Abstract
Lowering oxygen tension in free-living Bradyrhizobium japonicum resulted in a dramatic switch of membrane chemistry in which phosphatidylcholine, the predominant lipid in aerated cultures, was no longer synthesized and phosphatidylethanolamine became the major lipid. Besides this change, phosphatidylinositol, a typical plant lipid rarely found in bacteria, was also synthesized.
The membrane and cell surface chemistry and antigenicity of Rhizobium species are dynamic and dependent on environmental factors such as oxygen tension and pH. Both chemical and immunochemical analyses support these facts (7, 9). We have been studying the effects of environmental conditions, such as oxygen tension and pH, on glycolipid biosynthesis in Rhizobium. These effects are likely to be very different once the bacterium enters the plant. We are especially interested in the effects of oxygen tension on membrane chemistry, since oxygen tension is critical to the physiology of the bacterium once it is inside the plant. Low oxygen concentrations trigger bacteroid differentiation in free-living cultures (7). Bacteroids are the nitrogen-fixing form of the bacterium when it resides inside the plant cell.
For these experiments, Bradyrhizobium japonicum USDA 110 was grown at 30°C in modified Bergensen’s medium as previously described (3). Low oxygen concentrations were obtained by stoppering 4-liter Erlenmeyer flasks containing 2 liters of culture media with butyl rubber stoppers at the time of inoculation to restrict oxygen exchange with the atmosphere. The concentration at the time of cell harvesting was typically between 500 and 1,000 ppm, as measured by an O2 electrode. Lipids were extracted and analyzed on thin-layer chromatography (TLC) silica plates with chloroform-acetone-methanol-acetic acid-water (10:4:2:1:1) as described earlier (2).
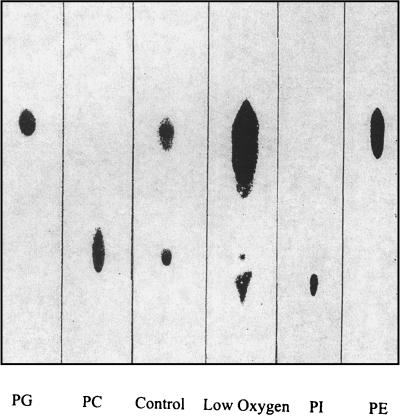
TLC indicated major differences in composition between lipids from bacteria grown under low-oxygen (microaerobic) conditions and those from bacteria grown under normal aeration (Fig. 1). Under low-oxygen conditions, there was a large increase in the amounts of phosphatidylglycerol and phosphatidylethanolamine and the amount of phosphatidylcholine synthesized was greatly reduced. The most striking difference between the TLC analyses of the two different lipid extracts was the presence of a slow-moving component in extracts from bacteria grown under microaerobic conditions. The retention time of this component was very similar to that of the phosphatidylinositol standard.
FIG. 1.
Orcinol-sprayed TLC plate of membrane lipid extracts from USDA 110 grown under both normal and low-oxygen conditions. Lanes: PG, phosphatidylglycerol; PC, phosphatidylcholine; Control, lipid extract of USDA 110 grown under normal conditions; Low Oxygen, lipid extract of USDA 110 grown under low-oxygen conditions; PI, phosphatidylinositol; PE, phosphatidylethanolamine. Note that under low-oxygen conditions, PC was no longer synthesized, but there was a marked increase in the amount of PE. Samples were normalized for total cell wet weight.
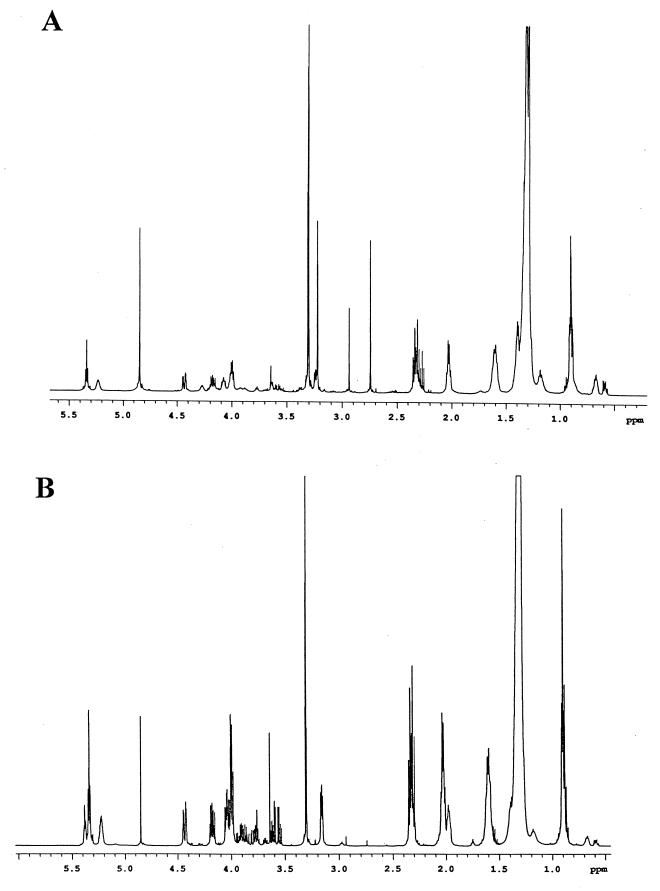
Proton nuclear magnetic resonance (NMR) spectroscopy (d-4-methanol, 30°C, 500 MHz) indicated that the major lipid species under normal aeration was phosphatidylcholine (Fig. 2A). This result was clear from the presence of strong signals at 3.23 ppm (the N-methyl groups). Also present in the spectrum were signals for phosphatidylethanolamine and the N-methyl derivatives of phosphatidylethanolamine. Signals for these N-methyl groups appeared as sharp singlets between 2.7 and 3.3 ppm.
FIG. 2.
(A) Proton NMR spectrum of the extracted membrane lipids of free-living USDA 110 grown under atmospheric oxygen. Signals between 2.8 and 4.8 ppm are due to carbohydrates and glyceryl components. The other signals between 0.5 and 2.4 ppm are due to resonances from the fatty acids. The strong resonances at 3.30 and 4.86 ppm are from residual CHD2OD and OH signals, respectively, of the solvent. (B) Spectrum of the membrane lipids of free-living USDA 110 grown under low-oxygen conditions. The signals at 3.23 and 4.27 ppm which correspond to phosphatidylcholine are gone. There are also a decrease in the peak intensity at 2.7 and 2.93 ppm (other N-methyl groups) and an increase in the peak intensity at 3.18 and 4.07 ppm (phosphatidylethanolamine). Note the appearance of new signals between 3.5 and 4.0 ppm.
The proton NMR spectrum (same conditions) of the total lipid extract from bacteria grown under microaerobic conditions was quite different (Fig. 2B). Changes observed in the latter were the disappearance of signals corresponding to phosphatidylcholine, a large decrease in the peak intensity for the N-methyl groups of monomethyl- and dimethylphosphatidylethanolamine, a large increase in the peak intensity corresponding to phosphatidylethanolamine at 3.18 and 4.07 ppm, and the appearance of new signals between 3.5 and 4.0 ppm. These new signals between 3.5 and 4.0 ppm were characteristic of a carbohydrate residue; however, no signal could be assigned to an anomeric proton, indicating that an inositol group was present, as indicated by the TLC analysis.
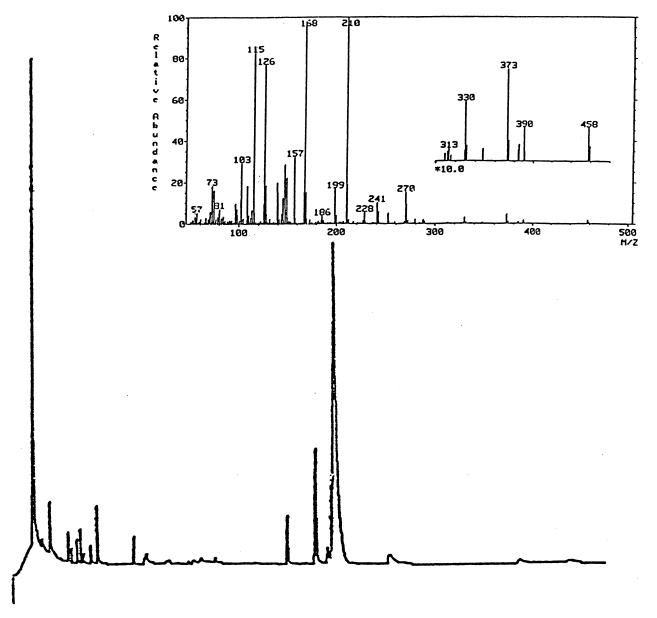
Inositol was conclusively identified by gas chromatography (GC) and GC-mass spectrometry (MS) analyses (JEOL 505 mass spectrometer with an accelerating voltage of 70 eV) of the lipid headspace group after hydrolysis (2 M trifluoroacetic acid, 120°C, 2 h) and peracetylation (acetic anhydride-pyridine, 24 h, room temperature). Separation was effected with a J and W DB225 column. The initial temperature was 180°C, and the temperature was raised at the rate of 2°C/min to a final temperature of 220°C. The column was kept at 220°C for 50 min. GC analysis (Fig. 3) showed that the predominant glycosyl component of the membrane lipid of bacteria grown under microanaerobic conditions had the same retention time as the peracetate of the inositol standard. The mass spectrum (Fig. 3, inset) was also the same as that of the standard.
FIG. 3.
Gas chromatograph of alditol acetate derivatives of a lipid extract from B. japonicum USDA 110 grown under microanaerobic conditions. The predominant peak had the same retention time as standard myo-inositol. The inset shows the mass spectrum.
At least three previous studies (1, 4, 8) have been conducted on the membrane lipid composition of free-living B. japonicum. Although there was much agreement on the presence of phosphatidylcholine and phosphatidylethanolamine, there were still some conflicts with respect to the presence or absence of phosphatidylglycerol, phosphatidylinositol, and phosphatidylserine. However, these discrepancies could have arisen from differences in growth medium (nutrients) and/or aeration (oxygen tension) and therefore could have reflected the sensitivity of the bacteria to these factors. In unsparged liquid cultures, the extent of aeration is determined by the geometry of the culture flask and the speed of shaking, conditions that are not very standardized. N-Methyl derivatives of phosphatidylethanolamine and phosphatidylglycerol are not easily resolved by TLC, although they can be easily identified by NMR or MS. This fact may explain why in the previous studies (1, 4) phosphatidylglycerol or N-methyl derivatives of phosphatidylethanolamine were not detected.
It is of interest that B. japonicum synthesizes large amounts of inositol-containing lipids in low-oxygen growth media because phosphatidylinositol is rarely found in gram-negative bacteria (5). It is one of the major phospholipids of plant membranes and may comprise up to 19% of the total phospholipid of the plasma membrane (6). Its presence in B. japonicum is certainly noteworthy, since it provides further evidence of the uniqueness of rhizobial membrane chemistry among bacteria and the close similarity of the latter to plant membrane chemistry.
Acknowledgments
This work was supported by grant DE-FG02-89ER 14029 from the U.S. Department of Energy to R.I.H. The GC-MS and fast atom bombardment mass spectral data were obtained at the Michigan State University Mass Spectrometry Facility, which is supported in part by grant DRR-00480 from the Biotechnology Research Technology Program, National Center for Research Resources, NIH. The NMR data were obtained with instruments purchased in part with funds from NIH grant 1-S10-RR04750, NSF grant CHE-88-00770, and NSF grant CHE-92-13241.
REFERENCES
- 1.Bunn C R, Elkan G H. The phospholipid composition of Rhizobium japonicum. Can J Microbiol. 1970;17:291–295. doi: 10.1139/m71-048. [DOI] [PubMed] [Google Scholar]
- 2.Cedergren R A, Hollingsworth R I. Occurrence of sufoquinovosyl diacylglycerol in some members of the family Rhizobiaceae. J Lipid Res. 1994;35:1452–1461. [PubMed] [Google Scholar]
- 3.Dazzo F B. Leguminous root nodules. In: Burns R G, Slater J H, editors. Experimental microbial ecology. Oxford, England: Blackwell Scientific Publications Ltd.; 1982. pp. 431–446. [Google Scholar]
- 4.Gerson T, Patel J J. Neutral lipids and phospholipids of free-living and bacteroid forms of two strains of Rhizobium infection on Lotus pedunculatus. Appl Microbiol. 1975;30:193–198. doi: 10.1128/am.30.2.193-198.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldfine H. Lipids of prokaryotes—structure and distribution. Curr Top Membr Transp. 1982;17:2–44. [Google Scholar]
- 6.Hetherington A M, Drobak B K. Inositol-containing lipids in higher plants. Prog Lipid Res. 1992;21:53–63. doi: 10.1016/0163-7827(92)90015-b. [DOI] [PubMed] [Google Scholar]
- 7.Kannenberg E L, Brewin N J. Expression of a cell surface antigen from Rhizobium leguminosarum 3841 is regulated by oxygen and pH. J Bacteriol. 1989;171:4543–4548. doi: 10.1128/jb.171.9.4543-4548.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller K J, Shon B C, Gore R S, Hunt W P. The phospholipid composition of Bradyrhizobium spp. Curr Microbiol. 1990;21:205–210. [Google Scholar]
- 9.Tao H, Brewin N J, Noel K D. Rhizobium leguminosarum CFN42 lipopolysaccharide antigenic changes induced by environmental conditions. J Bacteriol. 1992;174:2222–2229. doi: 10.1128/jb.174.7.2222-2229.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]