Abstract
Genomic DNA was isolated from Cryptosporidium parvum oocysts by a specific immunomagnetic separation-in vitro excystation procedure and subjected to randomly amplified polymorphic DNA analysis using sequence-independent primers. An estuary C. parvum isolate was easily differentiated from several bovine isolates, while five bovine isolates of the same origin were indistinguishable from each other.
Cryptosporidium parvum is a coccidian parasite that can cause gastrointestinal illness in humans and a broad range of other mammals (6). The diarrheal disease, usually self-limiting in immunocompetent individuals, can be prolonged and life threatening among immunocompromised people such as AIDS patients because an effective treatment is not available (3). Cross-transmission of C. parvum oocysts among humans and animals has been reported, and oocysts shed from one species of mammal are infectious to other species of mammals (5, 6). To define the transmission route of C. parvum and to design preventive measures to minimize the potential for cryptosporidiosis outbreaks, a reliable method to determine the genetic diversities within C. parvum and to differentiate isolates of various sources is in imminent need.
Randomly amplified polymorphic DNA (RAPD), a technique based on the PCR using primers of arbitrary sequence, has been used increasingly for taxonomic identification and isolate differentiation in a variety of organisms (7, 12, 13). It has also been introduced for genetic characterization of C. parvum isolates (2, 9). Unlike the earlier phenotypic and genotypic characterization methods, such as two-dimensional gel electrophoresis (8), antigenic analysis (10), or restriction fragment length polymorphism (11), RAPD uses small quantities of DNA, no sequence information is needed, and it is technically easier to perform (9). However, the validity of a RAPD analysis depends mainly on the purity of template DNA and on the primer chosen for arbitrarily primed PCR (AP-PCR) amplification. In previous C. parvum RAPD analyses, the purity of the parasite DNA was ensured by the absence of contaminant organisms determined by microscopical examination or bleach sterilization after laborious oocyst purification. On the other hand, primers used in AP-PCR were longer than standard 10-nucleotide RAPD primers and were primarily sequence dependent. Although the use of longer primers increased the reproducibility of band patterns, fewer bands could have been generated, possibly limiting the discrimination ability of the technique. In the present study, we simplified the RAPD technique by introducing a genomic DNA isolation method involving a specific immunomagnetic separation (IMS) and in vitro excystation of C. parvum oocysts and discriminated C. parvum isolates by RAPD analysis using sequence-independent primers.
A total of six bovine C. parvum isolates were acquired from symptomatic calves in a commercial dairy farm near Davis, Calif. They were collected in May 1996 (isolate CP6742), May 1997 (CP7265, CP7278, and CP7284), and July 1997 (CP7375 and CP7380), and the sample code referred to the individual calf from which the fecal sample was taken. Oocysts were purified from diarrheal feces by Sheather’s sucrose flotation, followed by a CsCl gradient centrifugation method (4). Four estuary samples (ERS1-2, ERS2-2, MRS1-2, and MRS2-2) were collected in June 1997 in Humboldt Bay, Calif.; the code referred to the sampling site. A 100-ml portion from each sample was passed through several sieves, the last one with a porosity of 63 μm, and centrifuged at 750 × g for 2 min to remove large particles. The oocysts were then pelleted at 1,000 × g for 8 min and resuspended in 1.0 ml of distilled water.
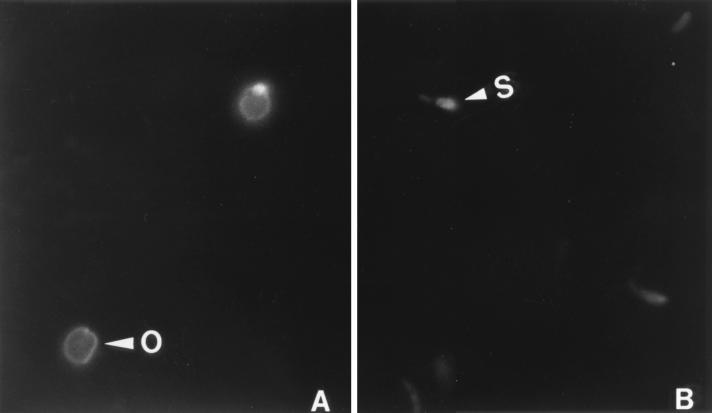
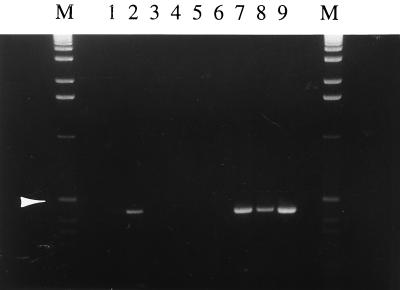
An immunomagnetic capture (IC) PCR was used to detect viable C. parvum oocysts from the estuary samples. Anti-C. parvum serum was prepared by immunizing a rabbit with formalin-treated C. parvum oocysts, and immunoglobulin G (IgG) was purified, biotinylated, and used to coat magnetic particles as described previously (4). The reactivity of anti-C. parvum serum was predetermined by an indirect fluorescent antibody staining procedure. Briefly, a mixture of oocysts and excysted sporozoites was fixed on a glass slide, reacted sequentially with a 1:400 dilution of the rabbit anti-C. parvum serum and a 1:160 dilution of a fluorescein isothiocyanate-conjugated goat anti-rabbit IgG (Sigma Chemical Co., St. Louis, Mo.), and examined with a standard fluorescence microscope at ×4,500 magnification. As shown in Fig. 1, both oocysts (Fig. 1A) and excysted sporozoites (Fig. 1B) were stained, confirming that they will be captured in IMS. By use of a previously described protocol (4), IC-PCR was performed to detect a C. parvum-specific 452-bp fragment. To minimize the effect of possible interference of inhibitory substances and to avoid false-negative judgments, three dilutions of the concentrated sample (500, 50, and 5.0 μl, respectively) were assayed. Two positive controls receiving, respectively, 10 and 100 oocysts were contained to monitor the IC, and another control using previous PCR product as the PCR template was included to ensure the PCR efficiency. Among the four samples, ERS1-2 was found to be positive (Fig. 2). Notably, a specific band was obtained by the IC-PCR using 50- and 5-μl concentrated samples (lanes 7 and 8) but not by the IC-PCR using a 500-μl concentrated sample (lane 6). Since results with both the PCR positive control (lane 9) and the IMS positive control containing 100 oocysts (lane 2) were positive (although another IMS positive control using 10 oocysts gave a weak signal in lane 1), this phenomenon was interpreted as interference from inhibitory substances present in estuary samples.
FIG. 1.
Reaction activity of rabbit anti-C. parvum serum with C. parvum oocysts (arrowhead labeled O) (A) and excysted C. parvum sporozoites (arrowhead labeled S) (B). Magnification, ×4,500.
FIG. 2.
IC-PCR to detect C. parvum oocysts from estuary samples. Lanes: M, DNA size marker (1-kb ladder), with an arrowhead indicating band of 506 bp; 1 and 2, IC positive controls inoculated with 10 and 100 oocysts, respectively; 3 to 5 and 6 to 8, estuary samples ERS2-2 and ERS1-2, with 500, 50, and 5.0 μl of concentrated sample used per lane, respectively; 9, PCR positive control.
For RAPD analysis, an IMS-in vitro excystation method was used to isolate genomic DNA from ERS1-2 and bovine isolates CP7278, CP7284, and CP6742. Briefly, a more-concentrated ERS1-2 sample or a purified oocyst suspension containing ∼5 × 105 oocysts was incubated with IgG-coated magnetic beads, separated from contaminating materials, and subjected to in vitro excystation; DNA was released from sporozoites or partially excysted oocysts by incubation at 95°C for 10 min, as in the IC-PCR. DNA was further extracted by phenol-chloroform-isoamyl alcohol (25:24:1, vol/vol/vol; Fisher Scientific, Fair Lawn, N.J.), precipitated by 100% isopropanol in the presence of 200 μg of d-glycogen (Fisher Scientific) per ml, and resuspended in 1× Tris-EDTA buffer (TE; pH 8.0), as described previously (4). The DNA concentration was estimated with a DNA DipStick (Invitrogen, Carlsbad, Calif.) as described in the manufacturer’s instructions and was referred to as the number of purified oocysts used for IMS and DNA extraction. AP-PCR was performed on a Progene 120 thermal cycler (Techne Inc., Princeton, N.J.) with 0.5-ml centrifuge tubes. A 25-μl PCR mixture, containing 1× PCR Buffer II (Perkin-Elmer Cetus Corp., Norwalk, Conn.), 4.0 mM MgCl2 solution (Perkin-Elmer), a 200 μM concentration of each of the four deoxynucleoside triphosphates (Perkin-Elmer), a 1.0 μM concentration of a 10-nt-long primer with 60% GC content, 2.5 U of AmpliTaq DNA polymerase (Perkin-Elmer), and approximately 1.0 ng of DNA template, was overlaid with 25 μl of mineral oil (Sigma) to prevent evaporation. The reaction mixture was heated at 94°C for 3 min and subjected to 45 cycles of denaturation at 94°C for 1 min, annealing at 36°C for 1 min, and extension at 72°C for 2 min. After an additional 7-min extension at 72°C, the reaction mixtures were held at 4°C. A 20-μl portion of the amplified products was separated on a 1.8% agarose gel and visualized on a UV transilluminator after being stained with 0.5 μg of ethidium bromide per ml. A 1.0-kb DNA ladder (GIBCO-BRL Life Technologies, Gaithersburg, Md.) was also included to estimate the size of amplified products. RAPD patterns were examined for the numbers, sizes, and intensities of generated DNA fragments.
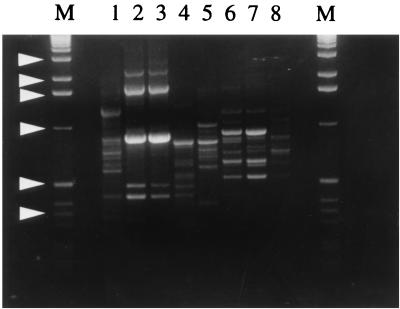
Figure 3 illustrates the results obtained with primers GPD62 (CAATGCCCGA; GeneMed Biotechnologies, San Francisco, Calif.) (lanes 1 to 4) and GPD63 (CAATGCCCGA, GeneMed) (lane 5 to 8). Both primers produced multiple bands, usually ranging from 0.3 to 2.0 kb. With either primer, the profile of ERS1-2 (lanes 1 and 5) was clearly different from those of the bovine isolates. Band patterns of CP7278 and CP7284, two bovine isolates apparently of the same origin (since they were collected from neighboring calves), were identical, indicating the reliability of the DNA extraction method and the reproducibility of RAPD analysis (lanes 2, 3, 6, and 7). However, they were distinguishable from the band patterns of CP6742, another bovine isolate acquired 1 year earlier (lanes 4 and 8), suggesting that the origin of CP6742 might be different from that of CP7278 and CP7284.
FIG. 3.
RAPD profiles of C. parvum isolates. Lanes: M, 1-kb DNA ladder with arrowheads indicating bands (from top to bottom) of approximately 3.0, 2.0, 1.6, 1.0, 0.5, and 0.35 kb; 1 and 5, ERS1-2; 2 and 6, CP7278; 3 and 7, CP7284; 4 and 8, CP6742. Primers used were GPD62 (lanes 1 to 4) and GPD63 (lanes 5 to 8).
Effects of DNA isolation methods on the reproducibility of RAPD analysis were investigated by using five bovine C. parvum isolates, namely, CP7265, CP7278, CP7284, CP7375, and CP7380. Oocysts were purified from CsCl gradient centrifugation, and genomic DNAs were prepared by either a proteinase K-phenol procedure or a modified IMS-in vitro excystation procedure. The proteinase K-phenol procedure involved proteinase K digestion in the presence of N-lauroyl sarcosine, phenol-chloroform-isoamyl alcohol extraction, and isopropanol precipitation in the presence of glycogen (4). To enhance the efficiency of DNA extraction, the IMS-in vitro excystation procedure described above was modified, based on the facts that acidification is necessary to get maximum oocyst excystation efficiency (1) and that acidification will dissociate oocysts from IgG-coated particles and affect subsequent antigen-antibody reactions. Roughly 3 × 105 oocysts were incubated in 1.0 ml of acidified 1× Hanks’ balanced salts solution (HBSS, pH 2.0; Sigma), incubated at 37°C for 1 h, washed with 1× HBSS (pH 7.4), resuspended in 300 μl of 1× HBSS (pH 7.4), mixed with 600 μl of 1.0% bovine bile (Sigma) in Eagle’s minimum essential medium (Sigma) and 150 μl of 0.44% NaHCO3, and incubated at 37°C for 2 h. Then, 150 μl of IgG-coated magnetic particles was added, and the tubes were incubated at 37°C for another 2 h. Oocysts and excysted sporozoites were separated by IMS, washed three times with distilled water, and resuspended in 300 μl of 1× TE; DNA was isolated by heating at 95°C for 10 min, phenol-chloroform-isoamyl alcohol extraction, and precipitation with isopropanol and quantified as described above.
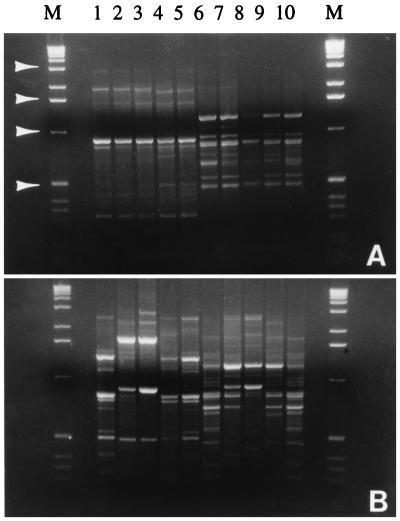
When DNA was isolated by the modified IMS-in vitro excystation procedure, the RAPD profiles of the five bovine isolates were almost identical, regardless of whether GPD62 (Fig. 4A, lanes 1 to 5) or GPD63 (Fig. 4A, lanes 6 to 10) was used in the AP-PCR, although there were minor differences in the intensities of some bands. The RAPD patterns of these isolates were also identical to those resulting from the original IMS-in vitro excystation method (data not shown). In contrast, when the proteinase K-phenol procedure was used, RAPD profiles of these isolates varied significantly; based on the band patterns, they could be divided into two groups, one consisting of CP7265, CP7375, and CP7380 and the other consisting of CP7278 and CP7284 (Fig. 4B), although they were seemingly from the same origin. Compared with the profiles in Fig. 4A, the absence or presence of some specific bands, as well as differences in intensities of some common bands, was not unusual. Besides, the profile of a certain isolate was unstable from batch to batch, even when isolated under identical conditions (data not shown). Such instability was attributed to contamination from bacterial DNA in parasite genomic DNA. After CsCl gradient centrifugation and enumeration in a hemocytometer, a 10- to 30-μl portion of the oocyst stock suspension was inoculated into 2.0 ml of brain heart infusion medium (Difco Laboratories, Detroit, Mich.) or 2.0 ml of fluid thioglycolate medium (Difco Laboratories), in which bacterial growth was observed after 24 to 72 h of incubation at 37°C, even though the suspension was free of bacteria by microscopic criteria. However, due to the specificity of anti-C. parvum IgG, contaminating bacteria or other organisms will not be isolated by IMS, which consequently precluded the presence of their DNAs in AP-PCR. In addition, application of IMS bypasses the time-consuming oocyst purification and allows more rapid analysis, since a test sample can be subjected to IMS after fast processing.
FIG. 4.
Effect of genomic DNA preparation methods on RAPD reproducibility. DNA was extracted by modified IMS-in vitro excystation (A) or the proteinase K-phenol method (B). Lanes: M, 1-kb DNA ladder, with arrowheads indicating bands (from top to bottom) of approximately 3.0, 1.6, 1.0, and 0.5 kb; 1 and 6, CP7265; 2 and 7, CP7278; 3 and 8, CP7284; 4 and 9, CP7375; 5 and 10, CP7380. Primers used were GPD62 (lanes 1 to 5) and GPD63 (lanes 6 to 10).
The optimal DNA template amount for AP-PCR was determined to be 0.5 to 1.0 ng, which was equivalent to 3 × 103 to 1 × 104 oocysts, comparable to results from other studies. The requirement of fewer organisms for the RAPD technique allows quick analysis of C. parvum isolates, especially when a clinical or environmental sample contains an insufficient number of oocysts for traditional characterization methods (typically 107 to 109 oocysts) (2, 9). This advantage becomes more significant when an outbreak is being studied, since timely analysis is important to take necessary precautions to prevent the spread of cryptosporidiosis.
Although a limited number of isolates were analyzed due to the unavailability of more diversified C. parvum isolates at the time, the study described herein demonstrates that the IMS-in vitro excystation procedure was efficient in specifically purifying C. parvum genomic DNA from environmental samples and that the RAPD technique using standard primers was useful in C. parvum isolate differentiation. We anticipate the further evaluation of this simplified RAPD technique, using more C. parvum isolates of various origins, and its use in investigating the epidemiology of a cryptosporidiosis outbreak or tracing environmental contamination sources. For example, since cattle have long been viewed as the leading source of waterborne cryptosporidiosis and grazing of cattle on watershed regions is considered to put humans at high risk of cryptosporidiosis, the application of the RAPD technique should help address these claims.
Acknowledgments
This study was supported by the Center for Food Animal Health, School of Veterinary Medicine, University of California, Davis.
We thank Teresa Wistrom for providing the estuary samples and Tadesse W. Mariam and Sophakhonterry Son for laboratory assistance.
REFERENCES
- 1.Campbell A T, Robertson L J, Smith H V. Viability of Cryptosporidium parvum oocysts: correlation of in vitro excystation with inclusion or exclusion of fluorogenic vital dyes. Appl Environ Microbiol. 1992;58:3488–3493. doi: 10.1128/aem.58.11.3488-3493.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carraway M, Tzipori S, Widmer G. Identification of genetic heterogeneity in the Cryptosporidium parvum ribosomal repeat. Appl Environ Microbiol. 1996;62:712–716. doi: 10.1128/aem.62.2.712-716.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Current W L, Garcia L S. Cryptosporidiosis. Clin Microbiol Rev. 1991;4:325–358. doi: 10.1128/cmr.4.3.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deng M Q, Cliver D O, Mariam T W. Immunomagnetic capture PCR to detect viable Cryptosporidium parvum oocysts from environmental samples. Appl Environ Microbiol. 1997;63:3134–3138. doi: 10.1128/aem.63.8.3134-3138.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupont H L, Chappell C L, Sterling C R, Okhuysen P C, Rose J B, Jakubowski W. The infectivity of Cryptosporidium parvum in healthy volunteers. N Engl J Med. 1995;332:855–859. doi: 10.1056/NEJM199503303321304. [DOI] [PubMed] [Google Scholar]
- 6.Fayer R, Speer C A, Dubey J P. General biology of Cryptosporidium. In: Dubey J P, Speer C A, Fayer R, editors. Cryptosporidiosis of man and animals. Boca Raton, Fla: CRC Press, Inc.; 1990. pp. 1–30. [Google Scholar]
- 7.Hadrys H, Balick M, Schierwater B. Applications of random amplified polymorphic DNA (RAPD) in molecular ecology. Mol Ecol. 1992;1:55–63. doi: 10.1111/j.1365-294x.1992.tb00155.x. [DOI] [PubMed] [Google Scholar]
- 8.Mead J R, Humphreys R C, Sammons D W, Sterling C R. Identification of isolate-specific sporozoite proteins of Cryptosporidium parvum by two-dimensional gel electrophoresis. Infect Immun. 1990;58:2071–2075. doi: 10.1128/iai.58.7.2071-2075.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan U M, Constantine C, O’Donoghue P J, Meloni B P, O’Brien P A, Thompson R C A. Molecular characterization of Cryptosporidium isolates from humans and other animals using RAPD (random amplified polymorphic DNA) analysis. Am J Trop Med Hyg. 1995;52:559–564. doi: 10.4269/ajtmh.1995.52.559. [DOI] [PubMed] [Google Scholar]
- 10.Nina J M S, McDonald V, Deer R M A, Wright S E, Dyson D A, Chiodini P L, McAdam K P W J. Comparative study of the antigenic composition of oocyst isolates of Cryptosporidium parvum from different hosts. Parasite Immunol (Oxford) 1992;14:227–232. doi: 10.1111/j.1365-3024.1992.tb00463.x. [DOI] [PubMed] [Google Scholar]
- 11.Ortega Y R, Sheehy R R, Cama V A, Oishi K K, Sterling C R. Restriction fragment length polymorphism analysis of Cryptosporidium parvum isolates of bovine and human origin. J Protozool. 1991;38:40s–41s. [PubMed] [Google Scholar]
- 12.Welsh J, Petersen P, McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990;18:7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams J G K, Hanafey M K, Rafalski J A, Tingey S V. Genetic analysis using random amplified polymorphic DNA markers. Methods Enzymol. 1993;218:704–740. doi: 10.1016/0076-6879(93)18053-f. [DOI] [PubMed] [Google Scholar]