Abstract
Recently, updated guidelines for post-polypectomy surveillance have been published by the U.S. Multi‐Society Task Force (USMSTF), the British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England (BSG/ACPGBI/PHE), the European Society of Gastrointestinal Endoscopy (ESGE), the Japan Gastroenterological Endoscopy Society (JGES), and the Korean Multi-Society Taskforce Committee. This review summarizes and compares the updated recommendations of these 5 guidelines. There are some differences between the guidelines for the recommended post-polypectomy surveillance intervals. In particular, there are prominent differences between the guidelines for 1–4 tubular adenomas < 10 mm with low-grade dysplasia (nonadvanced adenomas [NAAs]) and tubulovillous or villous adenomas. The USMSTF, JGES, and Korean guidelines recommend colonoscopic surveillance for patients with 1–4 NAAs and those with tubulovillous or villous adenomas, whereas the BSG/ACPGBI/PHE and ESGE guidelines do not recommend endoscopic surveillance for such patients. Surveillance recommendations for patients with serrated polyps (SPs) are limited. Although the USMSTF guidelines provide specific recommendations for patients who have undergone SPs removal, these are weak and based on very lowquality evidence. Future studies should examine this topic to better guide the surveillance recommendations for patients with SPs. For countries that do not have separate guidelines, we hope that this review article will help select the most appropriate guidelines as per each country’s healthcare environment.
Keywords: Polypectomy, Surveillance, Guidelines, Colonoscopy, Colorectal polyp
INTRODUCTION
Adenomas and serrated polyps (SPs; sessile serrated lesion [SSL] and traditional serrated adenoma [TSA]) are precancerous lesions that can progress to colorectal cancer (CRC) [1-3]. Therefore, polypectomy through colonoscopy is the most effective method to reduce the incidence and mortality of CRC [4,5]. However, since patients who undergo colonoscopic polypectomy have an increased risk of developing colorectal polyps and CRC in the future, these patients require appropriate surveillance using colonoscopy after polypectomy.
The aim of the post-polypectomy surveillance guidelines is to determine the appropriate follow-up for patients based on the results of the index colonoscopy. Since it is important to determine the appropriate follow-up for patients with colorectal polyps, guidelines for surveillance after polypectomy have been established. These guidelines recommend surveillance colonoscopy at appropriate intervals for patients at a high risk of developing metachronous advanced colorectal neoplasia (ACRN) and minimize the surveillance colonoscopy burden for low-risk patients, allowing a balance between the risk of further development of colorectal neoplasia and the burden of colonoscopy.
As more studies on the risk of metachronous ACRN according to baseline polyp characteristics and new evidence on this topic, especially on the risk of metachronous CRC, are being reported, post-polypectomy surveillance guidelines have recently been updated by organizations in various countries. For example, in 2020, the U.S. Multi-Society Task Force (USMSTF) on CRC [6], British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England (BSG/ACPGBI/PHE) [7], and European Society of Gastrointestinal Endoscopy (ESGE) [8] published updated post-polypectomy surveillance guidelines. More recently, updated post-polypectomy surveillance guidelines were published by the Japan Gastroenterological Endoscopy Society (JGES) [9] in 2021 and by the Korean Multi-Society Taskforce Committee (the Korean Society of Gastroenterology, Korean Society of Gastrointestinal Endoscopy, Korean Association for the Study of Intestinal Diseases, and Korean Society of Abdominal Radiology jointly) [10] in 2022. It is now time to review these latest guidelines. This review summarizes and compares the recommendations of 5 recently updated guidelines. This review focuses on the recommendations themselves rather than on their evidence or background.
DEFINITIONS OF TERMS
The USMSTF guidelines classify the types of polyps as advanced adenoma (AA), advanced neoplasia, low-risk adenoma, and high-risk adenoma. In these guidelines, AA is defined as adenoma ≥ 10 mm or with tubulovillous/villous histology or high-grade dysplasia (HGD), and advanced neoplasia is defined as AA or CRC. Low‐risk adenomas are defined as 1–2 nonadvanced adenomas (NAAs) and high‐risk adenomas are defined as AAs or ≥ 3 adenomas. The USMSTF proposes that instead of using categories such as “high-risk adenoma” or “lowrisk adenoma,” studies specify individual criteria that are captured by the categories (e.g., use 3–4 adenomas < 10 mm instead of high-risk adenoma) because the evidence supporting the level of risk for the different criteria is constantly evolving.
The BSG/ACPGBI/PHE guidelines use the term “premalignant polyp,” and this term includes adenomas and SPs (excluding diminutive [1–5 mm] rectal hyperplastic polyps). In these guidelines, high-risk findings comprise either: ≥ 2 premalignant polyps including ≥ 1 advanced colorectal polyp, or ≥ 5 premalignant polyps. Advanced colorectal polyps are advanced adenomatous polyps or advanced SPs. An advanced adenomatous polyp refers to an adenoma measuring at least 10 mm in size or with HGD. Advanced SP refers to an SP measuring at least 10 mm in size or with dysplasia. Contrary to the USMSTF guidelines, the BSG/ACPGBI/PHE guidelines do not consider tubulovillous or villous histology as advanced adenomatous polyps.
The ESGE guidelines categorize polyps as those that require surveillance and those that do not. In these guidelines, polyps not requiring surveillance are defined as 1–4 adenomas < 10 mm in size with low‐grade dysplasia (LGD), irrespective of villous components or any SP < 10 mm without dysplasia, whereas polyps requiring surveillance are defined as adenoma ≥ 10 mm or with HGD or ≥ 5 adenomas, or any SP that is either with dysplasia or ≥ 10 mm.
Similar to the USMSTF guidelines, the JGES guidelines and the Korean guidelines define AAs as adenomas ≥ 10 mm or with HGD or tubulovillous/villous histology.
SURVEILLANCE OF CONVENTIONAL ADENOMA
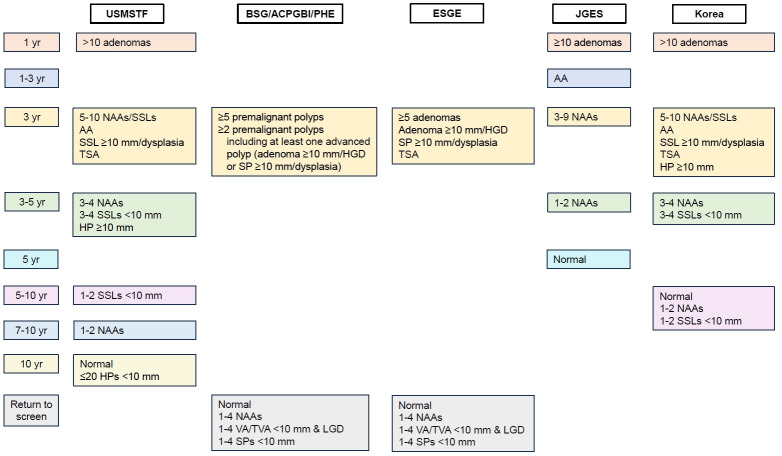
A summary and comparison of the updated surveillance recommendations for conventional adenomas from the 5 guidelines is presented in Table 1 and Fig. 1.
Table 1.
Comparison of Surveillance Interval Recommendations for Conventional Adenomas Provided in the 5 Guidelines
| Baseline colonoscopy finding | 2020 USMSTF | 2020 BSG/ACPGBI/PHE | 2020 ESGE | 2021JGES | 2022 Korea |
|---|---|---|---|---|---|
| 1–2 tubular adenomas < 10 mm | 7–10 yr | Return to screening | Return to screening | 3–5 yr | 5–10 yr |
| 3–4 tubular adenomas < 10 mm | 3–5 yr | Return to screening | Return to screening | 3 yr | 3–5 yr |
| 5–10 tubular adenomas < 10 mm | 3 yr | 3 yr | 3 yr | 3 yr | 3 yr |
| Adenoma ≥ 10 mm | 3 yr | 3 yra | 3 yr | 1–3 yr | 3 yr |
| Adenoma with high-grade dysplasia | 3 yr | 3 yra | 3 yr | 1–3 yr | 3 yr |
| Adenoma with villous histology < 10 mm | 3 yr | Return to screening | Return to screening | 1–3 yr | 3 yr |
| > 10 adenomas | 1 yr and consider genetic testing | Referred to BSG hereditary CRC guidelines (1–2 yr) | Genetic counseling | 1 yr | 1 yr and consider genetic testing |
| Piecemeal resection of adenoma > 20 mm | 6 mo | 2–6 mob | 3–6 mo | 6 mo | 6 mo |
The BSG/ACPGBI/PHE recommends surveillance after 3 years in the presence of 2 or more precancerous polyps.
The BSG/ACPGBI/PHE recommends checking the site once more 18 months after the original excision.
USMSTF, U.S. Multi-Society Task Force; BSG, British Society of Gastroenterology; ACPGBI, Association of Coloproctology of Great Britain and Ireland; PHE, Public Health England; ESGE, European Society of Gastrointestinal Endoscopy; JGES, Japan Gastroenterological Endoscopy Society; CRC, colorectal cancer.
Fig. 1.
Summary of main recommendations from the 5 guidelines. Advanced adenomas (AAs) refer to adenomas ≥10 mm in size or those with high-grade dysplasia or tubulovillous/villous histology. Nonadvanced adenomas (NAAs) refer to tubular adenomas <10 mm and with low-grade dysplasia. Serrated polyps (SPs) include hyperplastic polyps (HPs), sessile serrated lesions (SSLs), and traditional serrated adenomas (TSAs). USMSTF, U.S. Multi-Society Task Force; BSG, British Society of Gastroenterology; ACPGBI, Association of Coloproctology of Great Britain and Ireland; PHE, Public Health England; ESGE, European Society of Gastrointestinal Endoscopy; JGES, Japan Gastroenterological Endoscopy Society; HGD, high-grade dysplasia; VA, villous adenoma; TVA, tubulovillous adenoma; LGD, low-grade dysplasia.
1. Surveillance for 1–4 Tubular Adenomas <10 mm in Size and with LGD
In most guidelines, surveillance intervals for patients with tubular adenomas < 10 mm in size and with LGD (NAAs) were extended from those in previous guidelines. However, the recommended intervals for these lesions differ slightly among the guidelines. The USMSTF recommends intervals of 7–10 years and 3–5 years for patients with 1–2 and 3–4 NAAs, respectively. Compared to the 2012 USMSTF guidelines [11] which recommended 5–10 years and 3-year intervals for patients with 1–2 and 3–4 NAAs, the intervals were slightly extended to 7–10 years and 3–5 years, respectively. The shift to an extended interval is based on new studies confirming that individuals with low-risk adenomas are not at increased risk of CRC as well as ACRN during follow-up.
In contrast to the USMSTF guidelines, the BSG/ACPGBI/PHE and ESGE guidelines do not recommend endoscopic surveillance for patients with 1–4 adenomas < 10 mm in size with LGD. Instead, the BSG/ACPGBI/PHE and ESGE guidelines recommend that the patients return to screening programs (e.g., fecal immunochemical tests). The ESGE recommends performing colonoscopies after 10 years in countries without organized screening programs. The BSG/ACPGBI/PHE recommends colonoscopy after 5 or 10 years if the patient is more than 10 years younger than the national bowel screening lower age limit and has polyps but no high-risk findings. These recommendations are in contrast to the previous 2010 BSG/ACPGBI/PHE [12] and the 2013 ESGE guidelines [13], which recommended a 3-year surveillance interval for patients with 3–4 adenomas < 10 mm. The 2013 ESGE guidelines recommend a 3-year surveillance interval for patients with ≥ 3 adenomas.
Among the 5 guidelines, the JGES guidelines recommend the shortest interval for 1–4 NAAs. The JGES recommends intervals of 3–5 years and 3 years for patients with 1–2 and 3–4 NAAs, respectively. In contrast, the Korean guidelines recommend intervals of 5–10 years and 3–5 years for patients with 1–2 and 3–4 NAAs, respectively.
2. Surveillance for 5–10 Adenomas
All 5 guidelines consistently recommend a 3-year surveillance interval for patients with 5–10 adenomas.
3. Surveillance for >10 Adenomas
For patients with > 10 adenomas, the USMSTF, JGES, and Korean guidelines recommend surveillance colonoscopy after 1 year. Additionally, the USMSTF and Korean guidelines recommend genetic testing for these patients based on the absolute/ cumulative adenoma number, patient age, family history of CRC, and personal history of features related to polyposis. The ESGE also recommends genetic counseling for these patients. The BSG/ACPGBI/PHE recommends the BSG hereditary CRC guidelines for managing patients with 10 or more adenomas.
The BSG hereditary CRC guidelines published In 2020 suggest colonoscopic surveillance at 1- to 2-year intervals until 75 years of age for patients with ≥ 10 adenomas without MUTYH or APC gene mutations [14]. Furthermore, the BSG hereditary CRC guidelines suggest gene panel testing for patients aged < 60 years with ≥ 10 adenomas, ≥ 60 years with ≥ 20 adenomas, or those with ≥ 10 adenomas with a family history of polyposis or CRC [14].
4. Surveillance for Adenoma ≥10 mm or with HGD
All 5 guidelines agree that patients with adenoma ≥ 10 mm or with HGD require a surveillance colonoscopy after 3 years. However, for these patients, the BSG/ACPGVI/PHE recommends a 3-year surveillance interval only in patients with 2 or more premalignant polyps and not in those with a single premalignant polyp.
5. Surveillance for Adenoma with Tubulovillous/Villous Histology
The USMSTF and Korean guidelines recommend a 3-year surveillance interval for patients with adenomas < 10 mm with tubulovillous/villous histology. The JGES recommends surveillance intervals of 1–3 years for these patients. However, the BSG/ACPGVI/PHE and ESGE guidelines consider that patients with adenomas with tubulovillous/villous histology do not require surveillance.
The USMSTF, JGES, and Korean guidelines recommend a shorter surveillance interval for patients with adenomas containing tubulovillous/villous histology because several studies have reported that villous histology is a potential risk factor for ACRN on follow-up [15,16]. However, tubulovillous/villous histology is not included in the BSG/ACPGVI/PHE and ESGE guidelines based on recent studies showing that tubulovillous/villous histology was not significantly associated with long-term risk of CRC incidence or mortality [17-19], and given the high interobserver variability among pathologists in the assessment of villous architecture [20].
6. Piecemeal Resection of Adenoma >20 mm
As the risk of local recurrence increases after piecemeal resection of colorectal polyps [21], all guidelines recommend repeat endoscopies at short intervals. Recent studies have reported a higher risk of recurrence associated with piecemeal resection than en-bloc resection, especially for polyps > 20 mm [22,23]. The USMSTF, JGES, and Korean guidelines recommend conducting colonoscopic surveillance after 6 months for patients with piecemeal resection of adenomas > 20 mm. The ESGE recommends surveillance intervals of 3 to 6 months for these patients. Meanwhile, the BSG/ACPGVI/PHE recommends performing a site check 2–6 months and 18 months after piecemeal resection of adenomas > 20 mm.
SURVEILLANCE OF SP
A summary and comparison of the updated surveillance recommendations for SPs from the 5 guidelines is presented in Table 2 and Fig. 1.
Table 2.
Comparison of Surveillance Interval Recommendations for Serrated Polyps Provided in the 5 Guidelines
| Baseline colonoscopy finding | 2020 USMSTF | 2020 BSG/ACPGBI/PHE | 2020 ESGE | 2021 JGESc | 2022 Korea |
|---|---|---|---|---|---|
| ≤ 20 HPs in rectum or sigmoid colon < 10 mm or ≤ 20 HPs proximal to sigmoid colon < 10 mm | 10 yr | No recommendation | No recommendation | No recommendation | No recommendation |
| HP > 10 mm | 3–5 yr | No recommendation | No recommendation | No recommendation | 3 yrd |
| 1–2 SSLs < 10 mm | 5–10 yr | Return to screening | Return to screening | No recommendation | 5–10 yr |
| 3–4 SSLs < 10 mm | 3–5 yr | Return to screening | Return to screening | No recommendation | 3–5 yr |
| 5–10 SSLs < 10 mm | 3 yr | 3 yr | No recommendation | No recommendation | 3 yr |
| SSL ≥ 10 mm | 3 yr | 3 yra | 3 yr | No recommendation | 3 yr |
| SSL with dysplasia | 3 yr | 3 yra | 3 yr | No recommendation | 3 yr |
| TSA | 3 yr | 3 yra | 3 yr | No recommendation | 3 yr |
| Piecemeal resection of SSL > 20 mm | 6 mo | 2–6 mob | 3–6 mo | No recommendation | 6 mo |
| SPS | No recommendation | Referred to BSG hereditary CRC guidelines (1–2 yr) | No recommendation | 1 yr | No recommendation |
The BSG/ACPGBI/PHE recommends surveillance after 3 years in the presence of 2 or more precancerous polyps.
The BSG/ACPGBI/PHE recommends checking the site once more 18 months after the original excision.
The JGES proposes surveillance intervals of 3–5 years for SSL without considering size and number.
The Korean guidelines recommend a 3-year surveillance interval for serrated polyps ≥10 mm regardless of whether they are HPs or SSLs.
USMSTF, U.S. Multi-Society Task Force; BSG, British Society of Gastroenterology; ACPGBI, Association of Coloproctology of Great Britain and Ireland; PHE, Public Health England; ESGE, European Society of Gastrointestinal Endoscopy; JGES, Japan Gastroenterological Endoscopy Society; HP, hyperplastic polyp; SSL, sessile serrated lesion; TSA, traditional serrated adenoma; SPS, serrated polyposis syndrome; CRC, colorectal cancer.
1. SP Terminology
Colorectal carcinogenesis can occur via the serrated pathway in addition to the adenoma-carcinoma sequence [2,3]. SPs are considered to give rise to 15% of CRC via the serrated neoplastic pathway [3]. SP is an umbrella term that includes hyperplastic polyp (HP), SSL, and TSA [24]. HPs have little malignant potential, whereas SSLs and TSAs are known to be precursors of CRC [24]. Because HPs and SSLs have different malignancy risks, surveillance strategies for these 2 lesions may have to be different. Therefore, it is clinically important to differentiate these 2 types of lesions. The key feature that differentiates SSL from HP is their architectural distortion, which is most likely due to changes in the proliferative zone of the crypts [24]. The updated 2019 guidelines from the World Health Organization (WHO) proposed using the term “SSL” instead of “sessile serrated adenoma, sessile SP, or sessile serrated adenoma/polyp (SSA/P)” [24]. Previously, “SSA/P” was vaguely described histopathologically as an SP with overall distortion of normal architecture in 2 or 3 continuous crypts. Accordingly, in actual clinical practice, there has been a great interobserver variation among pathologists in distinguishing between HPs and SSA/Ps [25]. To reduce these variations, the updated 2019 WHO guidelines recommended the use of the term “SSL” and defined it as an SP with at least one unequivocal aberrant crypt. Although interobserver variation in discrimination between HP and SSL is expected to improve by adopting the one crypt rule, variations between pathologists are likely to persist [26,27]. This background has made it difficult to study the risk of metachronous ACRN after the resection of SPs; therefore, it remains challenging to determine the surveillance strategies for SPs.
2. Surveillance for HP
Except for the USMSTF guidelines, the remaining 4 guidelines do not provide detailed surveillance strategies for HPs because data regarding the risk of metachronous ACRN associated with HPs are lacking. In the USMSTF guidelines, patients with ≤ 20 HPs < 10 mm in size in the rectum or sigmoid colon are recommended to undergo CRC screening after 10 years with strong recommendation strength and moderate-quality evidence, while patients with ≤ 20 HPs < 10 mm in size proximal to the sigmoid colon are recommended to undergo repeat colonoscopy after 10 years with weak recommendation strength and low-quality evidence. For patients with HPs ≥ 10 mm, the USMSTF recommends surveillance colonoscopy after 3–5 years. The USMSTF specifically proposes a 3-year surveillance interval if there are concerns about consistency in the distinction between SSP and HP, complete resection, or bowel preparation, and a 5-year interval if there are fewer concerns about these 3 aspects. The Korean guidelines recommend a 3-year follow-up interval for SPs ≥ 10 mm regardless of whether they are HPs or SSLs. The BSG/ACPGVI/PHE, ESGE, and JGES do not provide specific recommendations for HPs.
3. Surveillance for SSL and TSA
In the 2020 USMSTF guidelines, the revised term “SSL” was not reflected, and the old term, “SSP,” was used. In this review, the “SSP” used in the USMSTF guidelines is referred to as “SSL.” Detailed surveillance guides for SSLs are provided by the USMSTF. The Korean guidelines for SSLs are the same as those of the USMSTF. Only these 2 guidelines considered the number of SSL < 10 mm. The USMSTF and Korean guidelines recommend surveillance colonoscopy after 5–10, 3–5, and 3 years for patients with 1–2, 3–4, and 5–10 SSLs < 10 mm, respectively. The recommended surveillance intervals for SSLs < 10 mm are similar to those for conventional adenomas < 10 mm.
Except for the JGES guidelines, the remaining 4 guidelines consistently regard SSLs ≥ 10 mm, SSLs with dysplasia, and TSAs as high-risk SPs and recommend surveillance colonoscopy after 3 years for patients with these lesions. However, the JGES proposes surveillance intervals of 3–5 years for SSL without considering their size and number. The recommended surveillance intervals for patients who undergo piecemeal resection of SSL > 20 mm are the same as those for patients who undergo piecemeal resection of adenomas > 20 mm.
In summary, most guidelines recommend similar surveillance intervals for SSLs and conventional adenomas. A recent meta-analysis of 11 studies with 1,079,315 patients supports these guidelines. This meta-analysis demonstrated that the risks of metachronous ACRN and CRC were very similar between patients with SSLs and those with conventional adenomas (odds ratio [95% confidence interval]: ACRN, 0.91 [0.23–3.63]; CRC, 1.11 [0.42–2.97]) [28].
Because data on the risk of metachronous ACRN associated with SPs are extremely limited, most surveillance recommendations for SPs are weak and based on very low-quality evidence. Nevertheless, the USMSTF and Korean guidelines, which provide detailed recommendations, are very helpful to clinicians.
4. Surveillance for Serrated Polyposis Syndrome
In patients with > 20 HPs, the possibility of serrated polyposis syndrome (SPS) should be considered. The 2019 updated WHO diagnostic criteria for SPS are as follows: ≥ 5 SPs proximal to the rectum, all ≥ 5 mm in size, with ≥ 2 being ≥ 10 mm in size or > 20 SPs of any size distributed throughout the colon, with ≥ 5 being proximal to the rectum [29]. The JGES recommends annual surveillance for patients with SPS. The BSG/ACPGBI/PHE guidelines refer to the BSG hereditary CRC guidelines for the management of SPS. The BSG hereditary CRC guidelines recommend colonoscopic surveillance every 1–2 years until the age of 75 years for patients with SPS [14]. This guideline also recommends that patients with SPS should undergo surveillance colonoscopy annually once all lesions > 5 mm in size in the colon have been removed, and the interval can be extended to 2 years if no polyps ≥ 10 mm in size are identified at subsequent surveillance examinations [14].
SURVEILLANCE FOR YOUNG ADULTS AGED <50 YEARS
Since the target age for screening colonoscopy is over 50 years, post-polypectomy surveillance guidelines have focused on those over 50 years of age. However, the 2022 USMSTF guidelines recommend lowering the starting age for CRC screening from 50 to 45 years [30]. This is because the incidence of CRC in young adults under the age of 50 has been rapidly increasing and the prevalence of ACRN in adults aged 45–49 years is similar to that in adults aged 50–59 years [30-32]. The percentage of young adults with colorectal adenoma is not negligible. Summarizing the results of several studies, the prevalence of colorectal neoplasia in adults aged 30–39 and 40–49 years is 8.0%–13.4% and 13.8%–28.1%, respectively [33-41]. Nonetheless, none of the 5 updated post-polypectomy surveillance guidelines provide specific recommendations for young adults under the age of 50.
A recent meta-analysis of 8 studies found a lower risk of metachronous ACRN in patients with sporadic adenomas aged < 50 years than in those aged ≥ 50 years (patients aged ≥ 50 years vs. < 50 years: odds ratio, 1.62; 95% confidence interval, 1.34–1.96) [42]. These results suggest that patients aged < 50 years with sporadic adenoma without the possibility of hereditary syndrome do not require more intensive surveillance. However, given the small number of studies included in this meta-analysis, it is difficult to conclude whether the surveillance interval can be extended to young patients. Until more evidence is accumulated on this issue, it seems reasonable to apply the current post-polypectomy surveillance guidelines to young patients aged < 50 years.
AGE TO STOP SURVEILLANCE
The age at which post-polypectomy surveillance is discontinued differs slightly according to the guidelines; however, in most cases, the recommended age is 75 to 80 years. The USMSTF notes that more research is needed to determine whether the potential CRC prevention and early detection benefits in individuals older than 75 years or those with multiple comorbidities outweigh the risks of colonoscopy-related adverse events. The BSG/ACPGVI/PHE also suggests that surveillance colonoscopy should not be performed in individuals aged > 75 years or in those with a life expectancy of less than 10 years. Similarly, the ESGE suggests discontinuing colonoscopic surveillance at the age of 80 years or earlier, if life expectancy is limited by comorbidities. The Korean guidelines state that surveillance colonoscopy should be performed in elderly patients based on the individual physician’s judgment, considering various factors such as the patient’s health condition and medical resources in each country rather than applying a uniform standard.
CONCLUSIONS
Though some recommendations for post-polypectomy surveillance are similar to the 5 guidelines, different surveillance intervals have been recommended for specific types of polyps. In particular, there are significant differences between the guidelines for 1–4 NAAs and tubulovillous or villous adenomas. The USMSTF, JGES, and Korean guidelines recommend colonoscopic surveillance for patients with 1–4 NAAs and those with tubulovillous or villous adenomas, whereas the BSG/ACPGBI/PHE and ESGE guidelines do not.
Although the detection rate of SPs continues to increase owing to the widespread use of high-definition colonoscopies and the development of image-enhanced endoscopic technology, surveillance strategies for SPs are not as definite as for conventional adenomas and are insufficient. As studies on the risk of metachronous ACRN according to the type, number, and size of SPs are sparse, some guidelines do not provide recommendations on the surveillance of patients with SPs. Although the USMSTF and Korean guidelines provide specific recommendations for surveillance intervals depending on the number and size of SSLs, these recommendations are based on low-quality evidence. To better guide surveillance recommendations for patients with SPs, more studies on outcomes after SP removal should be conducted.
Healthcare environments fundamentally differ among countries. For example, there can be considerable differences in terms of the cost and accessibility of colonoscopy, the number and skill of colonoscopists, health insurance coverage, and healthcare policies. It is optimal to adhere to the guidelines created in each country using their specific clinical data; however, in reality, it is difficult to create individual guidelines. For countries that do not have separate guidelines, we hope that this review article will help select the most appropriate guidelines as per their healthcare environment.
Footnotes
Funding Source
The author received no financial support for the research, authorship, and/or publication of this article.
Conflict of Interest
Jung YS is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Data Availability Statement
Not applicable.
Author Contributions
Writing and approval of the final manuscript: Jung YS.
REFERENCES
- 1.Rubio CA. Two intertwined compartments coexisting in sporadic conventional colon adenomas. Intest Res. 2021;19:12–20. doi: 10.5217/ir.2019.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim SY, Kim TI. Serrated neoplasia pathway as an alternative route of colorectal cancer carcinogenesis. Intest Res. 2018;16:358–365. doi: 10.5217/ir.2018.16.3.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nguyen LH, Goel A, Chung DC. Pathways of colorectal carcinogenesis. Gastroenterology. 2020;158:291–302. doi: 10.1053/j.gastro.2019.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hong SW, Byeon JS. Endoscopic diagnosis and treatment of early colorectal cancer. Intest Res. 2022;20:281–290. doi: 10.5217/ir.2021.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park CH, Yang DH, Kim JW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Intest Res. 2021;19:127–157. doi: 10.5217/ir.2020.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta S, Lieberman D, Anderson JC, et al. Recommendations for follow-up after colonoscopy and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2020;115:415–434. doi: 10.14309/ajg.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rutter MD, East J, Rees CJ, et al. British Society of Gastroenterology/Association of Coloproctology of Great Britain and Ireland/Public Health England post-polypectomy and post-colorectal cancer resection surveillance guidelines. Gut. 2020;69:201–223. doi: 10.1136/gutjnl-2019-319858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassan C, Antonelli G, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline: update 2020. Endoscopy. 2020;52:687–700. doi: 10.1055/a-1185-3109. [DOI] [PubMed] [Google Scholar]
- 9.Saito Y, Oka S, Kawamura T, et al. Colonoscopy screening and surveillance guidelines. Dig Endosc. 2021;33:486–519. doi: 10.1111/den.13972. [DOI] [PubMed] [Google Scholar]
- 10.Kim SY, Kwak MS, Yoon SM, et al. Korean Guidelines for Postpolypectomy Colonoscopic Surveillance: 2022 revised edition. Intest Res. 2023;21:20–42. doi: 10.5217/ir.2022.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman DA, Rex DK, Winawer SJ, Giardiello FM, Johnson DA, Levin TR. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Cairns SR, Scholefield JH, Steele RJ, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–689. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 13.Hassan C, Quintero E, Dumonceau JM, et al. Post-polypectomy colonoscopy surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2013;45:842–851. doi: 10.1055/s-0033-1344548. [DOI] [PubMed] [Google Scholar]
- 14.Monahan KJ, Bradshaw N, Dolwani S, et al. Guidelines for the management of hereditary colorectal cancer from the British Society of Gastroenterology (BSG)/Association of Coloproctology of Great Britain and Ireland (ACPGBI)/United Kingdom Cancer Genetics Group (UKCGG) Gut. 2020;69:411–444. doi: 10.1136/gutjnl-2019-319915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fairley KJ, Li J, Komar M, Steigerwalt N, Erlich P. Predicting the risk of recurrent adenoma and incident colorectal cancer based on findings of the baseline colonoscopy. Clin Transl Gastroenterol. 2014;5:e64. doi: 10.1038/ctg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology. 2013;144:1410–1418. doi: 10.1053/j.gastro.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Atkin W, Wooldrage K, Brenner A, et al. Adenoma surveillance and colorectal cancer incidence: a retrospective, multicentre, cohort study. Lancet Oncol. 2017;18:823–834. doi: 10.1016/S1470-2045(17)30187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkin W, Brenner A, Martin J, et al. The clinical effectiveness of different surveillance strategies to prevent colorectal cancer in people with intermediate-grade colorectal adenomas: a retrospective cohort analysis, and psychological and economic evaluations. Health Technol Assess. 2017;21:1–536. doi: 10.3310/hta21250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wieszczy P, Kaminski MF, Franczyk R, et al. Colorectal cancer incidence and mortality after removal of adenomas during screening colonoscopies. Gastroenterology. 2020;158:875–883. doi: 10.1053/j.gastro.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 20.Mahajan D, Downs-Kelly E, Liu X, et al. Reproducibility of the villous component and high-grade dysplasia in colorectal adenomas <1 cm: implications for endoscopic surveillance. Am J Surg Pathol. 2013;37:427–433. doi: 10.1097/PAS.0b013e31826cf50f. [DOI] [PubMed] [Google Scholar]
- 21.Belderbos TD, Leenders M, Moons LM, Siersema PD. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy. 2014;46:388–402. doi: 10.1055/s-0034-1364970. [DOI] [PubMed] [Google Scholar]
- 22.Rex KD, Vemulapalli KC, Rex DK. Recurrence rates after EMR of large sessile serrated polyps. Gastrointest Endosc. 2015;82:538–541. doi: 10.1016/j.gie.2015.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Pellise M, Burgess NG, Tutticci N, et al. Endoscopic mucosal resection for large serrated lesions in comparison with adenomas: a prospective multicentre study of 2000 lesions. Gut. 2017;66:644–653. doi: 10.1136/gutjnl-2015-310249. [DOI] [PubMed] [Google Scholar]
- 24.Crockett SD, Nagtegaal ID. Terminology, molecular features, epidemiology, and management of serrated colorectal neoplasia. Gastroenterology. 2019;157:949–966. doi: 10.1053/j.gastro.2019.06.041. [DOI] [PubMed] [Google Scholar]
- 25.Niv Y. Changing pathological diagnosis from hyperplastic polyp to sessile serrated adenoma: systematic review and metaanalysis. Eur J Gastroenterol Hepatol. 2017;29:1327–1331. doi: 10.1097/MEG.0000000000000994. [DOI] [PubMed] [Google Scholar]
- 26.Jaravaza DR, Rigby JM. Hyperplastic polyp or sessile serrated lesion? The contribution of serial sections to reclassification. Diagn Pathol. 2020;15:140. doi: 10.1186/s13000-020-01057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boylan KE, Kanth P, Delker D, et al. Three pathologic criteria for reproducible diagnosis of colonic sessile serrated lesion versus hyperplastic polyp. Hum Pathol. 2023;137:25–35. doi: 10.1016/j.humpath.2023.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung YS, Park JH, Park CH. Serrated polyps and the risk of metachronous colorectal advanced neoplasia: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2022;20:31–43. doi: 10.1016/j.cgh.2020.09.051. [DOI] [PubMed] [Google Scholar]
- 29.Mankaney G, Rouphael C, Burke CA. Serrated polyposis syndrome. Clin Gastroenterol Hepatol. 2020;18:777–779. doi: 10.1016/j.cgh.2019.09.006. [DOI] [PubMed] [Google Scholar]
- 30.Patel SG, May FP, Anderson JC, et al. Updates on age to start and stop colorectal cancer screening: recommendations from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2022;162:285–299. doi: 10.1053/j.gastro.2021.10.007. [DOI] [PubMed] [Google Scholar]
- 31.Bailey CE, Hu CY, You YN, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the United States, 1975-2010. JAMA Surg. 2015;150:17–22. doi: 10.1001/jamasurg.2014.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Siegel RL, Medhanie GA, Fedewa SA, Jemal A. State variation in early-onset colorectal cancer in the United States, 1995-2015. J Natl Cancer Inst. 2019;111:1104–1106. doi: 10.1093/jnci/djz098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byeon JS, Yang SK, Kim TI, et al. Colorectal neoplasm in asymptomatic Asians: a prospective multinational multicenter colonoscopy survey. Gastrointest Endosc. 2007;65:1015–1022. doi: 10.1016/j.gie.2006.12.065. [DOI] [PubMed] [Google Scholar]
- 34.Park HW, Byeon JS, Yang SK, et al. Colorectal neoplasm in asymptomatic average-risk Koreans: the KASID prospective multicenter colonoscopy survey. Gut Liver. 2009;3:35–40. doi: 10.5009/gnl.2009.3.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hong SN, Kim JH, Choe WH, et al. Prevalence and risk of colorectal neoplasms in asymptomatic, average-risk screenees 40 to 49 years of age. Gastrointest Endosc. 2010;72:480–489. doi: 10.1016/j.gie.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 36.Chung SJ, Kim YS, Yang SY, et al. Prevalence and risk of colorectal adenoma in asymptomatic Koreans aged 40-49 years undergoing screening colonoscopy. J Gastroenterol Hepatol. 2010;25:519–525. doi: 10.1111/j.1440-1746.2009.06147.x. [DOI] [PubMed] [Google Scholar]
- 37.Chang LC, Wu MS, Tu CH, Lee YC, Shun CT, Chiu HM. Metabolic syndrome and smoking may justify earlier colorectal cancer screening in men. Gastrointest Endosc. 2014;79:961–969. doi: 10.1016/j.gie.2013.11.035. [DOI] [PubMed] [Google Scholar]
- 38.Jung YS, Ryu S, Chang Y, et al. Risk factors for colorectal neoplasia in persons aged 30 to 39 years and 40 to 49 years. Gastrointest Endosc. 2015;81:637–645. doi: 10.1016/j.gie.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 39.Koo JE, Kim KJ, Park HW, et al. Prevalence and risk factors of advanced colorectal neoplasms in asymptomatic Korean people between 40 and 49years of age. J Gastroenterol Hepatol. 2017;32:98–105. doi: 10.1111/jgh.13454. [DOI] [PubMed] [Google Scholar]
- 40.Kim KO, Yang HJ, Cha JM, et al. Risks of colorectal advanced neoplasia in young adults versus those of screening colonoscopy in patients aged 50 to 54 years. J Gastroenterol Hepatol. 2017;32:1825–1831. doi: 10.1111/jgh.13798. [DOI] [PubMed] [Google Scholar]
- 41.Kim NH, Jung YS, Yang HJ, et al. Prevalence of and risk factors for colorectal neoplasia in asymptomatic young adults (20-39 years old) Clin Gastroenterol Hepatol. 2019;17:115–122. doi: 10.1016/j.cgh.2018.07.011. [DOI] [PubMed] [Google Scholar]
- 42.Jung YS, Park JH, Park CH. Comparison of risk of metachronous advanced colorectal neoplasia in patients with sporadic adenomas aged < 50 versus ≥ 50 years: a systematic review and meta-analysis. J Pers Med. 2021;11:120. doi: 10.3390/jpm11020120. [DOI] [PMC free article] [PubMed] [Google Scholar]