Abstract
Escherichia coli cells transformed with several carotenogenic genes to mediate the formation of ζ-carotene, neurosporene, lycopene, β-carotene, and zeaxanthin were exposed to UV-B radiation. Short-term kinetics revealed that endogenous levels of neurosporene and β-carotene protected E. coli against irradiation with UV-B. Zeaxanthin protected against only the photosensitized UV-B treatment. All other carotenoids were ineffective.
Due to the increasing exposure to UV-B radiation in the environment, it is of special interest to investigate the impact of UV-B radiation on higher and lower organisms and to understand the action of antioxidants. First attempts were made to assess the protective properties of carotenoids in carotenogenic Escherichia coli transformants. The chosen bacterium exhibits a high sensitivity to UV-B (3). Furthermore, it is devoid of carotenoids and suitable for transformation with genes mediating the formation of different carotenoids. In previous studies, it was found that zeaxanthin and its glucoside prevented cell death by UV-B in the presence of a photosensitizer (12), whereas lycopene was not effective (11). In the present investigation we extended the use of E. coli as a suitable model to evaluate the potential of structurally different carotenoids to protect against UV-B radiation.
Different E. coli transformants, accumulating the carotenoids ζ-carotene, neurosporene, lycopene, β-carotene, and zeaxanthin (Fig. 1), were exposed to UV-B directly or after the addition of the photosensitizer α-terthienyl. Plasmids which mediate the formation of these carotenoids are listed and described in Table 1.
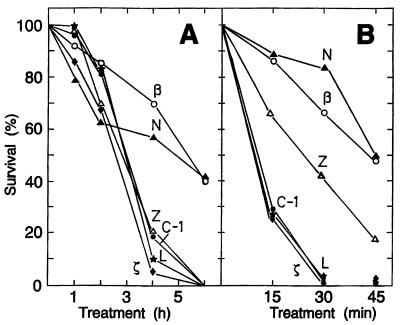
FIG. 1.
Kinetics of UV-B radiation and its effect on the viability of E. coli transformants which accumulate the following carotenoids: ζ-carotene (strain ζ), neurosporene (strain N), lycopene (strain L), β-carotene (strain β), and zeaxanthin (strain Z). Strain C-1 is a control transformant which is devoid of any carotenoid. In the experiments represented in panel A, no photosensitizer was added and an intensity of 0.73 W/m2 was used. UV-B treatment (B) was conducted after the addition of 10 μg of α-terthienyl per ml at an intensity of 0.55 W/m2.
TABLE 1.
Characteristics and carotenoid contents of the E. coli transformants used in this studya
| Transformant | Plasmid(s) | Carotenoid(s) (mg/g dry wt) | Reference(s) |
|---|---|---|---|
| Part A | |||
| C-1 | pACYC184 | None | 8 |
| ζ | pACCRT-EBP | ζ-Carotene (0.20) | 4 |
| N | pACCRT-EBIRc | Neurosporene (0.29) | 4 |
| L | pACCRT-EBIEu | Lycopene (0.33) | 9 |
| β | pACCAR16ΔcrtX | β-Carotene (0.24) | 6 |
| Z | pACCAR25ΔcrtX | Zeaxanthin (0.29) | 6 |
| Part B | |||
| C-2 | pACYC184 + pUC18 | None | 8, 13 |
| C-2+ | pACYC184 + pKJ21 | None + binding protein | 7, 8 |
| β-h (= β) | pACCAR16ΔcrtX + pUC18 | β-Carotene (0.24) | 6, 13 |
| β-l | pACCRT-EBIEu + pUC-crtY | β-Carotene (0.12) | 9 |
| Z-h (= Z) | pACCAR25ΔcrtX + pUC18 | Zeaxanthin (0.27) | 5, 13 |
| Z-l | pCAR25ΔcrtX + pACYC184 | Zeaxanthin (0.14) | 6, 7 |
| Z-G | pCAR25 + pACYC184 | Zeaxanthin diglucoside (0.30) | 5, 8 |
| Z-P | pACCAR25ΔcrtX + pKJ21 | Zeaxanthin + binding protein (0.28) | 6, 7 |
Enzymes encoded by the genes used for construction of the plasmids: crtE, geranylgeranyl pyrophosphate synthase; crtB, phytoene synthase; crtIRc, three-step phytoene desaturase; crtIEu, four-step phytoene desaturase; crtY, lycopene cyclase; crtZ, β-carotene hydroxylase.
E. coli cells from overnight cultures were concentrated in 50 mM phosphate buffer, pH 7.5, to a density of 4 × 108 cells/ml, α-terthienyl was added as indicated, and the samples were exposed to UV-B radiation. The radiation chamber was equipped with a bank of four Philips TL40W/12 fluorescent UV-B lamps, which exhibit an emission maximum at 306 nm and have a cutoff at 275 nm. Aliquots of 5 μl were taken, diluted 102- and 104-fold, and plated out. After one day of growth, the colonies were counted. All values are means of five to eight independent determinations. Carotenoid content in the transformants prior to UV-B treatment was analyzed as described previously (9).
The concentrations of all carotenoids in the strains used in the experiments in Fig. 1 were in the same range, from 0.20 to 0.33 mg/g dry weight (Table 1). Figure 1A represents a time course of UV-B irradiation without the addition of a photosensitizer. Within 6 h, an intensity of 0.73 W of UV-B per m2 killed all cells of the control strain and most of the transformants which accumulate either lycopene, zeaxanthin, or ζ-carotene. When the tranformants which synthesize β-carotene or neurosporene were irradiated for 6 h, about 40% of the cells survived. In the experimental results shown in Fig. 1B, α-terthienyl was added as a photosensitizer and the UV-B intensity was lowered to 0.55 W/m2. Under these conditions, survival of the control cells and the ζ-carotene- or lycopene-accumulating transformants decreased with increasing irradiation time and reached zero after 30 min. For the transformant which synthesizes zeaxanthin, a moderate protection was observed, in contrast to the sample with no α-terthienyl present. In the case of the neurosporene- and β-carotene-forming transformants, survival after 45 min of UV-B irradiation was similar to that in the experiment without added photosensitizer, around 50%. When a transformant (β-1) with half the carotenoid content was used for α-terthienyl-sensitized treatment, survival was only about 25% compared with survival of transformant β (data not shown).
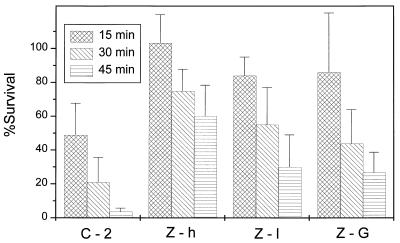
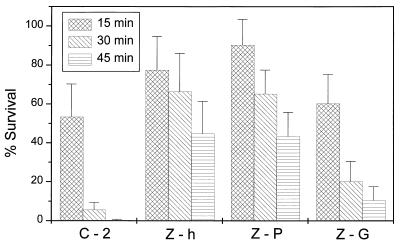
The following experiments were carried out to determine the protective effect of carotenoids on cells containing high (Z-h; 0.30 mg/g dry weight) and low (Z-1; 0.14 mg/g dry weight) concentrations of zeaxanthin or the glucoside of this carotenoid (Fig. 2). At an intensity of 0.55 W/m2, the viability of the control transformant C-2 decreased in 15-min intervals to about 40, 20, and 5%. Cells of transformant Z-h showed, over this whole period of irradiation, a decrease of survival values to only 60%. This protective effect was less pronounced, with a survival of 30% in case of the transformant Z-1. Glycosylation of zeaxanthin in the E. coli transformant Z-G, which accumulated 0.30 mg of zeaxanthin diglucoside per g dry weight, resulted in survival rates two- to fivefold higher than the control. However, the survival rates of the zeaxanthin glucoside-accumulating transformant were lower than those for the cells which synthesized similar amounts of zeaxanthin. An increase in the irradiation intensity, from 0.55 to 0.73 W/m2, resulted (in all transformants) in a higher killing rate of the cells (Fig. 3). In the control transformants C-2, which contains both cloning vectors without inserts, and C-2+, which expressed the carotenoid-binding protein (7) alone without any carotenoids, survival was less than 1% after 45 min. Even at the higher intensity, cells of transformant Z-P, with the expression of carotenoid-binding protein being simultaneous with the synthesis of zeaxanthin, exhibited the same response to UV-B irradiation as cells accumulating a similar amount of zeaxanthin alone. In comparing the results of Fig. 2 and 3, a clear quantitative relationship between the degree of killing of the transformants on one hand and the irradiation time and/or the increased intensity on the other is evident as a linear dose effect, especially when the first values obtained after 15 min are disregarded.
FIG. 2.
Survival of E. coli transformants which accumulate large or small amounts (0.27 or 0.14 mg/g dry weight, respectively) of zeaxanthin (Z-h, Z-l) or zeaxanthin diglucoside (Z-G) after treatment with UV-B at an intensity of 0.55 W/m2. C-2 is a carotenoid-free control containing the same plasmids as the other transformants but without insertions of carotenoid genes.
FIG. 3.
Survival of E. coli transformants which accumulate zeaxanthin (Z-h), zeaxanthin diglucoside (Z-G), or zeaxanthin in combination with a binding protein (Z-P) after UV-B treatment at an intensity of 0.73 W/m2. C-2 is a carotenoid-free control containing the same plasmids as the other transformants but without insertions of carotenoid genes.
Carotenoids differ structurally by the length of the polyene chromophore and the nature of the end groups (2). These factors determine their interaction with other molecules and their integration into membranes (1, 10). It is difficult to figure out what the acyclic neurosporene and the cyclic β-carotene have in common and how they differ from inactive phytoene, ζ-carotene, and lycopene. Obviously, a certain length of the polyene chain, which is reflected by the spectral properties, is important for a UV-B-protective carotenoid compound. In our case, the best effect was reached with neurosporene and β-carotene, which both show a central absorbance maximum around 440 nm. The lower protective efficiency of zeaxanthin (Fig. 1B), which has the same spectral properties as β-carotene, may be due to its increased polarity caused by the two additional hydroxy groups at positions 3 and 3′. It is more easily transferred from a lipid environment into the aqueous phase than β-carotene (1). This could be a disadvantage which is increased by glycosylation. The zeaxanthin diglucoside was significantly less protective than zeaxanthin alone (Fig. 3).
From the present investigation it can be concluded that the protective potential of different carotenoids is determined by their amounts in the cell relative to the UV-B dose, by their accumulation in the membrane, and by the structural features of the carotenoid molecule, such as the length of the polyene chain, for direct interaction with sensitizing molecules.
Acknowledgments
This work was supported by a grant from the German BMBF (GSF), no. 07UVB07.
Due thanks are expressed to N. Misawa, Kirin Research Laboratories, Yokohama, Japan, for providing us with the genes from Erwinia uredovora and most of the corresponding plasmids and to K. J. Reddy, State University of New York, Binghamton, for supplying plasmid pKJ21.
REFERENCES
- 1.Borel P, Grolier P, Armand M, Partier A, Lafont H, Lairon D, Azais-Braesco V. Carotenoids in biological emulsions: solubility, surface-to-core distribution, and release from lipid droplets. J Lipid Res. 1996;37:250–261. [PubMed] [Google Scholar]
- 2.Britton G. Structure and properties of carotenoids in relation to function. FASEB J. 1995;9:1551–1558. [PubMed] [Google Scholar]
- 3.Degiorgio C F, Fernandez R O, Pizarro R A. Ultraviolet-B lethal damage on Pseudomonas aeruginosa. Curr Microbiol. 1996;33:141–146. doi: 10.1007/s002849900090. [DOI] [PubMed] [Google Scholar]
- 4.Linden H, Vioque A, Sandmann G. Isolation of a carotenoid biosynthesis gene coding for ζ-carotene desaturase from Anabaena PCC7120 by heterologous complementation. FEMS Microbiol Lett. 1993;106:99–104. [Google Scholar]
- 5.Misawa N, Nakagawa M, Kobayashi K, Yamano S, Izawa Y, Nakamura K, Harashima K. Elucidation of the Erwinia uredovora carotenoid biosynthetic pathway by functional analysis of gene products expressed in Escherichia coli. J Bacteriol. 1990;172:6704–6712. doi: 10.1128/jb.172.12.6704-6712.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Daito T, Ohtani T, Miki W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol. 1995;177:6575–6584. doi: 10.1128/jb.177.22.6575-6584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reddy K J, Masamoto K, Sherman D M, Sherman L A. DNA sequence and regulation of the gene (cbpA) encoding the 42-kilodalton cytoplasmic membrane carotenoprotein of the cyanobacterium Synechococcus sp. strain PCC 7942. J Bacteriol. 1989;171:3486–3493. doi: 10.1128/jb.171.6.3486-3493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schnurr G, Misawa N, Sandmann G. Expression, purification and properties of lycopene cyclase from Erwinia uredovora. Biochem J. 1996;315:869–874. doi: 10.1042/bj3150869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strzalka K, Gruszecki W I. Effect of β-carotene on structural and dynamic properties of model phosphatidylcholine membranes. I. An EPR spin label study. Biochim Biophys Acta. 1994;1194:138–142. doi: 10.1016/0005-2736(94)90212-7. [DOI] [PubMed] [Google Scholar]
- 11.Tuveson R W, Sandmann G. Protection by cloned carotenoid genes expressed in Escherichia coli against phototoxic molecules activated by near-ultraviolet light. Methods Enzymol. 1993;214:323–330. doi: 10.1016/0076-6879(93)14075-t. [DOI] [PubMed] [Google Scholar]
- 12.Tuveson R W, Larson R A, Kagan J. Role of cloned carotenoid genes expressed in Escherichia coli in protecting against inactivation by near-UV light and specific phototoxic molecules. J Bacteriol. 1988;170:4675–4680. doi: 10.1128/jb.170.10.4675-4680.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]