Abstract
The rearranged during transfection (RET) gene is one of the receptor tyrosine kinases and cell‐surface molecules responsible for transmitting signals that regulate cell growth and differentiation. In non‐small cell lung cancer (NSCLC), RET fusion is a rare driver gene alteration associated with a poor prognosis. Fortunately, two selective RET inhibitors (sRETi), namely pralsetinib and selpercatinib, have been approved for treating RET fusion NSCLC due to their remarkable efficacy and safety profiles. These inhibitors have shown the ability to overcome resistance to multikinase inhibitors (MKIs). Furthermore, ongoing clinical trials are investigating several second‐generation sRETis that are specifically designed to target solvent front mutations, which pose a challenge for first‐generation sRETis. The effective screening of patients is the first crucial step in the clinical application of RET‐targeted therapy. Currently, four methods are widely used for detecting gene rearrangements: next‐generation sequencing (NGS), reverse transcription‐polymerase chain reaction (RT‐PCR), fluorescence in situ hybridization (FISH), and immunohistochemistry (IHC). Each of these methods has its advantages and limitations. To streamline the clinical workflow and improve diagnostic and treatment strategies for RET fusion NSCLC, our expert group has reached a consensus. Our objective is to maximize the clinical benefit for patients and promote standardized approaches to RET fusion screening and therapy.
Keywords: NSCLC, precision medicine, RET fusion, targeted therapy, tyrosine receptor kinase
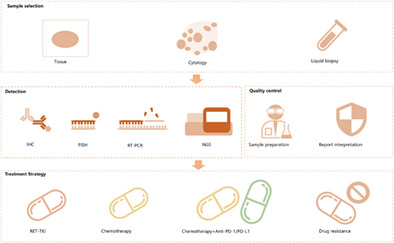
Diagnosis and treatment process of RET gene fusion non‐small cell lung cancer in China.
INTRODUCTION
In the initial management of advanced non‐small cell lung cancer (NSCLC), patients with specific gene alterations such as EGFR mutation, ALK or ROS1 rearrangements are categorized as oncogene‐addicted tumors. These patients are typically treated with kinase inhibitors as the appropriate therapy. Conversely, patients without driver gene mutations or with other gene alterations are classified as nononcogene‐addicted tumors. For these patients, the recommended first‐line treatment options for them include chemotherapy and/or immunotherapy. 1 , 2
However, emerging evidence suggests that patients with oncogene alterations may achieve higher response rates when treated with selective inhibitors as their first‐line therapy, compared to chemotherapy. Additionally, targeted therapies have shown promise in extending survival and improving safety outcomes. Among the various oncogene drivers in NSCLC, rearranged during transfection (RET) rearrangement is a rare mutation found in only 1%–2% of NSCLC patients. Due to the structurally high similarity of RET and VEGFR2 ATP binding sites, 3 several multikinase inhibitors (MKIs) have been tried for RET fusion NSCLC, such as cabozantinib, vandetanib, lenvatinib, and alectinib. However, these inhibitors have shown lower efficacy with a high safety risk, as evidenced by a median objective response rate (ORR) of approximately 30%, a median progression‐free survival (PFS) of 2.3 months, and a median overall survival (OS) of 6.8 months. 4
Since 2016, BLU‐667 and LOXO‐292, two RET inhibitors, have presented promising preclinical data at international conferences, generating significant interest among clinical experts in RET‐altered tumors. 3 , 5 In 2020, both BLU‐667 and LOXO‐292 received FDA approval, marking the era of targeted therapy for RET fusion NSCLC.
Given the diversity of available testing platforms and the challenges associated within accumulating treatment experience due to the low incidence of RET fusion in NSCLC, it is crucial to standardize the screening of RET alterations and the application of RET inhibitors. In this expert consensus, we (1) recommend specific methods and platforms for RET gene testing, (2) propose treatment options based on these alterations, and (3) summarize the resistance mechanisms observed with first‐generation RET tyrosine kinase inhibitors (TKIs) and the progress in developing second‐generation RET TKIs.
THE BIOLOGICAL BASIS OF THE RET GENE
The gene structures and biological functions of the RET gene
The RET gene, located on the long arm of chromosome 10 (10q11.21), encodes a membrane tyrosine kinase receptor that plays a role in various cancers, including medullary thyroid carcinoma, papillary thyroid carcinoma, lung cancer, breast cancer, colorectal cancer, and so on. The RET protein consists of distinct regions, including an extracellular region encoded by exon 1–10 and part of exon 11, a transmembrane region encoded by part of exon 11, and a cytoplasmic region encoded by part of exon 11 and exon 12–19 or 12–20 (Figure 1). Notably, there is a cysteine‐rich domain (CRD, aa 515–634) that is crucial for receptor dimerization. While RET is primarily expressed in neural tissues such as the brain and autonomic nervous system, as well as in neuroendocrine cells and developing kidneys, it is also found in the lung, digestive tract, adult kidney, female and male reproductive organs, skin, and blood apparatus. In terms of physiological activation, the four RET ligands, namely GDNF, NRTN, PSPN, and ARTN, preferentially interact with coreceptors GFRA1, GFRA2, GFRA3, and GFRA4, respectively. These ligands form homodimers with a cystine knot at their center and require their respective coreceptors for RET activation. 6
FIGURE 1.
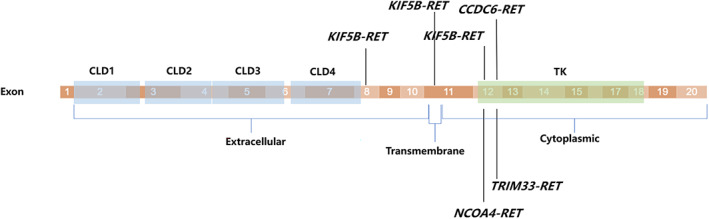
The structure of RET gene coding region. CRD, cysteine‐rich domain; GLD, 4 cadherin‐like domains; TK, tyrosine kinase domain.
RET gene alterations and their mechanism of carcinogenesis
To date, three primary mechanisms of aberrant RET activation have been identified in cancer: in‐frame RET gene fusions, 7 , 8 targeted mutations within the RET gene itself, 9 , 10 , 11 and aberrant overexpression of the RET gene. 12 , 13 These abnormalities lead to the inappropriate activation of the RET tyrosine kinase even in the absence of its ligand. Consequently, the RAS‐MAPK and PI3K‐AKT signaling pathways are activated through the binding of adaptor proteins to phosphorylated sites on the RET protein. 14 , 15
The frequency of RET fusion in NSCLC is relatively low, ranging from 1% to 2%, 16 , 17 , 18 with no significant difference observed between Asian and Western populations. 19 Among the RET fusion cases, the most common partners are KIF5B and CCDC6, accounting for approximately 90% of cases. 20 With the utilization of next‐generation sequencing (NGS) technology, over 50 distinct fusion partners have been identified, indicating a diverse and varied distribution in their occurrence. This demonstrates a clear long‐tail effect in the incidence distribution of RET fusion events. 21
Activating point alterations represent another significant oncogenic mutation associated with inherited diseases such as multiple endocrine neoplasia 2A (MEN2A), multiple endocrine neoplasia 2B (MEN2B), and familial medullary thyroid carcinomas. These mutations are distributed across exons 7 to 16, predominantly occurring in the cysteine‐rich domain and tyrosine kinase domains. Noteworthy examples of these alterations include C609, C611, C618, C620 (exon 10), C630, D631, C634, T636, K666, D707 (exon 11), E505 (exon 7), C515, C531, G533, G548 (exon 8), E768, L790, Q781 (exon 13), V804 (exon 14), A883, S891, S904 (exon 15), M918, and R912 (exon 16). 6
It is worth noting that RET fusion has been identified as one of the key mechanisms underlying acquired resistance to TKI treatments in cases involving other driver mutations. 4 Following resistance to EGFR TKIs, the occurrence of acquired RET fusion was observed in approximately 1%–4% of patients, with CCDC6 being the predominant fusion partner. 22 , 23
TYPES OF DETECTION METHOD AND THEIR LIMITATIONS
Next‐generation sequencing (NGS)
In Asian NSCLC, especially lung adenocarcinoma, 19 approximately 80% of patients carry driver gene mutations. Currently, more than 10 driver genes are known to be targetable or associated with resistance to targeted therapies. For patients with advanced NSCLC, tissue samples are typically obtained through biopsy, followed by histopathological examination using hematoxylin and eosin (HE) staining, immunohistochemical subtyping, and molecular testing. However, biopsies often yield limited amounts of tissue, necessitating the maximization of information extraction from scarce samples. The key advantage of NGS lies in its ability to simultaneously detect multiple genes and various types of mutations, including point mutations, fusions (rearrangements), and amplifications (copy number variations), even when sample quantities are limited.
With the increasing utilization and cost‐effectiveness of NGS technology, clinicians are increasingly acknowledging its value in identifying driver gene mutations in lung cancer patients, especially those experiencing drug resistance to targeted therapies. Nonetheless, the selection of an appropriate NGS panel necessitates careful consideration of clinical requirements, testing expenses, and turnaround time. For patients who have recently received a diagnosis, it is advisable to opt for a smaller panel comprising fewer than 100 genes. Conversely, for patients exhibiting treatment resistance, a larger panel may prove more advantageous.
DNA‐based NGS
NGS offers notable advantages in terms of sensitivity and specificity, enabling the identification of gene alterations in both tissue and liquid samples. Hybrid capture and amplicon‐based technologies are two commonly employed methods in DNA‐based NGS. Currently, the targeted gene hybrid capture approach is primarily utilized in DNA‐based NGS for the detection of RET gene rearrangements, encompassing both known and unknown fusion partners. This is due to the absence of complex or repetitive sequences within the RET gene. In terms of RET fusion detection, NGS exhibits a sensitivity ranging from 87.2% to 100% and a specificity ranging from 98.1% to 100%. 24 , 25 However, it is crucial to acknowledge that the accuracy of gene fusion detection through NGS is influenced by critical factors such as the coverage of capture probes, sequencing depth, specimen quality, complexity of gene sequences, and bioinformatic analysis. 26 , 27
NGS detection utilizing amplicon technology offers the advantage of minimal DNA requirement and facilitates gene mutation testing. Nonetheless, it is important to acknowledge the limitations of this approach, as it can solely identify genes for which corresponding primer sequences have been designed. Consequently, amplicon‐based NGS may not be suitable for detecting unknown fusion partners or genes that lack identifiable primer sequences, thereby restricting its application.
Given the enhanced sensitivity, specificity, and clinical accessibility of DNA‐based NGS, it is commonly advised to employ this method for the identification of RET fusion in both newly diagnosed patients and those who have developed treatment resistance.
RNA‐based NGS
RNA‐based NGS represents an optimal approach for the detection of gene fusions and exon skipping, as it focuses solely on retained exons at the RNA level, thereby simplifying the complexity of NGS testing. However, it is important to acknowledge the inherent instability and susceptibility to degradation of RNA, posing a significant challenge in maintaining high sample quality, particularly when working with formalin‐fixed paraffin‐embedded (FFPE) tissues. In a study, RNA‐based NGS sequencing was conducted on 55 samples with confirmed RET fusion positivity by DNA‐based NGS. Among these samples, 10 were excluded from the analysis due to inadequate sample quality. 28 Among the remaining 44 samples with confirmed RET fusion positivity by DNA‐based NGS, 41 (93.2%) were successfully validated at the RNA level. Notably, in two cases where only a RET 5′‐end fusion was identified by DNA‐based NGS, RNA‐based NGS detected KIF5B‐RET (K24:R10) transcripts in one case, suggesting that DNA‐based NGS may overlook 3′‐end fusions.
According to available research findings, both DNA‐based NGS and RNA‐based NGS exhibit a high level of concordance in detecting RET fusion alterations. However, when considering factors such as economic cost and clinical accessibility, routine implementation of RNA‐based NGS for RET fusion gene testing is not advised. Nonetheless, in cases where DNA‐based NGS identifies a RET 5′‐end fusion, it is recommended to conduct further validation of the presence of the RET kinase domain using RNA‐based NGS.
Reverse transcription‐polymerase chain reaction (RT‐PCR)
The rapid and straightforward detection of RET gene fusions is the primary advantage of RT‐PCR. However, its applicability is restricted to known fusion partners within the primer design range, thereby limiting its ability to identify unknown or novel fusion partners, which may result in false‐negative outcomes. 29 RT‐PCR detects RET gene fusion at the RNA level, making the accuracy of the test results highly reliant on the RNA sample quality. Moreover, in terms of cost‐effectiveness, PCR testing is more advantageous compared to NGS. Therefore, in situations where NGS is not readily available, routine utilization of multiplex RT‐PCR for RET gene testing is recommended.
Fluorescence in situ hybridization (FISH)
FISH is widely regarded as the gold standard technique for detecting gene rearrangements, regardless of the specific fusion partner involved. Its notable advantages include the ability to achieve single‐cell resolution and a rapid turnaround time. However, the cost of FISH testing remains relatively high, particularly when applied to single gene testing. Additionally, the interpretation of FISH results requires specialized technical expertise and is typically only accessible in larger medical centers. Furthermore, it is important to note that there is currently no universally standardized cutoff defining positivity in FISH analysis, as reported rates of RET split signals in positive cases range from 10% to 20% of cells. 30 , 31 , 32
A comparative study assessing the efficacy of frequently employed assays in detecting RET fusions demonstrated that FISH exhibited a sensitivity of 100% for both KIF5B and CCDC6 but displayed a lower sensitivity of 66.7% for NCOA4. 33 The diminished sensitivity observed for NCOA4 is attributed to the close proximity of the NCOA4 and RET genes.
In summary, although FISH demonstrates high sensitivity (100%) in detecting RET fusions, its specificity is suboptimal, ranging from 45% to 60%. As a result, FISH is not routinely recommended as a primary method for RET testing.
Immunohistochemistry (IHC)
While IHC is a cost‐effective method suitable for large‐scale screening of ALK‐positive NSCLC, 34 its effectiveness in detecting RET fusions is limited. A study demonstrated that RET IHC staining patterns showed no discernible differences between RET‐positive and RET‐negative specimens previously identified by RT‐PCR. The false‐negative (FN) and false‐positive (FP) rates for RET fusion detection by IHC were found to be 46% and 62%, respectively. 30 Furthermore, three additional retrospective analyses 31 , 35 , 36 yielded similar results, confirming the poor performance of IHC in detecting RET fusions. Consequently, IHC is not considered a reliable technique for the detection of RET fusions.
TREATMENTS FOR RET FUSION NSCLC
Since 2020, the Food and Drug Administration (FDA) has granted approval for two selective RET tyrosine kinase inhibitors (sRETi) for the treatment of advanced RET NSCLC. These sRETi drugs are specifically designed to possess high potency and selectivity in targeting oncogenic RET alterations, including RET fusions such as KIF5B‐RET and CCDC6‐RET, as well as RET activating mutations like C634W, M918T, and V804L/M. The antitumor mechanism of sRETi involves competitive binding to the ATP receptor, thereby inhibiting the activity of the RET protein kinase. 37 , 38
Pralsetinib
On September 4, 2020, pralsetinib (BLU‐667) received approval from the FDA for the treatment of adult patients with metastatic NSCLC positive for RET gene fusion. 39 Following this, in March 2021, the National Medical Products Administration (NMPA) in China also granted approval for pralsetinib as the first selective RET tyrosine kinase inhibitor (sRETi) for the treatment of locally advanced or metastatic NSCLC with RET gene fusion in patients who had previously received platinum‐based chemotherapy. Subsequently, in June 2023, the NMPA approved pralsetinib as a first‐line treatment option for locally advanced or metastatic NSCLC with RET gene fusion. 40
The ARROW study (NCT03037385) is a multicohort, open‐label, phase I/II clinical trial designed to assess the safety and effectiveness of pralsetinib in patients with advanced tumors harboring RET alterations. Updated data from the NSCLC cohorts, as published in the Annals of Oncology, revealed an overall response rate (ORR) of 79% (95% CI: 59–92) in treatment‐naïve patients and 59% (95% CI: 50–67) in patients who had previously received platinum‐based chemotherapy (data cutoff: November 6, 2020). The median duration of response (DoR) was not reached in treatment‐naïve patients and was 22.3 months in patients with prior platinum‐based chemotherapy. Among patients with measurable intracranial metastases, the intracranial response rate was 70% (7/10; 95% CI: 35–93), and the median DoR was 10.5 months (95% CI: 5.5–12.6). 41
Further analysis of the relationship between RET fusion partners and treatment outcomes from the ARROW study confirmed the efficacy of pralsetinib in patients with RET fusion NSCLC, regardless of the specific fusion partner. 42 The efficacy results of pralsetinib in Chinese patients were consistent with those reported in the global population. 43
Selpercatinib
Selpercatinib (LOXO‐292), another selective RET inhibitor (sRETi), received FDA and NMPA approval for the treatment of adult patients with metastatic NSCLC harboring RET gene fusions in May 2020 and October 2022, respectively. 44 , 45 Updated data was reported in the Journal of Clinical Oncology in 2022. 46 The updated data set included a larger number of patients (n = 316) compared to the original reported population (n = 144). In treatment‐naïve patients, the objective response rate (ORR) was 84% (95% CI: 73–92). The median duration of response (DoR) was 20.2 months (95% CI: 13.0–could not be evaluated). The median progression‐free survival (PFS) was 22.0 months. In patients who had previously received platinum‐based chemotherapy, the ORR was 61% (95% CI: 55–67). The median DoR was 28.6 months (95% CI: 20.4–could not be evaluated). The median PFS was 24.9 months (n = 247, median follow‐up of 24.7 months). In the registrational analysis set, the median PFS was 19.3 months with longer follow‐up (n = 105, median follow‐up of 30.3 months). Among the 26 patients with measurable baseline CNS metastasis, the intracranial ORR was 85% (95% CI: 65–96), and the median DoR was 9.4 months (95% CI: 7.4–15.3).
Options other than sRETi
Chemotherapy
Prior to the availability of selective RET TKIs (sRETi), platinum‐based chemotherapy was the standard treatment for RET fusion advanced NSCLC. However, there were no prospective chemotherapy trials specifically focused on RET‐fusion NSCLC due to the relatively low frequency of this genomic alteration.
A global, multicenter registry study was conducted to evaluate the efficacy of traditional chemotherapy in 84 patients who received platinum doublet as the first‐line treatment. The study showed that the overall response rate (ORR) was 51% (95% CI: 38.1–63.4), the median progression‐free survival (PFS) was 7.8 months (95% CI: 5.3–10.2), and the median overall survival (OS) was 24.8 months (95% CI: 13.6–32.3). Among the 66 patients who received a platinum agent in combination with pemetrexed, the ORR was 49% (95% CI: 35.4–62.9), the median PFS was 6.4 months (95% CI: 4.3–8.8), and the median OS was 23.6 months (95% CI: 13.6–32.3). 47 Similar results were observed in a retrospective study conducted in China. 48
These findings suggest that platinum‐based chemotherapy can lead to a certain level of response and survival benefit in patients with RET gene fusion NSCLC. However, the introduction of sRETi has revolutionized treatment options and has shown improved efficacy compared to traditional chemotherapy.
Is chemotherapy combined PD‐1/PD‐L1 inhibitors a better choice for RET‐fusion NSCLC?
The combination of platinum doublet and PD‐1/PD‐L1 inhibitors has become the standard treatment for patients without driver gene alterations. 49 , 50 However, previous phase III studies did not exclude patients with RET fusions from these trials. A post hoc subgroup analysis of patients treated with the CCP regimen (carboplatin, pemetrexed, and pembrolizumab) in the first‐line setting, including a small number of patients with RET fusions (N = 10), showed an ORR of 70% (7/10). 51 In real‐world settings, a study of 12 patients with RET fusion NSCLC reported a median PFS of 5.4 months (1.4–14.2) and a median OS of 19 months (6.9‐not reached). 52 These findings suggest that PD‐1/PD‐L1 inhibitors may not provide additional benefits beyond chemotherapy in patients with RET fusion NSCLC.
However, caution should be exercised when combining immune checkpoint inhibitor (ICI) therapy with targeted agents. Concurrent or sequential use of ICI therapy with targeted agents may increase the risk of severe immune‐related adverse events (irAEs). 53 In the ongoing phase 1/2 LIBRETTO‐001 trial (NCT03157128), 22 out of 329 patients (7%) experienced hypersensitivity reactions attributed to selpercatinib, with a higher incidence observed in patients previously treated with ICIs (n = 17, 77%) compared to ICI‐naïve patients (n = 5, 23%). 54 Therefore, our expert group has reached a consensus that ICI‐based therapy is not the optimal choice for RET fusion NSCLC, especially in patients who may consider selpercatinib treatment in the future.
Treatment strategy for acquired RET fusion NSCLC
RET fusion can also be found in cases after EGFR/ALK TKI progression and appears to become more prevalent after the administration of third‐generation EGFR TKIs. 4 , 22 Piotrowska et al. 23 demonstrated that the emergence of acquired RET fusion expression in NSCLC cell lines with EGFR mutations led to resistance against EGFR inhibitors, a challenge that could be overcome by combining EGFR and RET inhibition. Notably, two patients who harbored acquired RET fusion and were treated with pralsetinib and osimertinib exhibited PR, with ongoing treatment progress at the time the paper was authored. In a multicenter real‐world retrospective study involving 31 patients, two cohorts were formed based on whether pralsetinib‐based targeted therapy was initiated immediately upon detecting RET fusion subsequent to EGFR/ALK inhibitor progression. The results indicated a median time to treatment failure (mTTF) of 7.93 months for cohort 1 (n = 20) versus 4.24 months for cohort 2 (n = 11), with ORR of 35.0% and 18.2%, respectively. Generally, the combined treatment of pralsetinib and other TKIs was well tolerated, with adverse events aligning with the known profiles of the two drugs. 55 Another prospective expanded access clinical trial aimed at patients with acquired RET fusion post‐osimertinib treatment involved the use of osimertinib and selpercatinib. This trial enrolled 14 patients, achieving an ORR of 50% and a median treatment duration of 7.9 months. 56 These results underscore the potential of RET inhibitor‐based therapy as a promising strategy for overcoming acquired RET fusion in NSCLC patients.
Drug resistance and second‐generation RET inhibitors
In the context of targeted treatment, the emergence of resistance to RET tyrosine kinase inhibitors (TKIs) is a common occurrence among patients. However, due to the relatively recent introduction of these medications to the market, the mechanisms underlying resistance to selective RET inhibitors have not been extensively studied. Nevertheless, several known variants have been identified that are associated with resistance to first‐generation RET inhibitors: (1) Solvent‐front mutations, such as RET G810S/C; (2) hinge region mutations, such as RET Y806C/C; (3) mutations in the “roof” region of the ATP binding site, such as RET L730; (4) bypass pathway mutations, such as MET/ERBB2/EGFR amplifications, BRAF V600E, KRAS mutations, PIK3CA mutation, ALK/ROS1 fusion; (5) small cell transformation of NSCLC and (6) other or unknown resistance mechanisms. 57 , 58 , 59 , 60 , 61
Among these variants, solvent‐front mutations are the most common and recurring “on‐target” resistance mechanism, occurring in approximately 10% of patients who experience disease progression after sRETi treatment. 57 To provide precise therapy for patients who develop resistance to sRETi, a second biopsy and genetic testing are recommended.
In response to drug resistance to first‐generation sRETi, four second‐generation RET TKIs have initiated clinical trials. 62 , 63 , 64 , 65 These second‐generation drugs, such as APS03118, TPX0046, LOXO‐260, and KL590586, were designed to target and overcome solvent‐front mutations. Preclinical data has demonstrated potent activity against solvent‐front and gatekeeper mutations, except for TPX0046, which does not show activity against gatekeeper mutations. 66 , 67 , 68 , 69 In the 2023 ASCO meeting, KL590586 presented the results of a phase I study, reporting an overall response rate (ORR) of 60% in the study population. Moreover, significant reductions in target lesions were observed in seven patients who had previously received first‐generation sRETi treatment.
SUMMARY AND PROSPECTS
Although RET gene fusion is rare in NSCLC patients, its impact and burden should not be underestimated, particularly given the large population of advanced NSCLC patients in China. Our expert group thoroughly discussed the diagnosis and treatments of RET gene fusion NSCLC in China based on clinical practice (Table 1 ). The approval of sRETi by NMPA in China has significantly extended the lifespan of patients, emphasizing the importance of patient management.
TABLE 1.
Expert consensus on the diagnosis and treatment of NSCLC patients with RET fusion in China.
| Consensus number | Key points | Recommendation level | |
|---|---|---|---|
| Detection time point | Consensus 1 | RET fusion testing is recommended for patients with stage III/IV NSCLC | Strongly recommended |
| Consensus 2 | RET fusion testing is recommended to be performed in conjunction with testing for other driver genes such as EGFR, ALK, ROS1, BRAF, KRAS, HER2, and so on | Strongly recommended | |
| Consensus 3 | EGFR, ALK, and ROS1 were tested first, when negative, RET gene fusion should be tested | Strongly recommended | |
| Consensus 4 | RET gene fusion testing should be performed for patients who experience disease progression on targeted therapy with other driver gene mutations | Recommended | |
| Detection method | Consensus 5 | RET fusion testing is preferred to be performed using DNA‐based NGS | Strongly recommended |
| Consensus 6 | When NGS is unavailable, RET fusion testing can be performed using RT‐PCR | Recommended | |
| Consensus 7 | When the tissue is very limited, RET fusion testing by FISH can be considered as an alternative | Recommended | |
| Consensus 8 | When 5′‐end RET fusion is detected by DNA‐based NGS, RNA‐based NGS can be used to verify its activity | Recommended | |
| Consensus 9 | RET protein expression tested by IHC | Not recommended | |
| Detection strategy | Consensus 10 | Tumor tissue or cytology samples are preferred for RET fusion testing. If it cannot be obtained or is insufficient, peripheral blood, pleural effusion, or cerebrospinal fluid can be considered for ctDNA testing in advanced NSCLC patients. | Strongly recommended |
| Detecting quality control | Consensus 11 | Physicians should make every effort to obtain sufficient samples for both pathological and molecular diagnosis. | Strongly recommended |
| Consensus 12 | Laboratories should participate in annual quality control programs, such as the PQCC, CAP, CLIA, or other laboratory quality assessment initiatives. | Strongly recommended | |
| Treatment strategy | Consensus 13 | For RET gene fusion advanced NSCLC patients, treatment with sRETi drugs such as pralsetinib and selpercatinib is recommended. | Strongly recommended |
| Consensus 14 | For patients with acquired RET gene fusion, sequential treatment with sRETi following other TKIs could be a viable treatment option. | Recommended | |
| Consensus 15 | When sRETi is not available or intolerable, platinum‐based chemotherapy ± bevacizumab is recommended as the first‐line treatment option. | Recommended | |
| Consensus 16 | For RET gene fusion patients with resistance to sRETi, performing NGS testing to identify the mechanism of resistance is encouraged. This will help in determining whether second‐generation RET inhibitors or participation in related clinical trials are appropriate treatment options. | Recommended |
Abbreviations: CAP, College of American Pathologists; CLIA, Clinical Laboratory Improvement Amendments; FISH, fluorescence in situ hybridization; IHC, immunohistochemistry; NSCLC, non‐small cell lung cancer; NGS, next‐generation sequencing; PQCC, Pathology Quality Control Center; RET, rearranged during transfection; RT‐PCR, reverse transcription polymerase chain reaction.
In the context of rare gene mutations, cost‐effective identification of target patients becomes crucial. Our expert consensus systematically compared the advantages and disadvantages of different testing platforms for RET gene fusion. DNA‐based NGS was identified as the first preferred method, followed by RT‐PCR, while FISH was considered as an alternative. IHC, however, was not recommended. For the treatment of patients with RET gene fusion NSCLC, sRETi should be the preferred option for the first‐line setting. If sRETi is unavailable, platinum‐double chemotherapy can be considered, but ICI monotherapy or combination with chemotherapy is not recommended for the first‐line setting in patients who may receive targeted therapy in the future.
Several critical areas still require further exploration. First, it is essential to establish a national platform for collecting Chinese patients' gene mutation data and refining the map of RET gene alterations in China. This will effectively translate the progress of molecular biology research into clinical practice benefits. Second, more high‐quality real‐world studies are needed for the two sRETi drugs. Efficacy and safety data from real‐world analysis will provide reliable experiences for the drugs' application. Last but not least, the resistance mechanism of sRETi, especially in Chinese patients, remains unclear and is highly relevant to subsequent treatment decisions. Therefore, exploratory studies on sRETi resistance and personalized treatment for patients are also necessary.
AUTHOR CONTRIBUTIONS
Yuanzhi Lu, Wenfeng Fang, Ziming Li and Lin Wu participated in the design of the expert consensus. Xingxiang Pu, Chunwei Xu, Qian Wang, Wenxian Wang and Fang Wu conceived of the expert consensus, and participated in its design and other authors coordination and helped to draft the expert consensus. All authors read and approved the final manuscript.
FUNDING INFORMATION
This work was supported by the Science and Technology Innovation Program of Hunan Province (grant number 2023SK4024), the Natural Science Foundation of China (grant number 82002456), China Postdoctoral Science Foundation (grant number 2022M723207), and the Medical Scientific Research Foundation of Zhejiang Province of China (grant number 2023KY666).
CONFLICT OF INTEREST STATEMENT
There are no conflicts of interest to disclose.
Pu X, Xu C, Wang Q, Wang W, Wu F, Cai X, et al. Expert consensus on the diagnosis and treatment of RET gene fusion non‐small cell lung cancer in China . Thorac Cancer. 2023;14(31):3166–3177. 10.1111/1759-7714.15105
Xingxiang Pu, Chunwei Xu, Qian Wang, Wenxian Wang and Fang Wu contributed equally to the study.
Contributor Information
Yuanzhi Lu, Email: yuanzhi.lu@jnu.edu.cn.
Wenfeng Fang, Email: fangwf@sysucc.org.cn.
Ziming Li, Email: liziming1980@163.com.
Lin Wu, Email: wulin-calf@vip.163.com.
REFERENCES
- 1. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non‐small‐cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27(suppl 5):v1–v27. [DOI] [PubMed] [Google Scholar]
- 2. Bronte G, Rizzo S, La Paglia L, et al. Poster No 441, driver mutations and differential sensitivity to targeted therapies: a new approach to the treatment of lung adenocarcinoma. Cancer Treat Rev. 2010;36(Suppl 3):S21–S29. [DOI] [PubMed] [Google Scholar]
- 3. Brandhuber B, Haas J, Tuch B, et al. The development of LOXO‐292, a potent, KDR/VEGFR2‐sparing RET kinase inhibitor for treating patients with RET‐dependent cancers. presented at:EORTC‐NCI‐AACR Symposium 2016.
- 4. Bronte G, Ulivi P, Verlicchi A, Cravero P, Delmonte A, Crinò L. Targeting RET‐rearranged non‐small‐cell lung cancer: future prospects[J]. Lung Cancer (Auckl). 2019;10:27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rahal R, Maynard M, Hu W, et al. Poster No B151. BLU‐667 is a Potent and Highly Selective RET Inhibitor Being Developed for RET‐Driven Cancers. presented at: AACR‐NCI‐EORTC Symposium 2017.
- 6. Atlas of Genetics and Cytogenetics in Oncology and Hematology.
- 7. Takahashi M, Ritz J, Cooper GM. Activation of a novel human transforming gene, ret, by DNA rearrangement. Cell. 1985;42:581–588. [DOI] [PubMed] [Google Scholar]
- 8. Takahashi M, Cooper GM. Ret transforming gene encodes a fusion protein homologous to tyrosine kinases. Mol Cell Biol. 1987;7:1378–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mulligan LM, Kwok JB, Healey CS, Elsdon MJ, Eng C, Gardner E, et al. Germ‐line mutations of the RET proto‐oncogene in multiple endocrine neoplasia type 2A. Nature. 1993;363:458–460. [DOI] [PubMed] [Google Scholar]
- 10. Hofstra RM, Landsvater RM, Ceccherini I, et al. A mutation in the RET proto‐oncogene associated with multiple endocrine neoplasia type 2B and sporadic medullary thyroid carcinoma. Nature. 1994;367:375–376. [DOI] [PubMed] [Google Scholar]
- 11. Donis‐Keller H, Dou S, Chi D, Carlson KM, Toshima K, Lairmore TC, et al. Mutations in the RET proto‐oncogene are associated with MEN 2A and FMTC. Hum Mol Genet. 1993;2:851–856. [DOI] [PubMed] [Google Scholar]
- 12. Horibata S, Rice EJ, Mukai C, Marks BA, Sams K, Zheng H, et al. ER positive breast cancer cells are poised for RET‐mediated endocrine resistance. PLoS One. 2018;13:e0194023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mulligan LM. GDNF and the RET receptor in cancer: new insights and therapeutic potential. Front Physiol. 2018;9:1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 15. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. [DOI] [PubMed] [Google Scholar]
- 16. Feng J, Li Y, Wei B, Guo L, Li W, Xia Q, et al. Clinicopathologic characteristics and diagnostic methods of RET rearrangement in Chinese non‐small cell lung cancer patients. Transl Lung Cancer Res. 2022;11(4):617–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xia C, Dong X, Li H, Cao M, Sun D, He S, et al. Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl). 2022;135:584–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kato S, Subbiah V, Marchlik E, Elkin SK, Carter JL, Kurzrock R. RET aberrations in diverse cancers: next‐generation sequencing of 4,871 patients. Clin Cancer Res. 2017;23:1988–1997. [DOI] [PubMed] [Google Scholar]
- 19. Aaron CT, Tan DSW. Targeted therapies for lung cancer patients with oncogenic driver molecular alterations. J Clin Oncol. 2022;40(6):611–625. [DOI] [PubMed] [Google Scholar]
- 20. Saito M, Shimada Y, Shiraishi K, Sakamoto H, Tsuta K, Totsuka H, et al. Development of lung adenocarcinomas with exclusive dependence on oncogene fusions. Cancer Res. 2015;75(11):2264–2271. [DOI] [PubMed] [Google Scholar]
- 21. Parimi V, Tolba K, Danzigeret N, et al. Genomic landscape of 891 RET fusions detected across diverse solid tumor types. NPJ Precis Oncol. 2023;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu VW, Klempner SJ, Shong O, et al. Receptor tyrosine kinase fusions as an actionable resistance mechanism EGFR TKIs in EGFR‐mutant non‐small‐cell lung cancer. Trends in Cancer. 2019;5(11):677–692. [DOI] [PubMed] [Google Scholar]
- 23. Piotrowska Z, Isozaki H, Lennerz JK, Gainor JF, Lennes IT, Zhu VW, et al. Landscape of acquired resistance to Osimertinib in EGFR‐mutant NSCLC and clinical validation of combined EGFR and RET inhibition with Osimertinib and BLU‐667 for acquired RET fusion. Cancer Discov. 2018;8(12):1529–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chinese Society of Clinical Oncology, expert committee on non‐small cell lung cancer . Chinese expert consensus on next generation sequencing diagnosis for non‐small cell lung cancer (2020 Edition)[J]. Zhongguo Fei Ai Za Zhi. 2020;23(9):741–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Molecular pathology collaboration Group of Tumor Pathology Committee of Chinese anti‐cancer association, molecular pathology Group of Chinese Society of pathology, pathology quality control center . Expert consensus on clinical practice of RET fusion detection in non‐small cell lung cancer in China[J]. Zhonghua Bing Li Xue Za Zhi. 2021;50(6):583–591. [DOI] [PubMed] [Google Scholar]
- 26. Li W, Guo L, Liu Y, Dong L, Yang L, Chen L, et al. Potential unreliability of uncommon ALK, ROS1, and RET genomic breakpoints in predicting the efficacy of targeted therapy in NSCLC[J]. J Thorac Oncol. 2021;16(3):404–418. [DOI] [PubMed] [Google Scholar]
- 27. Song Z, Xu C, He Y, Li F, Wang W, Zhu Y, et al. Simultaneous detection of gene fusions and base mutations in cancer tissue biopsies by sequencing dual nucleic acid templates in unified reaction. Clin Chem. 2020;66(1):178–187. [DOI] [PubMed] [Google Scholar]
- 28. Xiang C, Guo L, Zhao R, Teng H, Wang Y, Xiong L, et al. Identification and validation of noncanonical RET fusions in non‐small‐cell lung cancer through DNA and RNA sequencing[J]. J Mol Diagn. 2022;24(4):374–385. [DOI] [PubMed] [Google Scholar]
- 29. Ferrara R, Auger N, Auclin E, Besse B. Clinical and translational implications of RET rearrangements in non‐small cell lung cancer[J]. J Thorac Oncol. 2018;13(1):27–45. [DOI] [PubMed] [Google Scholar]
- 30. Wang R, Hu H, Pan Y, et al. RET fusions define a unique molecular and clinicopathologic subtype of non‐small‐cell lung cancer. J Clin Oncol. 2012;30:4352–4359. [DOI] [PubMed] [Google Scholar]
- 31. Go H, Jung YJ, Kang HW, Park IK, Kang CH, Lee JW, et al. Diagnostic method for the detection of KIF5B‐RET transformation in lung adenocarcinoma. Lung Cancer. 2013;82:44–50. [DOI] [PubMed] [Google Scholar]
- 32. Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, et al. RET‐rearranged non‐small‐cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang SR, Aypar U, Rosen EY, Mata DA, Benayed R, Mullaney K, et al. A performance comparison of commonly used assays to detect RET fusions. Clin Cancer Res. 2021;27(5):1316–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pekar‐Zlotin M, Hirsch FR, Soussan‐Gutman L, Ilouze M, Dvir A, Boyle T, et al. Fluorescence in situ hybridization, immunohistochemistry, and next‐generation sequencing for detection of EML4‐ALK rearrangement in lung cancer. Oncologist. 2015;20:316–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Platt A, Morten J, Ji Q, Elvin P, Womack C, Su X, et al. A retrospective analysis of RET translocation, gene copy number gain and expression in NSCLC patients treated with vandetanib in four randomized phase III studies. BMC Cancer. 2015;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tsuta K, Kohno T, Yoshida A, Shimada Y, Asamura H, Furuta K, et al. RET‐rearranged nonsmall‐cell lung carcinoma: a clinicopathological and molecular analysis. Br J Cancer. 2014;110:1571–1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Subbiah V, Gainor JF, Rahal R, Brubaker JD, Kim JL, Maynard M, et al. Precision targeted therapy with BLU‐667 for RET‐driven cancers. Cancer Discov. 2018;8(7):836–849. [DOI] [PubMed] [Google Scholar]
- 38. Subbiah V, Velcheti V, Tuch BB, Ebata K, Busaidy NL, Cabanillas ME, et al. Selective RET kinase inhibition for patients with RET‐altered cancers. Ann Oncol. 2018;29(8):1869–1876. 10.1093/annonc/mdy137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-Pralsetinib-non-small-cell-lung-cancer-ret-gene-fusions.
- 40. https://www.cstonepharma.com/html/news/2859.html. Accessed June 10, 2023.
- 41. Griesinger F, Curigliano G, Thomas M, Subbiah V, Baik CS, Tan DSW, et al. Safety and efficacy of Pralsetinib in RET fusion‐positive non‐small‐cell lung cancer including as first‐line therapy: update from the ARROW trial[J]. Ann Oncol. 2022;33(11):1168–1178. [DOI] [PubMed] [Google Scholar]
- 42. Gadgeel SM, Gainor JF, Cappuzzo F, et al. Relationship between RET fusion partner and treatment outcomes in patients with non‐small cell lung cancer (NSCLC) from the phase 1/2 ARROW study and real‐world data (RWD). 2022 ESMO abstract 984P.
- 43. Zhou Q, Zhao J, Chang J, Wang H, Fan Y, Wang K, et al. Efficacy and safety of Pralsetinib in patients with advanced RET fusion‐positive non‐small cell lung cancer [J]. Cancer. 2023. Online ahead of print. 10.1002/cncr.34897 [DOI] [PubMed] [Google Scholar]
- 44. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves. Selpercatinib‐locally‐advanced‐or‐metastatic‐ret‐fusion‐positive‐non‐small‐cell‐lung.
- 45. https://www.nmpa.gov.cn/zwfw/sdxx/sdxxyp/yppjfb/20221009160008139.html.
- 46. Drilon A, Subbiah V, Gautschi O, Tomasini P, et al. Selpercatinib in patients with RET fusion‐positive non‐small‐cell lung cancer: updated safety and efficacy from the Registrational LIBRETTO‐001 phase I/II trial[J]. J Clin Oncol. 2023;41(2):385–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gautschi O, Milia J, Filleron T, Wolf J, Carbone DP, Owen D, et al. Targeting RET in patients with RET‐rearranged lung cancers: results from the global, multicenter RET registry[J]. J Clin Oncol. 2017;35(13):1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shen T, Pu X, Wang L, Yu Z, Li J, Zhang Y, et al. Association between RET fusions and efficacy of Pemetrexed‐based chemotherapy for patients with advanced NSCLC in China: a multicenter retrospective study[J]. Clin Lung Cancer. 2020;21(5):e349–e354. [DOI] [PubMed] [Google Scholar]
- 49. Chinese Society of Clinical Oncology (CSCO) . Guidelines for diagnosis and treatment of non‐small cell lung cancer (2023 edition) [M]. Beijing: People's Health Publishing House; 2023. [Google Scholar]
- 50. National Comprehensive Cancer Network . NCCN Clinical Practice Guidelines in Oncology: non‐small cell lung cancer, Version 3, 2023[DB/OL]. Available from: http://www.nccn.org/default.aspx Accessed June 10, 2023.
- 51. ClinicalTrials.gov . Study of Pemetrexed+Platinum Chemotherapy With or Without Pembrolizumab (MK‐3475) in Participants With First Line Metastatic Nonsquamous Non‐small Cell Lung Cancer (MK‐3475‐189/KEYNOTE‐189)[DB/OL]. Available from: https://clinicaltrials.gov/ct2/show/NCT02578680. Accessed June 12, 2023
- 52. Bhandari NR, Hess LM, Hanet Y, et al. Efficacy of immune checkpoint inhibitor therapy in patients with RET fusion‐positive non‐small‐cell lung cancer. Immunotherapy. 2021;13(11):893–904. [DOI] [PubMed] [Google Scholar]
- 53. Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, et al. Severe immune‐related adverse events are common with sequential PD‐(L)1 blockade and Osimertinib[J]. Ann Oncol. 2019;30(5):839–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McCoach CE, Rolfo C, Drilon A, et al. Hypersensitivity reactions to Selpercatinib treatment with or without prior immune checkpoint inhibitor therapy in patients with NSCLC in LIBRETTO‐001[J]. J Thorac Oncol. 2022;17(6):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hu J, Tang X, Guo R, et al. Poster No 37P, Pralsetinib in acquired RET fusion positive advanced non‐small cell lung cancer patients after resistance to EGFR/ALK‐TKI: a China multi‐center, real‐world data(RWD) analysis. presented at: European Lung Cancer Congress (ELCC) –29 Mar to 1 Apr 2023.
- 56. Rotow J, Patel J, Hanley M, et al. Osimertinib and Selpercatinib efficacy, safety, and resistance in a multicenter, prospectively treated cohort of EGFR‐mutant and RET fusion‐positive lung cancers. Clin Cancer Res. 2023;29(16):2979–2987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cooper AJ, Drilon AE, Rotow JK, Liu SV, Gautschi O, Smith KER, et al. First results from the RETgistry: a global consortium for the study of resistance to RET inhibition in RET‐altered solid tumors[J/OL]. J Clin Oncol. 2023;41(16_suppl):9065–9906. [Google Scholar]
- 58. Gainor JF, Curigliano G, Doebele RC, et al. Analysis of resistance mechanisms to Pralsetinib (BLU‐667) in patients with RET fusion–positive non‐small cell lung cancer (NSCLC) from the ARROW study[C]. Presented at: 2020 North American conference on lung cancer (NACLC). October 16–17, 2022. [Google Scholar]
- 59. Solomon BJ, Tan L, Lin JJ, Wong SQ, Hollizeck S, Ebata K, et al. RET solvent front mutations mediate acquired resistance to selective RET inhibition in RET‐driven malignancies[J]. J Thorac Oncol. 2020;15(4):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lin JJ, Liu SV, McCoach CE, et al. Mechanisms of resistance to selective RET tyrosine kinase inhibitors in RET fusion‐positive non‐small‐cell lung cancer[J]. Ann Oncol. 2020;31(12):1725–1733. 10.1016/j.annonc.2020.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Gazeu A, Aubert M, Pissaloux D, Lantuejoul S, Pérol M, Ikhlef N, et al. Small‐cell lung cancer transformation as a mechanism of resistance to Pralsetinib in RET‐rearranged lung adenocarcinoma: a case report[J]. Clin Lung Cancer. 2023;24(1):72–75. [DOI] [PubMed] [Google Scholar]
- 62. ClinicalTrials.gov . Study of TPX‐0046, A RET/SRC Inhibitor in Adult Subjects With Advanced Solid Tumors Harboring RET Fusions or Mutations[DB/OL]. Available from: https://clinicaltrials.gov/ct2/show/NCT04161391?term=TPX-0046&draw=2&rank=1. Accessed June 12, 2023
- 63. ClinicalTrials.gov . A Study of APS03118 in Advanced Solid Tumors Harboring RET Mutations or Fusions[DB/OL]. Available from: https://clinicaltrials.gov/ct2/show/NCT05653869?term=APS03118&draw=2&rank=1. Accessed June 12, 2023
- 64. ClinicalTrials.gov . A Study of LOXO‐260 in Cancer Patients With a Change in a Particular Gene (RET) That Has Not Responded to Treatment [DB/OL]. Available from: https://clinicaltrials.gov/ct2/show/NCT05241834. Accessed June 12, 2023
- 65. China drugtrials . Available from. http://www.chinadrugtrials.org.cn/clinicaltrials.searchlistdetail.dhtml. Accessed June 12, 2023
- 66. Drilon A, Zhong J, Lu Y, et al. Proceedings of the 113th annual meeting of the American Association for Cancer Research; 2021 April 8–13. New Orleans LA. Philadelphia(PA): AACR; 2022. Abstract nr {5363}. [Google Scholar]
- 67. Alexander ED, Da Z, Rogers E, et al. The next‐generation RET inhibitor TPX‐0046 is active in drug‐resistant and naïve RET‐driven cancer models. 2020ASCO abstract 3616.
- 68. Zhou Q, Wu YL, Zheng X, et al. A phase I study of KL590586, a next‐generation selective RET inhibitor, in patients with RET‐altered solid tumors. 2023 ASCO Abstract 3007.
- 69. Kolakowski GR et al. Pre‐clinical characterization of potent and selective next‐generation RET inhibitors. 2021 AACR Poster 1464, Abstract 1464: Pre‐clinical characterization of potent and selective next‐generation RET inhibitors, 81.


