G.10,21, 22, 23, 24 This is in line with data from animal studies using cobra venom factor (CVF) to decomplement animals via an exhaustive fluid phase consumption of C3 and C5 which apparently occurs without any ill effect to the animal.10,25, 26, 27 However, these conclusions from clinical practice in humans and animal studies using CVF are contrasted by a plethora of published data from in vitro or animal studies that suggest a direct mechanistic link between complement activation and coagulation and/or prothrombotic cell activation (reviewed previously4,10,11,28,29).
We propose that an important intermediate step between complement activation and prothrombotic cell activation had been overlooked (and/or conclusions were misguided because of a highly artificial in vitro setup). We hypothesize that this crucial intermediate step is the liberation of intracellular, prothrombotic danger signals due to complement-mediated cytolysis via MAC formation. Novel mechanistic insights into the cross talk between complement and hemostasis promise to (1) unravel mechanistically why eculizumab prevents thromboembolism in PNH, which is unclear to date,30 and (2) explain why antithrombotic therapy alone does not deliver the expected outcome in complement-associated thrombotic disorders, such as PNH, autoimmune hemolytic anemia, and CAPS. Tackling these important issues is expected to provide novel approaches to how these severe diseases may be better addressed in the future.
Using 2 different whole blood models, isolated platelets, and an established in vivo model of a mismatched erythrocyte transfusion reaction in Wistar rats, we prove our hypothesis that complement activation and its purified activation products have only very limited effect on direct platelet activation. Instead, complement activation provides the means to prothrombotic cell activation by liberating the intracellular danger signal adenosine 5′-diphosphate (ADP) that forcefully activates platelets among other cell types.
Material and methods
Collection of human blood
Venous blood from healthy donors was collected under the ethical approval by the ethics committee of Ulm University in either hirudin monovettes (S-Monovette Hirudin, 04.1959.001, Sarstedt, Nümbrecht, Germany) or neutral monovettes (S-Monovette neutral, 02.1726.021, Sarstedt) in which the specific factor Xa inhibitor fondaparinux (FPX; Arixtra, Aspen Pharma Trading Limited, Dublin, Ireland) was provided, leading to a final concentration of 8 μg/mL. Blood was carefully inverted and immediately used for the experiments.
Complement proteins and inhibitors
The monoclonal anti-C5 antibodies eculizumab (Soliris, Alexion) and ravulizumab (Ultomiris, Alexion) were obtained from remnants in infusion bags. A recombinant version of the natural tick-derived C5 inhibitor OmCI (endotoxin tested) was expressed in Pichia pastoris. C5 double inhibition was used as previously described.31,32 CVF (derived from Naja naja kaouthia) was purchased from Quidel and is known to convert C3 into C3 fragments and C5 into C5a and SC5b-9, per the manufacturer’s specifications. CVF (130 μg) was injected intraperitoneally per rat (as further detailed in “Results”). The C3 peptide inhibitor Cp40 was a kind gift from J.D. Lambris, University of Pennsylvania. All purified complement proteins C3a, C4a, C5a, C3b, and C4b were purchased from CompTech.
Preparation of erythrocytes
Rabbit erythrocytes (rRBCs; 36100010, Fiebig Nährstofftechnik, Düsseldorf, Germany) were washed 3 times in phosphate-buffered saline (PBS; −/− [containing no calcium or magnesium ions]) by centrifuging at 2000g. After the last centrifugation, the supernatant was discarded, and packed cells were kept at room temperature until further use. For preparation of shattered RBCs, packed rRBCs or human erythrocytes were disintegrated using an ultrasound stick and subsequently centrifuged at 10 000g for 20 seconds at room temperature. The supernatant was kept at room temperature until further use within 1 hour of the preparation. These erythrocytes were denominated shattered RBCs.
Blood count and laboratory parameters of hemostasis
Citrated and EDTA-supplemented human FPX–treated blood samples and rat blood samples were handed to the clinical routine laboratory of the University Hospital of Ulm for small blood count analysis (Coulter DxH 800; Beckman Coulter) and measurement of routine coagulation parameters (prothrombin time/Quick test; partial thromboplastin time; turbidimetry, Siemens-BCS XP, Siemens Healthineers, Erlangen, Germany).
EDTA-plasma generation
For plasma generation, EDTA-anticoagulated (10 mM) FPX-treated blood samples were centrifuged at 800g for 5 minutes at 4°C and again at 16 000g for 2 minutes at 4°C. Hirudin-treated blood was centrifuged at 2000g for 3 minutes. Finally, plasma was aliquoted and stored at −80°C until further use.
Platelet-rich plasma generation
Citrated or lepirudin-anticoagulated blood was centrifuged at 150g for 15 minutes, with the lowest possible adjustment of centrifuge acceleration and brake. The upper one-third was collected and kept at room temperature until further use.
Statistics
For descriptive statistics, statistical testing, and graphical depiction, Prism9 (GraphPad Software) was used. Results were considered statistically significant for P < .05. Note that, for the sake of visibility, depiction of statistically nonsignificant data, P values were omitted if not of relevance for the experimental hypotheses. For a detailed description of the statistical methodology, see supplemental Material.
Results
Artificial surface-induced complement activation induces activation of innate immune cells
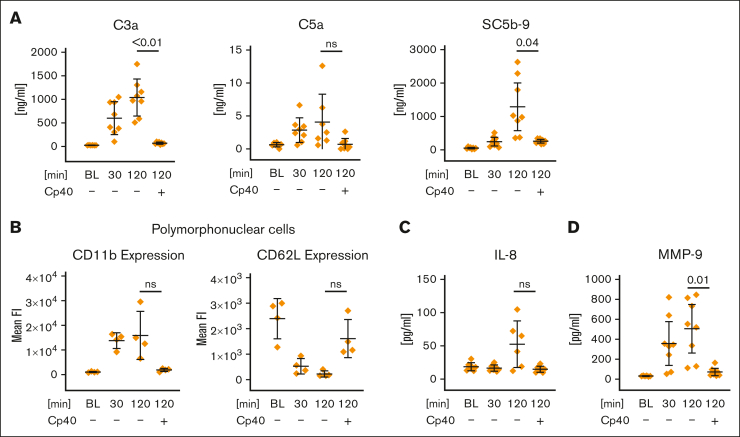
We used 2-methacryloyloxyethyl phos-phorylcholine–coated reaction tubes to attenuate complement activation on different artificial surfaces as previously demonstrated.33 However, we measured a time-dependent increase of the complement activation fragments C3a, C5a, and SC5b-9 in anticoagulated whole blood incubated in 2-methacryloyloxyethyl phosphorylcholine–coated tubes. Addition of the C3 peptide inhibitor Cp40, a member of the compstatin family,34 to a final concentration of 20 μM (C3 concentration in serum is ∼6 μM) completely inhibited the generation the anaphylatoxins and SC5b-9 (Figure 1A). In comparison, the addition of 2.5 mg/mL zymosan, a potent activator of the AP, produced ∼10 000–fold higher C3a plasma levels in this whole blood model (supplemental Figure 1A). Analysis of polymorphonuclear cells via flow cytometry revealed a complement-dependent surface expression of the activation markers CD11b and CD62L (Figure 1B). Complement-dependent cell activation occurs to a lesser extent for CD14+ monocytes as well but not for T cells (supplemental Figure 1B-C). In line with this, interleukin-8 and matrix metalloproteinase–9, which are mainly derived from polymorphonuclear cells or monocytes were found to be elevated in a complement-dependent manner (Figure 1C-D), whereas soluble interleukin-2 receptor, an activation marker of T cells, was unaltered (supplemental Figure 1D). Taken together, complement activation triggered by the reaction tube clearly induced cell activation of innate immune cells in our whole blood model, whereas adaptive immune cells remained unaffected within the observed 2-hour incubation time.
Figure 1.
Artificial surface-induced complement activation and its effect on polymorphonuclear cell activation in whole blood. FPX-anticoagulated blood was incubated for 0 (BL), 30, or 120 minutes at 37°C in the presence or absence of Cp40 (20 μM). (A) Levels of C3a, C5a, and SC5b-9 were determined via enzyme-linked immunosorbent assay (ELISA), using EDTA plasma that was obtained from the whole blood model after each time point. (B) Activation of polymorphonuclear cells. Surface expression of the activation markers CD11b and CD62L was measured via flow cytometry. The average of the mean fluorescence intensities is shown. (C-D) Inflammation marker interleukin-8 (IL-8) and matrix metalloproteinase-9 (MMP-9) were determined from EDTA-treated plasma (derived from the whole blood model) via ELISA. For all panels, the mean values from 4 to 8 independent assays ± standard deviation are shown. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). In the data set of panel A, 2 outliers were removed for each C3a and C5a before statistical testing; in the data set of panel C, 5 and 2 outliers were removed for IL-8 and MMP-9, respectively. Data sets were analyzed using either the Prism mixed-effects model (panels A [C3a, C5a] and C) or repeated measures one-way analysis of variance (ANOVA) (panels A [SC5b-9] and B). Experimental groups were post hoc tested for statistical significance against the 120 minutes + Cp40 group with correction for multiple comparisons (Tukey test, panel A; or Dunnett comparison in panels B-C). For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses. ns, not significant.
Artificial surface-induced complement activation does not alter hemostatic system parameters
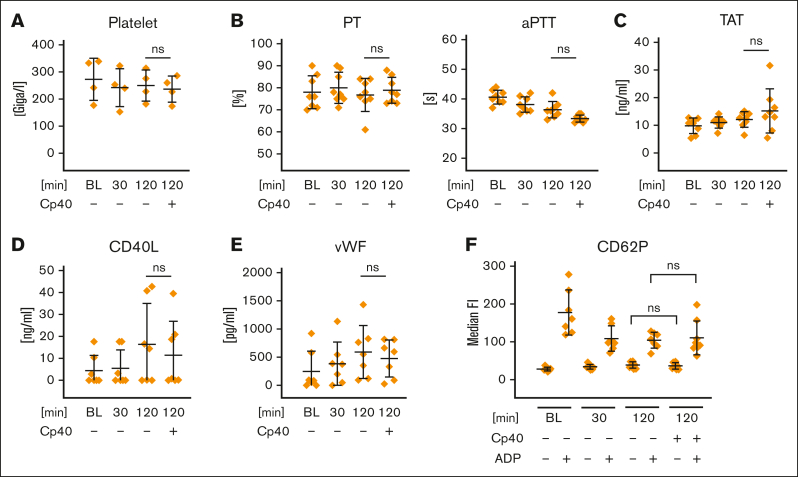
Next, we investigated whether complement activation would affect the cellular and/or humoral part of the hemostatic system. We observed no decrease in platelet count under any of the experimental whole blood conditions tested (Figure 2A). The routine laboratory hemostatic parameters prothrombin time (Quick; extrinsic pathway) and partial thromboplastin time (partial thromboplastin time; intrinsic pathway) revealed no significant differences between samples in which complement activation occurred or those in which it was inhibited by Cp40 (Figure 2B). Levels of thrombin/antithrombin complexes did not increase over time (Figure 2C). Rotational thromboelastometry (ROTEM) analysis of the aforementioned reactions, after specific activation of the extrinsic or intrinsic pathway, revealed no significant differences (supplemental Table 1). ROTEM analysis was also performed with freshly drawn citrated blood (without prior incubation) in the presence or absence of the C3 inhibitor Cp40. Although no significant difference was measured after extrinsic activation or after addition of cytochalasin D to exclude a platelet contribution (FIBTEM), a significant elongation of the clotting time in the intrinsic pathway was recorded when C3 activation was inhibited (supplemental Table 2). The same tendency (without reaching statistical significance) was observed with C5 blockage (supplemental Table 3). However, prolonged clotting time under C3 inhibition in the intrinsic pathway was not observed when conducting the experiment with platelet-rich plasma (supplemental Table 4), suggesting that this phenomenon is likely induced by leukocytes rather than being driven by a direct interaction of complement activation fragments with platelets. We also observed no significant differences when measuring either the soluble platelet activation marker CD40L or von Willebrand factor, both of which are reportedly released upon platelet activation35,36 (Figure 2D-E). Platelet activation was determined at different incubation time points by evaluating the surface expression of CD62P with or without stimulation with 5 μM ADP. Only addition of ADP led to platelet activation, whereas complement activation induced by the reaction conditions had no measurable effect (Figure 2F). Addition of Cp40 did not affect platelet activation after ADP stimulation either. In summary, artificial surface-induced complement activation triggered an innate immune response but induced neither a relevant activation nor any measurable impairment of the hemostatic system.
Figure 2.
Effect of artificial surface-induced complement activation on the hemostatic system in whole blood. FPX-anticoagulated blood was incubated for 0, 30, or 120 minutes at 37°C in the presence or absence of Cp40 (20 μM) and assessed for (A) platelet count and (B) global coagulatory parameters (prothrombin time [PT] and partial thromboplastin time [aPTT]). (C) The generation of thrombin/antithrombin complexes was measured in EDTA-treated plasma via ELISA. (D-E) EDTA-treated plasma derived from the whole blood reactions was analyzed for the concentrations of the soluble platelet activation markers CD40L and von Willebrand factor. (F) The activation status of platelets in whole blood was determined by CD62P surface expression using flow cytometry. After the indicated incubation time, blood was exposed for 10 minutes either to PBS (negative control [neg. Ctrl]) or ADP (5 μM). All graphs show mean values with standard deviation of at least 4 independent assays. For all panels, the mean values of at least 4 independent assays ± standard deviation are shown. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). Data sets were either analyzed using either Prism mixed-effects model (in case of missing values) or repeated measures one-way ANOVA. Experimental groups were post hoc tested for statistical significance against the 120 minutes + Cp40 group with Dunnett correction for multiple comparisons (panels A-E) or against each other experimental group (Tukey test with correction for multiple comparisons). For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses.
No direct effects of complement activation products on platelet activation
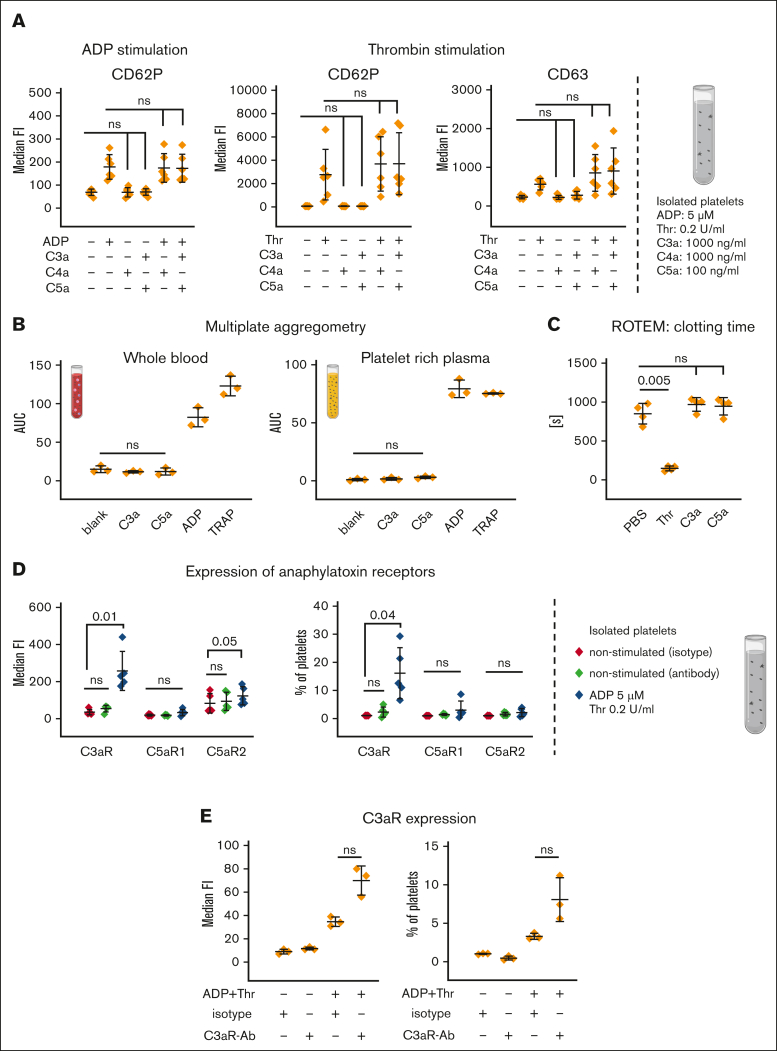
Because some reports in the literature described platelet activation based on the complement activation products (recently reviewed10,11), we performed a series of experiments to analyze the effect of purified complement activation products on platelet function. Isolated platelets and whole blood were exposed to purified anaphylatoxins as well as soluble C3b and C4b (Figure 3A; supplemental Figure 3A-B). None of these stimuli caused platelet activation. Concomitant incubation of platelets with ADP or thrombin and anaphylatoxins achieved no additional effect on the expression of surface activation markers as had been detected for ADP or thrombin stimulation alone (Figure 3A). By performing multiplate aggregometry analysis in lepirudin-anticoagulated whole blood or platelet-rich plasma, platelet aggregation was only observed after stimulation with ADP (6.5 μM) or thrombin-receptor activating peptide (TRAP; 32 μM) but not with C3a or C5a (Figure 3B). In another setup, prestimulation by anaphylatoxins was followed by ADP/TRAP addition (supplemental Figure 3C). Prestimulation with anaphylatoxins skewed toward increased platelet aggregation in whole blood but not in platelet-rich plasma, suggesting an indirect effect on aggregation that likely is mediated by anaphylatoxin-induced leukocyte activation. C3a and C5a also had no impact on clotting time in a modified ROTEM experiment without the addition of a specific trigger for the extrinsic or intrinsic pathways (Figure 3C). Preincubation of blood with C3a or C5a followed by stimulation with thrombin had also no impact in this modified ROTEM analysis (supplemental Figure 3D). Unable to observe any direct functional effect on platelet stimulation via anaphylatoxins, we re-examined the expression of the anaphylatoxin receptors C3aR and C5aR, which have recently been described to be present on human platelets.37,38 Analysis of isolated platelets via flow cytometry demonstrated a lack of the C3aR and both C5a receptors, that is, C5a receptor 1 (C5aR1) and C5aR2, on quiescent platelets. Interestingly, after activation with ADP and thrombin, we received a significant increase of the median fluorescence signal for C3aR and C5aR2, albeit the portion of positive platelets only increased significantly for C3aR (Figure 3D). However, when investigating this further, we observed an increase of the isotype signal upon cell activation, albeit not to the same level as the increase of the anti-C3aR antibody (Figure 3E). Staining of neutrophils using the same antibodies (even at 10-fold lower antibody concentrations than that used for platelet staining) demonstrated that the used antibodies successfully recognize the anaphylatoxin receptors (supplemental Figure 3E). Collectively, we could not identify any direct functional impact of purified complement activation products on platelets within whole blood or after being isolated. Consistent with this, we failed to detect signs for the surface expression of the C5a receptors, whereas the C3aR appeared to be expressed on a small proportion of platelets activated by ADP/thrombin.
Figure 3.
Influence of anaphylatoxins on platelet activation. (A) Anaphylatoxin stimulation of isolated platelets. Isolated platelets were exposed to either PBS−/− (neg Ctrl), ADP (5 μM), thrombin (Thr, 0.2 U/mL), C3a (1000 ng/mL), C4a (1000 ng/mL), or C5a (100 ng/mL) alone or in combination, as indicated. After stimulation for 10 minutes, cells were analyzed via flow cytometry for surface expression of the activation markers CD62P or CD63. (B) Multiplate aggregometry. Lepirudin-anticoagulated blood or platelet-rich plasma was mixed with NaCl (0.9%, neg. Ctrl), ADP (6.5 μM), TRAP (32 μM), C3a (1.8 μM), or C5a (0.18 μM), 2 minutes before analyzing platelet aggregation. Mean of the area under the curve with standard deviation is shown. (C) ROTEM without the addition of a specific reagent that activates the intrinsic or extrinsic pathway. Immediately before starting the reaction, citrated blood was exposed to Thr (1 U/mL), C3a (1.8 μM), or C5a (0.18 μM) and was recalcified without addition of the extrinsic or intrinsic pathway starting reagents. Mean values with standard deviation of the clotting time are shown. (D) Expression of anaphylatoxin receptors on platelets. Isolated platelets were stimulated with either PBS−/− (neg Ctrl) or ADP (5 μM) and Thr (0.2 U/mL) for 10 minutes before being analyzed for C3aR (final antibody concentration: 2 μg/mL, clone: hC3aRZ8), C5aR1 (final antibody concentration: 1 μg/mL; clone: S5/1), and C5aR2 (final antibody concentration: 4 μg/mL; clone: 1D9-M12) surface expression. Respective isotype controls were included in equimolar concentrations. Mean values with standard deviations are shown. (E) C3aR expression on isolated platelets. As in panel D but with isotype staining after platelet stimulation. At least 3 independent assays are shown in each panel. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). Data sets in panels A [CD62P ADP, CD62P Thr], B-C, and E were analyzed using repeated measures one-way ANOVA and data set in panel A [CD63 Thr]) by Prism mixed-effects model (because of a missing value). Experimental groups were post hoc tested for statistical significance with correction for multiple comparisons (panel A, Sidak; panel B, Dunnet comparison; and panels C,E, Tukey test). The data set in panel D was tested using a repeated measures two-way ANOVA test for adjusted multiple comparisons. For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses.
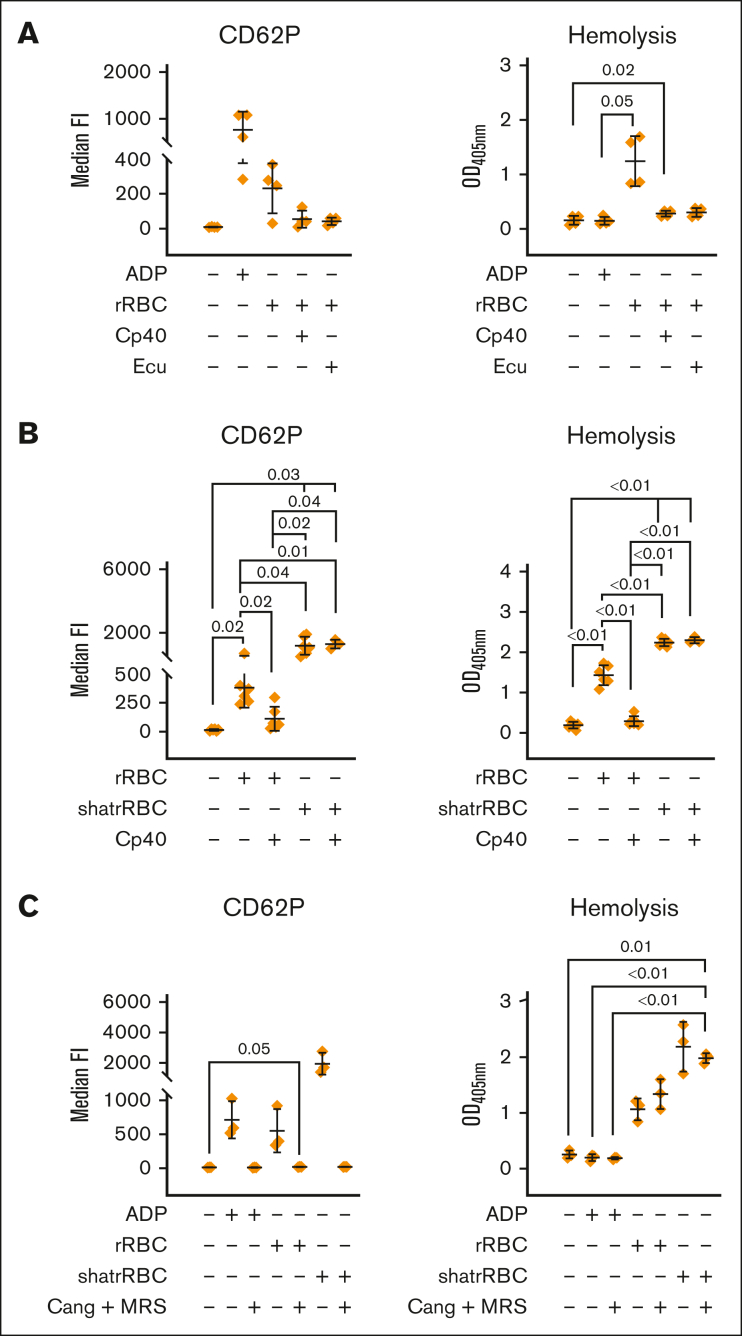
Complement-dependent and -independent lysis of rabbit erythrocytes causes platelet activation
In a hirudin-based whole blood model, we induced complement activation by the addition of rabbit red blood cells (foreign cells), which are known to be lysed by the AP. Platelet activation in this setup was confirmed by analyzing CD62P surface expression. Both proximal (at the level of C3) and terminal (at the level of C5) inhibition stopped the complement-mediated hemolysis of the rabbit erythrocytes and inhibited platelet activation (Figure 4A). We confirmed this by measuring the binding of PAC-1 recognizing a neoepitope in the activated integrin 2b/3a (supplemental Figure 4A). Because we failed to detect a platelet-activating effect via the addition of anaphylatoxins (Figure 3), we hypothesized that platelet activation in this model is solely triggered through intracellular danger signals, which are released upon the complement-mediated lysis of rabbit erythrocytes. Therefore, we investigated the effect of rabbit erythrocytes, which were lysed mechanically by sonication in a complement-independent manner. This resulted in elevated free hemoglobin, as expected, and led to CD62P surface expression on platelets (Figure 4B). Of note, this time, platelet activation could not be reversed by the addition of Cp40, indicating that complement-dependent or -independent lysis of cells is sufficient and necessary to activate platelets.
Figure 4.
Complement-dependent and independent lysis of rabbit erythrocytes cause platelet activation, which is abolished via ADP receptor antagonists. (A) Complement-mediated lysis of rRBCs induces platelet activation. Hirudin-anticoagulated blood was exposed to PBS−/− (neg Ctrl), ADP (5 μM), or rRBCs in presence or absence of proximal (Cp40, 4 μM) and terminal (eculizumab [Ecu], 0.4 μM) complement inhibitors. The final blood percentage was 20%. Surface expression of the activation marker CD62P was determined via flow cytometry. Hemolytic activity was determined in respective plasma samples measuring released hemoglobin from the supernatant (after blank subtraction). (B) Complement-dependent and -independent lysis of cells lead to platelet activation. As in panel A, but in addition to intact rRBCs, mechanically shattered rRBCs (shatrRBC) were also assayed with and without complement inhibition by Cp40. All graphs show mean values with standard deviation. (C) Hirudin-anticoagulated blood was exposed to PBS−/− (neg Ctrl), ADP (5 μM), rRBCs, or shatrRBC in presence or absence of ADP receptor antagonists cangrelor (1 μM) or MRS2179 (10 μM). Surface expression of CD62P on platelets was determined by flow cytometry. Hemolytic activity was measured in respective plasma samples. Mean values with standard deviation are shown. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). Data sets were either analyzed using a Prism mixed-effects model (in case of missing values) or repeated measures one-way ANOVA. Experimental groups were post hoc tested for statistical significance with Tukey correction for multiple comparisons. For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses.
ADP, and not complement activation, is the main trigger of platelet activation upon cell lysis
To substantiate our hypothesis, we focused on ADP as the intracellular danger signal known to trigger platelet activation. ADP can address 2 purinergic G-protein coupled receptors on platelets, P2Y12 and P2Y1, which can be inhibited by the receptor antagonists cangrelor and MRS2179, respectively.39,40 The addition of both purine receptor antagonists completely abolished ADP-induced platelet activation (Figure 4C), whereas activation by thrombin is still possible (supplemental Figure 4C). Cangrelor and MRS2179 did not influence the hemolytic readout (as expected) but completely suppressed hemolysis-induced platelet activation irrespective of erythrocytes being lysed in a complement-dependent or mechanical (complement independent) fashion (Figure 4C).
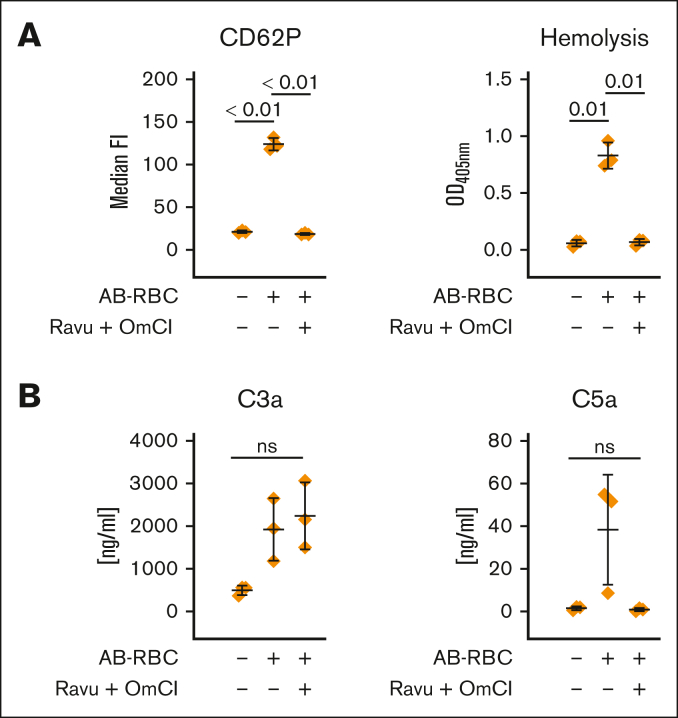
Complement-dependent cell lysis in a fully human AB/O mismatch model causes platelet activation
Using a completely human model, we simulated an AB/O blood transfusion mismatch by adding washed AB erythrocytes to hirudin-anticoagulated blood from a donor having blood group O. We observed substantial complement-dependent cell lysis, which correlated with platelet activation (Figure 5A). Further analysis of complement activation in the reaction mixtures proved the expected functionality of C5 double inhibition (using ravulizumab and OmCI). Although C5 activation was blocked in the presence of ravulizumab and OmCI, substantial activation of C3 is obvious as judged by the liberated C3a. Consistent with the aforementioned findings, strong proximal complement activation that failed to induce cytolysis also failed to cause platelet activation (Figure 5B).
Figure 5.
AB/O-mismatch–induced lysis of human RBCs causes platelet activation. Washed erythrocytes of a donor with AB-group blood were added to hirudin-anticoagulated whole blood of a donor with group O blood in absence or presence of ravulizumab and OmCI (both at final concentration of 0.8 μM). (A) Surface expression of the platelet activation marker CD62P was determined via flow cytometry, and hemolytic activity was determined in the respective plasma samples measuring released hemoglobin from the supernatant. (B) The level of complement activation in the reaction mixtures was investigated by measurement of C3a and C5a concentrations after the reactions had been stopped by the addition of EDTA. Data sets were either analyzed using a Prism mixed-effects model (in case of missing values) or repeated measures one-way ANOVA. Experimental groups were post hoc tested for statistical significance with Tukey correction for multiple comparisons. For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses.
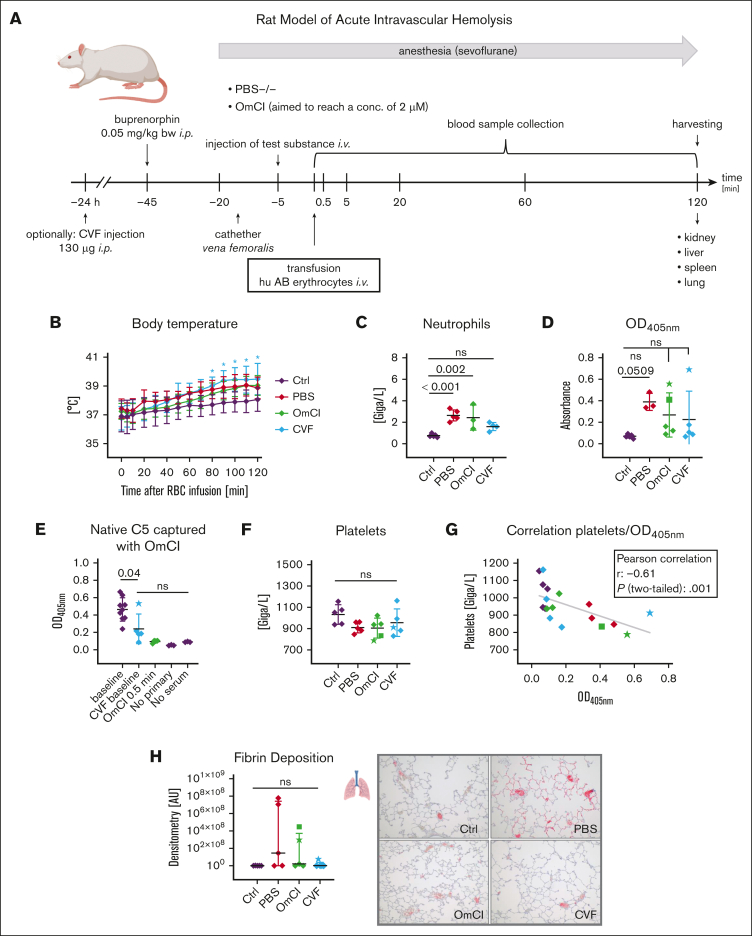
In vivo model of acute intravascular hemolysis
To confirm our findings in vivo, we used an established rat model of acute intravascular hemolysis, modeling a mismatched transfusion of erythrocytes including close monitoring of vital signs41,42 (Figure 6A-B). Human AB+ erythrocytes were infused into Wistar rats, which naturally contain antibodies against human AB antigens, thus, strongly activating the classical pathway via RBC agglutinating antibodies. Animals that received PBS instead of RBCs served as negative controls, and those with an RBC infusion without inhibitor (PBS group) served as positive controls. Specific complement inhibition at the level of C5 was achieved via the administration of the C5 inhibitor OmCI before RBC infusion. Alternatively, a CVF injection 24 hours before experimental start was administered to deplete the animals’ complement activity. End point blood count analysis indicated elevated neutrophil levels in the PBS group, which is slightly reduced under complement inhibition (Figure 6C). Unfortunately, few data points are missing in some of the graphs because of initial sample handling errors. Explanations for missing values are listed in supplemental Table 5. Erythrocyte lysis was determined from blood in the final bleed by measuring released hemoglobin via absorbance measurements as well as determining lactate dehydrogenase (LDH) levels. Hemolysis was most pronounced in the PBS group (Figure 6D; supplemental Figure 5A). As expected, determination of LDH levels via clinical analysis and absorbance measurements of released hemoglobin correlate well (supplemental Figure 5B). Animals treated with the C5 inhibitor OmCI or undergoing decomplementation with CVF exhibited substantially reduced hemolysis levels. However, especially in the inhibitor groups, a high variation of baseline values is noticeable for 1 (CVF group) or 2 (OmCI group) animals. To assess the levels of nonactivated C5 at certain time points in all animals, an in-house enzyme-linked immunosorbent assay detecting only native, nonactivated C5 was developed, using the desired binding property of OmCI.32,43 We observed that free, nonactivated C5 was absent in the OmCI group, indicating full C5 binding by OmCI in these animals. In contrast, in the CVF group, a significant reduction of C5 levels was obtained for all but 1 animal, which exhibited C5 levels comparable with those of baseline blood samples before the experiment (Figure 6E). This indicates a failure in complement depletion in this animal (likely because of a sample handling issue). Compared with the control animals, the animals experiencing substantial intravascular hemolysis exhibited a decreased platelet count although the differences did not reach statistical significance in this acute model, lasting only 2 hours (Figure 6F). However, platelet counts significantly correlated with the levels of released hemoglobin (optical density, 405 nm), pointing to a direct association of complement-mediated cell lysis and alterations in the hemostatic system (Figure 6G). Comparably, the animals in the OmCI group experiencing high levels of hemolysis despite C5 inhibition (marked as star and square) were among the animals with the lowest platelet counts. Immunohistochemical examination of lung tissue for fibrin deposition shows pronounced deposition in the PBS group in comparison with that in all other groups, although no statistically significant difference was reached (Figure 6H). Of note, elevated fibrin deposits in the OmCI and CVF groups were observed in the animals with the highest levels of cell lysis (optical density, 405 nm). Analyzing fibrin deposition and LDH levels for all animals exhibited a statistically significant positive correlation of these parameters (supplemental Figure 5C). Taken together, these in vivo results support the concept that complement-mediated lysis is associated with platelet activation and alterations in the hemostatic system, whereas massive generation of C3a and C5a in the CVF group (leading to exhaustive C3 and C5 consumption, as judged from the nonactivated C5 enzyme-linked immunosorbent assay) does not induce a prothrombotic phenotype per se.
Figure 6.
Rat model of acute intravascular hemolysis. (A) Experimental setup. Human AB erythrocytes were transfused in male Wistar rats simulating a 15% mismatch transfusion to induce an acute intravascular hemolysis. Animals were either pretreated with PBS−/− or OmCI (the aim was a 2-μM final concentration), alternatively, CVF (130 μg) was injected 24 hours before the start of transfusion to allow depletion of the complement system. Blood collection took place at indicated time points after transfusion. After 2 hours, animals were euthanized, and organs were extracted to assess end-organ analysis. (B) Body temperature. Animals were monitored for temperature after transfusion. Mean values with standard deviation are shown at each time point. A star indicates significant difference from control animals without RBC treatment. (C-D) End point clinical blood analysis of neutrophil count and hemolysis levels measured by spectrophotometric analysis of released hemoglobin. Mean values with standard deviation are shown. Star or square symbols in the OmCI and CVF group indicate outlier animals and correspond to star or square values in all other graphs. (E) ELISA-based measurement of nonactivated C5. EDTA-treated plasma samples were added to the surface-coated OmCI-FH8-15 fusion protein, which only binds native, nonactivated C5. Captured C5 was detected using a polyclonal anti-rat C5a antibody (PA5-78891). (F) End point clinical blood analysis of neutrophil count. (G) Correlation between platelets and released hemoglobin (optical density, 405 nm [OD405nm]). (H) Fibrin deposition in lungs was visualized via immunohistochemical staining against fibrinogen β chain and red signal intensity was quantified. From each group, 1 representative image (original magnification ×100) is shown. Data sets were tested for outliers using the ROUT outlier test (Q = 5%). In panel B, each time point was compared with the respective time point in the Ctrl group. Data sets in panels C-F and panel H were analyzed with one-way ANOVA. Experimental groups were post hoc tested for statistical significance against Ctrl/baseline with Dunnett correction for multiple comparisons (panels C-D,F,H) or against each other experimental group (Tukey test with correction for multiple comparisons). In panel G, data sets were tested for correlation using Pearson test. For the sake of visibility, nonsignificant P values were omitted from graphs, unless they were of relevance to the experimental hypotheses.
Discussion
We set out to mechanistically uncover why anticomplement therapy efficiently prevents thromboembolisms in the complement-mediated diseases PNH and aHUS but does not interfere negatively with hemostasis. Whole blood models in reaction tubes exhibited substantial levels of surface-induced complement activation but failed to induce prothrombotic platelet activation. However, innate immune cells (granulocytes and monocytes) were activated by these complement split products (Figures 1 and 2; supplemental Figure 1), demonstrating their functionality. ROTEM in whole blood showed that C3 inhibition (but not C5 inhibition) prolonged the clotting time after specific activation of the intrinsic pathway (Figures 2 and 3; supplemental Tables 1-4). This effect is likely due to the complement activation products activating leukocytes within the whole blood setting. In addition, the results from aggregometry experiments in whole blood and platelet-rich plasma support this concept. C3a and C5a alone did not activate platelets in the whole blood. However, with consecutive stimulation by anaphylatoxins and the known platelet activators TRAP and ADP, a trend (but no significant change) toward increased platelet aggregation was noticeable in the whole blood setup but not in platelet-rich plasma lacking leukocytes (Figure 3B; supplemental Figure 3C). This indicates that platelets fail to respond to C3a and C5a. Although there was no sign for the expression of the C5aR1 on nonstimulated or ADP-stimulated platelets, a signal for C3aR appeared only on ADP-stimulated platelets (Figure 3D). Because ADP-stimulated platelets also bound more of the C3aR isotype control antibody (Figure 3E), the difference for C3aR staining was not statistically significant but represented a clear signal. An increased staining signal for C3aR upon platelet activation has been described previously.37 Binding studies with radioactively labeled ligands failed to detect C3aR and C5aR1 on nonstimulated human platelets,44,45 and an RNA-sequencing analysis of human platelet transcriptomes revealed very low copy numbers of C3aR and C5aR1.46 A recent study also failed to observe an effect of C3a on platelet activation.47
To understand how complement activation, then, may induce prothrombotic platelet activation, we resorted to well-controlled in vitro models of hemolysis.31,32,48 Taken together, data on hemolysis of rabbit or human erythrocytes suggest that it is not the complement activation products themselves that activate the platelets but rather the MAC-mediated hemolysis reaction (Figures 4A and 5). To test this, mechanically shattered rabbit or human RBCs were added to whole blood with or without Cp40 addition (to control that complement activation is stopped in case it was to occur unexpectedly also on the shattered rRBCs) (Figure 4B; supplemental Figure 4B). Shattered RBCs induced strong platelet activation in whole blood irrespective of the presence of complement inhibition. This clearly shows that complement activation only provides the means for releasing intracellular danger signals via MAC-mediated cell lysis, which in turn activate platelets. This hypothesis was confirmed by repeating the experimental setup, but this time the ADP receptor antagonists cangrelor and MRS2179, which inhibit the purine receptors P2Y12 and P2Y1, respectively, were included39,40 (Figure 4C; supplemental Figure 4C). The ADP receptor antagonists completely blocked prothrombotic cell activation in conditions that produced strong complement activation.
Finally, we crossvalidated these findings using an in vivo rat model of acute intravascular hemolysis. Human erythrocytes were administered IV into Wistar rats, which is known to result in complement-dependent lysis of the transfused erythrocytes.41,42 The complement inhibitor OmCI (inhibiting C5) or CVF (known to deplete the animals of C3 and C5 in the fluid phase49), were administered minutes or 24 hours before the transfusion of the human erythrocytes, respectively. Rats that did not receive transfusion did not exhibit signs of intravascular hemolysis or reduced platelet numbers, as expected (Figure 6D,F; supplemental Figure 5A), but animals that received transfusion but did not receive a complement inhibitor did show, for example, high LDH levels and low platelet counts, albeit differences not reaching statistical significance (within 120 minutes of the experimental procedure). Of the 5 animals receiving OmCI, erythrocyte lysis was only inhibited in 3. All but 1 of the CVF-treated rats exhibited nearly complete C5 consumption, whereas in 1 animal, complement depletion by CVF apparently did not work (Figure 6E). CVF-mediated complement depletion did not, per se, lead to a prothrombotic phenotype in the animals. That the level of intravascular hemolysis correlates inversely with the platelet count (Figure 6G) but positively with the fibrin deposition levels in the lungs (supplemental Figure 5C) is also consistent with our concept: complement activation only then leads to strong prothrombotic cell activation whenever its activation triggers the release of the intracellular danger signals by MAC-mediated cytolysis. Therefore, by extrapolating our data it can be explained why massive complement activation in C3G does not predispose to thrombotic complications in contrast to aHUS and PNH. In C3G, terminal pathway activation products are “quenched” and do not inflict cytolysis21,22 (and reviewed previously10). However, our data are at variance with a number of reports that suggest a direct impact of isolated complement components on prothrombotic cell activation (50, 51, 52, 53, 54, summarized here10,11). It is likely that, at least in some of these reports, it had been overlooked that complement activation had led to ADP release, which secondarily induced prothrombotic cell activation and/or purified plasma-derived complement components might have been contaminated with minuscule amounts of thrombin.
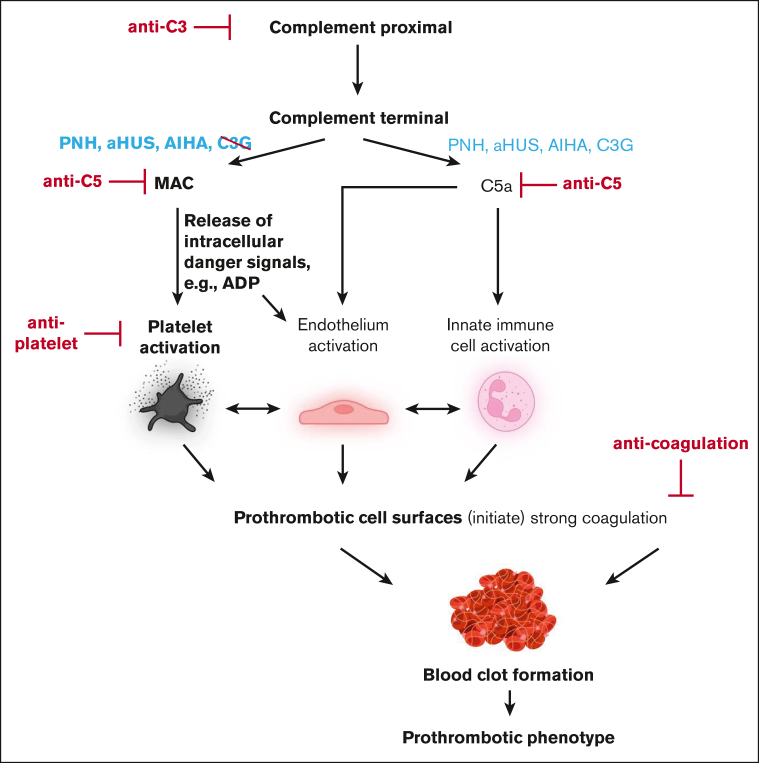
Our findings have several important clinical implications. Widespread fluid phase complement activation not leading to MAC-mediated cytolysis is not a risk factor for complement-mediated thrombosis, but surface-targeted complement activation liberating intracellular ADP is. Complement-induced thrombotic complications do not readily respond to anticoagulation therapy as is the case for PNH. The reason is that coagulation is neither the upstream nor the central event in the pathophysiology of complement-mediated thrombosis (Figure 7). Tentatively, our results suggest that inhibition of ADP receptor signaling may be clinically more efficient in stopping thrombotic complications in these conditions. However, preventing cytolysis by anticomplement therapy represents the most upstream and likely most successful therapeutic intervention route. Regarding prothrombotic mechanisms in PNH, it has to be stated that there is a small number of patients with PNH with a rather small erythrocytes clone who still experiencing an increased thrombotic risk.55 Potentially, other prothrombotic mechanisms are at play in these patients, like complement-induced shedding of microparticles from the endothelium or effects introduced by free hemoglobin.56,57 In any case, the clinical experience with patients with C3G unequivocally demonstrates that strong proximal and terminal complement activation alone is insufficient to cause clinically relevant thrombosis.
Figure 7.
Schematic diagram of complement-induced prothrombotic phenotype. Strong proximal complement activation culminates in terminal pathway activation liberating C5a and inducing MAC assembly. Although C5a is liberated in C3G, PNH, aHUS, and autoimmune hemolytic anemia (AIHA), cytolytic MAC complexes are reported for the latter 3 but not C3G. MAC-mediated release of the intracellular danger signal ADP induces prothrombotic activation states of platelets and endothelial cells, culminating in thrombosis. Potential therapeutic intervention strategies are shown in red. Elements in bold font depict concepts derived from data of this study. The illustration was created with BioRender.com.
Our study focuses mainly on the prothrombotic cell activation mechanisms of peripheral blood cells. Building on current studies,58 the contributions of the endothelium to thrombotic complications need to be addressed from a similar angle in future studies. In line with our observations, however, it is well established that ADP functions also as a danger signal for endothelial cells. In response to ADP the endothelium upregulates P-selectin expression.59 Because anticomplement therapy does not interfere with coagulation or platelet activation as such (Figure 7), inhibiting complement is of course not expected to be beneficial in conditions characterized by “pure” coagulation as it occurs in atrial fibrillation. However, not interfering with coagulation and platelet activation is the very reason why anticomplement therapy is not associated with bleeding risks. Our results, therefore, call to investigate anticomplement therapy (potentially in combination with ADP receptor antagonists) in diseases in which complement-mediated cytolysis associates with thrombotic complications that cannot be adequately addressed through anticoagulation and/or antiplatelet therapy. This includes, among others, warm agglutinins-mediated autoimmune hemolytic anemia, CAPS, and transplantation-associated thrombotic microangiopathy.
Conflict-of-interest disclosure: C.Q.S., H.S., and M.H.-L. are listed as inventors on (a) patent application(s) that describes the use of complement inhibitors for therapeutic applications. C.Q.S. has received research funding from pharmaceutical companies. M.H.-L., C.Q.S., and H.S. received honoraria for speaking at symposia organized by Alexion Pharmaceuticals. H.S. served on advisory committees for Alexion AstraZeneca Rare Diseases, Sanofi, Sobi, and Novartis; received research funding from Alexion Pharmaceuticals (all to the University of Ulm); and served on an advisory committee for Ra Pharmaceuticals and Alnylam. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank John D. Lambris (Department of Pathology and Laboratory Medicine, University of Pennsylvania) for his kind gift of Cp40. The authors thank Anke Schultze for her excellent technical assistance, and Sonja Braumueller and Annette Palmer for assistance in animal modeling.
This work was supported by Deutsche Forschungsgemeinschaft grants SCHM 3018/4-1 (C.Q.S.) and CRC1149 A01 (M.H.-L.).
Authorship
Contribution: C.Q.S., M.H.-L., and C.K.B. conceived the study; M.M., V.P., S.H., A.D., V.A.M., O.E., and C.Q.S. planned and performed the experiments; M.M., C.K.B., M.H.-L., V.P., S.H., H.S., E.-M.J., O.E., K.N.E., B.N., and C.Q.S. analyzed the data; H.S., M.H.-L., C.K.B., M.H., and B.N. advised on experimental design and data analysis; M.M., A.D., C.Q.S., and H.S. prepared or provided important reagents; M.M., C.K.B., and C.Q.S. wrote the manuscript; and all authors contributed to the discussion of the data and critically revised the manuscript.
Footnotes
∗C.K.B. and C.Q.S. contributed equally to this study.
Data and protocols or information on specialized reagents are available on request from the corresponding authors, Christoph Q. Schmidt (christoph.schmidt@uni-ulm.de) and Christian K. Braun (christian.braun@uniklinik-ulm.de).
The full-text version of this article contains a data supplement.
Contributor Information
Christian K. Braun, Email: christian.braun@uniklinik-ulm.de.
Christoph Q. Schmidt, Email: christoph.schmidt@uni-ulm.de.
Supplementary Material
References
- 1.Luzzatto L, Gianfaldoni G, Notaro R. Management of paroxysmal nocturnal haemoglobinuria: a personal view. Br J Haematol. 2011;153(6):709–720. doi: 10.1111/j.1365-2141.2011.08690.x. [DOI] [PubMed] [Google Scholar]
- 2.Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811. doi: 10.1182/blood-2014-02-522128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parker CJ. The pathophysiology of paroxysmal nocturnal hemoglobinuria. Exp Hematol. 2007;35(4):523–533. doi: 10.1016/j.exphem.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 4.Hill A, Kelly RJ, Hillmen P. Thrombosis in paroxysmal nocturnal hemoglobinuria. Blood. 2013;121(25):4985–4996. doi: 10.1182/blood-2012-09-311381. quiz 5105. [DOI] [PubMed] [Google Scholar]
- 5.Audebert HJ, Planck J, Eisenburg M, Schrezenmeier H, Haberl RL. Cerebral ischemic infarction in paroxysmal nocturnal hemoglobinuria report of 2 cases and updated review of 7 previously published patients. J Neurol. 2005;252(11):1379–1386. doi: 10.1007/s00415-005-0871-3. [DOI] [PubMed] [Google Scholar]
- 6.Azevedo L, Costa MR, Fonseca AC, Pinho e Melo T. Recurrent cerebral ischaemic events in the setting of paroxysmal nocturnal haemoglobinuria. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-213603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillmen P, Muus P, Dührsen U, et al. Effect of the complement inhibitor eculizumab on thromboembolism in patients with paroxysmal nocturnal hemoglobinuria. Blood. 2007;110(12):4123–4128. doi: 10.1182/blood-2007-06-095646. [DOI] [PubMed] [Google Scholar]
- 8.Legendre CM, Licht C, Muus P, et al. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368(23):2169–2181. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 9.Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25(11):1256–1264. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt CQ, Schrezenmeier H, Kavanagh D. Complement and the prothrombotic state. Blood. 2022;139(13):1954–1972. doi: 10.1182/blood.2020007206. [DOI] [PubMed] [Google Scholar]
- 11.Delvasto-Nuñez L, Jongerius I, Zeerleder S. It takes two to thrombosis: hemolysis and complement. Blood Rev. 2021;50:100834. doi: 10.1016/j.blre.2021.100834. [DOI] [PubMed] [Google Scholar]
- 12.Nayer A, Ortega LM. Catastrophic antiphospholipid syndrome: a clinical review. J Nephropathol. 2014;3(1):9–17. doi: 10.12860/jnp.2014.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rand JH, Wu X-X, Wolgast LR, Lei V, Conway EM. A novel 2-stage approach that detects complement activation in patients with antiphospholipid antibody syndrome. Thromb Res. 2017;156:119–125. doi: 10.1016/j.thromres.2017.06.014. [DOI] [PubMed] [Google Scholar]
- 14.Chaturvedi S, Braunstein EM, Yuan X, et al. Complement activity and complement regulatory gene mutations are associated with thrombosis in APS and CAPS. Blood. 2020;135(4):239–251. doi: 10.1182/blood.2019003863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaturvedi S, Braunstein EM, Brodsky RA. Antiphospholipid syndrome: complement activation, complement gene mutations, and therapeutic implications. J Thromb Haemost. 2021;19(3):607–616. doi: 10.1111/jth.15082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Audia S, Bach B, Samson M, et al. Venous thromboembolic events during warm autoimmune hemolytic anemia. PLoS One. 2018;13(11):e0207218. doi: 10.1371/journal.pone.0207218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barcellini W, Fattizzo B, Zaninoni A, et al. Clinical heterogeneity and predictors of outcome in primary autoimmune hemolytic anemia: a GIMEMA study of 308 patients. Blood. 2014;124(19):2930–2936. doi: 10.1182/blood-2014-06-583021. [DOI] [PubMed] [Google Scholar]
- 18.Lee JW, Jang JH, Kim JS, et al. Clinical signs and symptoms associated with increased risk for thrombosis in patients with paroxysmal nocturnal hemoglobinuria from a Korean Registry. Int J Hematol. 2013;97(6):749–757. doi: 10.1007/s12185-013-1346-4. [DOI] [PubMed] [Google Scholar]
- 19.Baek S-W, Lee M-W, Ryu H-W, et al. Clinical features and outcomes of autoimmune hemolytic anemia: a retrospective analysis of 32 cases. Korean J Hematol. 2011;46(2):111–117. doi: 10.5045/kjh.2011.46.2.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berentsen S, Barcellini W, D’Sa S, et al. Cold agglutinin disease revisited: a multinational, observational study of 232 patients. Blood. 2020;136(4):480–488. doi: 10.1182/blood.2020005674. [DOI] [PubMed] [Google Scholar]
- 21.Sethi S, Gamez JD, Vrana JA, et al. Glomeruli of Dense Deposit Disease contain components of the alternative and terminal complement pathway. Kidney Int. 2009;75(9):952–960. doi: 10.1038/ki.2008.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sethi S, Vrana JA, Fervenza FC, et al. Characterization of C3 in C3 glomerulopathy. Nephrol Dial Transplant. 2017;32(3):459–465. doi: 10.1093/ndt/gfw290. [DOI] [PubMed] [Google Scholar]
- 23.Pickering MC, Warren J, Rose KL, et al. Prevention of C5 activation ameliorates spontaneous and experimental glomerulonephritis in factor H-deficient mice. Proc Natl Acad Sci U S A. 2006;103(25):9649–9654. doi: 10.1073/pnas.0601094103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith RJH, Appel GB, Blom AM, et al. C3 glomerulopathy - understanding a rare complement-driven renal disease. Nat Rev Nephrol. 2019;15(3):129–143. doi: 10.1038/s41581-018-0107-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morser J, Shao Z, Nishimura T, et al. Carboxypeptidase B2 and N play different roles in regulation of activated complements C3a and C5a in mice. J Thromb Haemost. 2018;16(5):991–1002. doi: 10.1111/jth.13964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foley JH, Conway EM. Basic weapons to degrade C3a and C5a. J Thromb Haemost. 2018;16(5):987–990. doi: 10.1111/jth.13999. [DOI] [PubMed] [Google Scholar]
- 27.Schmidt CQ, Verschoor A. Complement and coagulation: so close, yet so far. Blood. 2017;130(24):2581–2582. doi: 10.1182/blood-2017-10-811943. [DOI] [PubMed] [Google Scholar]
- 28.Huber-Lang MS, Ignatius A, Köhl J, Mannes M, Braun CK. Complement in trauma—traumatised complement? Br J Pharmacol. 2021;178(14):2863–2879. doi: 10.1111/bph.15245. [DOI] [PubMed] [Google Scholar]
- 29.Pryzdial ELG, Leatherdale A, Conway EM. Coagulation and complement: key innate defense participants in a seamless web. Front Immunol. 2022;13:918775. doi: 10.3389/fimmu.2022.918775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Risitano AM, Marotta S, Ricci P, et al. Anti-complement treatment for paroxysmal nocturnal hemoglobinuria: time for proximal complement inhibition? A position paper from the SAAWP of the EBMT. Front Immunol. 2019;10:1157. doi: 10.3389/fimmu.2019.01157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harder MJ, Kuhn N, Schrezenmeier H, et al. Incomplete inhibition by eculizumab: mechanistic evidence for residual C5 activity during strong complement activation. Blood. 2017;129(8):970–980. doi: 10.1182/blood-2016-08-732800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannes M, Dopler A, Zolk O, et al. Complement inhibition at the level of C3 or C5: mechanistic reasons for ongoing terminal pathway activity. Blood. 2021;137(4):443–455. doi: 10.1182/blood.2020005959. [DOI] [PubMed] [Google Scholar]
- 33.Asif S, Asawa K, Inoue Y, et al. Validation of an MPC polymer coating to attenuate surface-induced crosstalk between the complement and coagulation systems in whole blood in in vitro and in vivo models. Macromol Biosci. 2019;19(5):e1800485. doi: 10.1002/mabi.201800485. [DOI] [PubMed] [Google Scholar]
- 34.Lamers C, Mastellos DC, Ricklin D, Lambris JD. Compstatins: the dawn of clinical C3-targeted complement inhibition. Trends Pharmacol Sci. 2022;43(8):629–640. doi: 10.1016/j.tips.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cognasse F, Duchez AC, Audoux E, et al. Platelets as key factors in inflammation: focus on CD40L/CD40. Front Immunol. 2022;13:825892. doi: 10.3389/fimmu.2022.825892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blair P, Flaumenhaft R. Platelet α-granules: basic biology and clinical correlates. Blood Rev. 2009;23(4):177–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauter RJ, Sauter M, Reis ES, et al. Functional relevance of the anaphylatoxin receptor C3aR for platelet function and arterial thrombus formation marks an intersection point between innate immunity and thrombosis. Circulation. 2018;138(16):1720–1735. doi: 10.1161/CIRCULATIONAHA.118.034600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nording H, Baron L, Haberthür D, et al. The C5a/C5a receptor 1 axis controls tissue neovascularization through CXCL4 release from platelets. Nat Commun. 2021;12(1):3352. doi: 10.1038/s41467-021-23499-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baurand A, Raboisson P, Freund M, et al. Inhibition of platelet function by administration of MRS2179, a P2Y1 receptor antagonist. Eur J Pharmacol. 2001;412(3):213–221. doi: 10.1016/s0014-2999(01)00733-6. [DOI] [PubMed] [Google Scholar]
- 40.Sible AM, Nawarskas JJ. Cangrelor: a new route for P2Y12 inhibition. Cardiol Rev. 2017;25(3):133–139. doi: 10.1097/CRD.0000000000000142. [DOI] [PubMed] [Google Scholar]
- 41.Shah TA, Mauriello CT, Hair PS, et al. Complement inhibition significantly decreases red blood cell lysis in a rat model of acute intravascular hemolysis. Transfusion (Paris) 2014;54(11):2892–2900. doi: 10.1111/trf.12695. [DOI] [PubMed] [Google Scholar]
- 42.Kumar PS, Pallera HK, Hair PS, et al. Peptide inhibitor of complement C1 modulates acute intravascular hemolysis of mismatched red blood cells in rats. Transfusion (Paris) 2016;56(8):2133–2145. doi: 10.1111/trf.13674. [DOI] [PubMed] [Google Scholar]
- 43.Jore MM, Johnson S, Sheppard D, et al. Structural basis for therapeutic inhibition of complement C5. Nat Struct Mol Biol. 2016;23(5):378–386. doi: 10.1038/nsmb.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kretzschmar T, Kahl K, Rech K, Bautsch W, Köhl J, Bitter-Suermann D. Characterization of the C5a receptor on guinea pig platelets. Immunobiology. 1991;183(5):418–432. doi: 10.1016/S0171-2985(11)80526-7. [DOI] [PubMed] [Google Scholar]
- 45.Kretzschmar T, Pohl M, Casaretto M, et al. Synthetic peptides as antagonists of the anaphylatoxin C3a. Eur J Biochem. 1992;210(1):185–191. doi: 10.1111/j.1432-1033.1992.tb17407.x. [DOI] [PubMed] [Google Scholar]
- 46.Rowley JW, Oler AJ, Tolley ND, et al. Genome-wide RNA-seq analysis of human and mouse platelet transcriptomes. Blood. 2011;118(14):e101–e111. doi: 10.1182/blood-2011-03-339705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Landsem A, Emblem Å, Lau C, et al. Complement C3b contributes to Escherichia coli-induced platelet aggregation in human whole blood. Front Immunol. 2022;13:1020712. doi: 10.3389/fimmu.2022.1020712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Harder MJ, Höchsmann B, Dopler A, et al. Different levels of incomplete terminal pathway inhibition by eculizumab and the clinical response of PNH patients. Front Immunol. 2019;10:1639. doi: 10.3389/fimmu.2019.01639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vogel C-W, Fritzinger DC. In: Snake Venoms. Springer. Inagaki H, Vogel CW, Mukherjee A, Rahmy T, editors. 2017. Cobra venom factor: the unique component of cobra venom that activates the complement system; pp. 345–404. [Google Scholar]
- 50.Polley MJ, Nachman R. The human complement system in thrombin-mediated platelet function. J Exp Med. 1978;147(6):1713–1726. doi: 10.1084/jem.147.6.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Polley MJ, Nachman RL. Human platelet activation by C3a and C3a des-arg. J Exp Med. 1983;158(2):603–615. doi: 10.1084/jem.158.2.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiedmer T, Esmon CT, Sims PJ. Complement proteins C5b-9 stimulate procoagulant activity through platelet prothrombinase. Blood. 1986;68(4):875–880. [PubMed] [Google Scholar]
- 53.Wiedmer T, Hall SE, Ortel TL, Kane WH, Rosse WF, Sims PJ. Complement-induced vesiculation and exposure of membrane prothrombinase sites in platelets of paroxysmal nocturnal hemoglobinuria. Blood. 1993;82(4):1192–1196. [PubMed] [Google Scholar]
- 54.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263(34):18205–18212. [PubMed] [Google Scholar]
- 55.Griffin M, Hillmen P, Munir T, et al. Significant hemolysis is not required for thrombosis in paroxysmal nocturnal hemoglobinuria. Haematologica. 2019;104(3):e94–e96. doi: 10.3324/haematol.2018.198846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Singhal R, Annarapu GK, Pandey A, et al. Hemoglobin interaction with GP1b induces platelet activation and apoptosis: a novel mechanism associated with intravascular hemolysis. Haematologica. 2015;100(12):1526–1533. doi: 10.3324/haematol.2015.132183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helms CC, Marvel M, Zhao W, et al. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013;11(12):2148–2154. doi: 10.1111/jth.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aiello S, Gastoldi S, Galbusera M, et al. C5a and C5aR1 are key drivers of microvascular platelet aggregation in clinical entities spanning from aHUS to COVID-19. Blood Adv. 2022;6(3):866–881. doi: 10.1182/bloodadvances.2021005246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noris M, Galbusera M, Gastoldi S, et al. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124(11):1715–1726. doi: 10.1182/blood-2014-02-558296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.