Horner syndrome (HS) is a neurological or/and neurosurgical disorder characterized by common signs including miosis, ptosis, anhidrosis, and enophthalmos. Enophthalmos is abnormally posterior displacement of the globe due to degradation of orbital fat, or loss of function of the orbitalis muscle. The main landmark for its diagnosis is a difference of 2 mm or more in the anteroposterior (AP) axis between the two globes.1 Enophthalmos manifests in two congenital or acquired forms that can result from anatomical or physiological changes especially HS.2 It occurs as a result of damage to the carotid artery, superior cervical ganglion (SCG) sympathectomy, inflammatory diseases, drug abuse, and neurocristopathy (abnormal migration of neural crest cells).2 The smooth muscle of the upper eyelid (Müller's muscle) is innervated by the surrounding plexus of internal carotid artery, but the smooth muscle of the lower eyelid (Kakizaki's muscle) that is not a single muscle is innervated by the surrounding plexus of external carotid artery. Moreover, the Lockwood's ligament is isolated by a layer of fat or is united to the capsulopalpebral fascia (CPF) between the inferior tarsal muscle, and the CPF is innervated by the surrounding plexus of external carotid artery.3
Signaling and new concept
Therefore, any damage to this sympathetic (as a result of superior cervical ganglion sympathectomy) causes dysfunction of the lower eyelid components, including the CPF and its surrounding smooth muscle fibers, and finally leads to forward displacement of the lower eyelid as well as manifestation of the enophthalmos symptom.3,4
When adipocytes are stimulated by nerves or various hormones, stored lipids are mobilized and cells release fatty acids and glycerol. Norepinephrine (NE) released by postganglionic sympathetic nerves in adipose tissue activates cyclic adenosine monophosphate (cAMP), which in turn causes activation of hormone-sensitive lipase that breaks down triglycerides (TGs) at the surface of the stored lipid droplets;5 however, when there is no sympathetic innervation, how does orbital lipolysis of fat around the globe result in enophthalmos?
Furthermore, hormone-sensitive lipase activity is also stimulated by growth hormone (GH) from the pituitary gland.6 Somatotropic cells within pars distalis of the anterior pituitary affected by stimulatory or inhibitory neuropeptides of the hypothalamus secrete GH.7 GH is secreted as a result of sympathetic fibers from the surrounding plexus of internal carotid artery and induces lipolysis, but how does this cycle work and how does GH cause lipolysis when postganglionic sympathetic fibers in Horner's syndrome are interrupted and sympathetic function is lost?
In our opinion, another neural pathway is involved in the occurrence of enophthalmos sign after sympathetic interruption following HS:
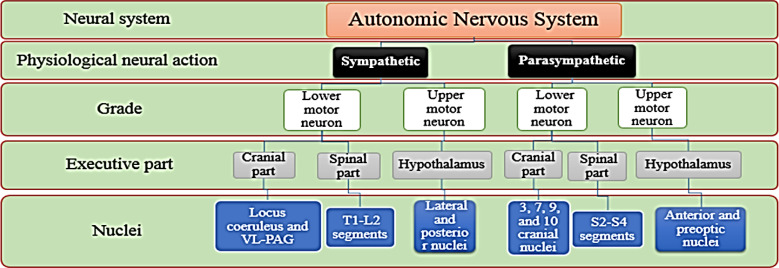
1) Generally, the autonomic nervous system has both upper and lower motor neuron control centers. The posterior and lateral nuclei of the hypothalamus are the centers of the upper motor neurons in the sympathetic part, but for the parasympathetic part, they are the anterior and preoptic nuclei (Figure 1).
Figure 1.
Division of the autonomic nervous system with the nuclei responsible for the sympathetic and parasympathetic parts
Locus coeruleus (LCo) nucleus and the ventrolateral-periaqueductal gray matter (VLPAG) as the cranial part of the lower motor neuron (LMN) of the sympathetic system are responsible for the release of norepinephrine. As one of the major descending pathways propagated by norepinephrine (NE), the VLPAG-LCo pathway exerts a compensatory effect to activate the hypothalamic-pituitary axis, and the adenohypophysis through thyroid-stimulating hormone (TSH) stimulates lipolysis. Based on our theory, “autonomic swapping” requires neurons with the ability of autonomic switch, and this fast and complex phenomenon occurs in the LCo nucleus.
2) The surrounding plexus of internal carotid artery innervates the distal part of the adenohypophysis. Since the GH causes lipolysis, if the sympathetic nerves are interrupted, lipolysis stops and enophthalmos does not occur. The parasympathetic nerves innervating the carotid and vertebrobasilar arteries as well as circle of Willis utilize a co-transmitter known as “non-cholinergic parasympathetic innervation”.8 Parasympathetic fibers that originate from the diffuse cranial paraganglia (neural crest-derived neuroendocrine cells), cavernous sinus ganglia, and the internal carotid mini-ganglia wrap around the carotid branches and travel to the eye and the skull base.9,10 Nitric oxide (NO) and pituitary adenylate cyclase-activating polypeptide (PACAP) are co-transmitters in parasympathetic neurons and all are potent vasodilators.8 PACAP not only increases thermogenesis by acting at the hypothalamus to increase sympathetic output,11 but also via impact on PACAP receptors which are expressed in adipocytes causes elevation of the intracellular amounts of cAMP in adipocytes which results in increased activity of protein kinase A (PKA) and promotes phosphorylation and activation of hormone-sensitive lipase and subsequently leads to lipolysis.12
3) The locus coeruleus (LCo) is the major noradrenergic nucleus to activate the hypothalamic-pituitary axis and the anterior pituitary (adenohypophysis) secretes thyroid-stimulating hormone (TSH).
Since TSH receptor is expressed in orbital fat tissue and extra-ocular muscles,13 TSH stimulates lipolysis in this area.14 NE release in medial prefrontal cortex (mPFC) is increased as a result of acute stress (due to superior cervical ganglion sympathectomy).15
The fibers arise from mPFC (as an alternative route) directly, coursing in the medial forebrain bundle (MFB) to the lateral part of hypothalamus; then, the hypothalamomedullary tract establishes a direct connection between the hypothalamus and the noradrenergic autonomic nuclei (such as LCo) in the medulla.16-18
4) Therefore, it is likely that the lipolytic effect of sympathetic activation on the periocular fat is transmitted indirectly via the periaqueductal gray matter (PAG) and also the sympathetic signals after SCG sympathectomy are transmitted through the oculomotor and vagus nerves which bind to the two subclasses of receptors including α1 and β2 adrenergic receptors (ARs).16 Although both brown adipose and white adipose tissues are innervated by the sympathetic system via postsynaptic β3-AR on adipocytes,5 innervation of abdominal fat is different from subcutaneous fat.19 In addition, LCo projects to motoneurons in the brainstem and the spinal cord, facilitating motoneuron activity via the stimulation of α1-adrenoceptors. The motor neurons situated in the nuclei of the third (oculomotor), fourth (trochlear), and sixth (abducens) cranial nerves form the oculomotor nuclear complex responsible for innervating the external muscles of the eyes controlling the movements of the eye. Some cells in the LCo have been found to project to the oculomotor nuclear complex20 and high levels of α1-adrenoceptors have been identified within this area, indicating an excitatory noradrenergic input to these neurons.21 Finally, noradrenergic signal passing through 3, 4, and 6 cranial nerves leads to lipolysis of sub/retro eyelid fat (between the Lockwood's ligament and CPF) and medial/central infraorbital fat pad as well as atrophy of ocular muscles and then the manifestation of the enophthalmos symptom.
In conclusion, we believe that enophthalmos after SCG sympathectomy occurs through both parasympathetic fibers (which carry sympathetic neurotransmitters) and other alternative sympathetic neurohormonal pathways, especially via LCo and ventrolateral-periaqueductal gray matter (VLPAG). This new signaling pathway is called the "Abell’s Autonomic Feedback”, in honor of Auob Rustamzadeh, an Iranian neuroanatomist, which refers to the “Ponto-Peduncular Feedback”, because the LCo, oculomotor nuclei, and VLPAG are located in these areas and trigger and stimulate autonomic responses. The authors hope that in the future, molecular and cellular investigations using medical imaging technologies and receptor tracking techniques will be carried out on our proposed hypothesis, because a precise understanding of this feedback can clarify the causes of many neurological diseases and the occurrence of visceral-emotional states.
Acknowledgments
None.
Notes:
How to cite this article: Rustamzadeh A, Afshari D, Alizadeh-Otaghvar HR, Ahadi R, Raoofi A, Shabani R, et al. Horner syndrome: A new hypothesis for signaling pathway of enophthalmos sign. Curr J Neurol 2023; 22(3): 197-200.
Conflict of Interests
The authors declare no conflict of interest in this study.
References
- 1.Athanasiov PA, Prabhakaran VC, Selva D. Non-traumatic enophthalmos: A review. Acta Ophthalmol. 2008;86(4):356–64. doi: 10.1111/j.1755-3768.2007.01152.x. [DOI] [PubMed] [Google Scholar]
- 2.Martin TJ. Horner syndrome: A clinical review. ACS Chem Neurosci. 2018;9(2):177–86. doi: 10.1021/acschemneuro.7b00405. [DOI] [PubMed] [Google Scholar]
- 3.Codner MA, Hanna MK. Applied anatomy of the eyelids and orbit. In: Nahai F, editor. The art of aesthetic Surgery: Principles and techniques. St Louis, MO: Quality Medical Publishing; 2005. pp. 626–49. [Google Scholar]
- 4.Hwang K. Surgical anatomy of the lower eyelid relating to lower blepharoplasty. Anat Cell Biol. 2010;43(1):15–24. doi: 10.5115/acb.2010.43.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartness TJ, Liu Y, Shrestha YB, Ryu V. Neural innervation of white adipose tissue and the control of lipolysis. Front Neuroendocrinol. 2014;35(4):473–93. doi: 10.1016/j.yfrne.2014.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kopchick JJ, Berryman DE, Puri V, Lee KY, Jorgensen JOL. The effects of growth hormone on adipose tissue: Old observations, new mechanisms. Nat Rev Endocrinol. 2020;16(3):135–46. doi: 10.1038/s41574-019-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wasinski F, Frazao R, Donato J. Effects of growth hormone in the central nervous system. Arch Endocrinol Metab. 2019;63(6):549–56. doi: 10.20945/2359-3997000000184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevan JA, Moscowitz M, Said SI, Buga G. Evidence that vasoactive intestinal polypeptide is a dilator transmitter to some cerebral and extracerebral cranial arteries. Peptides. 1984;5(2):385–8. doi: 10.1016/0196-9781(84)90239-0. [DOI] [PubMed] [Google Scholar]
- 9.Bleys RL, Thrasivoulou C, Cowen T. Cavernous sinus ganglia are sources for parasympathetic innervation of cerebral arteries in rat. J Cereb Blood Flow Metab. 2001;21(2):149–56. doi: 10.1097/00004647-200102000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Suzuki N, Hardebo JE. The cerebrovascular parasympathetic innervation. Cerebrovasc Brain Metab Rev. 1993;5(1):33–46. [PubMed] [Google Scholar]
- 11.Cline DL, Short LI, Forster MAM, Gray SL. Adipose tissue expression of PACAP, VIP, and their receptors in response to cold stress. J Mol Neurosci. 2019;68(3):427–438. doi: 10.1007/s12031-018-1099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holm C, Langin D, Manganiello V, Belfrage P, Degerman E. Regulation of hormone-sensitive lipase activity in adipose tissue. Methods Enzymol. 1997;286:45–67. doi: 10.1016/s0076-6879(97)86004-1. [DOI] [PubMed] [Google Scholar]
- 13.Neumann S, Krieger CC, Gershengorn MC. Targeting TSH and IGF-1 receptors to treat thyroid eye disease. Eur Thyroid J. 2020;9(Suppl 1):59–65. doi: 10.1159/000511538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagnon A, Antunes TT, Ly T, Pongsuwan P, Gavin C, Lochnan HA, Sorisky A. Thyroid-stimulating hormone stimulates lipolysis in adipocytes in culture and raises serum free fatty acid levels in vivo. Metabolism. 2010;59(4):547–53. doi: 10.1016/j.metabol.2009.08.018. [DOI] [PubMed] [Google Scholar]
- 15.Finlay JM, Zigmond MJ, Abercrombie ED. Increased dopamine and norepinephrine release in medial prefrontal cortex induced by acute and chronic stress: Effects of diazepam. Neuroscience. 1995;64(3):619–28. doi: 10.1016/0306-4522(94)00331-x. [DOI] [PubMed] [Google Scholar]
- 16.McKlveen JM, Myers B, Herman JP. The medial prefrontal cortex: Coordinator of autonomic, neuroendocrine and behavioural responses to stress. J Neuroendocrinol. 2015;27(6):446–56. doi: 10.1111/jne.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sawchenko PE, Swanson LW. Central noradrenergic pathways for the integration of hypothalamic neuroendocrine and autonomic responses. Science. 1981;214(4521):685–7. doi: 10.1126/science.7292008. [DOI] [PubMed] [Google Scholar]
- 18.Van Eden CG, Buijs RM. Functional neuroanatomy of the prefrontal cortex: autonomic interactions. Prog Brain Res. 2000;126:49–62. doi: 10.1016/S0079-6123(00)26006-8. [DOI] [PubMed] [Google Scholar]
- 19.Adler ES, Hollis JH, Clarke IJ, Grattan DR, Oldfield BJ. Neurochemical characterization and sexual dimorphism of projections from the brain to abdominal and subcutaneous white adipose tissue in the rat. J Neurosci. 2012;32(45):15913–21. doi: 10.1523/JNEUROSCI.2591-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carpenter MB, Periera AB, Guha N. Immunocytochemistry of oculomotor afferents in the squirrel monkey (Saimiri sciureus) J Hirnforsch. 1992;33(2):151–67. [PubMed] [Google Scholar]
- 21.Samuels ER, Szabadi E. Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: Principles of functional organisation. Curr Neuropharmacol. 2008;6(3):235–53. doi: 10.2174/157015908785777229. [DOI] [PMC free article] [PubMed] [Google Scholar]