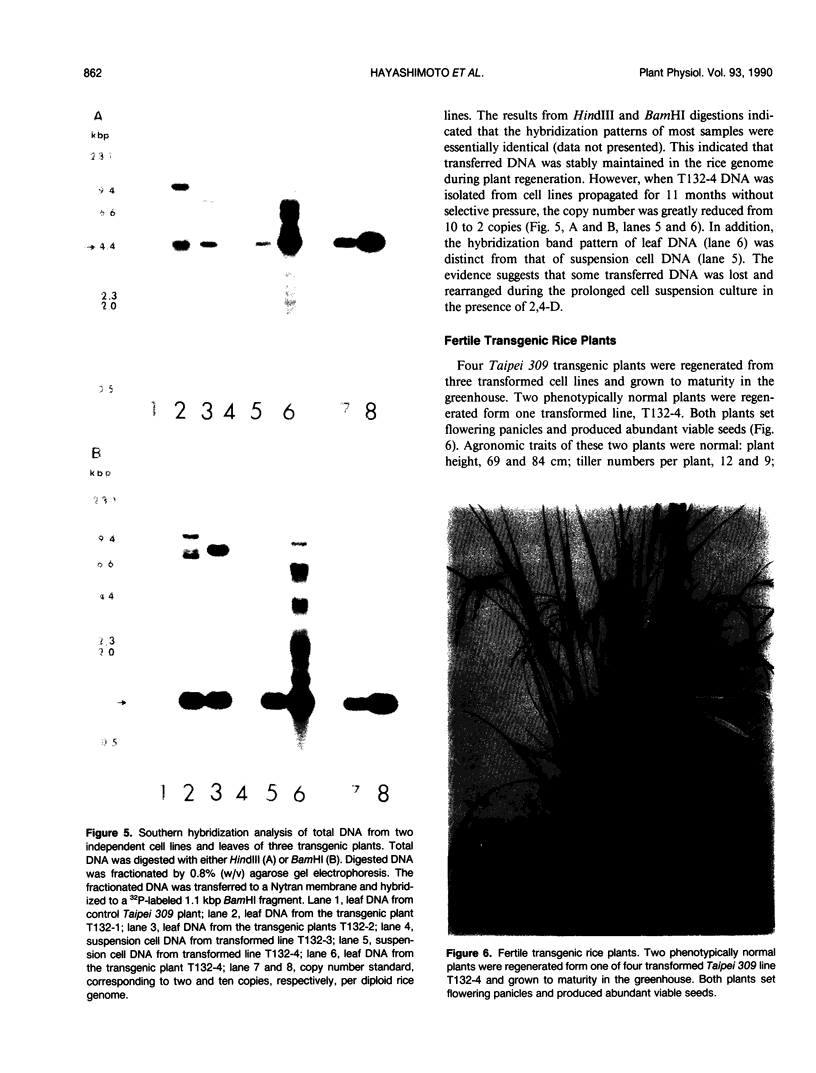
Abstract
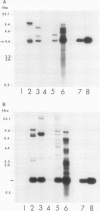
We have established an efficient procedure for protoplast transformation and regeneration of fertile transgenic plants of rice (Oryza sativa L.) cultivars Nipponbare and Taipei 309. Protoplasts were mixed with a plant-expressible hygromycin resistance gene and treated with 25% (w/v) polyethylene glycol. Stringent selection of transformed colonies was applied to 14-day-old regenerated protoplasts in the presence of 95 micromolar of hygromycin B for 12 days. After selection, 450 and 200 resistant colonies were recovered per million treated Taipei 309 and Nipponbare protoplasts, respectively. Southern hybridization analysis of hygromycin-resistant cell lines and regenerated plants indicated that 1 to 10 copies of transferred DNA were integrated at 1 to 4 loci of the rice genome. Southern DNA analysis suggests that the introduced plasmid DNA may form concatemers by intermolecular recombination prior to integration. Four Taipei 309 and 39 Nipponbare transgenic rice plants were regenerated and grown to maturity in the greenhouse. Two Taipei 309 and 35 Nipponbare plants set viable seeds. Agronomic traits of Taipei 309 transgenic plants and inheritance of the hygromycin resistance trait by progeny of the selfed transgenic plants were analyzed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gritz L., Davies J. Plasmid-encoded hygromycin B resistance: the sequence of hygromycin B phosphotransferase gene and its expression in Escherichia coli and Saccharomyces cerevisiae. Gene. 1983 Nov;25(2-3):179–188. doi: 10.1016/0378-1119(83)90223-8. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauptmann R. M., Vasil V., Ozias-Akins P., Tabaeizadeh Z., Rogers S. G., Fraley R. T., Horsch R. B., Vasil I. K. Evaluation of selectable markers for obtaining stable transformants in the gramineae. Plant Physiol. 1988 Feb;86(2):602–606. doi: 10.1104/pp.86.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirochika H., Takatsuji H., Ubasawa A., Ikeda J. E. Site-specific deletion in cauliflower mosaic virus DNA: possible involvement of RNA splicing and reverse transcription. EMBO J. 1985 Jul;4(7):1673–1680. doi: 10.1002/j.1460-2075.1985.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai N., Kemp J. D., Sutton D. W., Murray M. G., Slightom J. L., Merlo D. J., Reichert N. A., Sengupta-Gopalan C., Stock C. A., Barker R. F., Hall T. C. Phaseolin gene from bean is expressed after transfer to sunflower via tumor-inducing plasmid vectors. Science. 1983 Nov 4;222(4623):476–482. doi: 10.1126/science.222.4623.476. [DOI] [PubMed] [Google Scholar]
- Riggs C. D., Bates G. W. Stable transformation of tobacco by electroporation: evidence for plasmid concatenation. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5602–5606. doi: 10.1073/pnas.83.15.5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert L. S., Thompson R. D., Flavell R. B. Tissue-specific expression of a wheat high molecular weight glutenin gene in transgenic tobacco. Plant Cell. 1989 Jun;1(6):569–578. doi: 10.1105/tpc.1.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders P. R., Winter J. A., Barnason A. R., Rogers S. G., Fraley R. T. Comparison of cauliflower mosaic virus 35S and nopaline synthase promoters in transgenic plants. Nucleic Acids Res. 1987 Feb 25;15(4):1543–1558. doi: 10.1093/nar/15.4.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Weising K., Schell J., Kahl G. Foreign genes in plants: transfer, structure, expression, and applications. Annu Rev Genet. 1988;22:421–477. doi: 10.1146/annurev.ge.22.120188.002225. [DOI] [PubMed] [Google Scholar]