Abstract
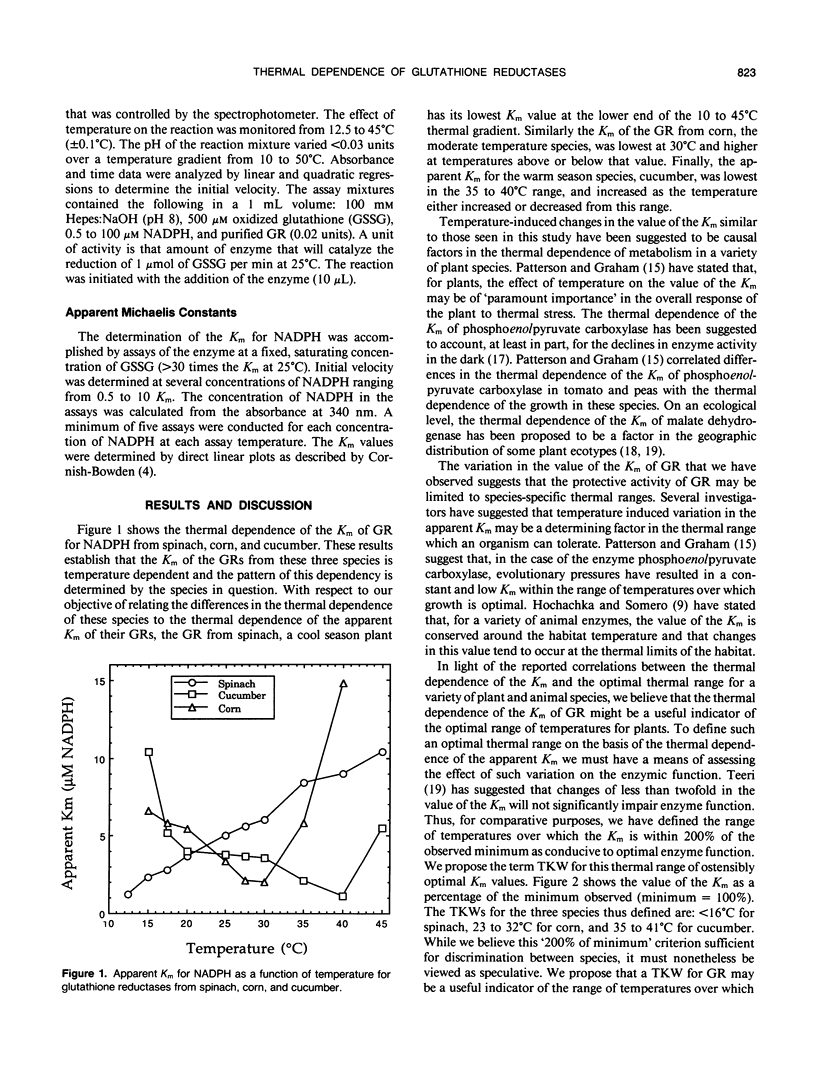
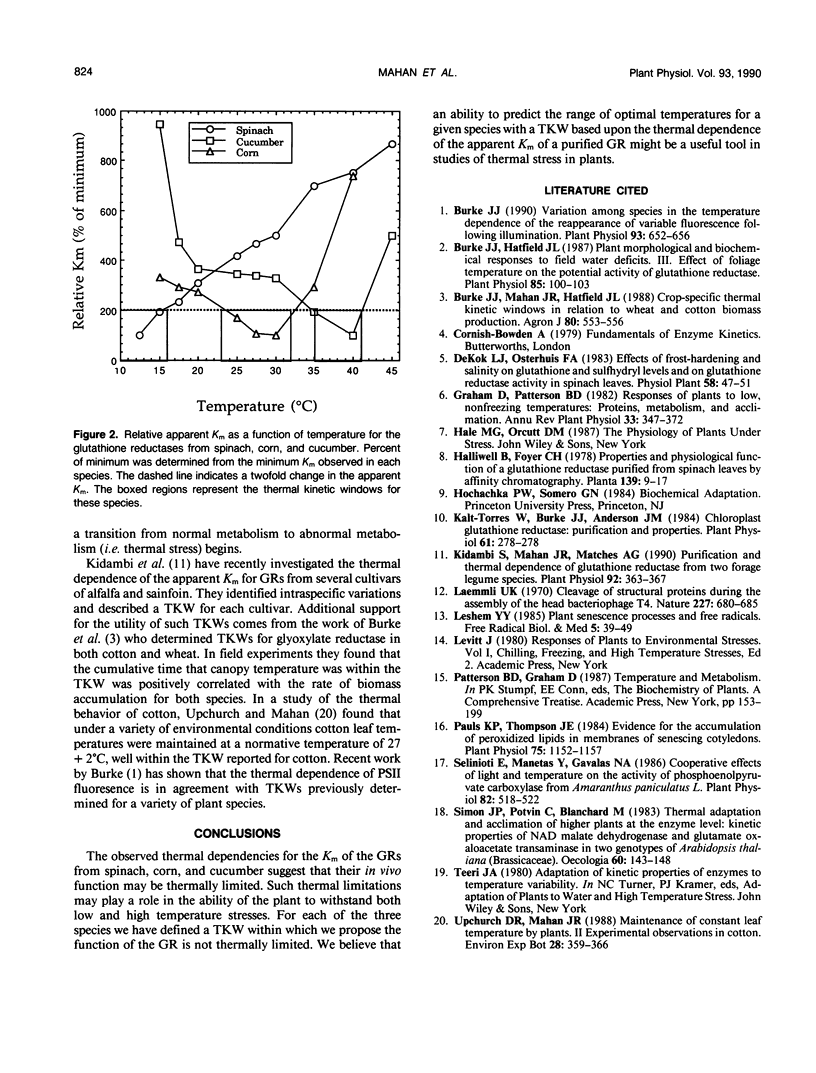
The thermal dependencies of the apparent Km of the glutathione reductases from spinach (Spinacia oleracea L.) corn (Zea mays L.), and cucumber (Cucumis sativus L.) were determined. The apparent Km of the enzymes were found to vary up to 9-fold between 12.5 and 45°C. Values of the apparent Km in excess of 200% of the observed minimum are suggested to be detrimental to the normal function of the enzyme. We propose the term “thermal kinetic window” to describe to the range of temperatures over which the apparent Km of the glutathione reductase is within 200% of its minimum and suggest that it may be a useful indicator of the limits of thermal stress for a given species. The thermal kinetic windows determined in this study are: <16°C for spinach, 23 to 32°C for corn, and 35 to 41°C for cucumber.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burke J. J., Hatfield J. L. Plant Morphological and Biochemical Responses to Field Water Deficits: III. Effect of Foliage Temperature on the Potential Activity of Glutathione Reductase. Plant Physiol. 1987 Sep;85(1):100–103. doi: 10.1104/pp.85.1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke J. J. Variation among Species in the Temperature Dependence of the Reappearance of Variable Fluorescence following Illumination. Plant Physiol. 1990 Jun;93(2):652–656. doi: 10.1104/pp.93.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidambi S. P., Mahan J. R., Matches A. G. Purification and thermal dependence of glutathione reductase from two forage legume species. Plant Physiol. 1990 Feb;92(2):363–367. doi: 10.1104/pp.92.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leshem Y. Y. Plant senescence processes and free radicals. Free Radic Biol Med. 1988;5(1):39–49. doi: 10.1016/0891-5849(88)90060-3. [DOI] [PubMed] [Google Scholar]
- Pauls K. P., Thompson J. E. Evidence for the accumulation of peroxidized lipids in membranes of senescing cotyledons. Plant Physiol. 1984 Aug;75(4):1152–1157. doi: 10.1104/pp.75.4.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selinioti E., Manetas Y., Gavalas N. A. Cooperative Effects of Light and Temperature on the Activity of Phosphoenolpyruvate Carboxylase from Amaranthus paniculatus L. Plant Physiol. 1986 Oct;82(2):518–522. doi: 10.1104/pp.82.2.518. [DOI] [PMC free article] [PubMed] [Google Scholar]


