Abstract
Carotenoids are tetraterpene pigments that are present in photosynthetic bacteria, some species of archaea and fungi, algae, plants, and animals. Carotenoids are essential pigments in photosynthetic organs along with chlorophylls. Carotenoids also act as photo-protectors, antioxidants, color attractants, and precursors of plant hormones in plants. Carotenoids in animals play important roles, such as precursors of vitamin A, photo-protectors, antioxidants, enhancers of immunity, and contributors to reproduction. More than 850 kinds of carotenoids are present in nature. The structures are similar and all of them are labile. Analysis of natural carotenoids requires the establishment of reliable methods for analyzing them. Liquid chromatography–mass spectrometry (LC-MS) and mass spectrometry/mass spectrometry (MS/MS) coupled with photodiode array detector (DAD) is an important tool for analysis of natural carotenoids. Electrospray ionization and atmospheric pressure chemical ionization are commonly used for ionization of LC-MS of carotenoids. MS and MS/MS provide not only molecular weight information but also some structural information on carotenoids. Ultraviolet-visible spectra from DAD provide information on chromophore systems, which cannot be provided by MS spectral data. In the present review, I report the structural diversity and function of natural carotenoids, and also describe the techniques for analysis of natural carotenoids using the LC-DAD-MS and MS/MS system.
Keywords: carotenoids, analysis, LC-MS, MS/MS, photodiode array detector
1. INTRODUCTION
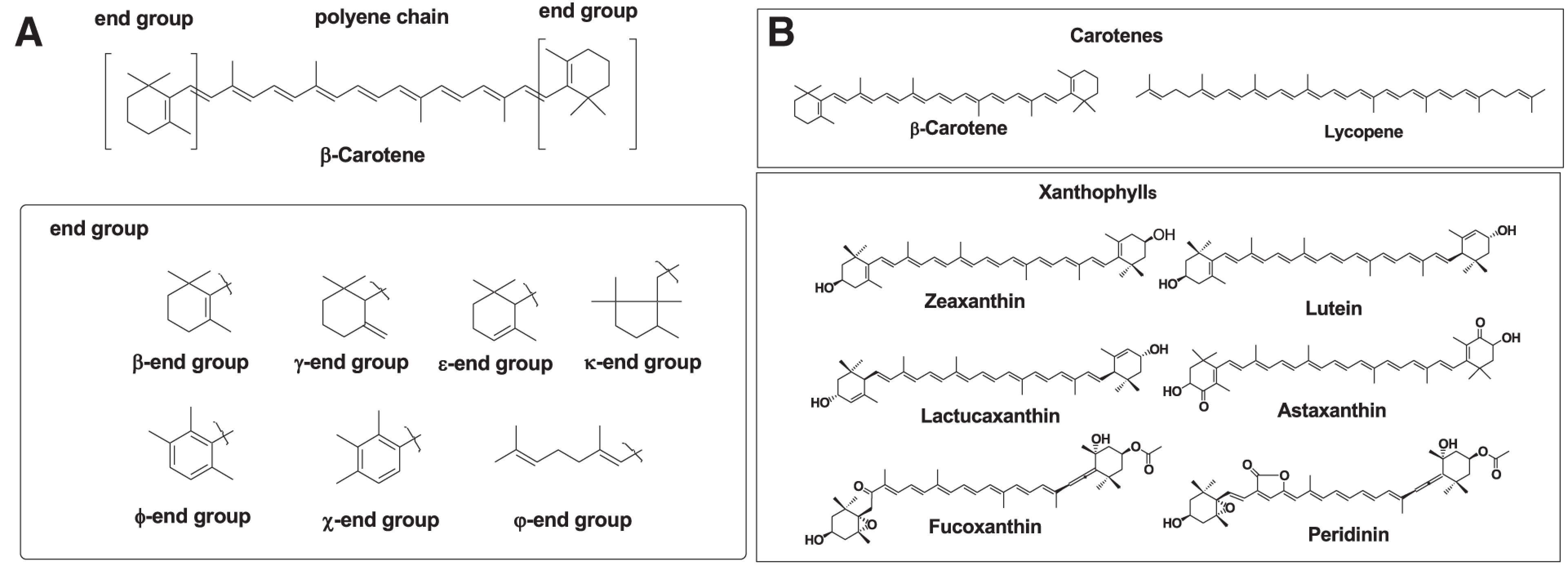
Carotenoids are tetraterpene pigments that exhibit yellow, orange, red, and purple colors. Carotenoids are distributed in photosynthetic bacteria, some species of archaea and fungi, algae, plants, and animals. Generally, carotenoids consist of a polyene chain with nine conjugated double bonds and an end group at both ends of the polyene chain. The structures of the polyene chain and end groups of carotenoids are shown in Fig. 1A.1) Carotenoids are divided into two groups: carotenes and xanthophylls. Carotenes are hydrocarbons. About 50 kinds of carotenes, such as α-carotene, β-carotene, and lycopene, are present in nature.1) On the other hand, xanthophylls are carotenoids containing oxygen atoms as hydroxy, carbonyl, aldehyde, carboxylic, epoxide, and furanoxide groups in these molecules. Therefore, the structure of xanthophylls shows marked diversity. About 800 kinds of xanthophylls, such as β-cryptoxanthin, lutein, zeaxanthin, lactucaxanthin, astaxanthin, fucoxanthin, and peridinin, have been reported in nature till now.1–3) Some xanthophylls are present as fatty acid esters, glycosides, sulfates, and protein complexes in nature. Figure 1B shows structures of typical carotenes and xanthophylls.
Fig. 1. (A) Basic structures of carotenoids and end groups. (B) Structures of typical carotenes and xanthophylls. Reprinted from Ref. 3.
In this review, I describe structural diversity and function of natural carotenoids.2–6) Then, I also describe the techniques for analysis of natural carotenoids using the liquid chromatography (LC)-photodiode array detector (DAD)-mass spectrometry (MS) and mass spectrometry/mass spectrometry (MS/MS) system.
2. CAROTENOIDS IN PLANTS
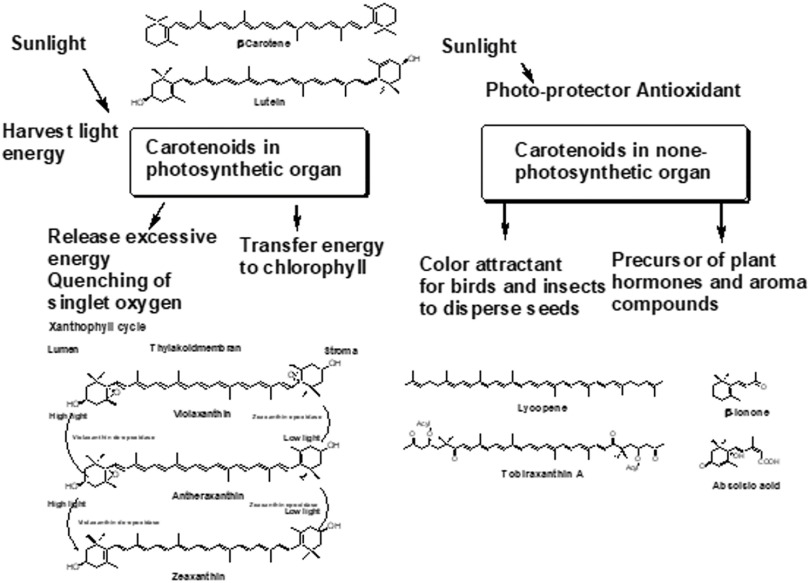
Carotenoids are essential compounds along with chlorophylls in photosynthetic organisms and are involved in photosynthesis and photo-protection. Carotenoids harvest light energy and transfer this energy to chlorophylls through singlet–singlet excitation transfer (Fig. 2). Carotenoids with more than eleven conjugated double bonds show a marked capacity to quench singlet oxygen.2–5) Xanthophyll cycles protect plants against oxidative stress generated by high light intensity. Xanthophyll cycles have in common the light-dependent transformation of epoxidized xanthophylls (violaxanthin and antheraxanthin) to de-epoxidized one (zeaxanthin) in high light, which facilitates the dissipation of excitation energy and their reversion to epoxidized xanthophylls in low light (Fig. 2).2–5)
Fig. 2. Role of carotenoids in plants. Reprinted from Ref. 3.
Carotenoids are also present in non-photosynthetic organs of plants such as fruits, pericarps, seeds, roots, and flowers. Carotenoids in these none-photosynthetic organs show structural diversity and are formed by secondary metabolic reactions.2–5) Carotenoids in non-photosynthetic organs act as photo-protectors, antioxidants, color attractants, and precursors of plant hormones such as abscisic acid (Fig. 2).2–5)
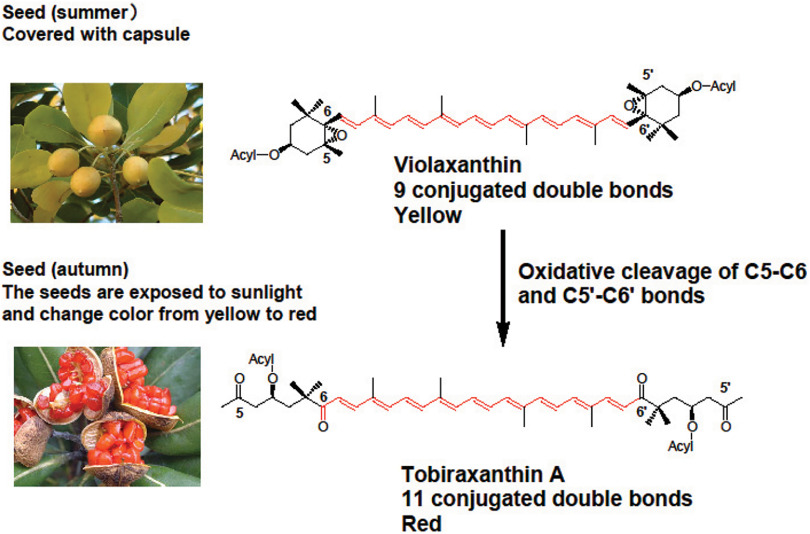
Many fruits and seeds turn red or purple during the ripening stage due to the formation of carotenoids and/or anthocyanins. These pigments protect seeds from photo-oxidation. Here, I describe examples of structural conversion and function of violaxanthin in the seeds of Pittosporum tobira (Tobera in Japanese) during the ripping stage.
P. tobira is a small, slender, evergreen tree growing in southern Japan. In summer, the seeds have a pale yellow color and are covered with a capsule. In autumn, the seeds are exposed to sunlight and change color from yellow to red. The major carotenoid in the yellow seeds is violaxanthin, with a pale yellow color, and related epoxy carotenoids. On the other hand, the major carotenoid in the red seeds is a series of red seco-carotenoids named as tobiraxanthin A. The formation mechanism of tobiraxanthin from violaxanthin is shown in Fig. 3. Tobiraxanthin A shows an approximately 30-nm longer wavelength shift than violaxanthin. Therefore, tobiraxanthin A shows strong activity to quench of singlet oxygen induced by sunlight. Furthermore, the red color of the seed acts as an attractant for birds to eat seeds in order to disperse them.2,3)
Fig. 3. Formation of tobiraxanthin from violaxanthin in the seeds of Pittosporum tobira. Reprinted from Ref. 3.
The seed of P. tobira contains interesting structural carotenoids such as violaxanthin–α-tocophel complex. These carotenoids’ formation mechanisms were described in my review.2,3)
3. CAROTENOIDS IN ANIMALS
The important role of carotenoid in animals is precursor of vitamin A. Carotenoids that contain unsubstituted β-ionone rings such as β-carotene, α-carotene, γ-carotene, and β-cryptoxanthin are precursor of retinoids and are called pro-vitamin A. Furthermore, carotenoids in animals play important roles such as photo-protectors, antioxidants, enhancers of immunity, and contributors to reproduction. Carotenoids are also used as signals for intra-species (sexual signaling, social status signaling, and parent–offspring signaling) and inter-species (species recognition, warning coloration, mimicry, and crypsis) communication in several animals.2,3,6)
Animals do not synthesize carotenoids de novo, and so those found in animals are either directly obtained from food or partly modified through metabolic reactions.2,3,6) Metabolic genes and enzymes of carotenoids in animals have not been revealed yet, except for few exceptions.2,3,6)
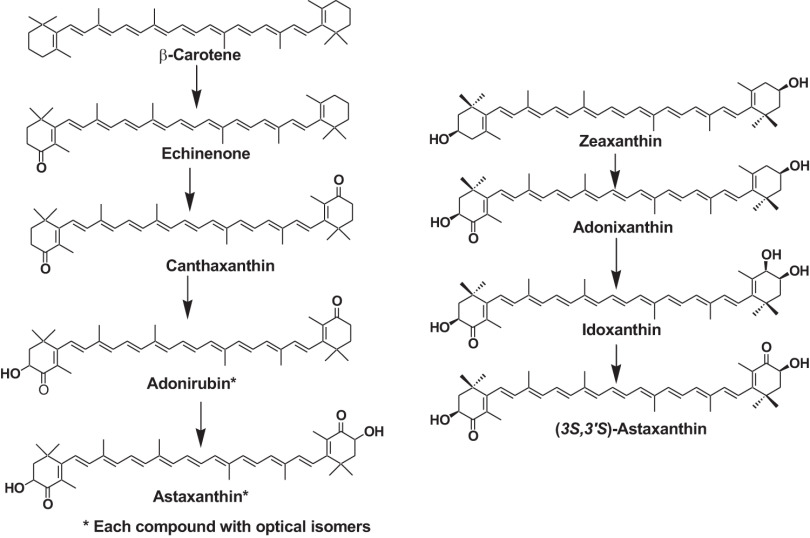
Many marine invertebrates such as crustaceans convert β-carotene to astaxanthin and accumulate it in integuments, carapaces, eggs, and ovaries. Zeaxanthin is also converted to astaxanthin (Fig. 4). Through the metabolic conversion, the carotenoid changes its color from yellow (β-carotene and zeaxanthin) to red (astaxanthin). Astaxanthin in marine invertebrates sometimes forms a carotenoid protein complex and is in a red, blue, or purple color. These colors may serve to camouflage the animals in the prevailing undersea light conditions, serve as general photoreceptors, or provide protection against possible harmful effects of light. Furthermore, through this metabolic conversion, the antioxidant effects of carotenoids such as the quenching of singlet oxygen, inhibiting lipid peroxidation, and protection against photo-oxidation are enhanced.2,3,6)
Fig. 4. Formation pathway of astaxanthin in marine animals. Reprinted from Ref. 3.
Salmon absorbs astaxanthin from dietary crustaceans and accumulates it in muscle during migration in ocean. In the breeding period, male salmon transfers astaxanthin from muscle to skin for breeding coloration. Female salmon transfers astaxanthin from muscle to egg for antioxidants. Astaxanthin also protects oxidative damage of muscle during upstream. Furthermore, spawned salmon eggs are exposed to sunlight; therefore, astaxanthin in the eggs acts as a photo-protector.
As with aquatic animals, most terrestrial animals cannot synthesize carotenoids de novo and so must obtain them from their diet. Therefore, carotenoids in terrestrial animals mainly originate from plants that they feed on. Many of the carotenoids present in terrestrial animals are β-carotene, β-cryptoxanthin, lutein, zeaxanthin, and their metabolites.3)
Here, I describe recent topics of animal carotenoids, which revealed carotenoid biosynthetic genes.
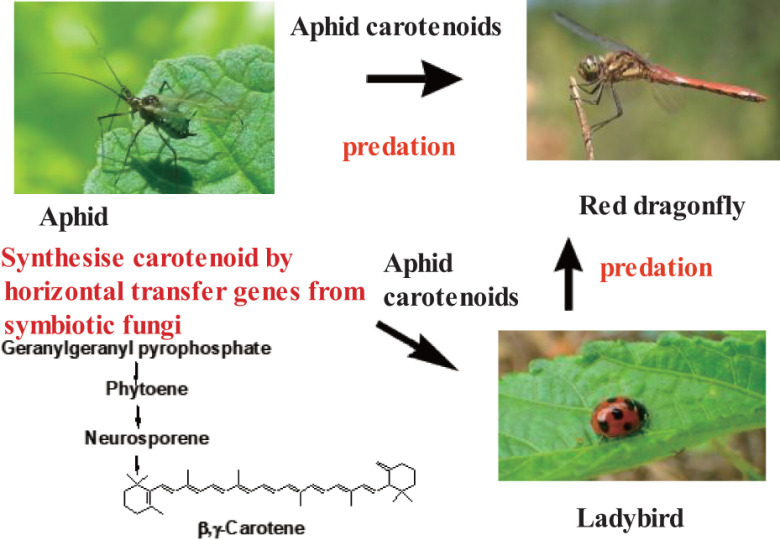
Insects are the most diverse group of animals. Therefore, carotenoids in insects show structural diversity. Carotenoids play important functional roles such as a photo-protector, an antioxidant, and for protective coloration in insects. Many of the carotenoids present in insects are β-carotene, β-cryptoxanthin, lutein, and zeaxanthin, which originate from their food. On the other hand, aphids and whitefly are sap-feeding insects that cannot intake carotenoids from their host plants. Therefore, aphid and whitefly synthesize carotenoids de novo by carotenoid biosynthesis genes that are acquired via horizontal gene transfer from fungi or endosymbiotic bacteria. These insects synthesize β-zeacarotene, β,ψ-carotene (γ-carotene), torulene, β,γ-carotene, and γ,γ-carotene by carotenoid biosynthesis genes transferred from fungi or endosymbiotic bacteria7,8) (Fig. 5). These carotenoids are also distributed in beetles and dragonflies through the food chain.8)
Fig. 5. Origin of β,γ-carotene in an aphid and distribution of this carotenoid through food chain.
In response to long nights and lower temperatures, the female spider mite Tetranychus urticae enters diapause and a marked change in body color from faint yellow to bright red-orange occurs. The red body color of spider mite is due to the presence of carotenoid such as astaxanthin. A recent investigation revealed that carotenoid cyclase/synthase and carotenoid desaturase genes are present in the two-spotted spider mite T. urticae. These carotenoid biosynthetic genes might be transferred from fungi into the spider mite genome.9,10)
Birds accumulate not only carotenes but also xanthophylls in their body. Carotenoids present in birds are lutein, zeaxanthin, canthaxanthin, astaxanthin and their metabolites.3) Zebra finch and red siskins convert β-carotene to canthaxanthin and astaxanthin by carotenoid 4-ketolase. Recently, carotenoid 4-ketolase gene was identified in zebra finch, red siskins, and canary. CYP2J19 loci were most likely to encode carotenoid 4-ketolases that generate red ketocarotenoids. CYP2J19 loci were considered to be involved in both red coloration and red retinal oil droplets. Such an involvement of cytochrome P450s may provide a novel mechanism of signal honesty.11–13)
4. ANALYSIS OF NATURAL CAROTENOIDS USING LC-DAD-MS AND MS/MS
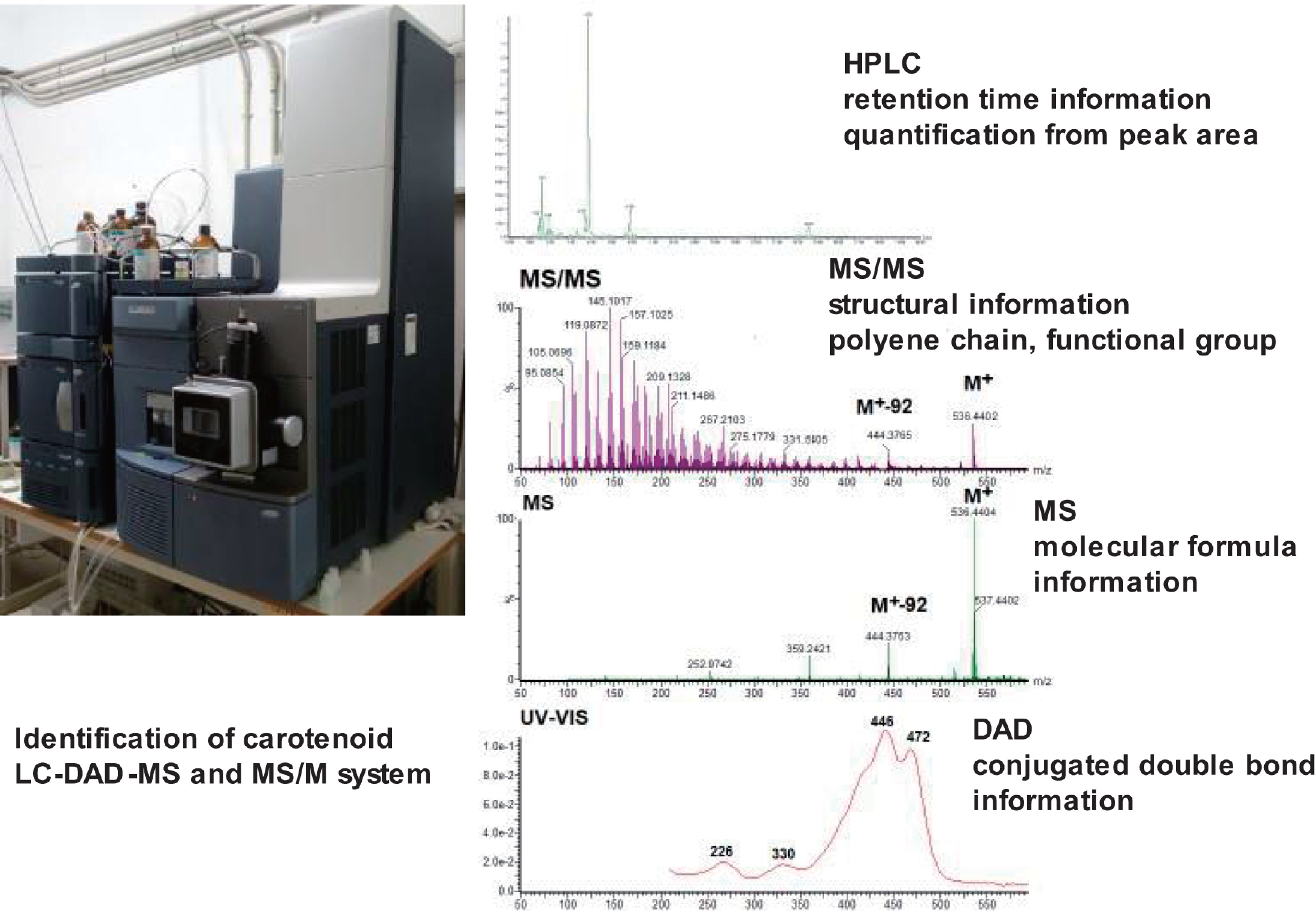
As described previously, more than 850 kinds of carotenoids are present in nature. The structures are similar and all of them are labile. Analysis of natural carotenoids requires the establishment of reliable methods for analyzing them. The LC-MS and MS/MS instrument coupled with DAD is a powerful tool for the identification and quantification of natural carotenoids.14–24) This method allows carotenoids to be characterized by the retention time in high performance liquid chromatography (HPLC), MS and MS/MS spectral data, and ultraviolet (UV)-visible (Vis) absorption spectral data provided by DAD, and be quantified by the peak area of MS and/or DAD chromatograms (Fig. 6).
Fig. 6. Identification and quantification of natural carotenoids by LC-DAD-MS and MS/MS system. DAD, photodiode array detector; HPLC, high performance liquid chromatography; LC, liquid chromatography; MS, mass spectrometry.
4.1. Extraction and pre-preparation of carotenoids from biological samples
Carotenoids are labile compounds for oxidation, heat, and light. Therefore, extraction and purification procedures should be carried out rapidly below 40°C and exposure to strong light should be avoided. Methanol and ethanol are used for extraction from plant tissues. Acetone is commonly used for the extraction of carotenoids from biological materials, especially animal tissues.
Acetone, methanol, or ethanol extracts of animal organs contain marked amounts of polar lipids. The extracts are transferred to a two-layer solution composed of hexane, diethyl ether–hexane (1:1), or ethyl acetate solution and water. The organic phase, which contains carotenoids, is washed with water several times to remove acetone and water solve contaminants. The organic phase solvent is evaporated to dryness below 40°C or evaporated directly by a stream of nitrogen or inactive gas. Generally, steroids and neutral lipids (triglycerides, wax) are contained together with carotenoids in extracts. These lipid impurities are removed as much as possible before carotenoid analysis. Although LC/MS is a high-sensitivity tool, lipid contaminants sometimes prevent detection of carotenoids. It is important to remove lipid impurities as much as possible during pre-preparation of samples. In my experience, the sensitivity of carotenoid detection can be increased by about 10 to 100 times by removing lipid impurities during pre-preparation.
Same natural carotenoids exist as fatty acid esterified forms. Saponification with KOH/MeOH is commonly used for hydrolysis of carotenoid esters and also used on carotenoid extracts to remove triglycerides from lipid-rich samples. Many carotenoids are stable toward bases. However, saponification causes several side reactions for unstable alkaline carotenoids, such as astaxanthin and fucoxanthin. To avoid these side reactions, enzymatic hydrolysis using cholesterol esterase or lipase has been employed for hydrolysis of labile alkaline carotenoid esters. It is also possible that carotenoids are analyzed as fatty acid esterified forms.14)
Supercritical fluid extraction (SFE) is also applied for extraction of carotenoid from several vegetables, fruits, and food stuff.
4.2. HPLC system for LC-MS analysis of natural carotenoids
Reverse-phase HPLC using C8, C18 octadecylsilyl (ODS), and C30 bonded-phase columns are commonly used for natural carotenoid analysis. The C30 column is effective for separation of carotenes, including their geometrical isomers. Normal-phase HPLC with a silica gel column is also used for carotenoid analysis. However, it has been considered that the hexane solvent cannot be used for the ionization of LC-MS. We found that LC-atmospheric pressure chemical ionization (APCI) MS could be done with a normal-phase HPLC system using tetrahydrofuran as a polar solvent in hexane.14,19)
4.3. LC-MS spectra of carotenoids
Electrospray ionization (ESI) and APCI are commonly used for ionization methods of LC-MS.
ESI provides several molecular mass ions of carotenoids. For example, the molecular ion M+ is predominantly observed in carotenes. Both M+ and [M+H]+ ions are predominantly observed in hydroxy carotenoids, such as β-cryptoxanthin and zeaxanthin. Keto-carotenoids, such as astaxanthin, predominantly provide sodium adduct ions [M+Na]+ along with [M+H]+. ESI MS of violaxanthin provides alkaline metal adduct ions, [M+Na]+ and [M+K]+, predominantly along with the protonated molecule [M+H]+. Fragment ions on elimination of water from M+, [M+H]+, [M+Na]+, and [M+K]+ are also observed (Table 1).14,15,18,21)
Table 1. Typical ions observed in the positive ion mode ESI MS and products ions in ESI MS/MS of carotenoids.
| Compound | Molecular formula |
Molecular ion species | MS/MS precursor ion |
Product ions |
|---|---|---|---|---|
| Carotene | ||||
| β-Carotene | C40H56 | 536 (M+) | 536 (M+) | 444 ([M-92]+), 430 ([M-106]+), 203, 177 |
| α-Carotene | C40H56 | 536 (M+) | 536 (M+) | 480 ([M-56]+), 444 ([M-92]+), 430( [M-106]+), 388, 321, 267 |
| γ-Carotene | C40H56 | 536 (M+) | 536 (M+) | 467 ([M-69]+), 444 ([M-92]+), 375( [M-69-92]+), 269, 177 |
| β,γ-Carotene | C40H56 | 536 (M+) | 536 (M+) | 444 ([M-92]+), 430 ([M-106]+), 413, 399, 177 |
| Lycopene | C40H56 | 536 (M+) | 536 (M+) | 467( [M-69]+), 444 ([M-92]+), 375 ([M-69-92]+), 157, 145 |
| Torulene | C40H54 | 534 (M+) | 534 (M+) | 465 ([M-69]+), 442 ([M-92]+), 397, 177, 145, 119 |
| β-Zeacarotene | C40H58 | 538 (M+) | 538 (M+) | 446 ([M-92]+), 401, 309, 177, 119 |
| Xanthophyll | ||||
| Lutein | C40H56O2 | 591 ([M+Na]+), 569([M+H]+), 568 (M+) | 568 (M+) | 550 ([M-H2O]+), 512 ([M-56]+), 476 ([M-92]+), 430, 366, 338, 175, 145 |
| Zeaxanthin | C40H56O2 | 591 ([M+Na]+), 569([M+H]+), 568 (M+) | 568 (M+) | 550 ([M-H2O]+), 476 ( M-92]+), 434, 366, 338, 175, 145 |
| Diatoxanthin | C40H54O2 | 589 ([M+Na]+), 567([M+H]+), 566 (M+) | 567 ([M+H]+) | 548 ([M+H-H2O]+), 475 ([M+H-92]+), 459, 413, 199, 175, 157, 145, 119 |
| Alloxanthin | C40H52O2 | 587 ([M+Na]+), 565 ([M+H]+), 564 (M+) | 565 ([M+H]+) | 547 ([M+H-H2O]+), 473 ([M+H-92]+), 465, 199, 176, 157, 145, 119 |
| Nostoxanthin | C40H56O4 | 623 ([M+Na]+), 601([M+H]+), 600 (M+) | 601 ([M+H]+) | 583 ([M+H-H2O]+), 565 ([M+H-2H2O]+), 509 ([M+H-92]+), 493, 197, 173, 159 |
| 2,2′-Dihydroxy-astaxanthin | C40H52O6 | 651 ([M+Na]+), 629 ([M+H]+), 628 (M+) | 629 ([M+H]+) | 611 ([M+H-H2O]+), 593 ([M+H-2H2O]+), 575, 537 ([M+H-92]+), 523 ([M+H-106]+), 453, 189, 147 |
| Astaxanthin | C40H52O4 | 619 ([M+Na]+), 597 ([M+H]+), 596 (M+) | 597 ([M+H]+) | 579 ([M+H-H2O]+), 561, 505 ([M+H-92]+), 473, 379, 285, 201, 173, 147 |
| 619 ([M+Na]+) | 601 ([M+Na-H2O]+), 575 ([M+Na-44]+), 527 ([M+Na-92]+), 513 ([M+Na-106]+) | |||
| Canthaxanthin | C40H52O2 | 587 ([M+Na]+), 565 ([M+H]+), 564 (M+) | 565 ([M+H]+) | 473 ([M+H-92]+), 459 ([M+H-106]+), 427, 361, 347, 215, 203, 133 |
| 587 ([M+Na]+) | 495 ([M+Na-92]+), 481 ([M+Na-106]+) | |||
| Echinenone | C40H54O | 573 ([M+Na]+), 551 ([M+H]+), 550 (M+) | 551 ([M+H]+) | 459 ([M+H-92]+), 447 ([M+H-106]+), 203, 157, 133, 119 |
| Capsanthin | C40H56O3 | 607 ([M+Na]+), 585([M+H]+), 584 (M+) | 585 ([M+H]+) | 567 ([M+H-H2O]+), 432, 413, 388, 159, 145, 109 |
| Antheraxanthin | C40H56O3 | 607 ([M+Na]+), 585 ([M+H]+), 584 (M+) | 585 ([M+H]+) | 567 ([M+H-H2O]+), 541, 505 ([M+H-80]+), 492 ([M+H-92]+), 171, 159, 145, 123, 119 |
| Diadinoxanthin | C40H54O3 | 605 ([M+Na]+), 583 ([M+H]+), 582 (M+) | 583 ([M+H]+) | 565 ([M+H-H2O]+), 539, 503 ([M+H-80]+), 491 ([M+H-92]+), 223, 171, 157 |
| Violaxanthin | C40H56O4 | 623 ([M+Na]+), 601([M+H]+), 600 (M+) | 601 ([M+H]+) | 583 ([M+H-H2O]+), 565 ([M+H-2H2O]+), 521 ([M+H-80]+), 509 ([M+H-92]+), 491 ([M+H-92-H2O]+), 221, 171 |
| Fucoxanthin | C42H58O6 | 681 ([M+Na]+), 659 ([M+H]+), 658 (M+) | 659 ([M+H]+) | 641 ([M+H-H2O]+), 523 ([M+H-2H2O]+), 599 ([M+H-80]+), 581, 567 ([M+H-92]+), 549, 563, 489, 433, 441, 149 |
ESI, electrospray ionization; MS, mass specrometry.
In APCI MS, protonated molecule [M+H]+ is observed as a molecular mass ion for both carotenes and xanthophylls. The same fragment ions, such as [M+H-H2O]+ and [M+H-AcOH]+, are observed in xanthophylls19,23) (Table 2). Furthermore, intensities of these dehydrated fragment ions reflect the structural characteristics of the hydroxylated end group in carotenoids. Zeaxanthin (β,β-carotene-3,3′-diol), lutein (β, ε-carotene-3,3′-diol), and lactucaxanthin (ε,ε-carotene-3,3′-diol), which possess the same molecular formula of C40H56O2, showed significant differences in the intensities of ion peaks at m/z 569 [M+H]+, m/z 551 [M+H-H2O]+, and m/z 533 [M+H-2H2O]+, as shown in Table 2. Zeaxanthin showed a base peak at m/z 569 [M+H]+ with weak dehydrated ion at m/z 551 [M+H-H2O]+. In contrast, both lutein and lactucaxanthin possessing a 3-hydoxy-ε-end group showed m/z 551 [M+H-H2O]+ as the most abundant ion and also showed a fragment ion at m/z 533 [M+H-2H2O]+, which was hardly observed in zeaxanthin possessing a 3-hydoxy-β-end group. These fragmentation patterns were in good agreement with EI MS data (structures of zeaxanthin, lutein, and lactucaxanthin are shown in Fig. 1B). Carotenoid glycosides, such as myxol fucoside, showed fragment ions at m/z 567, with elimination of the sugar moiety from [M+H]+, as the most abundant ion.20)
Table 2. Typical ions observed in the positive ion mode APCI MS of carotenoids.
| Molecular formula | Major ions (abundance %) |
|---|---|
| C40H56 | 537 ([M+H]+) (100) |
| C40H56 | 537 ([M+H]+) (100) |
| C40H56 | 537 ([M+H]+) (100) |
| C40H56 | 537 ([M+H]+) (100) |
| C40H56O2 | 569 ([M+H]+) (100), 551 ([M+H-H2O]+) (10) |
| C40H56O2 | 569 ([M+H]+) (21), 551 ([M+H-H2O]+) (100), 533 ([M+H-2H2O]+) (5) |
| C40H56O2 | 569 ([M+H]+) (5), 551 ([M+H-H2O]+) (100), 533 ([M+H-2H2O]+) (10) |
| C40H52O4 | 597 ([M+H]+) (100) |
| C40H52O2 | 565 ([M+H]+) (100) |
| C40H54O | 551 ([M+H]+) (100) |
| C40H56O3 | 585 ([M+H]+) (100), 567 [M+H-H2O]+) (20) |
| C40H56O3 | 585 ([M+H]+) (100), 584 ([M+H-H2O]+) (20) |
| C40H56O4 | 601 ([M+H]+) (100), 583 ([M+H-H2O]+) (20) |
| C42H58O6 | 659 ([M+H]+) (42), 641 ([M+H-H2O]+) (100), 623 ([M+H-2H2O]+) (6), 641 ([M+H-H2O]+) (100), 581 ([M+H-H2O-AcOH]+) (70), 563 ([M+H-2H2O-AcOH]+) (6) |
| C39H50O7 | 631 ([M+H]+) (10), 613 ([M+H-H2O]+) (34), 596 ([M+H-2H2O]+) (6), 553 ([M+H-H2O-AcOH]+) (100), 535 ([M+H-2H2O-AcOH]+) (18) |
| C46H66O7 | 731 ([M+H]+) (10), 713 ([M+H-H2O]+) (14), 567 ([M+H-Fucose]+) (100) |
APCI, atmospheric pressure chemical ionization; MS, mass spectrometry.
Atmospheric pressure photo ionization (APPI) has recently been introduced as a new ionization method for LC-MS. In APPI MS, [M+H]+ was observed in the case of polar xanthophylls, such as astaxanthin, violaxanthin, and zeaxanthin. M+ was observed in the case of less polar xanthophylls such as echinenone, β-carotene, and carotenes.23)
4.4. LC-MS/MS spectra of carotenoids
Quadrupole–quadrupole and quadrupole-time-of-flight (Q-TOF) instruments with ESI or APCI have been widely used for MS/MS measurement of several natural products. Figure 7A shows ESI Q-TOF MS/MS of astaxanthin using the protonated molecule [M+H]+ at m/z 597 as a precursor ion. Product ions on elimination of water at m/z 579 [M+H-H2O]+, at m/z 561 [M+H-2H2O]+, elimination of the toluene moiety at m/z 505 [M+H-92]+, and m/z 147 were observed. On the other hand, ESI Q-TOF MS/MS of astaxanthin using the sodium adduct ion [M+Na]+ m/z 619 as a precursor ion led to elimination of the toluene moiety at m/z 527 [M+Na-92]+ and elimination of the xylene moiety at m/z 513 [M+Na-106]+ from the sodium adduct ion of astaxanthin (Fig. 7B). They were diagnostic products in EI MS of carotenoids.14,21,24) These product ions are also observed in the case of MS/MS spectrum of [M+K]+. However, intensities of these productions are very weak.
Fig. 7. ESI Q-TOF MS/MS spectrum of astaxanthin using (A) [M+H]+ and (B) [M+Na]+ as a precursor ion. ESI, electrospray ionization; MS, mass spectrometry; Q-TOF, quadrupole-time-of-flight.
![Fig. 7. ESI Q-TOF MS/MS spectrum of astaxanthin using (A) [M+H]+ and (B) [M+Na]+ as a precursor ion. ESI, electrospray ionization; MS, mass spectrometry; Q-TOF, quadrupole-time-of-flight.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/801a/10626154/6817994a75e6/massspectrometry-12-1-A0133-figure07.jpg)
Our research group measured ESI MS/MS spectral data of more than 100 natural carotenoids. These data are published in MassBank.JP.25)
Table 1 shows ESI MS/MS spectral data of typical natural carotenoids. The following characteristic product ions were observed.
Product ion from polyene chain
Eliminations on toluene (92 u) and xylene (106 u) moieties from [M+Na]+, [M+H]+, and M+ were generally observed in several carotenoids. Eliminations of H2O and AcOH from [M+H]+ and M+ were observed in several xanthophylls having hydroxy and/or acetyl groups.
Product ion from β-end group
Product ions on elimination of 137 u from [M+H]+ and M+ were observed in carotenoids with the β-end group, such as β-carotene. These ions were formed by cleavage between C-7 and C-8 bond.
Product ion from ε-end group
Product ions on elimination of 56 u from [M+H]+ and M+ were observed in carotenoids with the ε-end group, such as α-carotene. These ions were formed by cleavage between C-7 and C-8 bond.
Product ion from ϕ-end group
In the case of carotenoids having ϕ-end group, such as γ-carotene and lycopene, product ions on elimination of 69 and 137 u from [M+H]+ and M+ were observed. These product ions were formed by cleavage between C-3 and C-4 bond and C-7 and C-8 bond, respectively.
Product ion of epoxy carotenoid
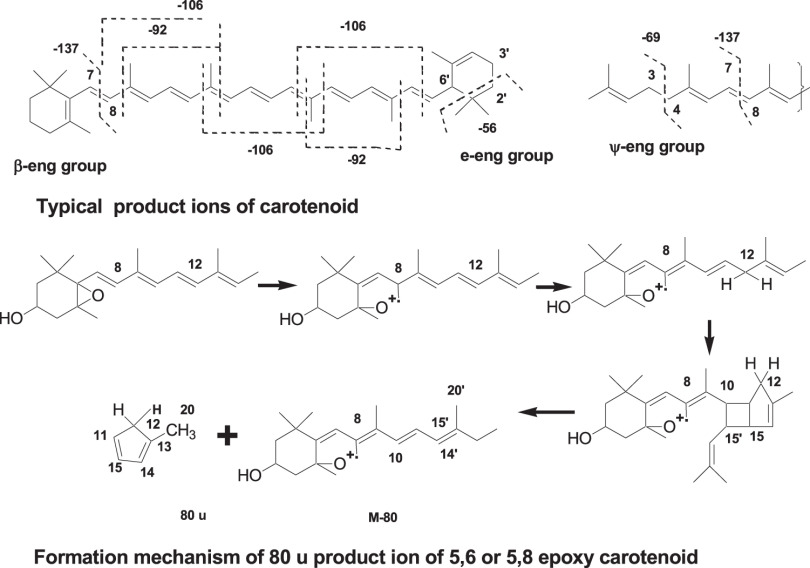
In epoxy carotenoids, such as antheraxanthin and violaxanthin, elimination of 80 u from [M+H]+ and M+ was observed. The formation mechanism of these products ions is presented in Fig. 8.14)
Fig. 8. Typical products ions of ESI MS/MS spectra of carotenoids. ESI, electrospray ionization.
4.5. DAD spectra of carotenoids
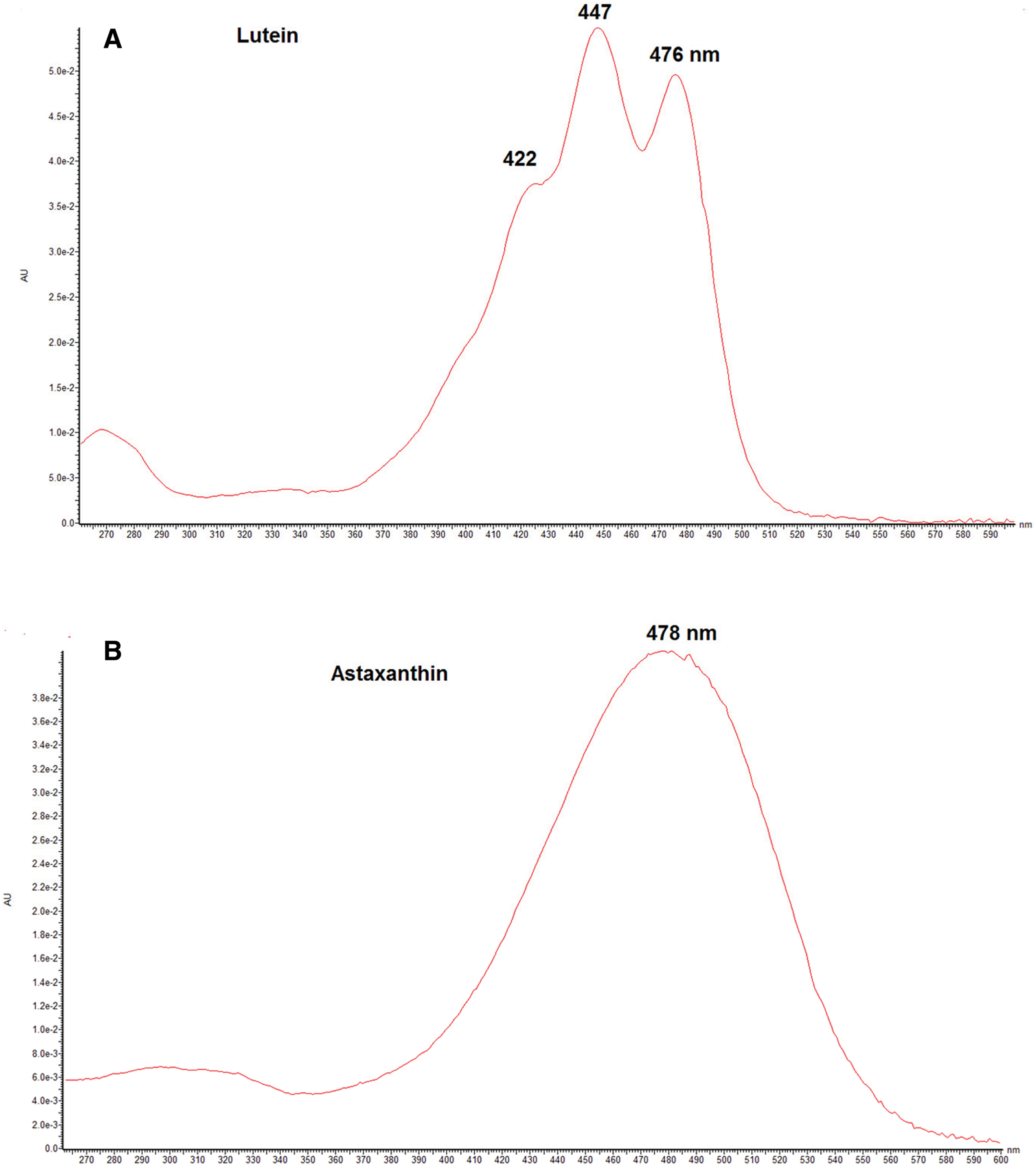
The UV-Vis absorption maxima obtained by online LC-DAD of typical carotenoids are shown in Table 3. These UV-Vis spectra provide chromophore information on carotenoids, which cannot be provided by MS spectral data. Therefore, α-carotene, β-carotene, γ-carotene, and lycopene, with the same molecular formula of C40H56 but having different conjugated double bond systems, can easily be characterized from the DAD spectrum. Many carotenoids show trimodal absorption spectra. On the other hand, carotenoids having a conjugated carbonyl group at C-4 in the β-end group, such as astaxanthin, show broad bell-shaped spectra (Fig. 9). Many natural carotenoids exist as an all-trans (all-E) form along with minor cis (Z) geometrical isomers. DAD spectra can be used to distinguish the cis (Z) isomer from the corresponding trans (Z) carotenoids.19)
Table 3. DAD spectral maxima of carotenoids in mobile phase; mixtures of acetonitrile:water (85:15) and acetonitrile:methanol (65:35).
| Compound | DAD max (nm) |
|---|---|
| Carotene | |
| β-Carotene | 423, 448, 476 |
| α-Carotene | 425*, 451, 476 |
| γ-Carotene | 440, 460, 490 |
| Lycopene | 448, 475, 506 |
| 9-cis-Lycopene | 360, 432, 461, 490 |
| 13-cis-Lycopene | 370, 430, 460, 448 |
| Xanthophyll | |
| Zeaxanthin | 425*, 451, 476 |
| Lutein | 423, 448, 476 |
| Lactucaxanthin | 415, 440, 465 |
| Astaxanthin | 477 |
| 9-cis-Astaxanthin | 368, 470 |
| 13-cis-Astaxanthin | 368, 468 |
| Canthaxanthin | 477 |
| Echinenone | 458 |
| Capsanthin | 472 |
| Antheraxanthin | 423, 448, 476 |
| Violaxanthin | 415, 440, 465 |
| Fucoxanthin | 446, 476 |
| Myxol fucoside | 450, 478, 510 |
*Shoulder.
DAD, photodiode array detector.
Fig. 9. DAD spectra of lutein (A) and astaxanthin (B). DAD, photodiode array detector.
4.6. Quantification of carotenoids using LC-DAD-MS
Carotenoids were quantified by the peak area of MS and/or DAD chromatogram.
The detection limit of LC-APCI MS is several nanograms.16,17) LC-ESI MS is highly sensitive and the detection limit is sub nanograms.15) We found the following detection limits of carotenoids in the LC-DAD-ESI MS system using a Waters Xevo G2S Q-TOF mass spectrometer equipped with an Acquity UPLC system (Waters, Milford, MA, USA). Full-scan ESI MS (m/z 100–1,500) and DAD (200–600 nm) spectra could be measured even with 0.5 ng of astaxanthin and α-carotene. Using the selected ion monitoring method, the detection limit for [M+H]+ of astaxanthin and M+ of α-carotene on chromatograms was approximately 0.05 ng.14) Using multiple reaction monitoring (MRM), the detection method remits and quantification remits of carotenoids are increased more.
4.7. Analysis of carotenoids in chlorella using LC-DAD-MS and MS/MS
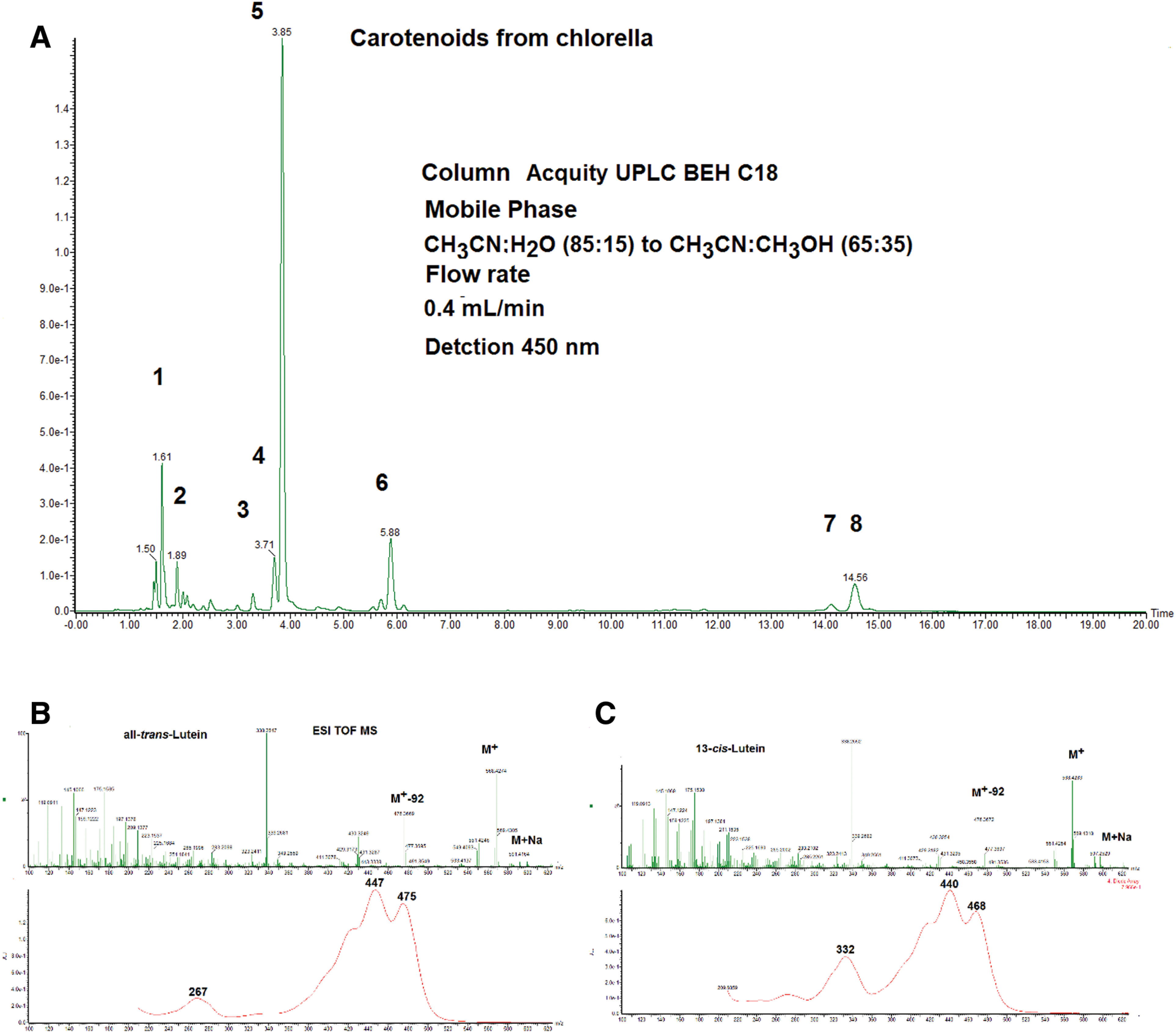
Figure 10 shows an HPLC chromatogram of carotenoids in chlorella. Seven carotenoids were identified by ESI MS, UV-Vis, and retention time in HPLC. Peaks 4 and 5 were identified as lutein and its 13-cis isomer, respectively. Lutein geometrical isomers, such as 9-cis and 13-cis, show the same ESI MS spectrum as that of all trans lutein. On the other hand, cis isomers of carotenoids show a characteristic absorption band in the UV-Vis spectrum around 332 nm with a different intensity (so-called cis peak). Peak 5 showed a characteristic absorption band at 332 nm and about 40% intensity of the main peak at 440 nm. This peak is a characteristic absorption band of 13-cis-isomer of carotenoids.14) Therefore, this lutein geometrical isomer was identified as 13-cis isomer. The UV-Vis spectrum obtained from DAD spectra was used for the characterization of geometrical isomers of carotenoids.14)
Fig. 10. (A) HPLC (detected at 450 nm) of carotenoids in Chlorella. Column: ACQUITY UPLC C18 1.7 μm 2.1 i.d. ×100 mm, mobile phase: acetonitrile:water (85:15) to acetonitrile:methanol (65:35), linear gradient, flow rate: 0.4 mL/min, and peak 1. deepoxyneoxanthin, 2. mutatoxanthin, 3. zeaxanthin, 4. all-trans-lutein, 5. 13-cis-lutein, 6. α-carotene, and 7. β-carotene. (B) UV-Vis and ESI MS spectra of all-trans-lutein. (C) UV-Vis and ESI MS spectra of 13-cis-lutein. ESI, electrospray ionization; HPLC, high performance liquid chromatography; MS, mass spectrometry; UV, ultraviolet; Vis, visible.
4.8. Analysis of carotenoids in red dragonfly using LC-DAD-MS and MS/MS
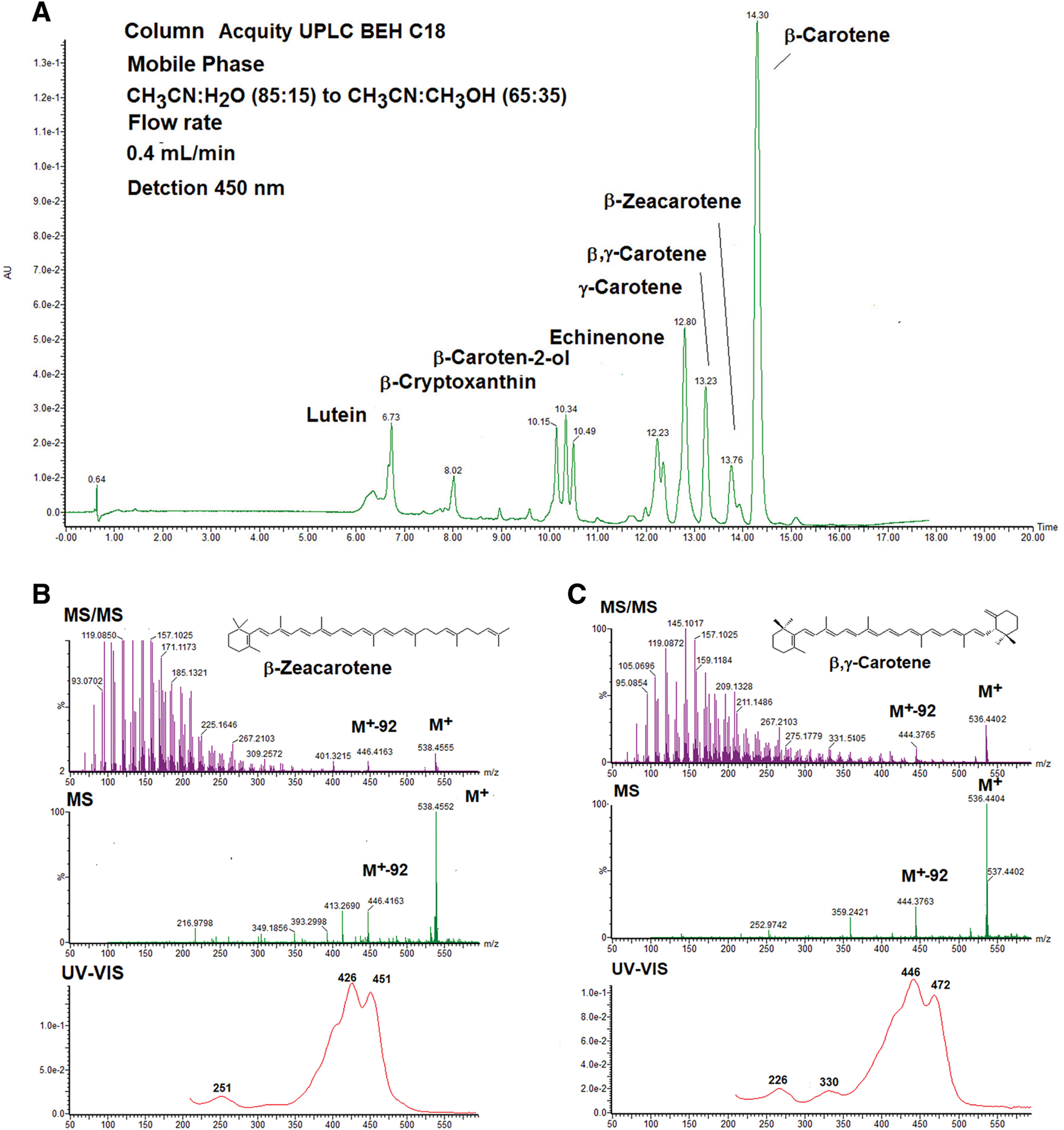
The second example shows carotenoid analysis in the red dragonfly Sympetrum frequens.26) Figure 11 shows an HPLC chromatogram of carotenoids in the red dragonfly S. frequens.26) Eight carotenoids were identified by ESI MS, MS/MS, and UV-Vis spectral data and retention time in HPLC, as shown in Fig. 11A. Figure 11B shows ESI MS, MS/MS, and UV-Vis spectra of β-zeacarotene. High-resolution MS of molecular mass ion of this carotenoid showed the molecular formula of C40H58. The UV-Vis spectrum showed that this carotenoid possessed eight conjugated double bonds in a polyene chain and one conjugated double bond in the end group. Product ions of MS/MS were also in agreement with published data of β-zeacarotene. Therefore, this carotenoid was identified as β-zeacarotene. Other carotenoids, excluding β,γ-carotene, were fully identified according to this method. Figure 11C shows ESI MS, MS/MS, and UV-Vis spectra of β,γ-carotene (molecular formula of C40H56). These spectral data closely resemble those of α-carotene, except for a slightly different retention time in HPLC. Therefore, this carotenoid could not be identified by LC-DAD-MS and MS/MS data. 1H-NMR (nuclear magnetic resonance) analysis was required to complete identification of this carotenoid.26)
Fig. 11. (A) HPLC (detected at 450 nm) of carotenoids in the red dragonfly Sympetrum frequens Column: ACQUITY UPLC C18 1.7 μm 2.1 i.d. ×100 mm, mobile phase: acetonitrile:water (85:15) to acetonitrile:methanol (65:35), linear gradient, and flow rate: 0.4 mL/min. (B) UV-Vis, ESI MS, and MS/MS spectra of β-zeacarotene. (C) UV-Vis, ESI MS, and MS/MS spectra of β,γ-carotene. ESI, electrospray ionization; HPLC, high performance liquid chromatography; MS, mass spectrometry; UV, ultraviolet; Vis, visible.
4.9. Analysis reaction products of carotenoids with reactive oxygen species using LC-DAD-MS and MS/MS
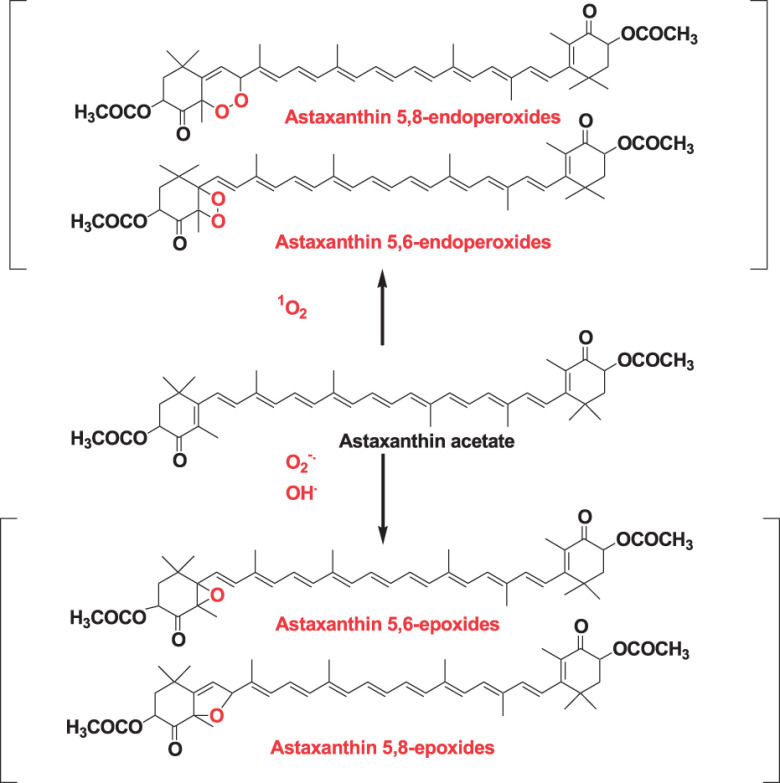
It was reported that the mechanism whereby carotenoids scavenge singlet oxygen was a physical reaction. Namely, carotenoids take up thermal energy from singlet oxygen and release this energy by polyene vibration.2,5) Recently, we investigated the reaction products of astaxanthin acetate with hydroxy radical, superoxide anion radical, and singlet oxygen by LC-DAD-ESI MS and MS/MS and electron spin resonance (ESR) spectrometry.27) The ESR study revealed that astaxanthin acetate could quench not only singlet oxygen but also superoxide anion radical and hydroxy radical. The reaction products were analyzed by LC-DAD-ESI MS and MS/MS. Astaxanthin acetate epoxides were identified as major reaction products of astaxanthin acetate with superoxide anion radical and hydroxyl radical. Similarly, astaxanthin acetate endoperoxides were identified as major reaction products of astaxanthin acetate with singlet oxygen, as shown in Fig. 12. The MS, MS/MS, and DAD spectral data of reaction products with reactive oxygen species with astaxanthin acetate are presented in Table 4.27) As an example, characterization of astaxanthin acetate 5,6-endoperoxide is described here. The molecular formula of astaxanthin acetate 5,6-endoperoxide as C44H56O8 was determined by high-resolution ESI MS ions of [M+H]+ and [M+Na]+. This molecular formula clearly indicated that two oxygen atoms attached to astaxanthin acetate (C44H56O6). Its UV-Vis showed an absorption maximum at 452 nm, consistent with the loss of two conjugated double bonds from 13 conjugated double bonds of astaxanthin acetate, which showed absorption maximum at 478 nm. Product ions of MS/MS spectra of [M+Na-92]+ and [M+Na-106]+ indicated that the structure of the polyene moiety of astaxanthin acetate was preserved. These spectral data indicated that the dioxetane moiety was attached to the C-5 and C-6 positions of astaxanthin acetate. Thus, the structure of astaxanthin acetate 5,6-endoperoxide can be characterized by ESI MS, MS/MS, and DAD spectral data. Similarly, structures of other compounds were characterized. Because of the instability of compounds, structures of these reaction products can be characterized using the LC-DAD-MS and MS/MS system.27) Similar results were also obtained in cases of astaxanthin, β-carotene, zeaxanthin, and capsanthin.27–29) These results indicate that carotenoids could take up singlet oxygen, superoxide anion radical, and hydroxyl radical by the formation of endoperoxide or epoxide.
Fig. 12. Reaction products of astaxanthin acetate with singlet oxygen, superoxide anion radicals, and hydroxyl radicals. Reprinted from Ref. 3.
Table 4. MS, MS/MS, and DAD spectral data of reaction products with reactive oxygen species with astaxanthin acetate.
| Compound | Molecular formula | ([M+H]+) | ([M+Na]+) | MS/MS product ion from ([M+Na]+) | DAD max (nm) |
|---|---|---|---|---|---|
| m/z | m/z | m/z | |||
| Astaxanthin acetate | C44H56O6 | 681.4157 | 703.3978 | 643, 611, 587, 583 | 478 |
| Astaxanthin acetate 5,6-epoxide | C44H56O7 | 697.4113 | 719.3909 | 659, 627, 613, 599 | 452 |
| Astaxanthin acetate 5,8-epoxide | C44H56O7 | 697.4113 | 719.3909 | 659, 627, 613, 599 | 430 |
| Astaxanthin acetate 5,6-endoperoxide | C44H56O8 | 713.4024 | 735.3873 | 675, 643, 629, 615 | 452 |
| Astaxanthin acetate 5,8-endoperoxide | C44H56O8 | 713.4024 | 735.3873 | 659, 627, 613, 599 | 430 |
DAD, photodiode array detector; MS, mass spectrometry.
4.10. Analysis of more complex carotenoids
Natural carotenoids, especially animal carotenoids, are presented as mixtures of closely similar structure compounds, such as geometrical, configurational, and optical isomers. In these cases, NMR and circular dichroism (CD) spectral data are needed for the identification of carotenoids. Chiral-phase HPLC analysis is also used for the identification of optical isomers of natural carotenoids.14)
4.11. Comprehensive metabolome analysis of natural carotenoids
Recently, comprehensive metabolome analysis using the LC-DAD-MS and MS/MS system has been widely used for several natural products. However, there are few reports on comprehensive metabolome analysis of natural carotenoids.30–32) More than 850 kinds of carotenoids are present in nature. Some of them are present as fatty acid esterified or glycoside forms. Therefore, it is difficult to construct an LC-DAD-MS and MS/MS database for comprehensive metabolome analysis of inclusive natural carotenoids. On the other hand, it is possible to construct an LC-DAD-MS and MS/MS database of carotenoids in certain species or geniuses of microorganisms, algae, plants, and animals. Indeed, our research group constructed an LC-DAD-MS and MS/MS database for analysis of carotenoids in dragonflies,26) aphids,33) and crustaceans.34) Furthermore, our research group has created a database of spectral data of carotenoids that are produced by several carotenoid biosynthetic genes transferred to Escherichia coli.35)
5. CONCLUSION
This review describes the natural distribution and function of carotenoids and techniques for analysis of natural carotenoids using the LC-DAD-MS and MS/MS system. The LC-DAD-MS and MS/MS systems are powerful tools for analysis of natural carotenoids. Progress of LC-DAD-MS and MS/MS system is advancing day by day. I hope that such progress of the LC-DAD-MS and MS/MS system will further reveal the distribution and function of minor natural carotenoids.
Acknowledgments
This review is based on my invited lecture at the 48th BMS conference, October 2022, Nara, Japan. I thank Dr. Takae Takeuchi of Nara Women’s University, all staff of the 48th BMS conference, and also Emeritus Professor Takaaki Nishioka, Kyoto University, for invitation MassBank project.
Mass Spectrom (Tokyo) 2023; 12(1): A0133
REFERENCES
- 1).G. Britton, S. Liaaen-Jensen, H. Pfander Eds. Carotenoids Hand Book, Birkhäuser, Basel, Switerland, 2004. [Google Scholar]
- 2).T. Maoka. Recent progress in structural studies of carotenoids in animals and plants. Arch. Biochem. Biophys. 483: 191–195, 2009. [DOI] [PubMed] [Google Scholar]
- 3).T. Maoka. Carotenoids as natural functional pigments. J. Nat. Med. 74: 1–16, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).G. Britton, S. Liaaen-Jensen, H. Pfander. Carotenoids Volume 3: Biosynthesis and Metabolism, Birkhäuser, Basel, Switzerland, 1998. [Google Scholar]
- 5).G. Britton, S. Liaaen-Jensen, H. Pfander. Carotenoids Volume 4: Natural Functions, Birkhäuser, Basel, Switzerland, 2008. [Google Scholar]
- 6).T. Maoka. Carotenoids in marine animals. Mar. Drugs 9: 278–293, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).N. A. Moran, T. Jarvik. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328: 624–627, 2010. [DOI] [PubMed] [Google Scholar]
- 8).M. Takemura, T. Maoka, T. Koyanagi, N. Kawase, R. Nishida, T. Tsuchida, M. Hironaka, T. Ueda, N. Misawa. Elucidation of the whole carotenoid biosynthetic pathway of aphids at the gene level and arthropodal food chain involving aphids and the red dragonfly. BMC Zool. 6: 19, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).B. Altincicek, J. L. Kovacs, N. M. Gerardo. Horizontally transferred fungal carotenoid genes in the two-spotted spider mite Tetranychus urticae. Biol. Lett. 8: 253–257, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).A. Bryon, A. H. Kurlovs, W. Dermauw, R. Greenhalgh, M. Riga, M. Grbic, L. Tirry, M. Osakabe, J. Vontas, R. M. Clark, T. Van Leeuwen. Disruption of a horizontally transferred phytoene desaturase abolishes carotenoid accumulation and diapause in Tetranychus urticae. Proc. Natl. Acad. Sci. USA 114(29): E5871–E5880, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).J. D. Blount, N. B. Metcalfe, T. R. Brikhead, P. F. Surain. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science 300: 125–127, 2003. [DOI] [PubMed] [Google Scholar]
- 12).N. R. Friedman, K. J. McGraw, K. E. Omland. Evolution of carotenoid pigmentation in caciques and meadowlarks (Icteridae): Repeated gains of red plumage coloration by carotenoid C4-oxygenase. Evolution 68: 791–801, 2014. [DOI] [PubMed] [Google Scholar]
- 13).N. I. Mundy, J. Stapley, C. Bennison, R. Tucker, H. Twyman, K.-W. Kim, T. Burke, T. R. Birkhead, S. Andersson, J. Slate. Red carotenoid coloration in the zebra finch is controlled by a cytochrome P450 gene cluster. Curr. Biol. 26: 1435–1440, 2016. [DOI] [PubMed] [Google Scholar]
- 14).T. Maoka. Structural studies of carotenoids in plants, animals, and food products, in Carotenoids Nutrition, Analysis and Technology (Ed: K. Agnieska, M. Baranska), Wiley Blackwell, UK, 2016, pp. 103–129. [Google Scholar]
- 15).R. B. van Breemen. Electrospray liquid chromatography-mass spectrometry of carotenoids. Anal. Chem. 67: 2004–2009, 1995. [Google Scholar]
- 16).R. B. van Breemen, C. R. Huang, Y. Tan, L. C. Sander, A. B. Schilling. Liquid chromatography/mass spectrometry of carotenoids using atmospheric pressure chemical ionization. J. Mass Spectrom. 31: 975–981, 1996. [Google Scholar]
- 17).P. A. Clarke, K. A. Barnes, J. R. Startin, F. I. Ibe, M. J. Shepherd. High performance liquid chromatography/atmospheric pressure chemical ionization-mass spectrometry for the determination of carotenoids. Rapid Commun. Mass Spectrom. 10: 1781–1785, 1996. [Google Scholar]
- 18).M. Careri, L. Elviri, A. Mangia. Liquid chromatography/electrospray mass spectrometry of β-carotene and xanthophylls: Validation of the analytical method. J. Chromatogr. A 854: 233–244, 1999. [DOI] [PubMed] [Google Scholar]
- 19).T. Maoka, Y. Fujiwara, K. Hashimoto, N. Akimoto. Rapid identification of carotenoids in a combination of liquid chromatography/UV-visible absorption spectrometry by photodiode-array detector and atmospheric pressure chemical ionization mass spectrometry (LC/PAD/APCI-MS). J. Oleo Sci. 51: 1–9, 2002. [Google Scholar]
- 20).V. V. de Rosso, A. Z. Mercadante. Identification and quantification of carotenoids, by HPLC-PDA-MS/MS, from Amazonian fruits. J. Agric. Food Chem. 55: 5062–5072, 2007. [DOI] [PubMed] [Google Scholar]
- 21).R. Frassanito, M. Cantonati, G. Flaim, I. Manchini, G. Guella. A new method for the identification and the structural characterisation of carotenoid esters in freshwater microorganisms by liquid chromatography/electrospray ionisation tandem mass spectrometry. Rapid Commun. Mass Spectrom. 22: 3531–3539, 2008. [DOI] [PubMed] [Google Scholar]
- 22).P. Crupi, R. A. Milella, D. Antonacci. Simultaneous HPLC-DAD-MS (ESI+) determination of structural and geometrical isomers of carotenoids in mature grapes. J. Mass Spectrom. 45: 971–980, 2010. [DOI] [PubMed] [Google Scholar]
- 23).S. Rivera, F. Vilaro, R. Canela. Determination of carotenoids by liquid chromatography/mass spectrometry: Effect of several dopants. Anal. Bioanal. Chem. 400: 1339–1346, 2011. [DOI] [PubMed] [Google Scholar]
- 24).Y. Weesepoel, J.-P. Vincken, P. M. Pop, K. Liu, H. Gruppen. Sodiation as a tool for enhancing the diagnostic value of MALDI-TOF/TOF-MS spectra of complex astaxanthin ester mixtures from Haematococcus pluvialis. J. Mass Spectrom. 48: 862–874, 2013. [DOI] [PubMed] [Google Scholar]
- 25).MSSJ MassBank. jp.
- 26).T. Maoka, N. Kawase, T. Ueda, R. Nishida. Carotenoids of dragonflies, from the perspective of comparative biochemical and chemical ecological studies. Biochem. Syst. Ecol. 89: 104001, 2020. [Google Scholar]
- 27).A. Nishino, T. Maoka, H. Yasui. Analysis of reaction products of astaxanthin and its acetate with reactive oxygen species using LC/PDA ESI-MS and ESR spectrometry. Tetrahedron Lett. 57: 1967–1970, 2016. [Google Scholar]
- 28).A. Nishino, H. Yasui, T. Maoka. Reaction of paprika carotenoids capsanthin and capsorubin with reactive oxygen species. J. Agric. Food Chem. 64: 4786–4792, 2016. [DOI] [PubMed] [Google Scholar]
- 29).A. Nishino, H. Yasui, T. Maoka. Reaction and scavenging mechanism of β-carotene and zeaxanthin with reactive oxygen species. J. Oleo Sci. 66: 77–84, 2017. [DOI] [PubMed] [Google Scholar]
- 30).B. P. Arathi, P. R.-R. Sowmya, K. Vijay, V. Baskaran, R. Lakshminarayana. Metabolomics of carotenoids: The challenges and prospects – A review. Trends Food Sci. Technol. 45: 105–117, 2015. [Google Scholar]
- 31).J. Zacarías-García, P. J. Cronje, G. Diretto, L. Zacarías, M. J. Rodrigo. A comprehensive analysis of carotenoids metabolism in two red-fleshed mutants of Navel and Valencia sweet oranges (Citrus sinensis). Front. Plant Sci. (New Haven) 13: 1034204, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32).J. Shi, W. Wu, Y. Zhang, S. Baldermann, Q. Peng, J. Wang, L. Xu, G. Yang, J. Fu, H. Lv, Z. Lin. Comprehensive analysis of carotenoids constituents in purple-coloured leaves and carotenoid-derived aroma differences after processing into green, black, and white tea. LWT-Food Sci. Technol. 173: 114286, 2023. [Google Scholar]
- 33).T. Maoka, N. Kawase, M. Hironaka, R. Nishida. Carotenoids of hemipteran insects, from the perspective of chemo-systematic and chemical ecological studies. Biochem. Syst. Ecol. 95: 104241, 2021. [Google Scholar]
- 34).T. Maoka. A new carotenoid, 5,6-dihydrocrustaxanthin, from prawns and the distribution of yellow xanthophylls in shrimps. Biochem. Syst. Ecol. 92: 104083, 2020. [Google Scholar]
- 35).N. Misawa, T. Maoka, M. Takemura. Carotenoids: Carotenoid and apocarotenoid analysis—Use of E. coli to produce carotenoid standards. in Methods in Enzymology, vol. 670 (Ed: E. T. Wurtzel), Academic Press, 2022, pp. 87–137. https://authors.elsevier.com/a/1eymzHRzCMNJC [DOI] [PubMed] [Google Scholar]