Abstract
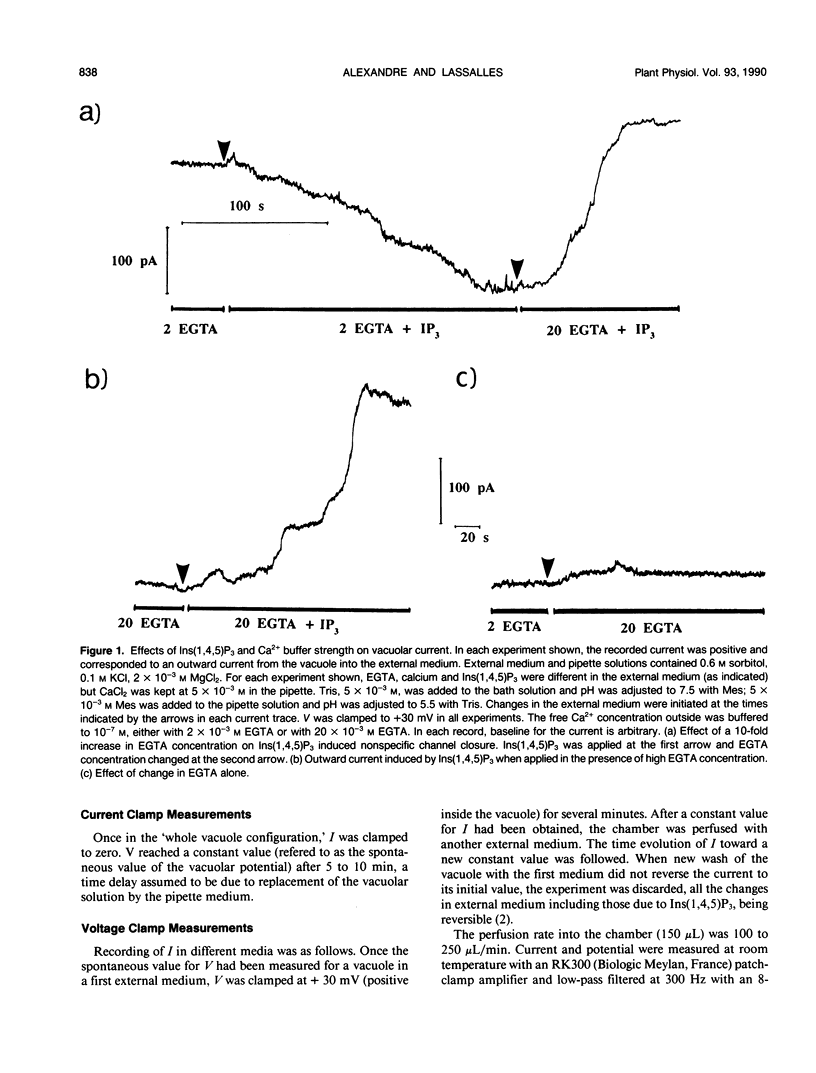
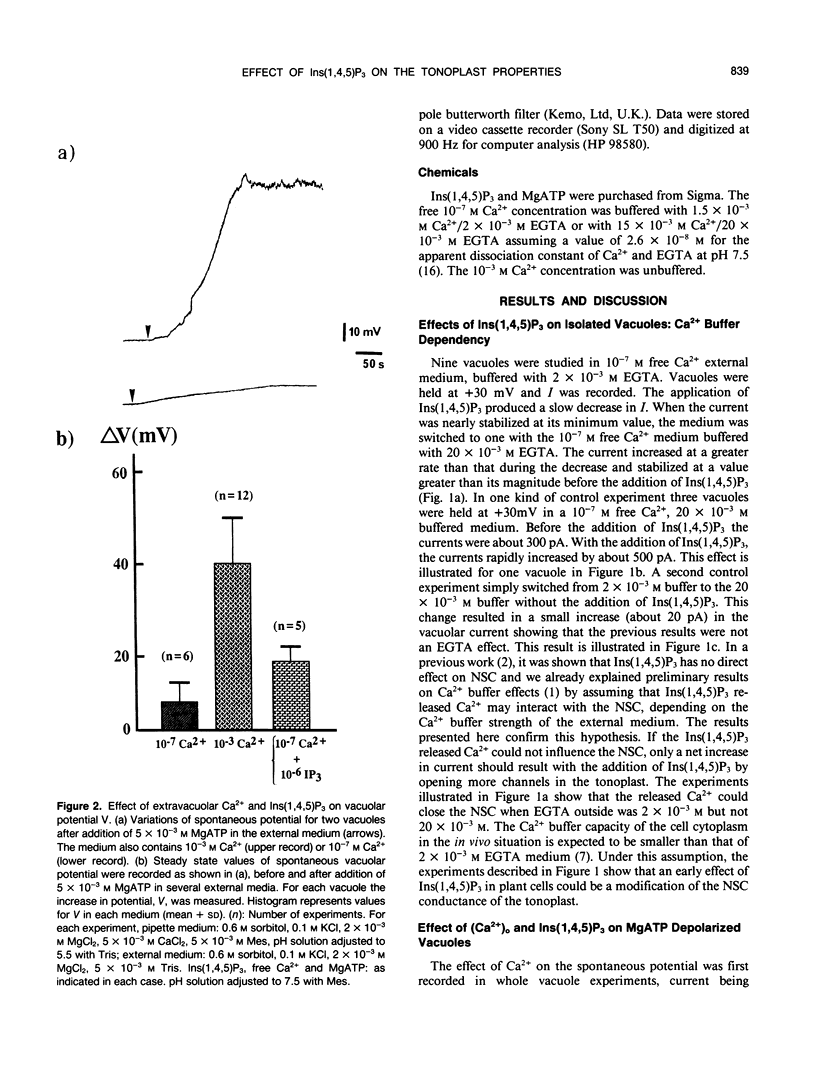
The effect of channel opening in the tonoplast by d-myo-inositol 1,4,5-trisphosphate [Ins(1,4,5)P3] has been examined on red beet (Beta vulgaris) vacuoles. Patch-clamp measurements of the vacuolar potential and current were performed on vacuoles isolated in 0.1 micromolar free Ca2+ medium. With vacuoles clamped at +30 millivolts, the Ins(1,4,5)P3 induced changes in current were depending on the Ca2+ buffer strength in the external medium. The spontaneous depolarization of vacuoles in which H+-pumps were activated by 5 millimolar MgATP was increased from +6 to +18 millivolts by 1 micromolar Ins(1,4,5)P3. We have interpreted our data by assuming that even with 2 millimolar EGTA to buffer Ca2+ at 0.1 micromolar in the external medium, Ins(1,4,5)P3 released enough Ca2+ from the vacuole to produce an accumulation of this ion near the tonoplast. Apart from their dependency with free Ca2+ in the cytoplasm, the electrical properties of the tonoplast could be depending on the Ins(1,4,5)P3 and Ca2+ buffer values in the cytoplasm.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Cornelius G., Gebauer G., Techel D. Inositol trisphosphate induces calcium release from Neurospora crassa vacuoles. Biochem Biophys Res Commun. 1989 Jul 31;162(2):852–856. doi: 10.1016/0006-291x(89)92388-7. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Time and calcium dependence of activation and inactivation of calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J Gen Physiol. 1985 Feb;85(2):247–289. doi: 10.1085/jgp.85.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A. Characterization of an anion-permeable channel from sugar beet vacuoles: effect of inhibitors. EMBO J. 1988 Dec 1;7(12):3661–3666. doi: 10.1002/j.1460-2075.1988.tb03247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R., Kurkdjian A., Guern J., Flügge U. I. Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J. 1989 Oct;8(10):2835–2841. doi: 10.1002/j.1460-2075.1989.tb08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumaker K. S., Sze H. Inositol 1,4,5-trisphosphate releases Ca2+ from vacuolar membrane vesicles of oat roots. J Biol Chem. 1987 Mar 25;262(9):3944–3946. [PubMed] [Google Scholar]
- Takagi S., Nagai R. Light-affected ca fluxes in protoplasts from vallisneria mesophyll cells. Plant Physiol. 1988 Sep;88(1):228–232. doi: 10.1104/pp.88.1.228. [DOI] [PMC free article] [PubMed] [Google Scholar]


