Abstract
Background
Surgery for adult spinal deformity (ASD) often involves long-segment posterior instrumentation that introduces stress at the proximal junction that can result in proximal junctional kyphosis (PJK) or proximal junctional failure (PJF). Recently, the use of tethers at the proximal junction has been proposed as a means of buffering the transitional stresses and reducing the risk of PJK/PJF. Our objectives are to summarize the clinical literature on proximal junctional tethers for PJK/PJF prophylaxis.
Methods
Articles published between 1 January 2000 and 10 November 2022 were identified via a PubMed search using combinations of the search terms “spine surgery,” “ASD,” “complication,” “surgery,” “PJK,” “PJF,” “tether,” “sublaminar band,” and “prophylaxis.” No restrictions were placed on the number of patients, surgical indications, or surgical procedures. Relevant articles were reviewed and summarized.
Results
Fifteen articles were identified, including 2 prospective cohorts (Level II), 10 retrospective cohorts (Level III), and 3 retrospective case series (Level IV). All studies were published between 2016 and 2022, and all focused on ASD patient populations. The mean age in each study ranged from 55 to 69 years, and most studies had a mean follow-up of at least 12 months (range, 5.5–45.4 months). Eleven studies used a polyethylene tether, 2 used soft sublaminar cables, and 2 used semitendinous allograft. The tether extended to the UIV+1 or UIV+2, passing either through or around the spinous processes, in 13 studies. In the remaining 2 studies, the tether was passed sublaminar at the UIV+1. Fourteen studies favored the use of tethers with regard to reduction of PJK/PJF rates, and one demonstrated similar rates of PJK between the tether and no-tether groups.
Conclusions
PJK/PJF remain major challenges in ASD surgery. Most early studies suggest that the use of tethers for ligamentous augmentation may help to mitigate the development of PJK/PJF. However, the multifactorial etiology of PJK/PJF makes it unlikely that any single technique will solve this complex problem. Further study is needed to address not only the effectiveness of junctional tethers but also to clarify whether there are optimal tether configurations, tether materials, and tether tension.
Level Evidence
3.
Keywords: adult spinal deformity, complications, ligamentous support, proximal junctional failure, proximal junctional kyphosis, sublaminar band, surgery, tether
Introduction
Significant advances in spinal instrumentation and techniques, along with improvements in anesthesia and critical care, have enabled spine surgeons to surgically treat a broad range of adult spinal deformity (ASD) patients who may have been considered inoperable just a few decades ago.1 Although multiple studies have shown the potential of modern surgical treatments to significantly improve pain, function, and quality of life in ASD patients, these procedures continue to have high complication rates.2–8
ASD surgery often necessitates long-segment posterior instrumentation that inherently introduces significant stress at the proximal termination that can produce a range of effects. The most common change at the proximal junction is the development of kyphosis, which is termed proximal junctional kyphosis (PJK) and can range from mild to severe. One of the earliest descriptions of PJK was from Glattes and colleagues.9 They defined PJK based on 2 criteria: (1) proximal junctional sagittal Cobb angle >+10° and (2) proximal junction sagittal Cobb angle at least 10° greater than the preoperative measurement. This definition, which continues to be the most commonly applied, has resulted in reported rates of PJK ranging from 17% to 61.7%.8,10,11 However, clinical implications based on this definition of PJK have been limited.12
The term proximal junctional failure (PJF) has been proposed to describe clinically significant junctional pathology.8,11,13 Patients with PJF exhibit more significant junctional kyphosis, fracture of the upper-most instrumented vertebrae (UIV) or UIV+1, disruption of the posterior ligaments, or failure of the UIV fixation/instrumentation. PJF has a reported incidence rate of 1.4% to 28.8%8,11,14 and frequently requires extensive revision surgery due to pain, disability, and neurological deficit.8,11,13
PJF remains one of the greatest unsolved problems in ASD surgery, prompting the development of a variety of techniques intended to provide junctional stability, including vertebroplasty at the junctional levels, use of hooks at the UIV, use of transitional rods or rods with reduced stiffness near the junction, and application of minimally invasive techniques for screw placement at the UIV to minimize soft tissue disruption.8,15–17 Recently, the use of tethers at the proximal junction has been proposed as a means of buffering the transitional stresses and reducing the risk of PJK/PJF. Multiple biomechanical studies support the potential benefits of junctional tethers,15,18–26 and several studies focused on the clinical application of these tethers have been published. Our objectives in the present review were to summarize the clinical literature on proximal junctional tethers for PJK/PJF prophylaxis and to describe the various reported tether techniques.
Methods
Relevant articles were identified by searching PubMed for articles published between 1 January 2000 through 10 November 2022 using combinations of the search terms “spine surgery,” “ASD,” “complication,” “surgery,” “PJK,” “PJF,” “tether,” “sublaminar band,” and “prophylaxis.” Titles and abstracts of the articles were reviewed to identify studies focused on clinical application of proximal junctional tethers used in combination with posterior spinal instrumentation for PJK/PJF prophylaxis in adult patients (age >18 years). No restrictions were placed on the number of patients, specific surgical indications, or other surgical procedures (eg, use of osteotomies or pelvic fixation). In addition, no restrictions were placed on the type of tether material or technique used. All relevant articles were reviewed, and the clinical outcomes, including rates of PJK/PJF and revision surgery for junctional failure, and tether techniques, were summarized.
Results
Fifteen articles meeting the criteria were identified (Table 1), including 2 prospective cohorts (Level II), 10 retrospective cohorts (Level III), and 3 retrospective case series (Level IV). All studies were published between 2016 and 2022, and all focused on ASD patient populations. The number of patients in each study ranged from 4 to 625, and the mean age ranged from 55 to 69 years. Most studies had a mean follow-up of at least 12 months.
Table 1.
Summary of proximal juntional tether studies include in the present review.
| Study | Study Design | Level of Evidence | Patients, n | Patient Population | Mean Age, y | Mean Follow-Up, mo | Tether | Clinical Outcome |
| Alluriu et al27 | Retrospective cohort | III | 83 | ASD patients | 64 | 20.3 | Semitendinous allograft | PJK present in 33% (16/49) of patients in tether group and 32% (11/34) of patients in control group (P = 0.31); PJF occurred in 18% (6/34) in control group but did not occur in tether group (P = 0.01) |
| Buell et al28 | Retrospective cohort | III | 120 | ASD patients with instrumentation at >6 motion segments without transitional rods or hooks at UIV; all had lower-thoracic UIV (T9-T11) | 67 | 28 | Mersilene polyethylene tape | Tethers significantly reduced PJK in ASD patients with lower-thoracic UIV (OR = 0.063, 95% CI = 0.016–0.247, P < 0.001); risk factors for PJK in patients with tether were greater postoperative lordosis of upper lumbar spine and greater UIV angle |
| Buell et al29 | Retrospective cohort | III | 184 | ASD patients with instrumentation at >6 motion segments without transitional rods, hooks at UIV, or vertebral augmentation | 66 | 20 | Mersilene polyethylene tape | PJK rates: 45.3% (29/64) in no-tether group; 34.4% (22/64) in tether-only group; and 17.9% (10/56) in tether with crosslink group; PJK rate was lower for all tethered (26.7%) vs no-tether groups (P = 0.011) |
| Iyer et al30 | Retrospective cohort | III | 108 | ASD patients with >5-level fusion to the pelvis | 55 | 17.6 | Mersilene polyethylene tape | Rates of PJK in tether group (27.3%) and no-tether group (28.6%) were similar (P = 0.827); tether was not protective against PJK |
| Line et al31 | Prospective cohort | II | 625 | ASD patients with >5 levels fused posteriorly | 58.6 | 31 | Polyethylene tether assessed in the context of other PJK preventive approaches (cement, hook, and avoidance of overcorrection) | If no PJF prophylaxis used and sagittal plane overcorrected, PJF rate was 24.2%; if PJF prophylaxis used, PJF rate was significantly lower (10.6%, P < 0.05) and further reduced to 9.9% if also not overcorrected; PJF rate similar (P > 0.05) for cement (12.1%), hooks (7.0%), and tether (16.1%) |
| Pham et al32 | Retrospective case series | IV | 4 | ASD patients with long-segment spinal fusion to the upper-thoracic spine | 60 | 5.5 | Semitendinous allograft | None of the 4 patients developed PJK at 5.5 mo follow-up; mean PJA increased by 3° |
| Rabinovich et al33 | Retrospective cohort | III | 184 | ASD patients with >5 level fusions to the pelvis | 67 | 45.4 | Mersilene polyethylene tape | PJK rates: 60.7% (37/61) in no-tether group; 35.7% (15/42) in tether-only group; and 23.3% (10/43) in tether with crosslink group; rate of PJK in no-tether group was significantly higher than in tether group (60.7% vs 29.4%, P < 0.001); PJK rate was lower in tether with crosslink vs no-tether group (P = 0.016); |
| Rabinovich et al34 | Retrospective case series | IV | 71 | ASD patients with long-segment spinal fusion | 66 | 14 | Polyethylene-terephthalate tape | PJK occurred in 15%; PJA increased by mean of 4°; rates of symptomatic PJK and revision for PJK were 8.8% and 2.9%, respectively |
| Rodnoi et al35 | Retrospective cohort | III | 43 | ASD patients with fusion from throacolumbar junction (T9–L1) to the pelvis | 69 | 24 | Mersilene polyethylene tape | Rate of PJK was significantly higher in no-tether group (17/20; 85%) vs tether group (10/23; 43.5%; P = 0.01); rate of PJF was significantly lower in tether group (0/23, 0%) vs no-tether group (7/20, 35%; P = 0.003) |
| Rodriguez-Fontan et al36 | Retrospective cohort | III | 80 | ASD patients with >3 levels fused posteriorly | 62.3 | 24 | Mersilene polyethylene tape | PJK rate at 2-y follow-up was 15% in tether group and 38% in no-tether group (OR = 0.28, P = 0.04); higher latent period to PJK for tether vs no-tether group (20 vs 7.5 mo, P = 0.018); tether reduced PJK risk after adjusting for confounders (age >55 y, 7–15 levels fused, thoracic UIV, BMI >27, osteoporosis) |
| Safaee et al37 | Retrospective cohort | III | 200 | ASD patients undergoing instrumented fusion | 64 | Minimum 6 | Soft sublaminar cable | Mean change in PJA was 6° in tether group vs 14° in no-tether group (P < 0.001); PJF rate in tether group was 4% (4/100) vs 18% (18/100) in no-tether group (P = 0.002) |
| Safaee et al38 | Retrospective cohort | III | 319 | ASD patients undergoing instrumented fusion | 65 | Minimum 12 | Soft sublaminar cable | Rate of reoperation for PJF significantly lower in tether group (8/242, 3.3%) vs no-tether group (12/77, 15.6%; P < 0.001); for patients with upper-throacic UIV, rate of PJF was 0% in tether group vs 6.7% in no-tether group (P = 0.014); for patients with lower-thoracic UIV, rate decreased from 21.3% to 5.3% (P = 0.001); on multivariate analysis, only use of tether and greater number of fused levels were associated with reductions in the rate of reoperation for PJF |
| Viswanathan et al39 | Prospective cohort | II | 40 | ASD patients undergoing thoracic to ilium instrumentation | 64 | 12 (median) | Braided sublaminar band | PJK developed in 3 of 40 (7.5%) patients; no instances of PJF; 3 procedure-related complications (2 CSF leaks and 1 transient neurological deficit) |
| Yagi et al40 | Retrospective cohort | III | 64 | ASD patients undergoing instrumented fusion from lower throacic spine to sacrum | 67 | Minimum 24 | Sublaminar polyethylene band | PJA was significantly greater in the no-tether group (17° vs 8°, P < 0.001); incidence of PJF was lower in the tether group (3% vs 25%, P = 0.03), with an OR of 0.1 (95% CI: 0.0–0.8, P = 0.03) |
| Zaghloul et al41 | Retrospective case series | IV | 23 | ASD patients treated with long-segment posterior instrumented fusion | 63 | 11.9 | Mersilene polyethylene tape | None of the patients had developed PJK (0%) as of last follow-up |
Abbreviations: ASD, adult spinal deformity; BMI, body mass index; CI, confidence interval; CSF, cerebrospinal fluid; OR, odds ratio; PJA, proximal junctional angle; PJF, proximal junctional failure; PJK, proximal junctional kyphosis; UIV, upper-most instrumented vertebra.
Tether material and technique varied across studies (Table 2). Eleven studies used a polyethylene tether, 2 used soft sublaminar cables, and 2 used a semitendinous allograft. The tether extended to the UIV+1 or UIV+2, passing either through or around the spinous processes, in 13 of the studies. In the remaining 2 studies, the tether was passed sublaminar at the UIV+1.
Table 2.
Summary of proximal junctional tether techniques from clinical outcomes studies.
| Study | Tether Technique |
| Pham et al32
Alluriu et al27 |
A No. 2 Ethibond double filament suture was used to create a modified locking Krackow weave at both ends of a cadaveric semitendinous tendon graft. This graft was then passed between the spinous processes from 1 level above the UIV to 1 to 2 levels below the UIV. The ends of the Ethibond suture were then tied together alone or tied together over a crosslink. |
| Buell et al28
Buell et al29 Line et al31 Rabinovich et al33 |
Two different techniques were used. (1) A high-speed drill was used to create holes through the base of the spinous processes at the UIV+1 and UIV-1. A polyetheylene Mersilene tape on a blunt needle was passed through the holes created in the UIV+1 and UIV-1 spinous processes and tied securely. (2) A high-speed drill was used to create a hole through the base of the spinous processes at the UIV+1. A polyetheylene Mersilene tape on a blunt needle was passed through the hole created in the UIV+1 spinous process. The tether was tied to a crosslink placed spanning the rods between UIV-1 and UIV-2. The crosslink was distracted caudally to tension the tether and secured to the rods. |
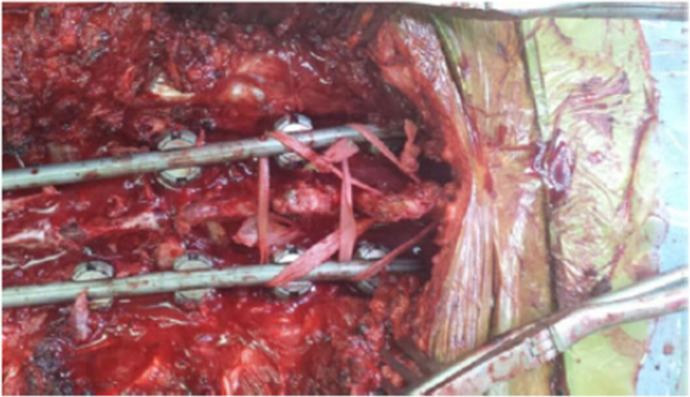
| Iyer et al30 | A 5-mm Mersilene tape on a curved needle was passed through the spinous process of the UIV+1 (Figure 1). A drill was used to create a hole through the spinous process if the needle could not be readily passed. The tether ends were then passed around the spinal rods below the pedicle screws at the UIV in a loop fashion, tensioned manually using a slip knot, and tied. This process was repeated, placing the tape through the spinous process of the UIV and securing the tape below the pedicle screws at the UIV-1. |
| Rabinovich et al34 | The VersaTie tether system (NuVasive) was used (Figure 2). A high-speed drill was used to create a hole through the base of the spinous process at the UIV+1 (and in some cases also at the UIV+2). A polyetheylene-terephthalate tape on a blunt needle was passed through the interspinous ligament between the UIV and UIV-1, then either passed through the spinous process at the UIV+1 alone or woven through UIV+1 and UIV+2, then passed back through the interspinous ligament between the UIV and UIV-1. The tether ends were then each passed through supplied tether rod connectors, which were then attached to the rods between the UIV and UIV-1 or between the UIV-1 and UIV-2. The VersaTie tower was then used to tension the tethers before final fixation of the tether to the connector device. |
| Rodnoi et al35 | A towel clamp was used to create a hole through the base of the spinous process at the UIV+1 (Figure 3). A polyethylene suture tape was passed through the spinous process at the UIV+1, and the ends of the tape were then tied over a crosslink placed spanning the rods between the UIV and UIV-1. A compressor was then used to distract the crosslink in order to tension the tape before the connector was final tightened. |
| Zaghloul et al41
Rodriguez-Fontan et al36 |
A 5-mm-wide Mersilene tape was passed through or looped around the supra-adjacent level spinous process (UIV+1), then looped in a figure-of-8 around the infra-adjacent spinous process (UIV), then tied to the rods below the screws at the UIV or tied to a crosslink attached to the rods between the UIV and UIV-1. |
| Safaee et al37
Safaee et al38 |
The Medicrea tether system was used (Figure 4). A high-speed drill was used to create holes through the base of spinous processes at UIV, UIV+1, and UIV-1. A soft sublaminar cable was passed through these holes in a mirrored weave pattern. Two cables were used (one on each side) and were pulled tightly to achieve the desired tension. The cables were then locked onto the rods using the supplied connectors. |
| Viswanathan et al39 | The Jazz system (Implanet America) was used. Bilateral hemilaminotomies were performed to widen the intralaminar spaces proximal and distal to the UIV+1 laminas with a power burr and Kerrison rongeur. The underlying ligamentum flavum was removed using Kerrison rongeurs until the underlying dura was exposed. The 2 braided polyester sublaminar bands were then carefully passed under the lamina from inferior to superior. Neuromonitoring was utilized. The sublaminar bands were connected to the rods at the UIV level and hand-tensioned using a tensioner device. |
| Yagi et al40 | Ligamentum flavum proximal and distal to the UIV lamina was partially removed using a Kerrison rongeur or high-speed drill (Figure 5). A 5-mm polethylene sublaminar band was then passed under the lamina of the UIV+1. The tether was attached to the rods bilaterally at a level distal to the upper-most pedicle screws with a torque of 200 Nm using a tape tighetener. |
Abbreviation: UIV, upper-most instrumented vertebra.
Studies Demonstrating Tether Benefit
The earliest study identified was a retrospective case series from Zaghloul et al.41 They reviewed 23 ASD patients with a mean follow-up of 11.9 months (range: 1–29 months). A 5-mm Mersilene polyethylene suture was passed either through or looped around the spinous process at the UIV+1 and then tied to the rods below the screws at the UIV or to a crosslink attached to the rods between the UIV and UIV-1 (Table 2). None of the patients had developed PJK as of the last follow-up, and they concluded that their technique might help prevent PJK. A subsequent retrospective matched cohort study by the same senior author using the same tether technique was published in 2020.36 At 2-year follow-up, the PJK rates were 15.0% and 38.4% in the tether (n = 60) and control (n = 20) groups, respectively (OR = 0.28, 95% CI = 0.07–1.1, P = 0.045). This protective effect of tethers remained significant after adjusting for potential confounding factors. They also noted that the time to development of PJK was longer in the tether group (20.0 [SD = 3.5] vs 7.5 [SD = 8.3] months, P = 0.018). They concluded that their tether technique effectively reduces PJK risk following posterior fusion for ASD.
Two studies published by surgeons at the University of Southern California27,32 utilized a semitendinous allograft for tethering. The graft was interwoven between the spinous processes at the UIV+1 and UIV, and the ends were then simply tied together or tied over a crosslink (Table 2). In the study from Pham et al,32 none of the 4 ASD patients who received interspinous ligament reinforcement using cadaveric semitendinous allograft developed PJK at a mean follow-up of 5.5 months (range, 4.2–6.6 months). The proximal junctional angle (PJA) increased by a mean of 3° (range, 1°–4°) at the last follow-up. They concluded that their approach was a feasible strategy to help prevent PJK. In a retrospective cohort study that included 83 ASD patients with a mean follow-up of 20.3 months from Alluri et al,27 PJK occurred in 33% (16/49) of the patients in the tether group and 32% (11/34) of patients in the control group (P = 0.31). PJF occurred in 18% (6/34) in the control group but did not occur in the tether group (P = 0.01). Moreover, although the preoperative Oswestry Disability Index was similar in both groups, the postoperative Oswestry Disability Index was significantly better in the tether group (P = 0.007). They concluded that their tether technique using semitendinosus allograft to augment the posterior ligament complex significantly decreased PJF incidence and improved functional outcomes scores.
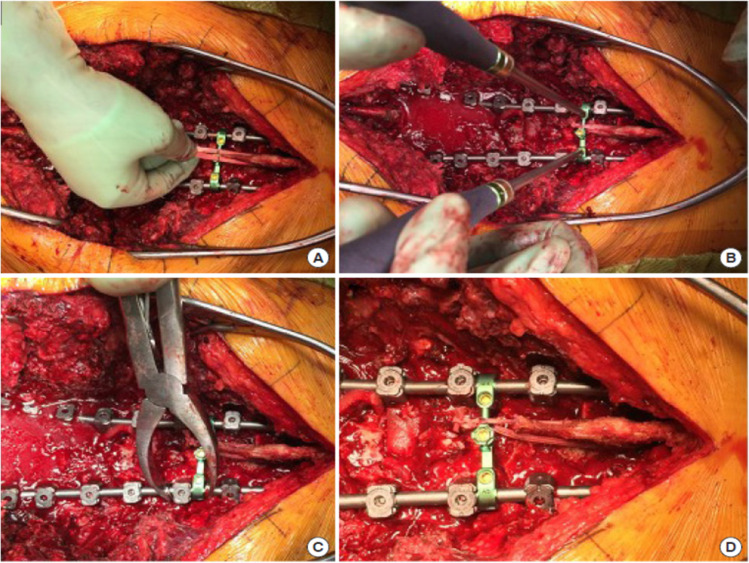
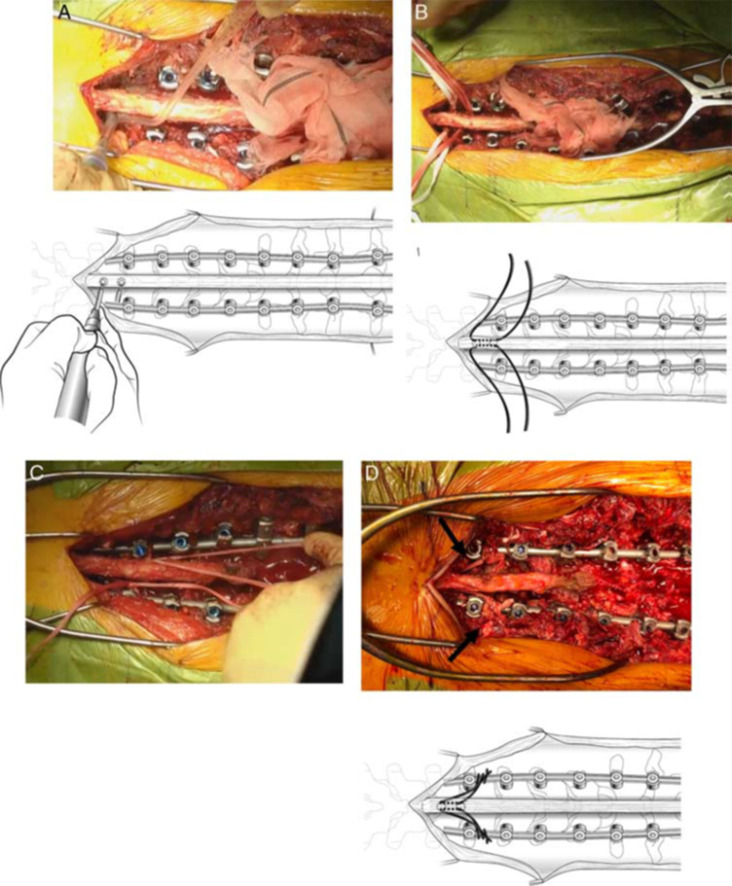
Two retrospective cohort studies were published by surgeons at the University of California, San Francisco.37,38 Both studies used a soft sublaminar cable that was passed through the spinous processes at the UIV, UIV+1, and UIV-1 in a mirrored weave pattern that employed the Medicrea system (Table 2, Figure 4). In their first study,37 100 patients who were treated for ASD with posterior ligament augmentation were compared with 100 historical controls without tether placement. At a minimum of 6-month follow-up, the mean change in PJA was 6° in the tether group and 14° in the no-tether group (P < 0.001). The PJF rate was significantly lower in the tether vs no-tether group (4% vs 18%, P = 0.002). After adjusting for the potential confounding effects of age and use of hook fixation at the UIV, the only variable with a significant association with PJF was use of ligament augmentation (OR = 0.193, 95 % CI = 0.053–0.701, P = 0.012). Subsequently, this group compared 242 patients treated for ASD with posterior ligament augmentation with 77 historical controls without tether placement.38 The rate of reoperation for PJF was significantly lower in the tether group (8/242, 3%) vs the no-tether group (12/77, 15.6%; P < 0.001). For patients with an upper-thoracic UIV, the rate of PJF in the tether group was 0% vs 6.7% in the no-tether group (P = 0.014), and for patients with a lower-thoracic UIV, the rate decreased from 21.3% to 5.3% (P = 0.001). On multivariate analysis, only the use of tether (OR = 0.184, 95 % CI = 0.071–0.478, P = 0.001) and a greater number of fused levels (OR = 0.762, 95 % CI = 0.620–0.937, P = 0.010) were associated with reductions in the rate of reoperation for PJF. They concluded that ligament augmentation might be useful for PJF reduction.
Figure 4.
Illustrations demonstrating proximal junctional tether technique as described by Safaee et al.37 A matchstick burr is used to create holes through the spinous processes of the upper-most instrumented vertebra (UIV), UIV+1, and UIV-1. A sublaminar cable is passed through each level (A) and then pulled to the side (B). The same process is repeated using a second cable on the opposite side (C). The cables are pulled distally to create desired tension (D). The cables are secured to the rods on each side using connectors. Source: Copyright Kenneth X. Probst. Reprinted with permission of XavierStudio.
Viswanathan et al43 prospectively assessed 40 ASD patients treated with long-segment posterior instrumented fusion with a median follow-up of 12 months (IQR = 6–15 months). All patients were treated with a sublaminar band (Jazz system, Implanet America) passed bilaterally under the lamina at UIV+1, with the tether ends then secured to the rods with the supplied connectors (Table 2). PJK developed in 7.5% (3/40) of patients, but there were no occurrences of PJF. They noted 3 procedure-related complications, including 2 cerebrospinal fluid leaks and 1 transient neurological deficit. They concluded that their technique is relatively safe and potentially protective against PJF.
Three retrospective cohort studies and 1 retrospective case series were published by surgeons at the University of Virginia.28,29,33,34 All 3 of the retrospective cohort studies utilized similar tether techniques (Table 2). A polyethylene Mersilene tape on a blunt needle was passed through the spinous process of the UIV+1 and then either passed through the spinous process at the UIV-1 and tied securely or tied to a crosslink attached to the rods between the UIV-1 and UIV-2. If the tether was attached to a crosslink, the crosslink was distracted caudally to further tension the tether. In their first study29 with 184 ASD patients treated with posterior instrumentation at >6 motion segments and mean 20-month (range = 3–56 months) follow-up, they reported PJK rates of 45.3% (29/64) in the no-tether group, 34.4% (22/64) in the tether-only group, and 17.9% (10/56) in the tether with crosslink group. The overall PJK rate for no-tether patients was significantly higher than for the tethered cohort (45.3% vs 26.7%, P = 0.011). The PJK rate was significantly lower in the tether with crosslink group compared with the no-tether group (P = 0.001). No effect on the revision rate for PJK was observed compared with the no-tether group. On multivariate analysis, older age (HR = 1.051, 95% CI = 1.016–1.088, P = 0.04) and greater correction of lumbar lordosis (HR = 1.022, 95% CI = 1.005–1.039, P = 0.013) were risk factors for PJK, while the use of tethers was protective (HR = 0.532, 95% CI = 0.318–0.892, P = 0.017).
Their second study33 assessed the same cohort but provided a minimum of 2-year follow-up. At a mean follow-up of 45.4 months, the PJK rates were 60.7% (37/61) in the no-tether group, 35.7% (15/42) in the tether-only group, and 23.3% (10/43) in the tether with crosslink group. The rate of PJK was significantly higher in the no-tether group compared with tether patients (60.7% vs 29.4%, P < 0.001), and the rate of PJK was lower in the tether with crosslink group vs the no-tether group (P = 0.016). On multivariate analysis, factors associated with increased risk of PJF included greater age (OR = 1.061, 95% CI = 1.011–1.118, P = 0.020), female sex (OR = 3.425, 95% CI = 1.359–9.288, P = 0.011), lower preoperative PJA (OR = 0.897, 95% CI = 0.827–0.965, P = 0.005), while the use of tethers was protective (OR = 0.140, 95% CI = 0.047–0.381, P = 0.0002).
Their third study28 explored the interplay of alignment and use of tethers on PJK development among ASD patients treated with long-segment posterior instrumentation and a UIV in the lower-thoracic spine. At a mean follow-up of 28 months (minimum 1 year), tether use was associated with a significant reduction in PJK (OR = 0.063, 95% CI = 0.016–0.247, P < 0.001). Among patients who developed PJK, those with a tether had greater pre- to postoperative change in upper segmental (L1–L4) lordosis (32.4° vs 19.4°, P = 0.017) and greater pre- to postoperative change in UIV angle (29.0° vs 16.7°, P = 0.007).
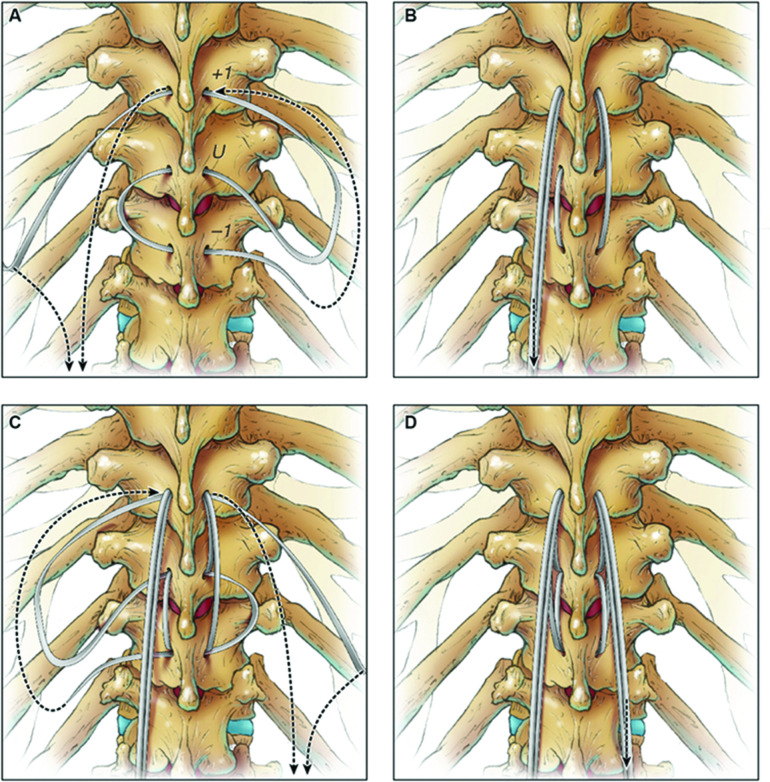
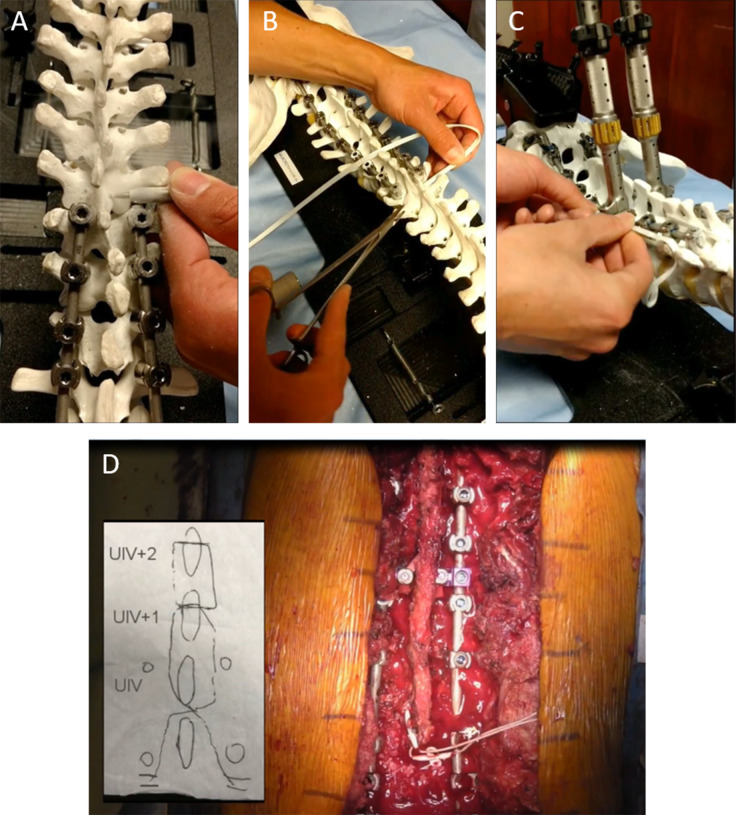
The fourth study34 reported a retrospective case series of 71 ASD patients treated with long-segment posterior instrumentation and tethering using the VersaTie system (NuVasive; Figure 2).42 A polyethylene-terephthalate tape on a blunt needle was passed through the interspinous ligament between the UIV and UIV-1, then either passed through the spinous process at the UIV+1 alone or woven through the spinous processes of the UIV+1 and UIV+2, then passed back through the interspinous ligament between the UIV and UIV-1. The tether ends were then secured to the rods using the supplied connectors and a tower system that facilitated tensioning of the tether. At a mean follow-up of 14 months, the PJA increased by a mean of 4°, and PJK occurred in 15% of the patients. The rates of symptomatic PJK and revision surgery for PJK were 8.8% and 2.9%, respectively. They noted that the rates of PJK and revision for PJK were favorable compared with the historical rates.
Figure 2.
Demonstration of proximal junctional tether technique as described by Rabinovich et al34 and Buell et al.42 A high-speed drill is used to create holes through the base of the spinous processes at the upper-most instrumented vertebra (UIV) and UIV+2 (A). The tether is passed through the interspinous ligament between the UIV and UIV-1, then through the spinous processes of UIV+1 (B), UIV+2, back through UIV+1, and then back through the interspinous ligament between the UIV and UIV-1. The tether is then tensioned and attached to the rods with a connector using the tower tensioning system (C). The general weave technique and a final intraoperative photo are shown in panel D.
Line et al31 used a propensity score matched analysis of 625 ASD patients to assess the use of polyethylene junctional tethers in the context of other PJK preventative approaches, including cement at the junctional level(s), hooks at the UIV, and avoidance of overcorrection relative to age-adjusted sagittal alignment goals. Their tether technique was similar to that of Buell et al29 (Table 2). They reported that if no PJF prophylaxis was used and the sagittal plane was overcorrected, the PJF rate was 24.2%. If PJF prophylaxis was used (tether, cement, or hooks), the PJF rate was significantly lower (10.6%, P < 0.05), and this rate was further reduced to 9.9% if the sagittal plane was not overcorrected. The overall PJF rate was similar (P < 0.05) for cement (12.1%), hooks (7.0%), and tethers (16.1%). They concluded that PJF implant prophylaxis alone was less effective than combining these implants with avoidance of sagittal overcorrection.
Rodnoi et al35 performed a retrospective cohort assessment of 43 ASD patients treated with posterior instrumentation that extended from the thoracolumbar junction (T9–L1) to the pelvis. For 23 patients, a Mersilene polyethylene tape was passed through the spinous process at the UIV+1, and the ends of the tape were then tied over a crosslink placed to span the rods between the UIV and UIV-1 (Table 2, Figure 3). Tension was applied to the tether before the crosslink was final-tightened. The rate of PJK was significantly higher in the no-tether group (85%, 17/20) vs the tether group (43.5%, 10/23; P = 0.01). The PJA was smaller for the tether group, and the rate of increase in PJA was slower in the tether group (P < 0.0001). The rate of PJF was significantly lower in the tether group (0%, 0/23) vs the no-tether group (35%, 7/20; P = 0.003). In addition, the time to revision surgery was lower in the no-tether group (P = 0.003). They concluded that their tether technique is effective in slowing the progression of the PJA and in lowering the risk for PJK.
Figure 3.
Intraoperative photos demonstrating proximal junctional tether technique as described by Rodnoi et al.35 (A) Polyethylene tape is passed through the base of the spinous process of the upper-most instrumented vertebra (UIV)+1. (B) The tape is tied around a crosslink, and excess tether is trimmed. (C) Compression between the crosslink and subjacent pedicle screw is performed to tension the tether. (D) The crosslink is final-tightened. Source: Rodnoi et al. Neurospine. 2021;18[3]:580–586. Copyright 2021. Reprinted with permission.
Yagi et al40 performed a retrospective matched cohort analysis of 67 severe ASD patients treated with long-segment posterior instrumentation. They used propensity score matching to generate 2 similar groups, with 32 patients treated with a sublaminar tether at the UIV+1 and 32 patients without a tether. Their tether technique involved passing a polyethylene band under the lamina of the UIV+1 (Table 2, Figure 5). The tether ends were then attached to the rods bilaterally at a level distal to the upper-most pedicle screws at a torque of 200 Nm using a tape tightener. At a minimum of 24-month follow-up, they reported that the PJA was significantly greater in the no-tether group (17 vs 8, P < 0.001), and the incidence of PJF was lower in the tether group (3% vs 25%, P = 0.03), with an OR of 0.1 (95% CI = 0.0–0.8, P = 0.03). They concluded that their technique is a promising procedure that may reduce the risk of PJF in surgery for severe ASD.
Figure 5.
Illustrations and intraoperative photos demonstrating the sublaminar tether technique as described by Yagi et al.40 Ligamentum flavum proximal and distal to the upper-most instrumented vertebra (UIV)+1 is partially removed using a high-speed burr (A). Two sublaminar bands are passed under the UIV+1 (B). The tethers are tied to the bilateral rods at a level distal to the UIV pedicle screws with a torque of 200 Nm with a tape tightener (C). Arrows show the tape passing under the UIV+1 lamina. Source: Yagi et al. Clin Spine Surg 2022;35(5):E496-E503. Copyright 2022. Reprinted with permission of Wolters Kluwer Health, Inc.
Study Demonstrating No Tether Benefit
Iyer et al30 performed a retrospective cohort study with 108 ASD patients treated with long-segment posterior instrumentation that extended to the pelvis. In 31 patients (38.7%), a Mersilene polyethylene tape on a curved needle was passed through the spinous process at the UIV+1, and the ends were looped around the rods below the pedicle screws at the UIV and tied securely using a slip knot. This was then repeated with another tape passed through the spinous process at the UIV, and the ends were looped around the rods and secured below the pedicle screws at the UIV-1 (Figure 1). The tether cohort was older and had larger initial sagittal corrections (P < 0.05). The rates of PJK for tether (27.3%) and no-tether (28.6%) groups were similar (P = 0.827) at a mean 17.6-month follow-up, and after controlling for degree of sagittal correction via propensity matching, tethering still had no statistically significant impact on PJK (29% vs 38.7%, P = 0.367). They concluded that ligamentous reinforcement at the UIV+1 using a hand-tensioned nylon tape does not reduce the incidence of PJK at minimum 1-year follow-up.
Figure 1.
Intraoperative photo demonstrating proximal junctional tether technique as described by Iyer et al.30 Image shows dual surgical nylon tape augmentation extending from a hole through the base of the spinous process at the upper-most instrumented vertebra (UIV) and tied to the rods and from a hole through the base of the spinous process at the UIV+1 and tied to the rods. Source: Iyer et al. Global Spine J. 2020;10[6]:692–699. Copyright 2019. Reprinted with permission of SAGE Publications.
Discussion
Despite significant advances in the surgical treatment for ASD, PJK and PJF remain major challenges. Ligamentous augmentation at the proximal junction has emerged as a promising technique to help reduce the occurrence of PJK/PJF. Fifteen clinical studies on the use of proximal junctional tethers in ASD surgery have been identified and summarized in the present review. Most of the studies are retrospective cohorts or case series from a single surgeon or institution, and all but one study suggest that proximal junctional tethers may be of benefit in reducing the incidence of PJK/PJF. Although early studies are favorable, there are many remaining questions, and higher-quality studies are needed.
In all but one reviewed article, the tether was extended to the UIV+1. In the study by Rabinovich et al,34 the tether was extended either to the UIV+1 or UIV+2. In a finite element analysis (FEA), Bess et al18 modeled extension of a proximal tether to the UIV+1, UIV+2, and UIV+3. They noted that posterior tethers created a more gradual transition of forces at the UIV, and the dissipation of forces was enhanced with tethering to a greater number of levels. Another potential advantage of extending tethers beyond the UIV+1 is the enhanced bony anchorage that potentially lowers the risk of tethers pulling through bony anchor points. However, a disadvantage of extending tethers to increasing numbers of levels above the UIV relates to the soft tissue disruption necessary for tether placement. Based on the study from Bess et al,18 an FEA from Buell et al,19 and a biomechanical study from Mar et al,22 a tether anchorage to the UIV+2 may be a preferred configuration.
In all but 2 of the reviewed studies, the tether was anchored proximally to the spinous process, either passing through or looping around it. Viswanathan et al44 and Yagi et al40 used a sublaminar banding technique extending to the UIV+1. In a separate biomechanical assessment, Viswanathan et al39 demonstrated that sublaminar banding, compared with passing a Mersilene tape through the spinous process, was significantly more effective in creating a transition zone and mitigating stresses at the proximal junction. In addition, the strength of the lamina is likely considerably greater than that of the spinous process, especially in the setting of osteopenia or osteoporosis. However, there are likely greater inherent risks when passing a tether sublaminar than through a spinous process. Notably, in the series from Viswanathan et al,44 out of 40 patients, 3 tether-related complications were reported, including 2 cerebrospinal fluid leaks and 1 transient neurological deficit.
Most reviewed studies reported some form of tether tensioning. Some studies simply noted that the tether was pulled tightly, or a slip knot technique was used to help tension the tether.27–30,32,33 Other studies used commercially available systems that incorporate more powerful and controlled tensioning devices.34,40 An alternative tensioning method was to secure the tether ends to a crosslink and then pull the crosslink distally before securing it to the rods.28,29,31,33,35 Yagi et al40 used a tape tightener, and this was the only study that specified objectively the tension applied. Biomechanical24 and FEA19 studies suggested that tensioning is important but did not provide clear clinical guidance as to optimal tensioning. The potential importance of tensioning is suggested by 2 clinical studies29,33 that included both a technique of hand-tightening the tether and the use of a crosslink to enable a distractor to increase the tension. Only the group that included the crosslink demonstrated a significant reduction in PJK incidence compared with the no-tether group.
All but one study in the current review favored the use of tethers as a means of reducing the incidence of PJK/PJF. In the study from Iyer et al,30 they noted no difference in the rate of PJK between their tether and no-tether groups. Although it is unclear why this study reached a different conclusion, there are potential explanations. As the authors noted, there were significant differences between the cohorts, including a significantly larger sagittal correction in tether patients. Although they performed a matched subgroup analysis and multivariate regression model to control for these differences, these analyses are imperfect at approximating a randomized study. In addition, the tethering technique used in this study was unique, with separate tethers anchored to the UIV and UIV+1 and directly attached to the rods. No tensioning, other than a slip knot technique, was used. Regardless, this study found no association between tethering and PJK/PJF and supports the need for further research.
Conclusions
PJK and PJF remain major challenges in ASD surgery. Most early studies suggest that use of ligamentous augmentation may be protective against the development of PJK/PJF. However, the multifactorial etiology of PJK/PJF makes it unlikely that any single technique will solve this complex problem. Further study is needed to address not only the effectiveness of junctional tethers but also to clarify if there are optimal tether configurations, tether materials, and tether tension.
References
- 1. Smith JS, Shaffrey CI, Ames CP, Lenke LG. Treatment of adult thoracolumbar spinal deformity: past, present, and future. J Neurosurg Spine. 2019;30(5):551–567. 10.3171/2019.1.SPINE181494 [DOI] [PubMed] [Google Scholar]
- 2. Kelly MP, Lurie JD, Yanik EL, et al. Operative versus nonoperative treatment for adult symptomatic lumbar scoliosis. J Bone Joint Surg Am. 2019;101(4):338–352. 10.2106/JBJS.18.00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Smith JS, Kelly MP, Yanik EL, et al. Operative versus nonoperative treatment for adult symptomatic lumbar scoliosis at 5-year follow-up: durability of outcomes and impact of treatment-related serious adverse events. J Neurosurg Spine. 2021;35(1):67–79. 10.3171/2020.9.SPINE201472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith JS, Lafage V, Shaffrey CI, et al. Outcomes of operative and nonoperative treatment for adult spinal deformity: a prospective, multicenter, propensity-matched cohort assessment with minimum 2-year follow-up. Neurosurgery. 2016;78(6):851–861. 10.1227/NEU.0000000000001116 [DOI] [PubMed] [Google Scholar]
- 5. Bridwell KH, Glassman S, Horton W, et al. Does treatment (nonoperative and operative) improve the two-year quality of life in patients with adult symptomatic lumbar scoliosis: a prospective multicenter evidence-based medicine study. Spine (Phila Pa 1976). 2009;34(20):2171–2178. 10.1097/BRS.0b013e3181a8fdc8 [DOI] [PubMed] [Google Scholar]
- 6. Elias E, Bess S, Line BG, et al. Operative treatment outcomes for adult cervical deformity: a prospective multicenter assessment with mean 3-year follow-up. J Neurosurg Spine. 2022;37(6):855–864. 10.3171/2022.6.SPINE22422 [DOI] [PubMed] [Google Scholar]
- 7. Smith JS, Klineberg E, Lafage V, et al. Prospective multicenter assessment of perioperative and minimum 2-year postoperative complication rates associated with adult spinal deformity surgery. J Neurosurg Spine. 2016;25(1):1–14. 10.3171/2015.11.SPINE151036 [DOI] [PubMed] [Google Scholar]
- 8. Sardi JP, Lazaro B, Smith JS, et al. Rod fractures in thoracolumbar fusions to the sacrum/pelvis for adult symptomatic lumbar scoliosis: long-term follow-up of a prospective, multicenter cohort of 160 patients. J Neurosurg Spine. 2023;38(2):217–229. 10.3171/2022.8.SPINE22423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Glattes RC, Bridwell KH, Lenke LG, Kim YJ, Rinella A, Edwards C. Proximal junctional kyphosis in adult spinal deformity following long instrumented posterior spinal fusion: incidence, outcomes, and risk factor analysis. Spine (Phila Pa 1976). 2005;30(14):1643–1649. 10.1097/01.brs.0000169451.76359.49 [DOI] [PubMed] [Google Scholar]
- 10. Kim YJ, Bridwell KH, Lenke LG, Glattes CR, Rhim S, Cheh G. Proximal junctional kyphosis in adult spinal deformity after segmental posterior spinal instrumentation and fusion: minimum five-year follow-up. Spine (Phila Pa 1976). 2008;33(20):2179–2184. 10.1097/BRS.0b013e31817c0428 [DOI] [PubMed] [Google Scholar]
- 11. Lau D, Clark AJ, Scheer JK, et al. Proximal junctional kyphosis and failure after spinal deformity surgery: a systematic review of the literature as a background to classification development. Spine (Phila Pa 1976). 2014;39(25):2093–2102. 10.1097/BRS.0000000000000627 [DOI] [PubMed] [Google Scholar]
- 12. Kim HJ, Bridwell KH, Lenke LG, et al. Proximal junctional kyphosis results in inferior SRS pain subscores in adult deformity patients. Spine (Phila Pa 1976). 2013;38(11):896–901. 10.1097/BRS.0b013e3182815b42 [DOI] [PubMed] [Google Scholar]
- 13. Hart RA, McCarthy I, Ames CP, Shaffrey CI, Hamilton DK, Hostin R. Proximal junctional kyphosis and proximal junctional failure. Neurosurg Clin N Am. 2013;24(2):213–218. 10.1016/j.nec.2013.01.001 [DOI] [PubMed] [Google Scholar]
- 14. Buell TJ, Nguyen JH, Mazur MD, et al. Radiographic outcome and complications after single-level lumbar extended pedicle subtraction osteotomy for fixed sagittal malalignment: a retrospective analysis of 55 adult spinal deformity patients with a minimum 2-year follow-up. J Neurosurg Spine. 2018;30(2):242–252. 10.3171/2018.7.SPINE171367 [DOI] [PubMed] [Google Scholar]
- 15. Doodkorte RJP, Vercoulen TFG, Roth AK, de Bie RA, Willems PC. Instrumentation techniques to prevent proximal junctional kyphosis and proximal junctional failure in adult spinal deformity correction-a systematic review of biomechanical studies. Spine J. 2021;21(5):842–854. 10.1016/j.spinee.2021.01.011 [DOI] [PubMed] [Google Scholar]
- 16. Shlobin NA, Le N, Scheer JK, Tan LA. State of the evidence for proximal junctional kyphosis prevention in adult spinal deformity surgery: a systematic review of current literature. World Neurosurg. 2022;161(179–189):179–189. 10.1016/j.wneu.2022.02.063 [DOI] [PubMed] [Google Scholar]
- 17. Vercoulen TFG, Doodkorte RJP, Roth A, de Bie R, Willems PC. Instrumentation techniques to prevent proximal junctional kyphosis and proximal junctional failure in adult spinal deformity correction: a systematic review of clinical studies. Global Spine J. 2022;12(6):1282–1296. 10.1177/21925682211034500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bess S, Harris JE, Turner AWL, et al. The effect of posterior polyester tethers on the biomechanics of proximal junctional kyphosis: a finite element analysis. J Neurosurg Spine. 2017;26(1):125–133. 10.3171/2016.6.SPINE151477 [DOI] [PubMed] [Google Scholar]
- 19. Buell TJ, Bess S, Xu M, et al. Optimal tether configurations and preload tensioning to prevent proximal junctional kyphosis: a finite element analysis. J Neurosurg Spine. 2019:1–11. 10.3171/2018.10.SPINE18429 [DOI] [PubMed] [Google Scholar]
- 20. Cho SK, Caridi J, Kim JS, Cheung ZB, Gandhi A, Inzana J. Attenuation of proximal junctional kyphosis using sublaminar polyester tension bands: a biomechanical study. World Neurosurg. 2018;120:e1136–e1142. 10.1016/j.wneu.2018.08.244 [DOI] [PubMed] [Google Scholar]
- 21. Kim JS, Cheung ZB, Arvind V, Caridi J, Cho SK-W. Role of posterior ligamentous reinforcement in proximal junctional kyphosis: a cadaveric biomechanical study. Asian Spine J. 2019;13(1):68–76. 10.31616/asj.2018.0102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mar DE, Burton DC, McIff TE. Biomechanics of prophylactic tethering for proximal junctional kyphosis: comparison of posterior tether looping techniques. Spine Deform. 2019;7(2):197–202. 10.1016/j.jspd.2018.07.001 [DOI] [PubMed] [Google Scholar]
- 23. Mar DE, Clary SJ, Ansley B, Bunch JT, Burton DC, McIff TE. Biomechanics of prophylactic tethering for proximal junctional kyphosis: effects of cyclic loading on tether strength and failure properties. Spine Deform. 2020;8(5):863–870. 10.1007/s43390-020-00111-7 [DOI] [PubMed] [Google Scholar]
- 24. Mar DE, Clary SJ, Burton DC, McIff TE. Biomechanics of prophylactic tethering for proximal junctional kyphosis: characterization of spinous process tether pretensioning and pull-out force. Spine Deform. 2019;7(2):191–196. 10.1016/j.jspd.2018.06.017 [DOI] [PubMed] [Google Scholar]
- 25. Yagi M, Nakahira Y, Watanabe K, Nakamura M, Matsumoto M, Iwamoto M. The effect of posterior tethers on the biomechanics of proximal junctional kyphosis: the whole human finite element model analysis. Sci Rep. 2020;10(1):3433. 10.1038/s41598-020-59179-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ou-Yang D, Moldavsky M, Wessell N, et al. Evaluation of spinous process tethering at the proximal end of rigid constructs: in vitro range of motion and Intradiscal pressure at instrumented and adjacent levels. Int J Spine Surg. 2020;14(4):571–579. 10.14444/7076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alluri R, Kim A, Ton A, Kang H, Acosta F, Hah R. Semitendinosus tendon augmentation for prevention of proximal junctional failure. Spine (Phila Pa 1976). 2021;46(4):241–248. 10.1097/BRS.0000000000003765 [DOI] [PubMed] [Google Scholar]
- 28. Buell TJ, Chen C-J, Quinn JC, et al. Alignment risk factors for proximal junctional kyphosis and the effect of lower thoracic junctional tethers for adult spinal deformity. World Neurosurg. 2019;121:e96–e103. 10.1016/j.wneu.2018.08.242 [DOI] [PubMed] [Google Scholar]
- 29. Buell TJ, Buchholz AL, Quinn JC, et al. A pilot study on posterior polyethylene tethers to prevent proximal junctional kyphosis after multilevel spinal instrumentation for adult spinal deformity. Oper Neurosurg (Hagerstown). 2019;16(2):256–266. 10.1093/ons/opy065 [DOI] [PubMed] [Google Scholar]
- 30. Iyer S, Lovecchio F, Elysée JC, et al. Posterior ligamentous reinforcement of the upper Instrumented vertebrae +1 does not decrease proximal junctional kyphosis in adult spinal deformity. Global Spine Journal. 2020;10(6):692–699. 10.1177/2192568219868472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Line BG, Bess S, Lafage R, et al. Effective prevention of proximal junctional failure in adult spinal deformity surgery requires a combination of surgical implant prophylaxis and avoidance of sagittal alignment overcorrection. Spine (Phila Pa 1976). 2020;45(4):258–267. 10.1097/BRS.0000000000003249 [DOI] [PubMed] [Google Scholar]
- 32. Pham MH, Tuchman A, Smith L, et al. Semitendinosus graft for interspinous ligament reinforcement in adult spinal deformity. Orthopedics. 2017;40(1):e206–e210. 10.3928/01477447-20161006-05 [DOI] [PubMed] [Google Scholar]
- 33. Rabinovich EP, Snyder MH, McClure JJ, et al. Posterior polyethylene tethers reduce occurrence of proximal junctional kyphosis after multilevel spinal instrumentation for adult spinal deformity: a retrospective analysis. Neurosurgery. 2021;89(2):227–235. 10.1093/neuros/nyab123 [DOI] [PubMed] [Google Scholar]
- 34. Rabinovich EP, Buell TJ, Sardi JP, Lazaro BCR, Shaffrey CI, Smith JS. A novel weave tether technique for proximal junctional kyphosis prevention in 71 adult spinal deformity patients: a preliminary case series assessing early complications and efficacy. Oper Neurosurg (Hagerstown). 2021;21(6):393–399. 10.1093/ons/opab305 [DOI] [PubMed] [Google Scholar]
- 35. Rodnoi P, Le H, Hiatt L, et al. Ligament augmentation with mersilene tape reduces the rates of proximal junctional kyphosis and failure in adult spinal deformity. Neurospine. 2021;18(3):580–586. 10.14245/ns.2142420.210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodriguez-Fontan F, Reeves BJ, Noshchenko A, et al. Strap stabilization for proximal junctional kyphosis prevention in Instrumented posterior spinal fusion. Eur Spine J. 2020;29(6):1287–1296. 10.1007/s00586-020-06291-0 [DOI] [PubMed] [Google Scholar]
- 37. Safaee MM, Deviren V, Dalle Ore C, et al. Ligament augmentation for prevention of proximal junctional kyphosis and proximal junctional failure in adult spinal deformity. J Neurosurg Spine. 2018;28(5):512–519. 10.3171/2017.9.SPINE1710 [DOI] [PubMed] [Google Scholar]
- 38. Safaee MM, Haddad AF, Fury M, et al. Reduced proximal junctional failure with ligament augmentation in adult spinal deformity: a series of 242 cases with a minimum 1-year follow-up. J Neurosurg Spine. 2021;35(6):752–760. 10.3171/2021.2.SPINE201987 [DOI] [PubMed] [Google Scholar]
- 39. Viswanathan VK, Ganguly R, Minnema AJ, et al. Biomechanical assessment of proximal junctional semi-rigid fixation in long-segment thoracolumbar constructs. J Neurosurg Spine. 2018;30(2):184–192. 10.3171/2018.7.SPINE18136 [DOI] [PubMed] [Google Scholar]
- 40. Yagi M, Suzuki S, Okada E, et al. Sublaminar tethers significantly reduce the risk of proximal junctional failure in surgery for severe adult spinal deformity: a propensity score-matched analysis. Clin Spine Surg. 2022;35(5):E496–E503. 10.1097/BSD.0000000000001294 [DOI] [PubMed] [Google Scholar]
- 41. Zaghloul KM, Matoian BJ, Denardin NB, Patel VV. Preventing proximal adjacent level kyphosis with strap stabilization. Orthopedics. 2016;39(4):e794–e799. 10.3928/01477447-20160503-05 [DOI] [PubMed] [Google Scholar]
- 42. Buell TJ, Mullin JP, Nguyen JH, et al. A novel junctional tether weave technique for adult spinal deformity: 2-dimensional operative video. Oper Neurosurg (Hagerstown). 2019;16(2):45–46. 10.1093/ons/opy148 [DOI] [PubMed] [Google Scholar]
- 43. Viswanathan VK, Kukreja S, Minnema AJ, Farhadi HF. Prospective assessment of the safety and early outcomes of sublaminar band placement for the prevention of proximal junctional kyphosis. J Neurosurg Spine. 2018;28(5):520–531. 10.3171/2017.8.SPINE17672 [DOI] [PubMed] [Google Scholar]
- 44. Viswanathan VK, Minnema AJ, Viljoen S, Farhadi HF. Sublaminar banding as an adjunct to pedicle screw-rod constructs: a review and technical note on novel hybrid constructs in spinal deformity surgery. J Neurosurg Spine. 2019;30(6):1–7. 10.3171/2018.11.SPINE181154 [DOI] [PubMed] [Google Scholar]