Abstract
Ethyl methanesulfonate (EMS) killed wild-type Bacillus subtilis spores as rapidly as spores lacking small, acid-soluble proteins (SASP) of the α/β type (α−β− spores), and 20% of the survivors had obvious mutations. A recA mutation increased the EMS sensitivity of wild-type and α−β− spores similarly but reduced their mutagenesis; EMS treatment of dormant spores also resulted in the induction of RecA synthesis during spore germination. EMS generated similar levels of alkylated bases in wild-type and α−β− spore DNAs, in purified DNA, or in DNA saturated with α/β-type SASP. Ethylene oxide (EtO) also generated similar levels of base alkylation in wild-type and α−β− spore DNAs. These data indicate that EMS and EtO kill spores at least in part by DNA damage but that α/β-type SASP, which protect DNA against many types of damage, do not protect spore DNA from base alkylation.
Spores of Bacillus species are much more resistant than their corresponding growing cells to a variety of treatments, including heat, UV radiation, and oxidizing agents (7, 11). A major factor contributing to spore resistance to these treatments is the saturation of spore DNA with a group of proteins termed small, acid-soluble proteins (SASP) of the α/β type (25, 26). These DNA binding proteins alter spore DNA UV photochemistry, thus contributing to spore resistance to UV radiation, and greatly slow DNA depurination as well as hydroxyl radical-induced DNA backbone cleavage, thus contributing to spore resistance to heat and oxidizing agents (25, 26). The effects of α/β-type SASP on DNA properties in spores generally are quite similar to the effects of purified α/β-type SASP on DNA properties in vitro (5, 12, 21). Studies of alkylation of DNA by dimethyl sulfate have indicated that α/β-type SASP do not significantly protect against this type of DNA damage in vitro (24). However, the effects of these proteins on DNA alkylation in spores have not been studied. Since DNA-alkylating agents, in particular ethylene oxide (EtO), are used for the sterilization of some types of materials (16, 17), we decided to examine the role of α/β-type SASP in the protection of DNA in spores against alkylation.
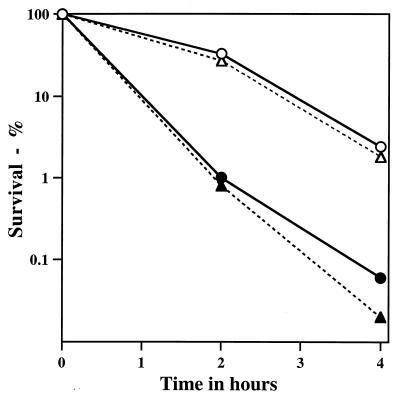
The alkylating agent we chose to use in most work was ethyl methanesulfonate (EMS), because of both its ease and its relative safety of use and the large amount of knowledge on its mechanism of action (1). The wild-type Bacillus subtilis strain (PS832) used for most experiments was a derivative of strain 168; the isogenic strain lacking the genes coding for the majority of spore α/β-type SASP was PS356 (12) (referred to as α−β−). Vegetative cells were prepared by growth at 37°C in 2×YT medium (21) to an optical density at 600 nm (OD600) of ∼1.0; spores of various strains were prepared at 37°C in 2×SG medium and purified as described previously (15). Incubation of vegetative cells at 30°C in 0.4 M KPO4 (pH 7.0) with 0.45 M EMS resulted in >99% killing in 5 min; similar incubation without EMS resulted in <50% killing (data not shown). In contrast, incubation of wild-type B. subtilis spores with EMS under these conditions resulted in only ∼93% killing in 15 h at 30°C (Table 1); even at 37°C there was only 98% killing in 4 h (Fig. 1). Removal of spore coats as described previously (21) had no obvious effect on spore EMS resistance (data not shown). Spores of strain PS356, which lack ∼75% of total α/β-type SASP and which are much more sensitive than wild-type spores to heat, UV radiation, and oxidizing agents (5, 12, 21), exhibited EMS resistance essentially identical to that of wild-type spores (Fig. 1).
TABLE 1.
Survival and mutagenesis of spores after treatment with alkylating agents
| Strain | Treatment | Survival (%) | % of survivors with the following mutation(s)c:
|
||
|---|---|---|---|---|---|
| aux | spo | aux spo | |||
| PS832 (wild type)a | —d | 100 | <0.5 | <0.5 | <0.5 |
| EMS | 3 | 2.9 | 11.5 | 4.8 | |
| PS356 (α−β−)a | — | 65 | <0.5 | <0.5 | <0.5 |
| EMS | 5 | 9.7 | 8.7 | 3.4 | |
| PS2318b (recA)a | — | 90 | <0.5 | <0.5 | <0.5 |
| EMS | 1 | <0.5 | <0.5 | <0.5 | |
| PS2319 (α−β−recA)a | — | 95 | <0.5 | 1 | <0.5 |
| EMS | 1.2 | <0.5 | 3 | <0.5 | |
| PS832b | — | 30 | 1 | 2.9 | 1.6 |
| EtO | 0.5 | 7.9 | 9.2 | 4.8 | |
Spores were incubated in 0.4 M KH2PO4 (pH 7.0) with or without 0.45 M EMS. Spores of strains PS832 and PS356 were incubated for 15 h at 30°C, and spores of strains PS2318b and PS2319 were incubated for 2 h at 37°C. In all cases, spores were diluted at least 200-fold into 2.4 ml of 0.1 M Na2S2O3 and then diluted further for analyses of survival on LB medium plates (5, 23). Survivors (at least 200) were picked onto minimal medium or sporulation medium plates to test for the presence of auxotrophic (aux) or sporulation (spo) mutations as described previously (5).
Spores of strain PS832 were treated with or without EtO for 5 min at 55°C and 60% relative humidity in a Joslyn EO Gas Biological Indicator Evaluator Resistometer Vessel (Joslyn Sterilization Corporation, Farmington, N.Y.).
The values for the percentage of survivors with the indicated mutations in a replicate of these experiments were essentially identical (±20%) to those shown here, and values for spores not treated with EMS were also essentially identical to values reported previously (5, 21).
—, no EMS treatment.
FIG. 1.
EMS resistance of wild-type and α−β− spores with or without a recA mutation. Spores of various strains were incubated at an OD600 of ∼1 and 37°C in 0.4 M KPO4 (pH 7.0)–0.45 M EMS. At various times, aliquots were diluted ∼100-fold in 2.4 ml of 0.1 M Na2S2O3 and then diluted further in 50 mM KPO4 (pH 7)–0.1 M NaCl prior to analysis of viable counts on LB medium plates (4). Symbols: ○, PS832 (wild type); ▵, PS356 (α−β−); •, PS2318 (recA); ▴, PS2319 (α−β− recA). Essentially identical results were obtained in a replicate experiment.
EMS killing of wild-type and α−β− spores occurred at least in part through DNA damage, as there was a high percentage of mutants among the survivors (Table 1), as observed previously (9, 14). A recA mutation decreased the EMS resistance of wild-type and α−β− spores by similar amounts (Fig. 1), and the percentage of mutants among the survivors of EMS treatment of recA spores, whether α−β− or otherwise wild type, decreased more than sevenfold (Table 1). A recA mutation also decreases the EMS resistance and mutagenesis of Escherichia coli, and these findings have been interpreted to indicate that some of the EMS resistance and much of the mutagenesis are due to the repair of alkylation damage by error-prone DNA repair, such as is induced during the SOS response (2, 6, 10, 19).
To obtain further evidence relative to this interpretation, we used spores of strain PS2271 (prepared as described above), which carry a recA-lacZ fusion but are otherwise wild type (23). Spores treated or not treated with EMS (2 h, 37°C) as described in Table 1 were germinated at 37°C to an initial OD600 of ∼0.5 to 0.6 in 25 ml of Spizizen minimal medium (28) with tryptophan (25 μg/ml) and Casamino Acids (0.1%) plus 4 mM l-alanine to stimulate spore germination. In this medium, >90% of spores had initiated germination after 30 min. l-[U-3H]leucine (10 μCi; 50 μCi/μmol) was also added to the medium to allow the measurement of protein accumulation during germination and outgrowth. At various times after the addition of spores to the medium, aliquots (500 μl) were removed for quantitation of the incorporation of 3H-leucine into protein (23). Samples (2 ml) were also harvested by centrifugation, and pellets were frozen for eventual assay of β-galactosidase with o-nitrophenyl-β-d-galactoside (23). The specific activity of β-galactosidase in the germinating and outgrowing spores was expressed as the ratio of the change in the OD420 per minute per milliliter of culture to the percentage of total leucine incorporated into protein per milliliter of culture (23).
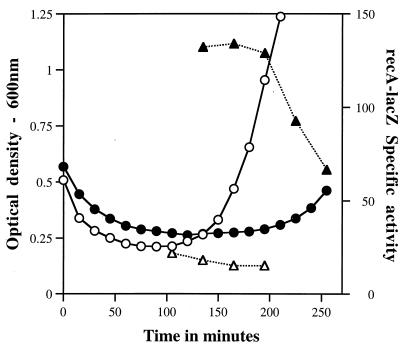
EMS treatment of dormant spores resulting in ∼50% killing caused no notable decrease in the rate or extent of subsequent initiation of spore germination, as measured by the initial reduction in the OD600 of the germinating spore culture (Fig. 2). However, this EMS treatment resulted in a large induction of recA-lacZ during subsequent spore germination (Fig. 2, compare specific activities with and without EMS treatment). Thus, EMS treatment of dormant spores almost certainly induces the SOS response during spore germination and outgrowth. Previous work showed that a number of other treatments of dormant B. subtilis spores that cause DNA damage result in the induction not only of recA but also of other DNA damage-inducible genes during subsequent spore germination and outgrowth (23). DNA repair has also been shown to be an important component of spore resistance to other agents that kill spores by causing DNA damage (23).
FIG. 2.
Level of expression of recA-lacZ during germination of spores with or without prior EMS treatment. Spores of strain PS2271 (recA-lacZ::amyE) were germinated as described in the text with or without prior treatment with EMS. This treatment resulted in ∼50% killing. At various times during spore germination, samples were taken for analysis of the level of recA-lacZ expression relative to protein accumulated during spore germination and outgrowth as described in the text. Symbols: ○ and •, OD600; ▵ and ▴, recA-lacZ specific activity; ○ and ▵, no EMS treatment; • and ▴, EMS treatment.
The data discussed above strongly suggested that EMS kills dormant spores at least in part by DNA damage and that α/β-type SASP play no role in the protection of spore DNA from this DNA damage. To test this latter point directly, we analyzed DNA from EMS-treated spores for alkylation damage. EMS can cause alkylation of DNA in a number of positions, including the N3 of adenine, the O6 and N7 of guanine, and the backbone phosphate, with by far the most abundant product being N7-ethylguanine (1). Treatment of DNA with 1 M piperidine at 90°C for 30 min cleaves DNA at sites of alkylguanines; consequently, such cleavage can be used as a test for the presence of these modified bases. For these analyses, we used spores of the wild-type (PS533) and α−β− (PS578) strains, which also carry plasmid pUB110, conferring kanamycin resistance. Spores of these strains were prepared as described above, and ∼8 mg (dry weight) was incubated at 30°C in 0.5 ml of 0.4 M KPO4 (pH 7.0)–0.45 M EMS for various times. To stop the reaction, 0.5 ml of 5% Na2S2O3 was added, and the spore pellet was harvested by centrifugation and washed twice with 1 ml of water. The final spore pellet was suspended in 0.5 ml of 50 mM Tris-HCl (pH 8)–1% sodium dodecyl sulfate–8 M urea–50 mM dithiothreitol–10 mM EDTA, incubated for 90 min at 37°C to remove spore coats, and washed extensively by centrifugation (5). The final spore pellet was suspended in 3.5 ml of Quiagen buffer B1 plus RNase as described by Qiagen Inc. (15a) for preparations of bacterial DNA, and DNA was purified on Qiagen columns according to the manufacturer’s instructions. Aliquots of the final DNA were dissolved in 10 mM Tris-HCl (pH 8)–1 mM EDTA or directly in 1 M piperidine. Samples in piperidine were incubated for 30 min at 90°C, and piperidine was removed by repeated lyophilization. Aliquots of the DNA (1 to 2 μg) were electrophoresed on agarose gels after denaturation with glyoxal (5), DNA was transferred to Hybond N membranes (Amersham), and pUB110 sequences were detected by hybridization (21).
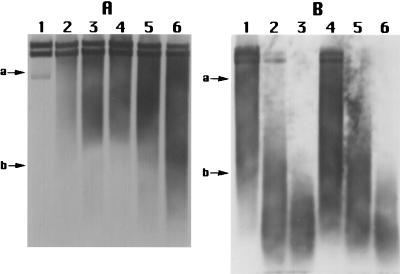
Analysis of DNA from spores treated with 0.45 M EMS at 30°C for up to 12 h did not reveal any obvious damage to either bulk chromosomal DNA or pUB110 when the spore DNA was analyzed without piperidine cleavage (data not shown). However, EMS treatment of spores for as little as 20 min resulted in an increase in piperidine-sensitive sites in pUB110, with the number of piperidine-sensitive sites increasing with increasing time of EMS treatment (Fig. 3). There was no obvious difference in the rate of generation of piperidine-sensitive sites in DNA upon EMS treatment of wild-type or α−β− spores (compare Fig. 3A and B). While piperidine treatment resulted in significant cleavage of DNA from EMS-treated spores, NaOH treatment (0.5 M, 1 h, 37°C) resulted in only minimal DNA cleavage (data not shown). Since the conditions used for NaOH treatment lead to DNA cleavage at alkyl phosphotriesters (27), the lack of NaOH cleavage of DNA from EMS-treated spores indicates that most of the piperidine-sensitive sites in this DNA were not alkyl phosphotriesters.
FIG. 3.
Analysis of piperidine-sensitive sites in plasmid pUB110 from EMS-treated wild-type and α−β− spores. Spores of strain PS533 (wild type) (A) and PS578 (α−β−) (B) were incubated at 30°C in 0.4 M KPO4 (pH 7) with (lanes 1, 2, 3, and 4) or without (lane 5) 0.45 M EMS. Spore DNA was isolated, treated with piperidine, denatured with glyoxal, and electrophoresed on agarose gels; the DNA was transferred to Hybond N membranes; and pUB110 sequences were detected as described in the text. Lanes: 1, 2, 3, and 4, incubated with EMS for 20 min, 90 min, 6 h, and 12 h, respectively; 5, incubated without EMS for 12 h. The positions of size markers of 3.5 and 1 kb are shown on the left. The two bands in the doublet seen at the top of the gel are circular pUB110 (upper band) and linear pUB110 (4.5 kb).
We also analyzed the effect of saturating levels of a purified α/β-type SASP, termed SspC, on the ability of EMS (50 mM in 20 mM KPO4 [pH 7.0], 8 h, 37°C) to generate piperidine-sensitive sites in plasmid pUC19 in vitro. Previous work has shown that in vitro saturating levels of SspC protect pUC19 DNA against damage caused by a number of agents (5, 21, 26). However, SspC resulted in no detectable protection against DNA ethylation in vitro (data not shown), confirming earlier work in which SspC did not protect the N7 of guanine in DNA against methylation in vitro (24).
As noted above, EMS also alkylates the phosphate backbone of DNA, forming phosphotriesters; these phosphotriesters are susceptible to alkaline cleavage (27). We might have expected α/β-type SASP to protect the DNA backbone against alkylation, as their binding protects the DNA backbone against many other types of damage (24). However, the level of all DNA phosphotriesters formed by EMS is generally <20% the level of alkylation at the N7 of guanine (1), and hot alkaline treatment only results in backbone cleavage at about one-third of total phosphotriesters (27). In addition, phosphotriesters are not thought to be mutagenesis-promoting lesions (18). Consequently, it appears likely that phosphotriester formation in DNA by EMS makes at most a small contribution to the deleterious effects of EMS on spores—even α−β− spores.
Initially, we had planned to carry out extensive analyses of the effect of α/β-type SASP on EtO alkylation of spore DNA, as EtO is both lethal and mutagenic for spores (16, 29). In initial work, we found that EtO killed spores at least in part by DNA damage, as there was a high level of mutants among the survivors of EtO treatment of wild-type spores (Table 1), although the mock-treated spores also underwent some mutagenesis. Unfortunately, the conditions used for EtO treatment, in particular the low relative humidity and elevated temperature, resulted in a very large degree of killing of the α−β− spores, even without EtO treatment (data not shown). This result was not totally unexpected, as previous work has shown that α−β− spores are quite sensitive to killing by dessication and dry heat (4, 22). However, the main DNA alkylation product with EtO is N7-hydroxyethylguanine (20), a modification that sensitizes the DNA backbone to piperidine cleavage. Consequently, we were able to assess the EtO-dependent generation of piperidine-sensitive sites in DNA from wild-type and α−β− spores carrying plasmid pUB110 essentially as described above for EMS treatment.
As found previously (4), dessication alone caused some fragmentation of the DNA in α−β− spores (Fig. 4A, lanes 1 and 4; DNA from untreated α−β− spores looked like that from dessicated wild-type spores) and resulted in significant (∼80%) killing. The mock EtO treatment also caused significant fragmentation of wild-type and α−β− spore DNAs and generated a large number of piperidine-sensitive sites in DNA (Fig. 4, lanes 2 and 5). This mock treatment killed <25% of wild-type spores but >99% of α−β− spores. Consequently, under these conditions, spore killing may not be due to the generation of piperidine-sensitive lesions in DNA. A 2-min EtO treatment which killed ∼95% of wild-type spores increased spore DNA fragmentation slightly (Fig. 4A, lanes 3 and 6) and also increased the number of piperidine-sensitive sites (Fig. 4B, lanes 3 and 6). However, the increase in the number of piperidine-sensitive sites generated by EtO treatment appeared similar in both wild-type and α−β− spores (Fig. 4B, compare lanes 2 and 3 and lanes 5 and 6). Thus, α/β-type SASP also did not block EtO alkylation of spore DNA.
FIG. 4.
DNA damage to wild-type and α−β− spores by EtO treatment. Spores of the wild-type (PS533) and α−β− (PS578) strains were lyophilized and subjected to EtO treatment (2 min, 55°C, 10% relative humidity) or a mock EtO treatment by use of the instrument described in Table 1, footnote b. DNA was isolated, denatured with glyoxal with or without prior piperidine treatment, and electrophoresed on agarose gels; the DNA was transferred to Hybond N membranes; and pUB110 sequences were detected as described in the text. (A) The samples were run without piperidine treatment. (B) The samples were run with piperidine treatment. The samples in lanes 1 to 3 are from spores of PS533 (wild type); those in lanes 4 to 6 are from spores of PS578 (α−β−). Samples in lanes 1 and 4 were only lyophilized and kept dry for ∼4 to 8 days; samples in lanes 2 and 5 were treated similarly but were subjected to mock EtO treatment; and samples in lanes 3 and 6 were subjected to EtO treatment. Arrows a and b denote the positions of 3.5- and 1-kb DNA size markers. Percentages of survival of the spores analyzed in the various lanes relative to untreated spores were as follows: 1, 100%; 2, 85%; 3, 4.5%; 4, 10%; 5, <0.05%; and 6, <0.05%.
The data in this communication allow a number of conclusions to be drawn about the killing of B. subtilis spores by alkylating agents. First, spores are much more resistant than cells to EMS. This result seems likely to be due largely to the very low permeability of spores to hydrophilic compounds (8). The barrier to these compounds seems likely to be a spore membrane, most likely the spore inner membrane, as decoated spores had essentially the same EMS resistance as untreated spores. In contrast, spores are often not more resistant than growing cells to small lipid-soluble gaseous disinfectants such as EtO (16). Second, the killing of spores by EMS and EtO appears to be due in large part to DNA damage, as evidenced by (i) the high degree of mutagenesis accompanying spore killing by these agents, (ii) the increased EMS sensitivity of recA spores, and (iii) the DNA damage accompanying spore killing. Third, the treatment of spores with EMS and EtO results in the generation of piperidine-sensitive sites in spore DNA. The major alkylation products generated in DNA by EMS and EtO are N7-alkylguanine residues, which are piperidine-sensitive lesions. However, alkylating agents generate a variety of other lesions in DNA, in particular O6-alkylguanine, which is an extremely mutagenic lesion in other systems (1, 20). If O6-ethylguanine is indeed the major mutagenesis-promoting lesion generated in spores by EMS, then the formation of this lesion is not slowed appreciably by the binding of α/β-type SASP to DNA. In other organisms, alkylation damage to DNA is repaired by alkyltransferase(s) and excision repair processes (2, 6, 19), and B. subtilis has both of these repair activities (13, 23). Excision repair, which can be error prone, does operate early in spore germination (23), but there is no information on whether alkyltransferases act in this period of development. The decreased survival and mutagenesis of recA spores following EMS treatment strongly suggest that some of the EMS-induced lesions in dormant spore DNA are repaired in an error-prone process during spore germination and outgrowth. Indeed, we found that EMS treatment of dormant spores induces the SOS response during spore germination, which can lead to error-prone repair (6). Presumably, during the germination of recA spores, EMS lesions are repaired only by a more error-free pathway, possibly via alkyltransferase(s) (2, 19). Fourth, the data in this communication clearly show that α/β-type SASP do not protect spore DNA from agents that attack either the O6 or the N7 of guanine. Clearly, the structure of the complex between α/β-type SASP and DNA does not hinder access to these two positions, which are both in the major groove of DNA, while at the same time blocking access to the DNA backbone and the glycosylic bond (5, 24) and altering DNA structure and DNA photochemistry (26). It is hoped that detailed analysis of the α/β-type SASP–DNA complex will clarify the mechanisms for these latter changes and the lack of effect of α/β-type SASP on the reactivity of DNA with alkylating agents.
Acknowledgments
This work was supported by a grant from the Army Research Office (to P.S.).
REFERENCES
- 1.Beranek D T. Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res. 1990;231:11–30. doi: 10.1016/0027-5107(90)90173-2. [DOI] [PubMed] [Google Scholar]
- 2.Couto L B, Chaudhuri I, Donahue B A, Demple B, Essingmann J M. Separation of SOS-dependent and SOS-independent components of alkylating agent mutagenesis. J Bacteriol. 1989;171:4170–4177. doi: 10.1128/jb.171.8.4170-4177.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dadd A H, Daley G M. Resistance of micro-organisms to inactivation by gaseous ethylene oxide. J Appl Bacteriol. 1980;49:89–91. doi: 10.1111/j.1365-2672.1980.tb01046.x. [DOI] [PubMed] [Google Scholar]
- 4.Fairhead H, Setlow B, Waites W M, Setlow P. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by freeze-drying. Appl Environ Microbiol. 1994;60:2647–2649. doi: 10.1128/aem.60.7.2647-2649.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fairhead H, Setlow B, Setlow P. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillus species. J Bacteriol. 1993;175:1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 7.Gerhardt P, Marquis R E. Spore thermoresistance mechanisms. In: Smith I, Slepecky R A, Setlow P, editors. Regulation of procaryotic development. Washington, D.C: American Society for Microbiology; 1989. pp. 43–63. [Google Scholar]
- 8.Gerhardt P, Scherrer R, Black S H. Molecular sieving by dormant spore structures. In: Halvorson H O, Hanson R, Campbell L L, editors. Spores V. Washington, D.C: American Society for Microbiology; 1972. pp. 68–74. [Google Scholar]
- 9.Ito J, Spizizen J. Increased rate of asporogenous mutations following treatment of Bacillus subtilis spores with ethyl methanesulfonate. Mutat Res. 1971;13:93–96. doi: 10.1016/0027-5107(71)90130-8. [DOI] [PubMed] [Google Scholar]
- 10.Kondo S, Ichikawa H, Iwo K, Kato T. Base-exchange mutagenesis and prophage induction in strains of Escherichia coli with different repair capacities. Genetics. 1970;66:187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marquis R E, Sim J, Shin S Y. Molecular mechanisms of resistance to heat and oxidative damage. J Appl Bacteriol. 1994;23:40S–48S. doi: 10.1111/j.1365-2672.1994.tb04356.x. [DOI] [PubMed] [Google Scholar]
- 12.Mason J M, Setlow P. Evidence for an essential role for small, acid-soluble spore proteins in the resistance of Bacillus subtilis spores to ultraviolet light. J Bacteriol. 1986;167:174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morohoshi F, Munakata N. Bacillus subtilis mutants deficient in the adaptive response to simple alkylating agents. J Bacteriol. 1985;161:825–830. doi: 10.1128/jb.161.3.825-830.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Necasek J, Pikalak P, Drobnik J. The mutagenic effect of prolonged treatment with ethyl methanesulfonate. Mutat Res. 1967;4:409–413. doi: 10.1016/0027-5107(67)90003-6. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson W L, Setlow P. Sporulation, germination and outgrowth. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons; 1990. pp. 391–450. [Google Scholar]
- 15a.Qiagen Inc. Genomic DNA handbook. Valencia, Calif: Qiagen Inc.; 1995. [Google Scholar]
- 16.Russell A D. The destruction of bacterial spores. London, England: Academic Press Ltd.; 1982. [Google Scholar]
- 17.Rutala W. Disinfection and sterilization of patient-care items. Infect Control Hosp Epidemiol. 1996;17:377–384. doi: 10.1086/647324. [DOI] [PubMed] [Google Scholar]
- 18.Saffhill R, Margison G P, O’Connor P J. Mechanisms of carcinogenesis induced by alkylating agents. Biochim Biophys Acta. 1985;823:111–145. doi: 10.1016/0304-419x(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 19.Schendel P F, Defais M. The role of umuC gene product in mutagenesis by simple alkylating agents. Mol Gen Genet. 1980;177:661–665. doi: 10.1007/BF00272677. [DOI] [PubMed] [Google Scholar]
- 20.Segerback D. Reaction products in hemoglobin and DNA after in vitro treatment with ethylene oxide and N-(2-hydroxyethyl)-N-nitrosourea. Carcinogenesis. 1990;11:307–312. doi: 10.1093/carcin/11.2.307. [DOI] [PubMed] [Google Scholar]
- 21.Setlow B, Setlow P. Binding of small, acid-soluble spore proteins to DNA plays a significant role in the resistance of Bacillus subtilis spores to hydrogen peroxide. Appl Environ Microbiol. 1993;59:3418–3423. doi: 10.1128/aem.59.10.3418-3423.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Setlow B, Setlow P. Small, acid-soluble proteins bound to DNA protect Bacillus subtilis spores from killing by dry heat. Appl Environ Microbiol. 1995;61:2787–2790. doi: 10.1128/aem.61.7.2787-2790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Setlow B, Setlow P. Role of DNA repair in Bacillus subtilis spore resistance. J Bacteriol. 1996;178:3486–3495. doi: 10.1128/jb.178.12.3486-3495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Setlow B, Sun D, Setlow P. Studies of the interaction between DNA and α/β-type small, acid-soluble spore proteins: a new class of DNA binding protein. J Bacteriol. 1992;174:2312–2322. doi: 10.1128/jb.174.7.2312-2322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Setlow P. Small. acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- 26.Setlow P. Mechanisms for the prevention of damage to the DNA in spores of Bacillus species. Annu Rev Microbiol. 1995;49:29–54. doi: 10.1146/annurev.mi.49.100195.000333. [DOI] [PubMed] [Google Scholar]
- 27.Shooter K V, Merrifeld R K. An assay for phosphotriester formation in the reaction of alkylating agents with deoxyribosenucleic acids in vitro and in vivo. Chem Biol Interact. 1976;13:223–236. doi: 10.1016/0009-2797(76)90076-4. [DOI] [PubMed] [Google Scholar]
- 28.Spizizen J. Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc Natl Acad Sci USA. 1958;44:1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanooka H. Application of Bacillus subtilis spores in the detection of gas mutagenesis: a case of ethylene oxide. Mutat Res. 1979;64:433–435. doi: 10.1016/0165-1161(79)90113-4. [DOI] [PubMed] [Google Scholar]