Abstract
Cancer therapy resistance and recurrence (CTRR) are the dominant causes of death in cancer patients. Recent studies have indicated that non-coding RNAs (ncRNAs) can not only reverse the resistance to cancer therapy but also are crucial biomarkers for the evaluation and prediction of CTRR. Herein, we developed CTRR-ncRNA, a knowledgebase of CTRR-associated ncRNAs, aiming to provide an accurate and comprehensive resource for research involving the association between CTRR and ncRNAs. Compared to most of the existing cancer databases, CTRR-ncRNA is focused on the clinical characterization of cancers, including cancer subtypes, as well as survival outcomes and responses to personalized therapy of cancer patients. Information pertaining to biomarker ncRNAs has also been documented for the development of personalized CTRR prediction. A user-friendly interface and several functional modules have been incorporated into the database. Based on the preliminary analysis of genotype–phenotype relationships, universal ncRNAs have been found to be potential biomarkers for CTRR. The CTRR-ncRNA is a translation-oriented knowledgebase and it provides a valuable resource for mechanistic investigations and explainable artificial intelligence-based modeling. CTRR-ncRNA is freely available to the public at http://ctrr.bioinf.org.cn/.
Keywords: Translational bioinformatics, Therapeutic resistance, Cancer recurrence, Non-coding RNA, Knowledgebase
Introduction
Cancer patients may develop resistance to previously effective treatments, including chemotherapy, radiotherapy, and immunotherapy [1], [2], [3]. Investigations of the mechanisms underlying cancer therapy resistance and recurrence (CTRR) are made complicated by the presence of confounding factors, such as heterogeneous genetic backgrounds, as well as diversity in cancer cells and tumor microenvironments [1], [2], [3], [4]. Reducing or eliminating the CTRR has been a tough challenge.
Protein-coding sequences only account for approximately 1.5% of the human genome, and the majority of the genome is associated with non-coding RNAs (ncRNAs), such as microRNAs (miRNAs), long ncRNAs (lncRNAs), and circular RNAs (circRNAs) [5], [6]. The evidence accumulated so far indicates that ncRNAs play crucial roles in the modulation of treatment resistance [7], [8]. For instance, miR-144-3p can regulate the resistance of lung cancer to cisplatin by targeting the gene encoding nuclear factor erythroid 2-related factor 2 (Nrf2) [9]. By inhibiting the Wnt2/β-catenin signaling pathway, LINC00968 can attenuate drug resistance in breast cancer [10]. Knockdown of hsa_circ_0081143 has been shown to reverse the cisplatin resistance by targeting the miR-646/CDK6 pathway in gastric cancer [11]. In addition, more and more evidence shows that ncRNAs have significant functions in regulating radiotherapeutic and immunotherapeutic resistance. Radiotherapeutic resistance in breast cancer can be modulated by miR-139-5p [12]. lncRNA CCAT2 and miR-191 promote resistance to radiotherapy in human oesophageal carcinoma cells and prostate cancer, respectively [13], [14]. The circRNA circFGFR1 can promote anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer [15]. As indicated in multiple reports, ncRNAs have been regarded as one of the most promising molecules to reverse therapeutic resistance [16], [17].
During the cancer treatment, some cancer cells may escape from their original locations and continue to survive. These cells may subsequently grow into large enough tumors to cause cancer recurrence, thereby increasing the complexity and difficulty of cancer treatment [18]. Previous research supports that ncRNAs could be proven to be biomarkers for diagnosis, treatment, and prognosis for recurrence in different types of cancer. For example, miRNA-125b could be a prognostic marker in recurrent hepatocellular carcinoma as its levels are significantly lower in the early stages than in the late stages of recurrence [19]. lncRNA CASC2a is identified as not only a prognosis biomarker but also a potential therapeutic target in the early recurrence of bladder cancer [20]. These data confirm the significance of ncRNAs in cancer recurrence [21], [22], [23], [24].
A large number of biological experiments and clinical trials have proven that many ncRNAs are involved in reversing treatment resistance and prevention of cancer recurrence [13], [25], [26], [27]. However, a public resource for ncRNAs related to CTRR is not unavailable. Indeed, some previously created databases focus on drug resistance, but they ignore the information regarding radiotherapeutic and immunotherapeutic resistance [28], [29], [30]. The data documented in these databases are derived from basic biological experiments without clinical records. With the accumulation of evidence for the correlation between ncRNAs and clinical traits observed in cancer patients, it is becoming increasingly necessary to build a database that integrates knowledge from biological and clinical domains. Such a database would be valuable for systematic modeling and personalized treatment of CTRR [31].
In recent decades, a variety of techniques are applied to identify biomarkers for CTRR. Most of the biomarker discovery methods are based on wet-lab experiments, such as small RNA sequencing and RT-qPCR assay [32], [33]. Computational approaches are also developed that are complementary to or integrated with experimental methods for efficient biomarker identification [34], such as, network-smoothed t-statistics-based biomarker detection [35] and network vulnerability-based identification of potential biomarkers [36], [37]. A competitive endogenous RNA (ceRNA) network has also been built to discover the critical position through network topology analysis for biomarker discovery [38]. However, until now, very few computational methods or knowledgebases have been developed that are specific for CTRR-associated ncRNA biomarker discovery.
To this end, we developed the CTRR-ncRNA knowledgebase to provide resources for CTRR-associated ncRNA biomarker discovery and customized analysis of the association between CTRR and ncRNAs. In this database, we have emphasized the regulatory mechanism of ncRNAs, the clinical characterization of ncRNAs associated with CTRR, and annotation of ncRNA biomarkers.
Data collection and processing
Data collection and knowledge extraction
We started by searching for literature in the PubMed database using the search terms listed in File S1. As a result, 3998 publications describing an association between CTRR and ncRNAs were obtained. These publications were further filtered using the following exclusion criteria: (1) research involving specimens other than human cell lines or tissues, (2) resistance alteration caused by epigenetic modification of ncRNAs, (3) resistance altered by the interaction between drugs and ncRNAs, and (4) reviews, meta-analyses, and bioinformatics analyses without experimental validation. Post filtering, biological and clinical information regarding ncRNAs and CTRR were manually curated.
For cancer therapy resistance (CTR), we retrieved the symbols of ncRNAs, cancer names, therapeutic methods, upstream regulatory factors and downstream targets of ncRNAs, biomarkers, and their clinical applications such as diagnosis, treatment, and prognosis, as well as the impact of ncRNA status, such as wildtype, knockdown, or overexpression on the treatment resistance. In addition, we paid much attention to the collection of clinical information related to CTR, including the difference in expression of an ncRNA between patients and healthy people, clinical sample size, sex ratio, and age ranges. Finally, the experimental details regarding the use of cell lines vs. patient’s tissues and the description of ncRNA’s role in CTR were collected. The following information was collated for the annotation of cancer recurrence: (1) cancer recurrence type, (2) overall survival of patients with altered ncRNA expression, (3) ratios of recurrence, (4) sex ratios of recurrence, (5) age ratios of recurrence, and (6) the differential expression between the recurrence and control groups.
Standardization of ncRNA nomenclature
To reduce the confusion caused by ncRNA synonyms, the names of miRNA and circRNA were standardized based on the names used in the miRbase (https://www.mirbase.org/) [39] and circBase (http://circbase.org/) [40], respectively. miRNA nomenclature has changed dramatically in recent years. The miRbase v22 was utilized as the reference for the standardization of miRNA nomenclature. For example, hsa-miR-24 used in the previous studies [41], [42] was renamed as “hsa-miR-24-3p” in CTRR-ncRNA. If the miRNAs were not included in miRbase v22, their original names were kept unchanged, such as “hsa-miR-128” [43]. Similar rules were applied for circRNA nomenclature, e.g., human circEIF6 [44] was standardized as hsa_circ_0060060 in accordance with the circBase. Both human circRNAs Cdr1as [45] and CDR1as [46] were standardized as hsa_circ_0001946. Currently, there are no unified nomenclature rules for lncRNA. Hence, we have used lncRNA names from the original resources. In addition, we have documented the body site of the cancers in the database as per International Classification of Diseases 11th Revision to provide information for personalized cancer diagnosis, prognosis, and treatment.
Online database implementation
The CTRR-ncRNA knowledgebase is available online at http://ctrr.bioinf.org.cn/. The web framework is Flask 1.0; the database is MySQL 5.7; the user interface framework is Bootstrap 4.4; and the website is deployed using Ngnix. It has been tested in Microsoft Edge, Mozilla, Chrome, and Safari browsers. A user-friendly interface is provided.
Cancer–ncRNA network construction and characterization
The cancer–ncRNA network was constructed based on the specific associations between different ncRNAs and cancer types. The different cancers were considered as phenotypes and the CTRR-related ncRNA statuses were regarded as the genotypes. Their relationships were analyzed at the systems biology level. The ncRNAs were classified as specific or universal, based on their relationship with cancers, i.e., if the association between the ncRNA and the cancer is unique, the ncRNA would be defined as specific. Otherwise, it was classified as universal. Cytoscape was used to visualize the cancer–ncRNA network [47]. The scale-free model [48] was applied to evaluate and characterize the cancer–ncRNA network.
Data content and usage
CTRR-ncRNA database statistics
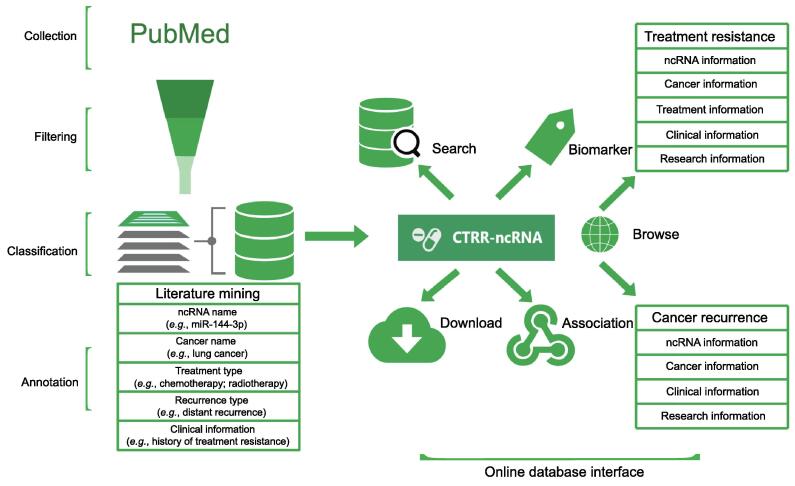
In total, 367 ncRNAs associated with CTR in 79 cancer types and 46 ncRNAs associated with cancer recurrence in 24 different cancer types are included in the CTRR-ncRNA knowledgebase. All the experimentally validated CTRR-associated ncRNAs, by RT-qPCR, Western blot, a luciferase reporter assay, etc., are included in the database. The ncRNA entries related to resistance in the database include 497 miRNAs (65%), 248 lncRNAs (32%), and 24 circRNAs (3%). Additionally, the ncRNA entries related to recurrence in the database include 33 miRNAs (69%) and 13 lncRNAs (31%). These statistics can be found on the website at http://ctrr.bioinf.org.cn/. The overview of the database construction and implementation is illustrated in Figure 1.
Figure 1.
An overview of data collection, filtering, classification, annotation, and online database interface
CTRR, cancer therapy resistance and recurrence; ncRNA, non-coding RNA.
Web pages and functions
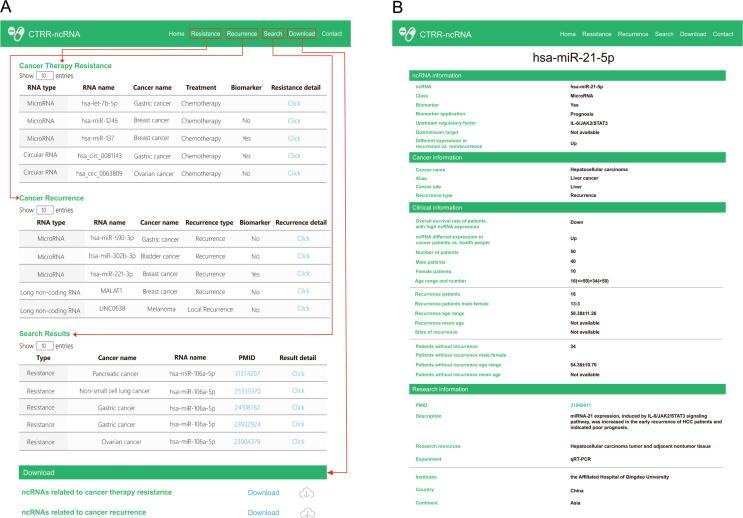
The CTRR-ncRNA knowledgebase includes multiple web pages including the Home page (Figure 2) that provide access to the information on CTRR, database search, data download, and contact information.
Figure 2.
A schematic design of CTRR-ncRNA
A. The “Resistance”, “Recurrence”, “Search”, and “Download” modules allow the users to browse, search, or download ncRNAs. B. Details of an ncRNA entry.
The Home page
The schematic diagram of the association between ncRNAs and CTRR is illustrated at the top of the page. A brief introduction of the CTRR-ncRNA database and its features are described at the bottom of the page.
The page for CTR
This page is designed to access information regarding CTR and the associated ncRNAs. Users can extract the information by ncRNAs, cancers, biomarkers, clinical applications, personalized treatments, upstream regulatory factors and downstream targets of ncRNAs, and CTR-associated ncRNA statuses. The entry details can be displayed by clicking on the “Click” button to check the information about the ncRNAs, cancer types, treatment, clinical information, and details about the original research.
The page for cancer recurrence
We have provided detailed clinical information regarding cancer recurrence cases including the overall survival of patients with abnormal ncRNA expression and the clinical information of cancer recurrence patients. Cancer patients’ overall survival values are correlated with the expression levels of the associated ncRNAs. Therefore, the CTRR-ncRNA would be proven to be helpful for the personalized prognosis of cancer recurrence cases.
The Search page
On this page, a quick fuzzy search function is provided for the users for efficient data retrieval. The contents of the CTRR-ncRNA knowledgebase have been classified structurally and users can quickly screen the data of interest by ncRNA names, cancer names, treatment or recurrence types, and biomarker applications.
The Download and Contact pages
The Download page is available for the users to download all entries of the CTRR-ncRNA knowledgebase. A tutorial for the usage of the database is provided on the Contact page.
Comparison with the existing databases
To date, several databases are available that have collected the ncRNAs related to drug resistance. They are well-structured and include information regarding ncRNAs and drug targets, but no databases were established about ncRNAs associated with CTRR. CTRR-ncRNA collected the 42 ncRNA records regarding radiotherapeutic and immunotherapeutic resistance which are useful for the research of different treatment. The advantages of our database as compared to other related databases are presented in Table 1.
Table 1.
Comparison of collections in different databases
|
Entity association |
Entity property |
CTRR-ncRNA |
NRDTD |
NoncoRNA |
| ncRNA association | ncRNA name | √ | √ | √ |
| Upstream regulatory factor | √ | × | × | |
| Downstream target | √ | × | √ | |
| Expression | √ | × | √ | |
| Biomarker | √ | × | × | |
| Biomarker type | √ | × | × | |
| Cancer association | Cancer type | √ | √ | √ |
| Cancer site | √ | × | × | |
| Resistance association | Chemotherapy resistance | √ | √ | √ |
| Radiotherapy resistance | √ | × | × | |
| Other therapy resistance | √ | × | × | |
| Clinical association | Basic patient information | √ | × | × |
| Overall survival rate of patients | √ | × | × | |
| Information for patients with cancer therapy resistance | √ | × | × | |
| Information for patients with or with no cancer recurrence | √ | × | × |
Note: CTRR, cancer therapy resistance and recurrence; ncRNA, non-coding RNA; NRDTD, ncRNA Drug Targets Database.
Personalized diagnosis, treatment, and prognosis of cancer are the key issues in this era of precision medicine. It is necessary to combine personalized clinical information with molecular omics profiles. However, most of the existing ncRNA databases are focused on the relationship between ncRNAs and drugs, but a lack of the information associated with clinical attributes limits the application of these databases for clinical use. For example, the ncRNA Drug Targets Database (NRDTD) has primarily collected descriptive information related to diseases, drugs, and ncRNAs, without clinical information or clinical applications [28]. It also lacks a description of the relationship between diseases, drugs, and ncRNA. Compared to the NRDTD database, the NoncoRNA database has a more detailed description of the relationship between diseases, drugs, and ncRNA [30]. However, it does not include details about the association between ncRNAs and therapy resistance or their clinical applications.
The CTRR-ncRNA contains information about the relationship between ncRNA expression and CTRR. The upstream regulatory factors and downstream targets of ncRNAs, and the clinical information of patients including sample sizes, sex ratios, age ranges, and expression of ncRNAs are included in the present database. We have also paid special attention to the ncRNA biomarkers, which have applications in the CTRR-related diagnosis, treatment, and prognosis.
In the design and implementation of our database, we have focused on the integration of clinical information with the classification of CTRR. The cancer recurrence was classified as local, distant, local and distant, early, and metastatic recurrence. The clinical information regarding overall survival and ncRNA expression was extracted from the literature.
Cancer–ncRNA network characterization
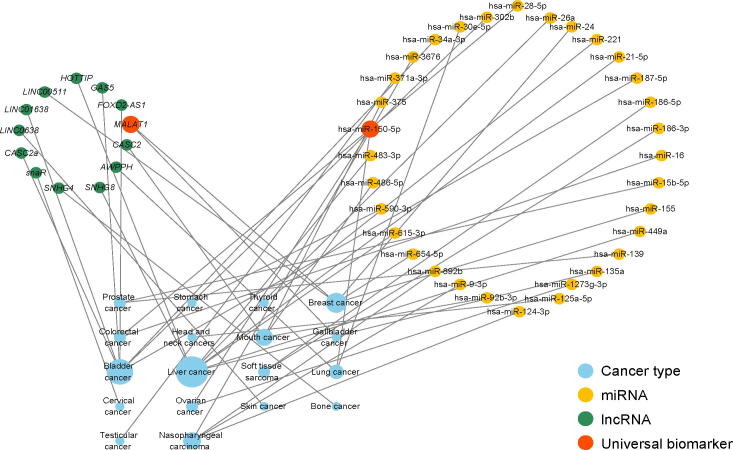
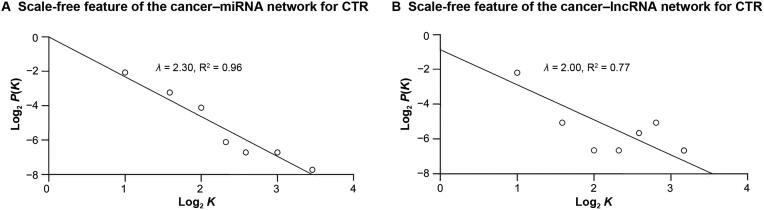
To investigate the genotype–phenotype relationship in CTRR, we first constructed cancer–ncRNA association network for cancer recurrence, as shown in Figure 3. The hsa-miR-150-5p and lncRNA MALAT1 are universal ncRNAs, which are associated with many types of cancers. The hsa-miR-150-5p is reported as a prognostic biomarker for recurrent ovarian cancer, and lncRNA MALAT1 is an indicator for the recurrence of gallbladder cancer [49]. As presented in Figure S1, most of the universal ncRNAs are reported as biomarkers in CTR, such as hsa-miR-31-5p [50], [51], [52], HOTAIR [53], MALAT1 [54], and LINC00152 [55]. The scale-free model [] that fitted our cancer–ncRNA network for CTR is presented in Figure 4. The λ values are 2.30 and 2.00 for the cancer–miRNA network and cancer–lncRNA network associated with CTR, respectively. The λ value between two and three indicates that the ncRNA nodes connected to many cancer types are crucial [56], and many experimental results support that universal ncRNAs are more likely to be biomarkers in CTR [57].
Figure 3.
An overview of the cancer–ncRNA network associated with cancer recurrence
The blue circles represent cancer types, and the size of the blue circles is proportional to the number of cancer-associated ncRNAs. The yellow circles represent miRNAs, the green circles represent lncRNAs, and the orange circles represent universal ncRNAs. miRNA, microRNA; lncRNA, long ncRNA.
Figure 4.
Distribution of degrees in the cancer–ncRNA network for CTR
A. Scale-free feature of the cancer–miRNA network for CTR. B. Scale-free feature of the cancer–lncRNA network for CTR. λ is the power exponent of the scale-free network, K is the node degree in the scale-free network, and P(K) is the probability that the node degree is K. CTR, cancer therapy resistance.
Discussion and conclusion
The CTRR-ncRNA can provide information for mapping the personalized relationship between ncRNA, cancer, and clinical information, for the pattern discovery in the genotype–phenotype relationship, and for supporting the clinical decision. Many ncRNAs are potential biomarkers for diagnosis, treatment, and prognosis of CTRR. We will update it routinely and further study the patterns hidden in the CTRR-ncRNA database.
Recent studies towards ncRNAs have successfully revealed the regulatory mechanisms of ncRNAs to CTRR. The ncRNA biomarkers can be very useful in the prediction of personalized CTRR. However, the ncRNA–CTRR association is very complex and personalized due to the heterogeneity of cancer. A comprehensive and reliable platform for ncRNAs and CTRR interaction is highly demanded and useful to precision CTRR medicine. The CTRR-ncRNA can provide knowledge for the mapping of the personalized relationship between ncRNA, cancer, and clinical information, for the pattern discovery in the genotype–phenotype relationship, as well as for the supporting of clinical decisions.
In summary, CTRR-ncRNA will be an informative and valuable resource for CTRR researchers. Further extensions will be the following aspects. First, we will extend the ncRNA types to small nucleolar RNAs (snoRNAs), piwi-interacting RNA (piRNA), small cajal body-specific RNAs (scaRNAs) and other ncRNAs not yet included in CTRR-ncRNA. Second, more putative ncRNA biomarkers with strong evidence supports will be added to the database. Third, the models for ncRNA biomarker combination and application will be developed based on our knowledge database to improve the prediction of CTRR.
Data availability
CTRR-ncRNA is publicly available at http://ctrr.bioinf.org.cn/.
Competing interests
The authors have declared no competing interests.
CRediT authorship contribution statement
Tong Tang: Data curation, Formal analysis, Methodology, Visualization, Writing – original draft. Xingyun Liu: Data curation, Methodology. Rongrong Wu: Data curation, Formal analysis. Li Shen: Visualization, Writing – review & editing. Shumin Ren: Formal analysis, Writing – review & editing. Bairong Shen: Conceptualization, Methodology, Supervision, Project administration, Writing – review & editing. All authors have read and approved the final manuscript.
Acknowledgments
We thank the members of the Institutes for Systems Genetics for the discussion and assistance with the data collection. This work was supported by the National Natural Science Foundation of China (Grant No. 32070671) and the regional innovation cooperation between Sichuan and Guangxi Provinces of China (Grant No. 2020YFQ0019).
Handled by Yi Zhao
Footnotes
Peer review under responsibility of Beijing Institute of Genomics, Chinese Academy of Sciences / China National Center for Bioinformation and Genetics Society of China.
Supplementary data to this study can be found online at https://doi.org/10.1016/j.gpb.2022.10.003.
Supplementary material
The following are the Supplementary material to this article:
Search terms used in PubMed database
An overview of the cancer–ncRNA network in CTR circRNA, circular RNA.
References
- 1.Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., et al. Drug resistance in cancer: an overview. Cancers. 2014;6:1769–1792. doi: 10.3390/cancers6031769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rycaj K., Tang D.G. Cancer stem cells and radioresistance. Int J Radiat Biol. 2014;90:615–621. doi: 10.3109/09553002.2014.892227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P., Hu-Lieskovan S., Wargo J.A., Ribas A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell. 2017;168:707–723. doi: 10.1016/j.cell.2017.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker H.E., Paget J.T.E., Khan A.A., Harrington K.J. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15:409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guttman M., Rinn J.L. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 7.Wang W.T., Han C., Sun Y.M., Chen T.Q., Chen Y.Q. Noncoding RNAs in cancer therapy resistance and targeted drug development. J Hematol Oncol. 2019;12:55. doi: 10.1186/s13045-019-0748-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He B., Zhao Z., Cai Q., Zhang Y., Zhang P., Shi S., et al. miRNA-based biomarkers, therapies, and resistance in cancer. Int J Biol Sci. 2020;16:2628–2647. doi: 10.7150/ijbs.47203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yin Y., Liu H., Xu J., Shi D., Zhai L., Liu B., et al. miR-144-3p regulates the resistance of lung cancer to cisplatin by targeting Nrf2. Oncol Rep. 2018;40:3479–3488. doi: 10.3892/or.2018.6772. [DOI] [PubMed] [Google Scholar]
- 10.Xiu D.H., Liu G.F., Yu S.N., Li L.Y., Zhao G.Q., Liu L., et al. Long non-coding RNA LINC00968 attenuates drug resistance of breast cancer cells through inhibiting the Wnt2/β-catenin signaling pathway by regulating WNT2. J Exp Clin Cancer Res. 2019;38:94. doi: 10.1186/s13046-019-1100-8. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Xue M., Li G., Fang X., Wang L., Jin Y., Zhou Q. hsa_circ_0081143 promotes cisplatin resistance in gastric cancer by targeting miR-646/CDK6 pathway. Cancer Cell Int. 2019;19:25. doi: 10.1186/s12935-019-0737-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pajic M., Froio D., Daly S., Doculara L., Millar E., Graham P.H., et al. miR-139-5p modulates radiotherapy resistance in breast cancer by repressing multiple gene networks of DNA repair and ROS defense. Cancer Res. 2018;78:501–515. doi: 10.1158/0008-5472.CAN-16-3105. [DOI] [PubMed] [Google Scholar]
- 13.Ray J., Haughey C., Hoey C., Jeon J., Murphy R., Dura-Perez L., et al. miR-191 promotes radiation resistance of prostate cancer through interaction with RXRA. Cancer Lett. 2020;473:107–117. doi: 10.1016/j.canlet.2019.12.025. [DOI] [PubMed] [Google Scholar]
- 14.Wang M., Wang L., He X., Zhang J., Zhu Z., Zhang M., et al. lncRNA CCAT2 promotes radiotherapy resistance for human esophageal carcinoma cells via the miR-145/p70S6K1 and p53 pathway. Int J Oncol. 2020;56:327–336. doi: 10.3892/ijo.2019.4929. [DOI] [PubMed] [Google Scholar]
- 15.Zhang P.F., Pei X., Li K.S., Jin L.N., Wang F., Wu J., et al. Circular RNA circFGFR1 promotes progression and anti-PD-1 resistance by sponging miR-381-3p in non-small cell lung cancer cells. Mol Cancer. 2019;18:179. doi: 10.1186/s12943-019-1111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cui C., Yang J., Li X., Liu D., Fu L., Wang X. Functions and mechanisms of circular RNAs in cancer radiotherapy and chemotherapy resistance. Mol Cancer. 2020;19:58. doi: 10.1186/s12943-020-01180-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller K.D., Siegel R.L., Lin C.C., Mariotto A.B., Kramer J.L., Rowland J.H., et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X., Xie K., Zhou H., Wu Y., Li C., Liu Y., et al. Role of non-coding RNAs and RNA modifiers in cancer therapy resistance. Mol Cancer. 2020;19:47. doi: 10.1186/s12943-020-01171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shimagaki T., Yoshizumi T., Harimoto N., Yoshio S., Naito Y., Yamamoto Y., et al. MicroRNA-125b expression and intrahepatic metastasis are predictors for early recurrence after hepatocellular carcinoma resection. Hepatol Res. 2018;48:313–321. doi: 10.1111/hepr.12990. [DOI] [PubMed] [Google Scholar]
- 20.Li Z., Wang K.E., Zhou X.L., Zhou J., Ye C.H. Long non-coding RNA cancer susceptibility candidate 2a (CASC2a) is a marker of early recurrence after radical cystectomy in patients with urothelial carcinoma of the bladder. Med Sci Monit. 2018;24:438–447. doi: 10.12659/MSM.905393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng Z., Gao Y. Down-regulation of lncRNA snaR is correlated with postoperative distant recurrence of HPV-negative cervical squamous cell carcinoma. Biosci Rep. 2018;38 doi: 10.1042/BSR20181213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S., Pu T., Xiao L., Gao H., Li L., Ye F., et al. Screening of recurrence related microRNA in ductal carcinoma in situ and functional study of microRNA-654-5p. J Breast Cancer. 2019;22:52–66. doi: 10.4048/jbc.2019.22.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vo D.T., Karanam N.K., Ding L., Saha D., Yordy J.S., Giri U., et al. miR-125a-5p functions as tumor suppressor microRNA and is a marker of locoregional recurrence and poor prognosis in head and neck cancer. Neoplasia. 2019;21:849–862. doi: 10.1016/j.neo.2019.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yuan S., Luan X., Han G., Guo K., Wang S., Zhang X. LINC01638 lncRNA mediates the postoperative distant recurrence of bladder cancer by upregulating ROCK2. Oncol Lett. 2019;18:5392–5398. doi: 10.3892/ol.2019.10924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun Q., Zhang S.Y., Zhao J.F., Han X.G., Wang H.B., Sun M.L. HIF-1α or HOTTIP/CTCF promotes head and neck squamous cell carcinoma progression and drug resistance by targeting HOXA9. Mol Ther Nucleic Acids. 2020;20:164–175. doi: 10.1016/j.omtn.2019.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong W., Xue M., Jiang J., Zhang Y., Gao X. Circular RNA circ-CPA4/let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC) J Exp Clin Cancer Res. 2020;39:149. doi: 10.1186/s13046-020-01648-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei Y., Lu C., Zhou P., Zhao L., Lyu X., Yin J., et al. EIF4A3-induced circular RNA ASAP1 promotes tumorigenesis and temozolomide resistance of glioblastoma via NRAS/MEK1/ERK1/2 signaling. Neuro Oncol. 2021;23:611–624. doi: 10.1093/neuonc/noaa214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen X., Sun Y.Z., Zhang D.H., Li J.Q., Yan G.Y., An J.Y., et al. NRDTD: a database for clinically or experimentally supported non-coding RNAs and drug targets associations. Database. 2017;2017:bax057. doi: 10.1093/database/bax057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H., Zhang W., Zou B., Wang J., Deng Y., Deng L. DrugCombDB: a comprehensive database of drug combinations toward the discovery of combinatorial therapy. Nucleic Acids Res. 2020;48:D871–D881. doi: 10.1093/nar/gkz1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li L., Wu P., Wang Z., Meng X., Zha C., Li Z., et al. NoncoRNA: a database of experimentally supported non-coding RNAs and drug targets in cancer. J Hematol Oncol. 2020;13:15. doi: 10.1186/s13045-020-00849-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen L., Bai J., Wang J., Shen B. The fourth scientific discovery paradigm for precision medicine and healthcare: challenges ahead. Precis Clin Med. 2021;4:80–84. doi: 10.1093/pcmedi/pbab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xing L., Xia M., Jiao X., Fan L. hsa_circ_0004831 serves as a blood-based prognostic biomarker for colorectal cancer and its potentially circRNA-miRNA-mRNA regulatory network construction. Cancer Cell Int. 2020;20:557. doi: 10.1186/s12935-020-01651-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pratama M.Y., Visintin A., Crocè L.S., Tiribelli C., Pascut D. Circulatory miRNA as a biomarker for therapy response and disease-free survival in hepatocellular carcinoma. Cancers. 2020;12:2810. doi: 10.3390/cancers12102810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen J., Zhang D., Zhang W., Tang Y., Yan W., Guo L., et al. Clear cell renal cell carcinoma associated microRNA expression signatures identified by an integrated bioinformatics analysis. J Transl Med. 2013;11:169. doi: 10.1186/1479-5876-11-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cun Y., Fröhlich H. Network and data integration for biomarker signature discovery via network smoothed t-statistics. PLoS One. 2013;8:e73074. doi: 10.1371/journal.pone.0073074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin Y., Wu W., Sun Z., Shen L., Shen B. MiRNA-BD: an evidence-based bioinformatics model and software tool for microRNA biomarker discovery. RNA Biol. 2018;15:1093–1105. doi: 10.1080/15476286.2018.1502590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qi X., Yu C., Wang Y., Lin Y., Shen B. Network vulnerability-based and knowledge-guided identification of microRNA biomarkers indicating platinum resistance in high-grade serous ovarian cancer. Clin Transl Med. 2019;8:28. doi: 10.1186/s40169-019-0245-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shao T., Wu A., Chen J., Chen H., Lu J., Bai J., et al. Identification of module biomarkers from the dysregulated ceRNA-ceRNA interaction network in lung adenocarcinoma. Mol Biosyst. 2015;11:3048–3058. doi: 10.1039/c5mb00364d. [DOI] [PubMed] [Google Scholar]
- 39.Kozomara A., Birgaoanu M., Griffiths-Jones S. miRBase: from microRNA sequences to function. Nucleic acids Res. 2019;47:D155–D162. doi: 10.1093/nar/gky1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Glažar P., Papavasileiou P., Rajewsky N. circBase: a database for circular RNAs. RNA. 2014;20:1666–1670. doi: 10.1261/rna.043687.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zheng X., Li J., Peng C., Zhao J., Chi J., Meng X., et al. MicroRNA-24 induces cisplatin resistance by targeting PTEN in human tongue squamous cell carcinoma. Oral Oncol. 2015;51:998–1003. doi: 10.1016/j.oraloncology.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Gong J.P., Yang L., Tang J.W., Sun P., Hu Q., Qin J.W., et al. Overexpression of microRNA-24 increases the sensitivity to paclitaxel in drug-resistant breast carcinoma cell lines via targeting ABCB9. Oncol Lett. 2016;12:3905–3911. doi: 10.3892/ol.2016.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yi D.Y., Su Q., Zhang F.C., Fu P., Zhang Q., Cen Y.C., et al. Effect of microRNA-128 on cisplatin resistance of glioma SHG-44 cells by targeting JAG1. J Cell Biochem. 2018;119:3162–3173. doi: 10.1002/jcb.26469. [DOI] [PubMed] [Google Scholar]
- 44.Liu F., Zhang J., Qin L., Yang Z., Xiong J., Zhang Y., et al. Circular RNA EIF6 (hsa_circ_0060060) sponges miR-144-3p to promote the cisplatin-resistance of human thyroid carcinoma cells by autophagy regulation. Aging. 2018;10:3806–3820. doi: 10.18632/aging.101674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao Z., Ji M., Wang Q., He N., Li Y. Circular RNA Cdr1as upregulates SCAI to suppress cisplatin resistance in ovarian cancer via miR-1270 suppression. Mol Ther Nucleic Acids. 2019;18:24–33. doi: 10.1016/j.omtn.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Yang W., Gu J., Wang X., Wang Y., Feng M., Zhou D., et al. Inhibition of circular RNA CDR1as increases chemosensitivity of 5-FU-resistant BC cells through up-regulating miR-7. J Cell Mol Med. 2019;23:3166–3177. doi: 10.1111/jcmm.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shannon P., Markiel A., Ozier O., Baliga N.S., Wang J.T., Ramage D., et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barabasi A.L., Albert R. Emergence of scaling in random networks. Science. 1999;286:509–512. doi: 10.1126/science.286.5439.509. [DOI] [PubMed] [Google Scholar]
- 49.Sun K.K., Hu P.P., Xu F. Prognostic significance of long non-coding RNA MALAT1 for predicting the recurrence and metastasis of gallbladder cancer and its effect on cell proliferation, migration, invasion, and apoptosis. J Cell Biochem. 2018;119:3099–3110. doi: 10.1002/jcb.26451. [DOI] [PubMed] [Google Scholar]
- 50.Zheng Y., Sun Y., Liu Y., Zhang X., Li F., Li L., et al. The miR-31-SOX10 axis regulates tumor growth and chemotherapy resistance of melanoma via PI3K/AKT pathway. Biochem Biophys Res Commun. 2018;503:2451–2458. doi: 10.1016/j.bbrc.2018.06.175. [DOI] [PubMed] [Google Scholar]
- 51.Korourian A., Roudi R., Shariftabrizi A., Madjd Z. MicroRNA-31 inhibits RhoA-mediated tumor invasion and chemotherapy resistance in MKN-45 gastric adenocarcinoma cells. Exp Bio Med. 2017;242:1842–1847. doi: 10.1177/1535370217728460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du Z., Niu S., Xu X., Xu Q. MicroRNA31-NDRG3 regulation axes are essential for hepatocellular carcinoma survival and drug resistance. Cancer Biomark. 2017;19:221–230. doi: 10.3233/CBM-170568. [DOI] [PubMed] [Google Scholar]
- 53.Li P., Zhang X., Wang L., Du L., Yang Y., Liu T., et al. lncRNA HOTAIR contributes to 5FU resistance through suppressing miR-218 and activating NF-κB/TS signaling in colorectal cancer. Mol Ther Nucleic Acids. 2017;8:356–369. doi: 10.1016/j.omtn.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xue D., Lu H., Xu H.Y., Zhou C.X., He X.Z. Long noncoding RNA MALAT1 enhances the docetaxel resistance of prostate cancer cells via miR-145-5p-mediated regulation of AKAP12. J Cell Mol Med. 2018;22:3223–3237. doi: 10.1111/jcmm.13604. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 55.Yue B., Cai D., Liu C., Fang C., Yan D. Linc00152 functions as a competing endogenous RNA to confer oxaliplatin resistance and holds prognostic values in colon cancer. Mol Ther. 2016;24:2064–2077. doi: 10.1038/mt.2016.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choromański K., Matuszak M., Miȩkisz J. Scale-free graph with preferential attachment and evolving internal vertex structure. J Stat Phy. 2013;151:1175–1183. [Google Scholar]
- 57.Wu R., Lin Y., Liu X., Zhan C., He H., Shi M., et al. Phenotype–genotype network construction and characterization: a case study of cardiovascular diseases and associated non-coding RNAs. Database. 2020;2020:baz147. doi: 10.1093/database/baz147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Search terms used in PubMed database
An overview of the cancer–ncRNA network in CTR circRNA, circular RNA.
Data Availability Statement
CTRR-ncRNA is publicly available at http://ctrr.bioinf.org.cn/.