Abstract
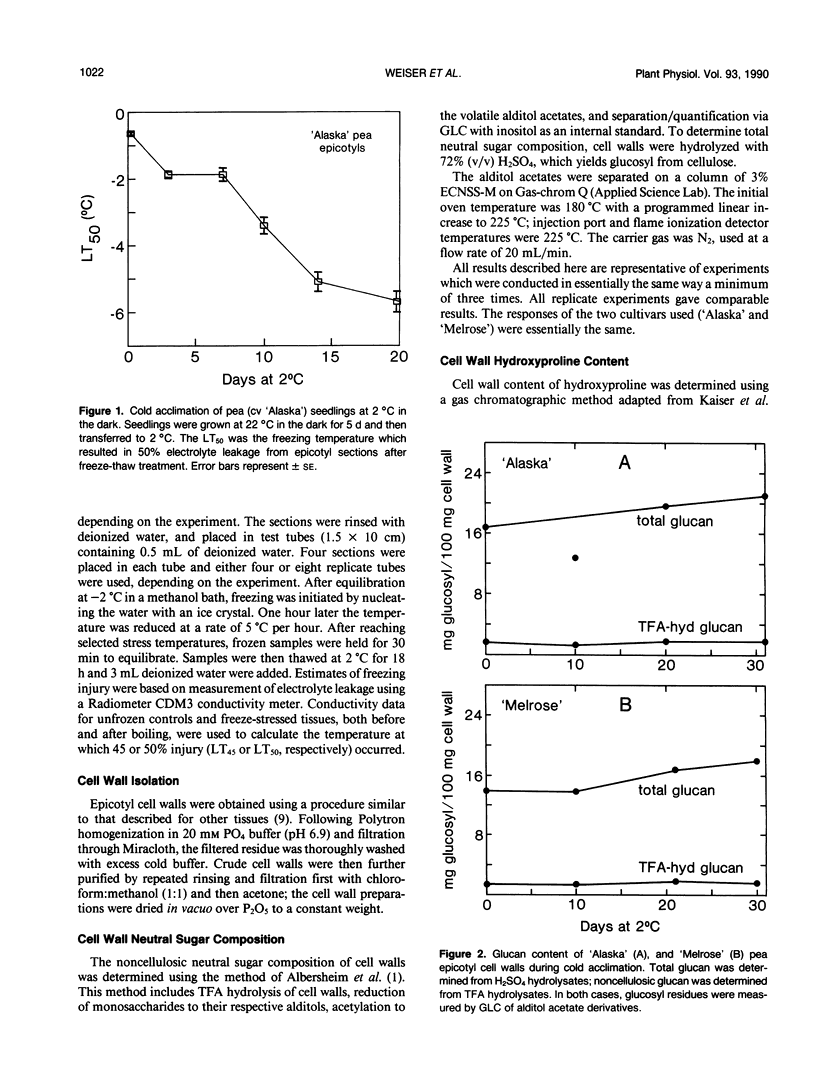
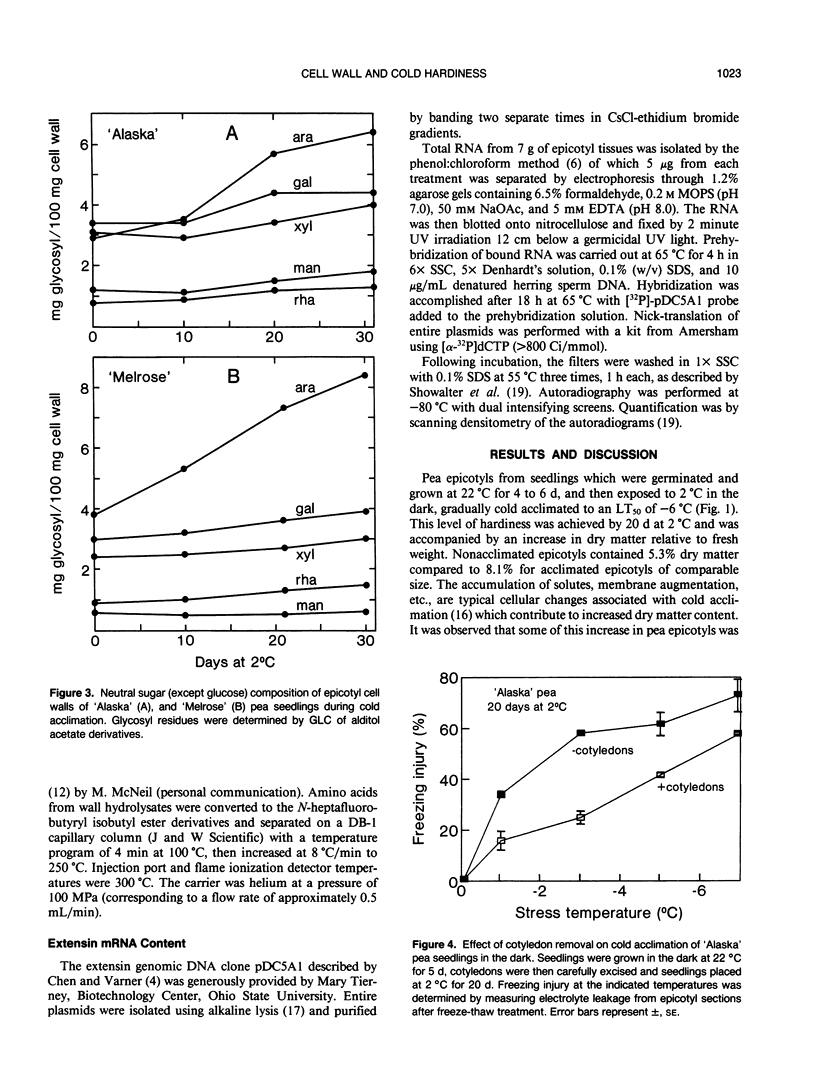
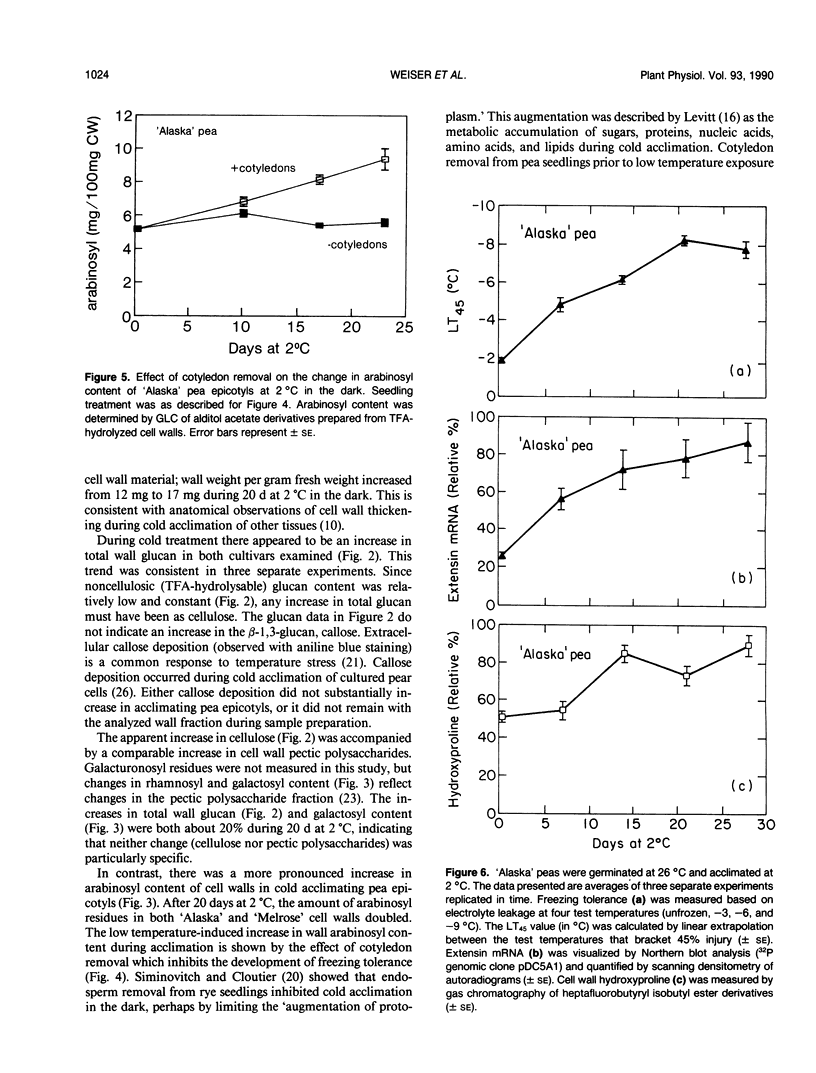
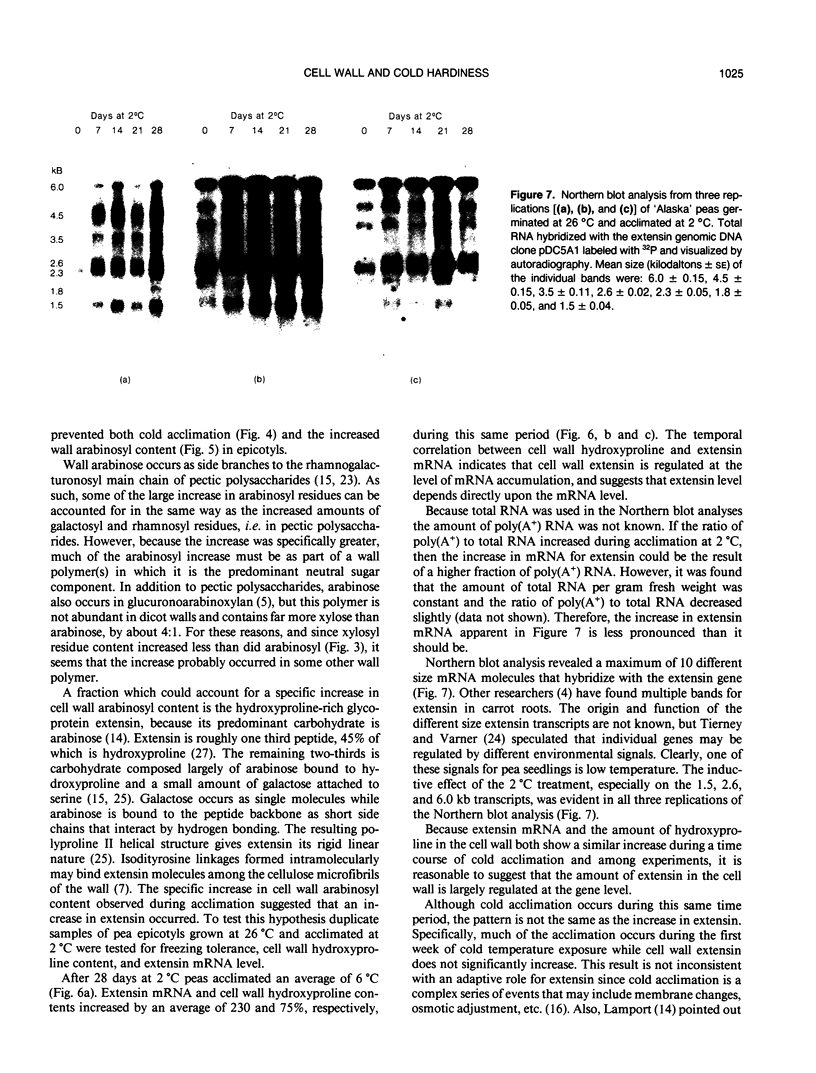
During exposure to 2 °C, pea (Pisum sativum) seedlings cold acclimated to a killing temperature of −6 °C. Associated with this increase in freezing resistance was an increase in the weight of cell walls and changes in wall composition. Arabinosyl content increased by 100%, while other cell wall glycosyl residues and cellulose increased by about 20%. The cell wall hydroxyproline content increased by 80%. Arabinose and hydroxyproline are both major components of the structural cell wall glycoprotein, extensin. The increase in these components indicates that the level of extensin in the cell wall increases during cold acclimation. Northern blot analysis, using the pDC5A1 genomic clone as a probe, revealed a more than three-fold increase in total extensin mRNA during exposure to cold temperature. Specific extensin transcripts of 6.0, 4.5, 3.5, 2.6, 2.3, 1.8, and 1.5 kilobases were identified. Those at 6.0, 2.6, and 1.5 kilobases were especially promoted by low temperature treatment. The rise in extensin during cold acclimation may be regulated, at least in part, at the gene level. The possible structural role of this protein in freezing protection is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolaños J. A., Longstreth D. J. Salinity Effects on Water Potential Components and Bulk Elastic Modulus of Alternanthera philoxeroides (Mart.) Griseb. Plant Physiol. 1984 Jun;75(2):281–284. doi: 10.1104/pp.75.2.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Varner J. E. An extracellular matrix protein in plants: characterization of a genomic clone for carrot extensin. EMBO J. 1985 Sep;4(9):2145–2151. doi: 10.1002/j.1460-2075.1985.tb03908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darvill J. E., McNeil M., Darvill A. G., Albersheim P. Structure of Plant Cell Walls: XI. GLUCURONOARABINOXYLAN, A SECOND HEMICELLULOSE IN THE PRIMARY CELL WALLS OF SUSPENSION-CULTURED SYCAMORE CELLS. Plant Physiol. 1980 Dec;66(6):1135–1139. doi: 10.1104/pp.66.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C., Elzen P., Tamaki S., Dunsmuir P., Bedbrook J. Differential expression of the eight genes of the petunia ribulose bisphosphate carboxylase small subunit multi-gene family. EMBO J. 1985 Dec 1;4(12):3055–3061. doi: 10.1002/j.1460-2075.1985.tb04045.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry S. C. Isodityrosine, a new cross-linking amino acid from plant cell-wall glycoprotein. Biochem J. 1982 May 15;204(2):449–455. doi: 10.1042/bj2040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M. F., Burke M. J. Cold hardiness and deep supercooling in xylem of shagbark hickory. Plant Physiol. 1977 Feb;59(2):319–325. doi: 10.1104/pp.59.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross K. C., Wallner S. J. Degradation of Cell Wall Polysaccharides during Tomato Fruit Ripening. Plant Physiol. 1979 Jan;63(1):117–120. doi: 10.1104/pp.63.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOLLAR S. J., JARAI M. Biochemical chlorination in Streptomvces aureofaciens. Nature. 1960 Nov 19;188:665–665. doi: 10.1038/188665a0. [DOI] [PubMed] [Google Scholar]
- Kaiser F. E., Gehrke C. W., Zumwalt R. W., Kuo K. C. Amino acid analysis. Hydrolysis, ion-exchange cleanup, derivatization, and quantitation by gas-liquid chromatography. J Chromatogr. 1974 Jul 17;94(0):113–133. doi: 10.1016/s0021-9673(01)92361-1. [DOI] [PubMed] [Google Scholar]
- Lamport D. T., Katona L., Roerig S. Galactosylserine in extensin. Biochem J. 1973 May;133(1):125–132. doi: 10.1042/bj1330125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showalter A. M., Bell J. N., Cramer C. L., Bailey J. A., Varner J. E., Lamb C. J. Accumulation of hydroxyproline-rich glycoprotein mRNAs in response to fungal elicitor and infection. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6551–6555. doi: 10.1073/pnas.82.19.6551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch D., Cloutier Y. Twenty-four-hour induction of freezing and drought tolerance in plumules of winter rye seedlings by desiccation stress at room temperature in the dark. Plant Physiol. 1982 Jan;69(1):250–255. doi: 10.1104/pp.69.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K. W., Keegstra K., Bauer W. D., Albersheim P. The Structure of Plant Cell Walls: I. The Macromolecular Components of the Walls of Suspension-cultured Sycamore Cells with a Detailed Analysis of the Pectic Polysaccharides. Plant Physiol. 1973 Jan;51(1):158–173. doi: 10.1104/pp.51.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney M. L., Varner J. E. The extensins. Plant Physiol. 1987 May;84(1):1–2. doi: 10.1104/pp.84.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst G. J., Varner J. E. Reinforced Polyproline II Conformation in a Hydroxyproline-Rich Cell Wall Glycoprotein from Carrot Root. Plant Physiol. 1984 Feb;74(2):247–251. doi: 10.1104/pp.74.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]



