Abstract
Background
Pulsed field ablation (PFA) has emerged as a novel energy source for the ablation of atrial fibrillation (AF) using ultrarapid electrical pulses to induce cell death via electroporation.
Objective
The purpose of this study was to compare the safety and acute efficacy of ablation for AF with PFA vs thermal energy sources.
Methods
We performed an extensive literature search and systematic review of studies that evaluated the safety and efficacy of ablation for AF with PFA and compared them to landmark clinical trials for ablation of AF with thermal energy sources. Freeman-Tukey double arcsine transformation was used to establish variance of raw proportions followed by the inverse with the random-effects model to combine the transformed proportions and generate the pooled prevalence and 95% confidence interval (CI).
Results
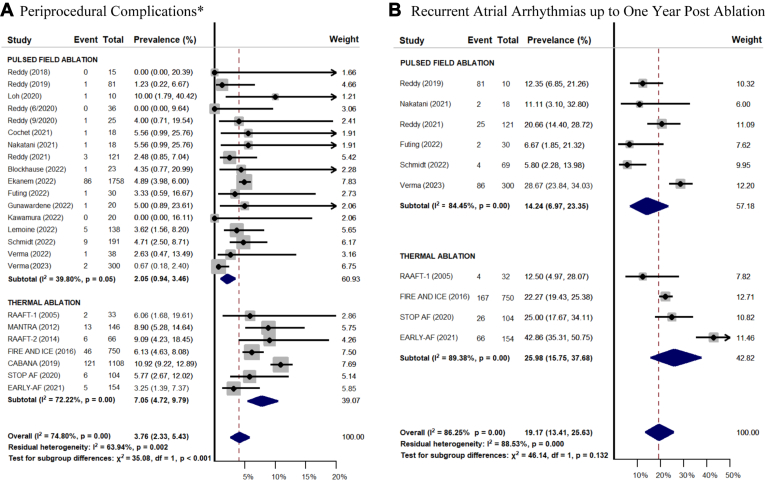
We included 24 studies for a total of 5203 patients who underwent AF ablation. Among these patients, 54.6% (n = 2842) underwent PFA and 45.4% (n = 2361) underwent thermal ablation. There were significantly fewer periprocedural complications in the PFA group (2.05%; 95% CI 0.94–3.46) compared to the thermal ablation group (7.75%; 95% CI 5.40–10.47) (P = .001). When comparing AF recurrence up to 1 year, there was a statistically insignificant trend toward a lower prevalence of recurrence in the PFA group (14.24%; 95% CI 6.97–23.35) compared to the thermal ablation group (25.98%; 95% CI 15.75–37.68) (P = .132).
Conclusion
Based on the results of this meta-analysis, PFA was associated with lower rates of periprocedural complications and similar rates of acute procedural success and recurrent AF with up to 1 year of follow-up compared to ablation with thermal energy sources.
Keywords: Atrial fibrillation, Pulsed field ablation, Thermal ablation, Meta-analysis, Safety
Key Findings.
-
▪
The results of this meta-analysis show that there are significantly fewer complications with pulsed field ablation (PFA) compared to thermal ablation.
-
▪
There is no statistically significant difference in the rate of recurrent atrial arrhythmias between PFA and thermal ablation when looking at studies with follow-up out to 1 year, although follow-up data with PFA are limited.
-
▪
Among the studies with both PFA and thermal ablation arms, there were no differences in fluoroscopy or procedure times. However, among studies that reported left atrial dwell times, the time was <1 hour in the PFA group.
Introduction
Atrial fibrillation (AF) is the most common arrhythmia and is associated with significant morbidity and mortality.1 Catheter ablation has been included in guidelines as a viable therapy in rhythm control treatment of AF, especially in symptomatic patients with significant AF burden refractory to antiarrhythmic drugs (AADs).2,3 Thermal energy using either radiofrequency or cryoballoon catheters is delivered to atrial cardiomyocytes to isolate the pulmonary veins as the mainstay of rhythm control therapy. However, thermal energy is not selective to cardiomyocytes and thus can lead to complications such as pulmonary vein stenosis, phrenic nerve palsy, and the extremely morbid atrioesophageal fistula.4
Pulsed field ablation (PFA) has emerged as a novel energy source for ablation of AF using ultrarapid electrical pulses to induce cell death via electroporation.5, 6, 7 In contrast to thermal ablation, different noncardiac tissues have characteristic thresholds of vulnerability to pulsed field energy. Thus, PFA has the advantage of being more selective to cardiac tissue relative to thermal ablation and potentially could result in less damage to periatrial structures such as the phrenic nerve or esophagus.8, 9, 10, 11 The purpose of our current study was to perform a systematic review of the literature and meta-analysis evaluating the safety and efficacy of PFA in comparison to thermal ablation.
Methods
Electronic databases were searched from inception up to March 2023 using the keywords “atrial fibrillation” and “pulsed field ablation” or “electroporation.” No language restriction was applied. The PRISMA statement for reporting systemic reviews and meta-analyses was applied to the methods for this study.12 The studies were required to fulfill the following criteria to be considered in the analysis: (1) include at least 10 patients undergoing PFA; (2) report the rates of periprocedural complications or recurrent AF; and (3) have been published in a peer-reviewed scientific journal. We subsequently compared clinical outcomes to thermal ablation from landmark clinical trials of thermal ablation.13, 14, 15, 16, 17, 18, 19
We aimed to compare the safety and efficacy of ablation for AF with PFA vs thermal energy sources. Two authors (OMA, AMA) independently performed the literature search and extracted data from eligible studies. Outcomes were extracted from original manuscripts and supplementary data. Information was gathered using standardized protocol and reporting forms. Disagreements were resolved by consensus. Two reviewers (OMA, AMA) independently assessed the quality items and discrepancies were resolved by consensus or involvement of a third reviewer (JCH), if necessary.
Two authors (OMA, CL) independently assessed the risk of bias of the included trials using standard criteria defined in the Cochrane Handbook for Systematic Reviews of Interventions. Discrepancies were resolved by discussion or adjudication by a third author (JCH).
Statistical analysis
Statistical analyses for studies including both PFA and thermal ablation arms were performed by the Review Manager (RevMan Version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014, The Netherlands). Data were summarized across treatment arms using the inverse variance mean difference (MD), where MD <0 favored the PFA group. Heterogeneity of effects was evaluated using the Higgins I2 statistic. Random-effects models for analyses were used with high heterogeneity (defined as I2 >25%); otherwise fixed effects models of DerSimonian and Laird were used. Statistical analyses involving the meta-analysis of single proportions were performed using Stata 11 (StataCorp LLC, College Station, TX) statistical software. We used the Freeman-Tukey double arcsine method to establish variance of raw proportions. The DerSimonian and Laird method with a random-effects model was used to generate a pooled estimate based on the transformed values and their variances. Finally, we back-transformed the pooled estimates and plotted the data on forest plots. Data was summarized as prevalence (%) with 95% confidence interval (CI). Heterogeneity of effects was evaluated using the Higgins I2 statistic. We used meta-regression to establish residual heterogeneity and test for subgroup differences between the PFA and thermal ablation groups, where P <.05 was considered significant. Descriptive statistics are presented as mean ± SD for continuous variables or number of cases (n) and percentage (%) for dichotomous and categorical variables.
Results
Study selection
The initial search resulted in 285 studies, of which 65 were duplications and 199 were excluded as outlined in Figure 1. Of the remaining 21 full-text articles, 4 were excluded because they did not meet eligibility criteria or did not have an outcome of interest. In our final analysis, we included 17 studies reviewing PFA20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36 and, for comparison, 7 landmark clinical trials of AF ablation with thermal energy sources (Table 1).13, 14, 15, 16, 17, 18, 19
Figure 1.
Selection of studies.
Table 1.
Patient demographics and characteristics
| Study | No. of patients | Age (y) | No. male | Persistent AF | LVEF (%) | CHA2DS2VASc score | AAD | Repeat AF ablation |
|---|---|---|---|---|---|---|---|---|
| Pulsed field ablation | ||||||||
| Reddy (2018)20 | 22 | 65 ± 5 | 12 (55) | — | 63 ± 3 | — | — | 0 (0) |
| Reddy (2019)21 | 81 | 58 ± 11 | 60 (74) | — | 63 ± 4 | — | 69 (85) | 0 (0) |
| Loh (2020)22 | 10 | 59 ± 11 | 7 (70) | 3 (30) | — | — | 9 (90) | 0 (0) |
| Reddy (June 2020)27 | 76 | 59 ± 10 | 50 (66) | 21 (28) | 58 ± 6 | — | 72 (95) | 0 (0) |
| Reddy (September 2020)26 | 25 | 67 | 20 (80) | 25 (100) | 60 | — | 24 (96) | 0 (0) |
| Cochet (2021)24 | 18 | 58 ± 9 | 15 (83) | — | 62 ± 6 | — | 14 (78) | 0 (0) |
| Nakatani (2021)25 | 18 | 56 ± 9 | 15 (83) | — | 62 ± 6 | 0.5 (0-1) | 13 (72) | 0 (0) |
| Reddy (2021)28 | 121 | 57 ± 10 | 89 (74) | — | 63 ± 6 | — | 118 (98) | 0 (0) |
| Blockhaus (2022)23 | 23 | 57 ± 10 | 15 (65) | 11 (48) | 56 ± 8 | 1.5 ± 1.1 | — | NR |
| Ekanem (2022)29 | 1758 | 62 | 1157 (66) | 619 (35) | 55 | 2.1 | — | 114 (6.5) |
| Futing (2022)30 | 30 | 63 ± 10 | 14 (47) | 0 (0) | 60 ± 6 | — | — | 0 (0) |
| Gunawardene (2022)31 | 20 | 70 ± 10 | 12 (60) | 13 (65) | — | 2.5 (2-4) | — | NR |
| Kawamura (2022)32 | 20 | 56 ± 12 | 15 (75) | — | 64 ± 4 | — | 19 (95) | 0 (0) |
| Lemoine (2022)33 | 138 | 57 ± 12 | 91 (66) | 86 (62) | 52 ± 10 | 2.7 ± 1.7 | 26 (19) | 0 (0) |
| Schmidt (2022)34 | 191 | 69 ± 12 | 111 (58) | 72 (38) | 60 ± 10 | — | — | 0 (0.0) |
| Verma (2022)35 | 38 | 62 ± 11 | 20 (53) | 3 (8) | 60 ± 5 | 1.9 ± 1.6 | — | 0 (0.0) |
| Verma (2023)36 | 300 | 65 ± 9 | 209 (70) | 150 (50) | — | — | 187 (62) | 0 (0.0) |
| Thermal ablation | ||||||||
| RAAFT-1 (2005)13 | 70 | 54 ± 8 | — | 3 (4) | 54 ± 6 | — | 0 (0) | 0 (0.0) |
| MANTRA (2012)14 | 294 | 55 ± 10 | 206 (70) | 0 (0) | — | 0 (0-1) | 0 (0) | 0 (0.0) |
| RAAFT-2 (2014)15 | 127 | 55 ± 10 | 96 (76) | 3 (2) | 61 ± 6 | 0 (0-1) | 0 (0) | 0 (0.0) |
| FIRE AND ICE (2016)16 | 750 | 60 ± 10 | 457 (61) | 0 (0) | — | 1.9 ± 1.4 | 461 (61) | 0 (0.0) |
| CABANA (2019)17 | 2204 | 68 (62-72) | 1385 (63) | 1258 (57) | — | 3 (2-4) | — | 0 (0.0) |
| STOP AF (2020)18 | 203 | 61 ± 11 | 120 (59) | 0 (0) | 61 ± 6 | 2 (1-3) | 0 (0) | 0 (0.0) |
| EARLY-AF (2021)19 | 303 | 59 ± 11 | 214 (71) | 16 (5) | 60 ± 7 | 1.9 ± 1.1 | 0 (0) | 0 (0.0) |
Values are given as mean ± SD or n (%).
AAD = antiarrhythmic drug; AF = atrial fibrillation; CHA2DS2VASc = risk score for thromboembolic events; LVEF = left ventricular ejection fraction.
Study characteristics
Baseline demographics of patients included in the 17 PFA studies and 7 thermal energy AF ablation trials are summarized in Table 1. Study characteristics and average follow-up are listed in Table 2. Among the PFA studies, 14 were single-arm studies and 7 were single-center studies.
Table 2.
Descriptions of studies included in meta-analysis
| Study | Study design | Study population | Catheter | Follow-up | Monitoring method | Quality assessment | ||
|---|---|---|---|---|---|---|---|---|
| PFA | ||||||||
| Selection | Comparability | Outcome | ||||||
| Reddy (2018)20 | Single-arm, multicenter, prospective clinical study | Patients with paroxysmal AF refractory or intolerant to at least 1 AAD. Had to have anteroposterior LA diameter <5.5 cm and LVEF ≥40%. | Farawave | 1 mo | NR | ∗∗∗ | ∗∗∗ | |
| Reddy (2019)21 | Combined analysis of prospective nonrandomized feasibility trials, multicenter | Patients with symptomatic paroxysmal AF resistant to class I to IV antiarrhythmic medications, with LVEF > 40% and LA diameter <5.5 cm for Trial 1 (IMPULSE) or LA diameter <5 cm for Trial 2 (PEFCAT). | Farawave | 12 mo | Transtelephonic monitor with weekly transmissions, 24-h Holter at 6 and 12 mo | ∗∗∗ | ∗∗∗ | |
| Loh (2020)22 | Single-arm, single-center, nonrandomized, prospective cohort study | Patients with symptomatic paroxysmal or persistent AF undergoing first ablation with pulmonary vein diameter <23 mm and no LA or LA appendage thrombus. | Custom nondeflectable 8F, 14-polar catheter with a variable hoop diameter (16– 27 mm) | N/A | N/A | ∗∗∗ | ∗∗∗ | |
| Reddy (June 2020)27 | Single-arm, multicenter, prospective clinical study | Patients with symptomatic paroxysmal or persistent AF resistant to class I to IV antiarrhythmic medications undergoing first ablation procedure with LVEF >40% and LA diameter ≤5.5 cm. | Sphere-9 lattice tip | 3 mo | NR | ∗∗∗ | ∗∗∗ | |
| Reddy (September 2020)26 | Single-arm, multicenter feasibility study | Patients with symptomatic persistent AF refractory or intolerant to at least one class I/III antiarrhythmic agent. | Farawave | 75 d | NR | ∗∗∗ | ∗∗∗ | |
| Cochet (2021)24 | Single-center, prospective clinical study | Patients with paroxysmal AF referred for first catheter ablation procedure without contraindication to gadolinium-enhanced cardiac MRI. | Farawave | 3 mo | N/A | ∗∗∗∗ | ∗∗∗ | |
| Nakatani (2021)25 | Single-center, prospective, feasibility study | Patients with paroxysmal AF undergoing first catheter ablation with no contraindication to gadolinium-enhanced cardiac MRI. | Farawave | 9 mo | 12-lead ECG at 1, 3, and 6 mo, 24-h Holter if symptomatic | ∗∗∗∗ | ∗∗∗ | |
| Reddy (2021)28 | Combined analysis of 3 prospective safety and feasibility trials, multicenter | Patients with symptomatic paroxysmal AF resistant to at least 1 class I to IV antiarrhythmic medication, with LVEF >40% and LA diameter <5.5 cm for Trial 1 (IMPULSE) or LA diameter <5 cm for Trials 2, 3 (PEFCAT I and II). | Farawave | 12 mo | Weekly transtelephonic ECGs and 24-h Holter at 6 and 12 mo | ∗∗∗ | ∗∗∗ | |
| Blockhaus (2022)23 | Single-center, retrospective analysis | Patients with AF who were previously selected for pulmonary vein isolation ablation at a single center. | Farawave | N/A | N/A | ∗∗∗ | ∗∗∗ | |
| Ekanem (2022)29 | Retrospective survey of all centers performing PFA | Patients with AF who underwent PFA at 1 of 24 centers after regulatory approval of PFA procedure. | Farawave | N/A | N/A | ∗∗∗ | ∗∗ | |
| Futing (2022)30 | Single-arm, single-center, prospective clinical study | Patients with paroxysmal AF refractory or intolerant to a class I or III antiarrhythmic agent or opted for first-line rhythm control therapy without a history of a previous ablation. | Farawave | 90 d | 12-lead ECG and 7-d Holter | ∗∗∗ | ∗∗∗ | |
| Gunawardene (2022)31 | Single-arm, single-center, prospective clinical study | Patients with AF who were eligible for catheter ablation of AF. | Farawave | N/A | N/A | ∗∗∗ | ∗∗∗ | |
| Kawamura (2022)32 | Retrospective analysis of single-arm, single-center feasibility study | Patients with symptomatic paroxysmal AF resistant to antiarrhythmic medications with LVEF >40% and LA diameter <5 cm. | Farawave | 84 d | N/A | ∗∗∗ | ∗∗ | |
| Lemoine (2022)33 | Retrospective analysis of single-arm, multicenter clinical study | Patients with symptomatic AF undergoing first time ablation with LA diameter <6 cm and without severe valvular heart disease or contraindications to oral anticoagulation. | Farawave | 12 mo | 12-lead ECG and Holter for symptoms at 3-, 6-, and 12-mo visits | ∗∗∗ | ∗∗∗ | |
| Schmidt (2022)34 | Single-arm, nonrandomized, multicenter real-world series | Patients with symptomatic AF refractory to treatment of at least 1 AAD undergoing first-time ablation without moderate or severe mitral valve disease, intracardiac thrombus, or contraindications to oral anticoagulation. | Farawave | 3 mo | 72-h Holter at 3 mo and 24-h Holter or external monitor for symptoms | ∗∗∗ | ∗∗∗ | |
| Verma (2022)35 | Single-arm, multicenter, prospective clinical trial | Patients with AF refractory to at least 1 AAD, LVEF ≥35%, and LA diameter < 5cm undergoing first-time ablation. | PulseSelect | 30 d | 12-lead ECG | ∗∗∗ | ∗∗∗ | |
| Verma (2023)36 | Paired, single-arm, multicenter, prospective nonrandomized study | Patients with paroxysmal or persistent AF refractory to class I or III AADs. | PulseSelect | 12 mo | Weekly and symptomatic transtelephonic monitoring, 3-, 6-, and 12-mo 12-lead ECGs, and 6- and 12-mo 24-h Holter | ∗∗∗ | ∗∗∗ | |
| Thermal ablation | ||||||||
| RAAFT-1 (2005)13 | Multicenter, prospective randomized trial | Patients experiencing monthly symptomatic AF episodes for at least 3 mo who had not been treated with AADs or ablation. | 8-mm-tip RF catheter | 12 mo | 1-mo loop event recorder at discharge and 3 mo, 24-h Holter at 3, 6, and 12 mo | N/A | ||
| MANTRA (2012)14 | Multicenter, randomized trial | Patients with symptomatic paroxysmal AF who had not been treated with AADs or ablation, but had LVEF ≥40%, LA diameter >5 cm, absence of moderate-to-severe mitral valve disease, and absence of severe heart failure. | 3.5-mm RF catheter with irrigated tip or 8-mm solid-tip RF catheter | 24 mo | 7-d Holter at 3, 6, 12, 18, and 24 mo | N/A | ||
| RAAFT-2 (2014)15 | Multicenter, randomized trial | Patients with symptomatic paroxysmal AF who had not been treated with AADs or ablation, but had LVEF ≥40%, LA diameter <5.5 cm, absence of moderate-to-severe left ventricular hypertrophy, absence of valvular heart disease, and absence of coronary artery disease. | Left to discretion of operator | 24 mo | Transtelephonic monitoring and biweekly recordings with 1-, 3-, 6-, 12-, and 24-mo follow-up | N/A | ||
| FIRE AND ICE (2016)16 | Multicenter, randomized trial | Patients with symptomatic paroxysmal AF that was refractory to class I or III AADs or beta-blockers. | First- and second-generation cryoballoon catheters, the combined first-generation RF catheters, or the advanced-generation RF catheter | 18 mo | Weekly transtelephonic monitoring, 12-lead ECG and 24-h Holter at 3, 6, and 12 mo then every 6 mo thereafter | N/A | ||
| CABANA (2019)17 | Multicenter, randomized trial | Patients with AF who were ≥65 years or <65 years with ≥1 risk factors for stroke. Patients were excluded if they had a history of ablation or failed ≥2 AADs. | Left to discretion of operator | 48.5 mo | ECG event recorder for symptoms, quarterly 24-h recordings, and 96-h Holter every 6 mo | N/A | ||
| STOP AF (2020)18 | Multicenter, randomized trial | Patients with recurrent symptomatic paroxysmal AF with LA diameter <5 cm, no previous treatment with AADs, and no previous history of ablation. | Second-generation cryoballoon catheter | 12 mo | 12-lead ECG at 1, 3, 5, and 12 mo and 24-h Holter at 6 and 12 mo | N/A | ||
| EARLY-AF (2021)19 | Multicenter, randomized trial | Patients with symptomatic AF who did not have a history of regular (daily) use of a class I or III AAD at therapeutic doses. | 23-mm or 28-mm cryoballoon catheter | 12 mo | Implantable cardiac monitor | N/A | ||
ECG = electrocardiogram; LA = left atrium; MRI = magnetic resonance imaging; N/A = not applicable; NR = not reported; PFA = pulsed field ablation; RF = radiofrequency; other abbreviations as in Table 1.
Quality assessment
The quality of observational studies was evaluated using the Newcastle-Ottawa Quality Assessment Scale. This scale assesses study selection, comparability, and outcomes/exposure. A good-quality study will have 3–4 stars in the selection domain, 1–2 in the comparability domain, and 2–3 in the outcomes/exposure domain. A fair-quality study will have 2 stars in the selection domain, 1–2 in the comparability domain, and 2–3 in the outcomes/exposure domain.37
Study endpoints
There were no statistically significant differences in fluoroscopy time (MD 2.12; 95% CI –2.33 to 6.58) or procedure time (MD –22.18; 95% CI –47.77 to 3.40) between groups in studies that had both PFA and thermal ablation arms. Study endpoints between the PFA and thermal ablation groups are summarized in Figure 2. The PFA group had significantly lower rates of periprocedural complications (2.05%, 95% CI 0.94–3.46 vs 7.75%, 95% CI 5.40–10.47; P = .001). Specific periprocedural complications in both PFA vs thermal ablation groups are summarized in Table 3. There was a statistically insignificant trend toward lower recurrent atrial arrhythmias up to 1 year postablation in the PFA group (11.40%; 95% CI 5.93–18.19) compared to the thermal ablation group (25.98%; 95% CI 15.75–37.68; P = .052), but with less follow-up on average in the PFA group.
Figure 2.
Forest plots comparing the prevalence of periprocedural complications (A) and recurrent atrial arrhythmias up to 1 year postablation (B). Prevalence is expressed as a percentage with corresponding 95% confidence intervals. ∗Complications include access site complications, cardiac effusion/tamponade, major bleeding, transient ischemic attack or stroke, coronary vasospasm, myocardial infarction, phrenic nerve palsy, esophageal injury, atrioesophageal fistula, and death.
Table 3.
Periprocedural complications
| Study | Access site complication | Cardiac effusion or tamponade | Major bleeding | TIA or Stroke | Coronary vasospasm | Myocardial infarction | Phrenic nerve injury | Pulmonary vein stenosis | Esophageal injury | AEF | Death |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PFA | |||||||||||
| Reddy (2018)20 | NR | NR | NR | NR | NR | NR | 0 (0.0) | 0 (0) | NR | NR | NR |
| Reddy (2019)21 | 0 (0.0) | 1 (1.2) | NR | 0 (0.0) | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) | NR | NR | 0 (0.0) |
| Loh (2020)22 | NR | NR | NR | 1 (10) | NR | NR | NR | NR | NR | NR | NR |
| Reddy (June 2020)27 | NR | 0 (0.0) | NR | 0 (0.0) | NR | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Reddy (September 2020)26 | 0 (0.0) | 1 (4.0) | NR | 0 (0.0) | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Cochet (2021)24 | 1 (5.6) | NR | NR | NR | NR | NR | 0 (0.0) | NR | 0 (0.0) | 0 (0.0) | NR |
| Nakatani (2021)25 | 1 (5.6) | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Reddy (2021)28 | 1 (0.8) | 2 (1.7) | NR | 0 (0.0) | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) | NR | 0 (0.0) | 0 (0.0) |
| Blockhaus (2022)23 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (4.3) | NR | NR | 0 (0.0) | NR | NR | NR | NR |
| Ekanem (2022)29 | 50 (2.8) | 17 (1.0) | NR | 9 (0.5) | 1 (0.1) | NR | 8 (4.6) | NR | 0 (0.0) | 0 (0.0) | 1 (0.1) |
| Futing (2022)30 | 0 (0.0) | 1 (3.3) | NR | NR | NR | NR | 0 (0.0) | NR | 0 (0.0) | NR | NR |
| Gunawardene (2022)31 | 0 (0.0) | 0 (0.0) | NR | NR | 1 (5.0) | NR | 0 (0.0) | NR | 0 (0.0) | NR | NR |
| Kawamura (2022)32 | NR | NR | NR | 0 (0.0) | NR | NR | 0 (0.0) | 0 (0.0) | NR | 0 (0.0) | NR |
| Lemoine (2022)33 | 3 (2.2) | 1 (0.7) | NR | NR | 1 (0.7) | NR | NR | NR | NR | NR | NR |
| Schmidt (2022)34 | 4 (2.1) | 1 (0.5) | NR | 2 (1.0) | NR | NR | 2 (1.0) | NR | 0 (0.0) | NR | NR |
| Verma (2022)35 | 1 (2.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NR | 0 (0.0) | 0 (0.0) | 0 (0.0) | NR | NR | 0 (0.0) |
| Verma (2023)36 | NR | 1 (0.3) | NR | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Thermal ablation | |||||||||||
| RAAFT-1 (2005)13 | NR | NR | 2 (6.1) | 0 (0.0) | NR | NR | NR | 0 (0.0) | NR | NR | NR |
| MANTRA (2012)14 | 2 (1.4) | 4 (2.7) | 1 (0.7) | 2 (1.4) | NR | NR | NR | 1 (0.7) | NR | NR | 3 (2.1) |
| RAAFT-2 (2014)15 | NR | 4 (6.1) | NR | 0 (0.0) | NR | NR | NR | 1 (1.5) | NR | 0 (0.0) | 0 (0.0) |
| FIRE AND ICE (2016)16 | 23 (3.1) | 6 (0.8) | NR | 4 (0.5) | NR | NR | 10 (1.3) | NR | 1 (0.1) | 0 (0.0) | 2 (0.3) |
| CABANA (2019)17 | 39 (3.5) | 8 (0.7) | 36 (3.2) | 30 (2.7) | NR | 1 (0.1) | 1 (0.1) | 1 (0.1) | 5 (0.5) | 0 (0.0) | 0 (0.0) |
| STOP AF (2020)18 | NR | 1 (1.0) | 1 (1.0) | 0 (0.0) | NR | 2 (1.9) | 2 (1.9) | 0 (0.0) | NR | 0 (0.0) | 0 (0.0) |
| EARLY-AF (2021)19 | 2 (1.3) | 0 (0.0) | NR | 0 (0.0) | NR | 0 (0.0) | 3 (1.9) | NR | 0 (0.0) | NR | 0 (0.0) |
Values are given as n (%).
AEF = atrioesophageal fistula; TIA = transient ischemic attack; other abbreviations as in Table 2.
Discussion
This is the first systematic review and meta-analysis of studies comparing safety and efficacy of PFA with thermal ablation. The results of this meta-analysis show that there are significantly fewer complications with PFA compared to thermal ablation. There is no statistically significant difference in rate of recurrent atrial arrhythmias when looking at studies with follow-up out to 1 year, but there was relatively shorter follow-up and higher use of AAD in the PFA group. Furthermore, among the studies with both PFA and thermal ablation arms, there were no differences in fluoroscopy or procedure times in these early PFA studies. However, skin-to-skin procedure times often are inaccurate, as sheaths are sometimes removed in the recovery area. Among studies that reported left atrial dwell times, the time was <1 hour in the PFA group.23,36
Electroporation occurs after a sufficiently strong electrical field results in increased membrane permeability and instability, resulting in cell death due to adenosine triphosphate exhaustion, ion channel failure, calcium overload, and general loss of cellular homeostasis.38, 39, 40, 41 Pulse amplitude, pulse width, number of pulses, waveform (monophasic or biphasic), pulse cycle length, and distance of the tissue from delivery electrodes all influence the increased membrane permeability and whether this hyperpermeability is reversible.42 The area of tissue where irreversible electroporation occurs forms the margins of the lesion being created. Despite the high amount of energy delivered to tissues, PFA has a negligible thermal effect because of the short duration and pulses <100 μs.43 The lack of thermal effects allows for safer delivery with potential for reduced collateral damage.
Although it has been hypothesized that PFA could result in fewer complications after ablation of AF due to its superior cardiac tissue selectivity relative to thermal ablation, concerns have been raised about whether this is outweighed by complications more common with PFA, such as coronary vasospasm. This is especially a concern when ablating beyond the pulmonary veins and closer to the coronary arteries. However, coronary vasospasm has been shown to be subclinical in the majority of cases and is effectively treated prophylactically or post hoc with nitroglycerin.44 Despite including coronary vasospasm in the composite safety outcome, there were still significantly fewer periprocedural complications in the PFA group compared to the thermal ablation group. Furthermore, fluoroscopy time, procedure time, and complications with PFA are only expected to decrease as operators become more familiar with this new technology. Although many studies have examined the effects of different catheters, power settings, ablation durations, and lesion sets with radiofrequency and cryoablation, PFA still is in its nascent stage, so the optimal ablation strategy using this energy is to be determined.
With regard to recurrent atrial arrhythmias, it is not surprising that PFA had similar acute procedural success as thermal ablation, with its rate of acute pulmonary vein isolation in both paroxysmal and persistent AF shown to be similar to that of thermal ablation.16,36,45 However, the physiology underlying this finding likely is more nuanced. It has been postulated that ganglionated plexuses, which are situated in the fat pads close to pulmonary vein ostia, may interact with the sympathetic and parasympathetic nervous systems in the development of AF.46 It is possible that PFA may result in more transmural lesions with less incidence of pulmonary vein reconnection but does not adequately ablate the ganglionated plexuses because of its attenuated effect on nervous tissue, resulting in a net atrial arrhythmia recurrence similar to that of thermal ablation. However, this remains to be tested, as 3 of the PFA studies included in this meta-analysis had follow-up <1 year and none >1 year.25,30,34 Thus, the durability of lesions created by PFA needs to be studied further.
However, it should be highlighted that the PFA group comprised a very heterogeneous population with multiple different catheters and waveforms used. Although this makes it difficult to know which catheter or waveform is optimal, it enhances the generalizability of the studied outcomes. Similarly, radiofrequency and cryoablation were evaluated as a conglomerate comparator arm to represent contemporary practice for AF ablation.
Study limitations
The current systematic review and meta-analysis has several important limitations that should be acknowledged. First, this was a meta-analysis of single proportions comparing proportions of events occurring between different populations and thus is subject to biases from uncontrolled confounders. Second, there were different study protocols, with single-arm studies making up the majority of the PFA studies and randomized controlled trials making up the thermal ablation studies. Third, some patients may have been counted in more than 1 study, as some of the included studies were at the same center or national surveys. Fourth, multiple different PFA catheters were used in the included studies, and what effect the heterogeneity of different catheter designs and PFA waveforms could have on the results studied is unknown. Fifth, there was not a standardized protocol for the detection of recurrent atrial arrhythmias, and follow-up was highly variable, with shorter follow-up on average in the PFA group.
Conclusion
Based on the results of this meta-analysis, PFA was associated with lower rates of periprocedural complications and similar rates of recurrent AF with up to 1 year of follow-up compared to ablation with thermal energy sources, but there was relatively shorter follow-up and higher use of AADs in the PFA group. Randomized controlled trials with longer follow-up comparing PFA to thermal ablation are needed.
Acknowledgments
Dr Hsu's research is funded by support from The Marouf Family and The Butler and Gratt Family.
Funding Sources
The authors have no funding sources to disclose.
Disclosures
Jonathan C. Hsu reports receiving honoraria from Medtronic, Abbott, Boston Scientific, Biotronik, Janssen Pharmaceuticals, Bristol-Myers Squibb, Pfizer, Sanofi, Zoll Medical, Hillrom, iRhythm, Acutus Medical, and Biosense Webster; equity interest in Vektor Medical; and research grants from Biotronik and Biosense Webster. Gordon Ho reports receiving a research grant from Abbott; equity in Vektor Medical; and fellowship support from Medtronic, Abbott, Boston Scientific, and Biotronik. Gregory K. Feld reports receiving fellowship training program stipends (as CCEP fellowship training program director) from Medtronic, Biotronik, Biosense Webster, and Abbott Medical; has equity interest in Vektor Medical; is co-founder and co-owner of Perminova; and is a consultant to Acutus Medical. Frederick T. Han receives research support from Abbott and honoraria from Abbott. All other authors have no conflicts of interest to disclose.
Authorship
All authors attest they meet the current ICMJE criteria for authorship.
Ethics Statement
The PRISMA statement for reporting systemic reviews and meta-analyses was applied to the methods for this study.
Footnotes
Given her role as Associate Editor, Ulrika Birgersdotter-Green had no involvement in the peer review of this article and has no access to information regarding its peer review. Full responsibility for the editorial process for this article was delegated to Editors Nazem Akoum and Jeanne E. Poole.
References
- 1.Iwasaki Y.K., Nishida K., Kato T., Nattel S. Atrial fibrillation pathophysiology: implications for management. Circulation. 2011;124:2264–2274. doi: 10.1161/CIRCULATIONAHA.111.019893. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G., Potpara T., Dagres N., et al. ESC Scientific Document Group 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC). Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 3.January C.T., Wann L.S., Alpert J.S., et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2014;64:e1–e76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 4.Gupta A., Perera T., Ganesan A., et al. Complications of catheter ablation of atrial fibrillation: a systematic review. Circ Arrhythm Electrophysiol. 2013;6:1082–1088. doi: 10.1161/CIRCEP.113.000768. [DOI] [PubMed] [Google Scholar]
- 5.Davalos R.V., Mir I.L., Rubinsky B. Tissue ablation with irreversible electroporation. Ann Biomed Eng. 2005;33:223–231. doi: 10.1007/s10439-005-8981-8. [DOI] [PubMed] [Google Scholar]
- 6.Rubinsky B., Onik G., Mikus P. Irreversible electroporation: a new ablation modality—clinical implications. Technol Cancer Res Treat. 2007;6:37–48. doi: 10.1177/153303460700600106. [DOI] [PubMed] [Google Scholar]
- 7.Edd J.F., Horowitz L., Davalos R.V., Mir L.M., Rubinsky B. In vivo results of a new focal tissue ablation technique: irreversible electroporation. IEEE Trans Biomed Eng. 2006;53:1409–1415. doi: 10.1109/TBME.2006.873745. [DOI] [PubMed] [Google Scholar]
- 8.van Driel V.J., Neven K.G., van Wessel H., et al. Pulmonary vein stenosis after catheter ablation: electroporation versus radiofrequency. Circ Arrhythm Electrophysiol. 2014;7:734–738. doi: 10.1161/CIRCEP.113.001111. [DOI] [PubMed] [Google Scholar]
- 9.du Pre B.C., van Driel V.J., van Wessel H., et al. Minimal coronary artery damage by myocardial electroporation ablation. Europace. 2013;15:144–149. doi: 10.1093/europace/eus171. [DOI] [PubMed] [Google Scholar]
- 10.Neven K., van Es R., van Driel V., et al. Acute and long-term effects of full-power electroporation ablation directly on the porcine esophagus. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.116.004672. [DOI] [PubMed] [Google Scholar]
- 11.van Driel V.J., Neven K., van Wessel H., et al. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015;12:1838–1844. doi: 10.1016/j.hrthm.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:W65–W94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
- 13.Wazni O.M., Marrouche N.F., Martin D.O., et al. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of symptomatic atrial fibrillation: a randomized trial. JAMA. 2005;293:2634–2640. doi: 10.1001/jama.293.21.2634. [DOI] [PubMed] [Google Scholar]
- 14.Cosedis Nielsen J., Johannessen A., et al. Radiofrequency ablation as initial therapy in paroxysmal atrial fibrillation. N Engl J Med. 2012;367:1587–1595. doi: 10.1056/NEJMoa1113566. [DOI] [PubMed] [Google Scholar]
- 15.Morillo C.A., Verma A., Connolly S.J., et al. RAAFT-2 Investigators. Radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal atrial fibrillation (RAAFT-2): a randomized trial. JAMA. 2014;311:692–700. doi: 10.1001/jama.2014.467. [DOI] [PubMed] [Google Scholar]
- 16.Kuck K.H., Brugada J., Furnkranz A., et al. Fire and ICE Investigators. Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374:2235–2245. doi: 10.1056/NEJMoa1602014. [DOI] [PubMed] [Google Scholar]
- 17.Packer D.L., Mark D.B., Robb R.A., et al. CABANA Investigators Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wazni O.M., Dandamudi G., Sood N., et al. STOP AF First Trial Investigators Cryoballoon ablation as initial therapy for atrial fibrillation. N Engl J Med. 2021;384:316–324. doi: 10.1056/NEJMoa2029554. [DOI] [PubMed] [Google Scholar]
- 19.Andrade J.G., Wells G.A., Deyell M.W., et al. EARLY-AF Investigators. Cryoablation or drug therapy for initial treatment of atrial fibrillation. N Engl J Med. 2021;384:305–315. doi: 10.1056/NEJMoa2029980. [DOI] [PubMed] [Google Scholar]
- 20.Reddy V.Y., Koruth J., Jais P., et al. Ablation of atrial fibrillation with pulsed electric fields: an ultra-rapid, tissue-selective modality for cardiac ablation. JACC Clin Electrophysiol. 2018;4:987–995. doi: 10.1016/j.jacep.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 21.Reddy V.Y., Neuzil P., Koruth J.S., et al. Pulsed field ablation for pulmonary vein isolation in atrial fibrillation. J Am Coll Cardiol. 2019;74:315–326. doi: 10.1016/j.jacc.2019.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Loh P., van Es R., Groen M.H.A., et al. Pulmonary vein isolation with single pulse irreversible electroporation: a first in human study in 10 patients with atrial fibrillation. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.119.008192. [DOI] [PubMed] [Google Scholar]
- 23.Blockhaus C., Guelker J.E., Feyen L., Bufe A., Seyfarth M., Shin D.I. Pulsed field ablation for pulmonary vein isolation: real-world experience and characterization of the antral lesion size compared with cryoballoon ablation. J Interv Card Electrophysiol. 2023;66:567–575. doi: 10.1007/s10840-022-01359-x. [DOI] [PubMed] [Google Scholar]
- 24.Cochet H., Nakatani Y., Sridi-Cheniti S., et al. Pulsed field ablation selectively spares the oesophagus during pulmonary vein isolation for atrial fibrillation. Europace. 2021;23:1391–1399. doi: 10.1093/europace/euab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nakatani Y., Sridi-Cheniti S., Cheniti G., et al. Pulsed field ablation prevents chronic atrial fibrotic changes and restrictive mechanics after catheter ablation for atrial fibrillation. Europace. 2021;23:1767–1776. doi: 10.1093/europace/euab155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reddy V.Y., Anic A., Koruth J., et al. Pulsed field ablation in patients with persistent atrial fibrillation. J Am Coll Cardiol. 2020;76:1068–1080. doi: 10.1016/j.jacc.2020.07.007. [DOI] [PubMed] [Google Scholar]
- 27.Reddy V.Y., Anter E., Rackauskas G., et al. Lattice-tip focal ablation catheter that toggles between radiofrequency and pulsed field energy to treat atrial fibrillation: a first-in-human trial. Circ Arrhythm Electrophysiol. 2020;13 doi: 10.1161/CIRCEP.120.008718. [DOI] [PubMed] [Google Scholar]
- 28.Reddy V.Y., Dukkipati S.R., Neuzil P., et al. Pulsed field ablation of paroxysmal atrial fibrillation: 1-year outcomes of IMPULSE, PEFCAT, and PEFCAT II. JACC Clin Electrophysiol. 2021;7:614–627. doi: 10.1016/j.jacep.2021.02.014. [DOI] [PubMed] [Google Scholar]
- 29.Ekanem E., Reddy V.Y., Schmidt B., et al. MANIFEST-PF Cooperative. Multi-national survey on the methods, efficacy, and safety on the post-approval clinical use of pulsed field ablation (MANIFEST-PF) Europace. 2022;24:1256–1266. doi: 10.1093/europace/euac050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Futing A., Reinsch N., Howel D., Brokkaar L., Rahe G., Neven K. First experience with pulsed field ablation as routine treatment for paroxysmal atrial fibrillation. Europace. 2022;24:1084–1092. doi: 10.1093/europace/euac041. [DOI] [PubMed] [Google Scholar]
- 31.Gunawardene M.A., Schaeffer B.N., Jularic M., et al. Pulsed-field ablation combined with ultrahigh-density mapping in patients undergoing catheter ablation for atrial fibrillation: practical and electrophysiological considerations. J Cardiovasc Electrophysiol. 2022;33:345–356. doi: 10.1111/jce.15349. [DOI] [PubMed] [Google Scholar]
- 32.Kawamura I., Neuzil P., Shivamurthy P., et al. Does pulsed field ablation regress over time? A quantitative temporal analysis of pulmonary vein isolation. Heart Rhythm. 2021;18:878–884. doi: 10.1016/j.hrthm.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 33.Lemoine M.D., Fink T., Mencke C., et al. Pulsed-field ablation-based pulmonary vein isolation: acute safety, efficacy and short-term follow-up in a multi-center real world scenario. Clin Res Cardiol. 2023;112:795–806. doi: 10.1007/s00392-022-02091-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmidt B., Bordignon S., Tohoku S., et al. 5S Study: safe and simple single shot pulmonary vein isolation with pulsed field ablation using sedation. Circ Arrhythm Electrophysiol. 2022;15 doi: 10.1161/CIRCEP.121.010817. [DOI] [PubMed] [Google Scholar]
- 35.Verma A., Boersma L., Haines D.E., et al. First-in-human experience and acute procedural outcomes using a novel pulsed field ablation system: the PULSED AF PILOT TRIAL. Circ Arrhythm Electrophysiol. 2022;15 doi: 10.1161/CIRCEP.121.010168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Verma A., Haines D.E., Boersma L.V., et al. Investigators P.A. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF pivotal trial. Circulation. 2023;147:1422–1432. doi: 10.1161/CIRCULATIONAHA.123.063988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wells G.A., O'Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp Available at:
- 38.Yarmush M.L., Golberg A., Sersa G., Kotnik T., Miklavcic D. Electroporation-based technologies for medicine: principles, applications, and challenges. Annu Rev Biomed Eng. 2014;16:295–320. doi: 10.1146/annurev-bioeng-071813-104622. [DOI] [PubMed] [Google Scholar]
- 39.Freeman S.A., Wang M.A., Weaver J.C. Theory of electroporation of planar bilayer membranes: predictions of the aqueous area, change in capacitance, and pore-pore separation. Biophys J. 1994;67:42–56. doi: 10.1016/S0006-3495(94)80453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen W., Zhongsheng Z., Lee R.C. Supramembrane potential-induced electroconformational changes in sodium channel proteins: a potential mechanism involved in electric injury. Burns. 2006;32:52–59. doi: 10.1016/j.burns.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 41.Frandsen S.K., Gissel H., Hojman P., Tramm T., Eriksen J., Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res. 2012;72:1336–1341. doi: 10.1158/0008-5472.CAN-11-3782. [DOI] [PubMed] [Google Scholar]
- 42.Sugrue A., Vaidya V., Witt C., et al. Irreversible electroporation for catheter-based cardiac ablation: a systematic review of the preclinical experience. J Interv Card Electrophysiol. 2019;55:251–265. doi: 10.1007/s10840-019-00574-3. [DOI] [PubMed] [Google Scholar]
- 43.Rubinsky B. Irreversible electroporation in medicine. Technol Cancer Res Treat. 2007;6:255–260. doi: 10.1177/153303460700600401. [DOI] [PubMed] [Google Scholar]
- 44.Reddy V.Y., Petru J., Funasako M., et al. Coronary arterial spasm during pulsed field ablation to treat atrial fibrillation. Circulation. 2022;146:1808–1819. doi: 10.1161/CIRCULATIONAHA.122.061497. [DOI] [PubMed] [Google Scholar]
- 45.Su W.W., Reddy V.Y., Bhasin K., et al. STOP Persistent AF Investigators. Cryoballoon ablation of pulmonary veins for persistent atrial fibrillation: results from the multicenter STOP Persistent AF trial. Heart Rhythm. 2020;17:1841–1847. doi: 10.1016/j.hrthm.2020.06.020. [DOI] [PubMed] [Google Scholar]
- 46.Khan A.A., Lip G.Y.H., Shantsila A. Heart rate variability in atrial fibrillation: the balance between sympathetic and parasympathetic nervous system. Eur J Clin Invest. 2019;49 doi: 10.1111/eci.13174. [DOI] [PubMed] [Google Scholar]