Abstract
AIM
To compare the postoperative binocular visual performance with an iTrace analyzer following femtosecond laser-assisted cataract surgery (FLACS) combined with bilateral implantation of two different types of diffractive trifocal intraocular lenses (IOL).
METHODS
During this retrospective observational study, patients who received bilateral FLACS combined with implantation of two different types of diffractive trifocal IOLs were evaluated. According to the IOLs' different types and design, the patients were divided into AT LISA tri839MP group (tri839 group) and AcrySof PanOptix TFNT00 group (TFNT group). Study parameters included preoperative and postoperative uncorrected distance visual acuity (UDVA) at 5 m, uncorrected near visual acuity (UNVA) at 30 cm and 40 cm, uncorrected intermediate visual acuity (UIVA) at 60 cm and 80 cm, postoperative refractive status, objective visual qualities and total high order aberrations (HOAs) postoperatively. The postoperative complications were also recorded.
RESULTS
Totally 56 eyes of 28 patients (tri839 group, n=26; TFNT group, n=30) were included. Preoperative baseline characteristics between groups were not statistically significantly different. UDVA was not significantly different between groups except for 1wk follow-up due to the postoperative corneal edema. TFNT group showed statistically significant better UNIA at 60 cm than tri839 group at the 1wk (0.05±0.19 vs 0.15±0.10 logMAR, P=0.013), 1mo (0.05±0.12 vs 0.15±0.09 logMAR, P=0.001) and 3mo (0.04±0.12 vs 0.15±0.11 logMAR, P=0.001) follow-up, while tri839 group showed statistically significant better UNIA at 80 cm than TFNT group at the 1d (0.14±0.15 vs 0.20±0.14 logMAR, P=0.041) and 1mo (0.09±0.07 vs 0.14±0.10 logMAR, P=0.042) follow-up. Postoperative refractive status showed stable at every visit. Modulated transfer function (MTF) values and strehl ratio (SR) values were improved and HOAs were lower significantly after surgery.
CONCLUSION
FLACS with bilateral implantations of both tri839 and TFNT00 can achieve satisfactory natural whole-course vision, high postoperative refractive stability and good visual quality but without significantly difference. iTrace aberration instrument can accurately evaluate the visual quality under different status.
Keywords: femtosecond laser-assisted cataract surgery, visual performance, binocular, AT LISA tri839MP, AcrySof PanOptix TFNT00, iTrace
INTRODUCTION
Cataract remains the most common cause of blindness in the world[1]–[2]. Surgery is the only effective treatment at present, which has a profound impact on patients' quality of life. Conventional phacoemulsification is the most prevalent way of cataract surgery in many countries. With the development of technology, femtosecond laser has been recently applied to cataract surgery, particularly for corneal incision, anterior capsulotomy and phacofragmentation. However, there are still some controversial viewpoints in the two main surgery methods, especially in the potential benefits and cost-effectiveness[3]–[5]. Intraocular lens (IOLs) implantation following cataract surgery can provide spectacle independence at all distances after surgery, which is also a refractive procedure[6]. The majority of patients undergoing cataract surgery also suffer from presbyopia, a refractive disorder that affects the ability to see close clearly due to the crystalline lens's physiological degenerative changes as they age (common in adults older than 40)[7]–[8]. Through advances in IOL technology, patients with presbyopia could be corrected or mitigated during cataract surgery and achieve a stable and functional uncorrected vision without spectacles at all distances[9]–[11]. Trifocal IOLs are designed to improve the whole-course vision. In China, there are two commonly used trifocal IOLs: AT LISA tri839MP (Carl Zeiss, German) and AcrySof IQ PanOptix TFNT00 (Alcon, USA). These two IOLs have demonstrated excellent visual performances after cataract surgery in multiple clinical trials around the world, with low rates of serious and non-serious complications[12]–[16]. The postoperative visual performances includes not only subjective ones such as visual acuity at different distances, contrast sensitivity and visual functioning questionnaires, but also objective ones such as aberrations, modulated transfer function (MTF), point spread function (PSF), Strehl ratio (SR) and so on.
At present, there are few reports about the comparison of both subjective and objective binocular visual performance after bilateral implantation of these two different types of diffractive trifocal IOLs especially following femtosecond laser-assisted cataract surgery (FLACS). In this study, we aim to compare the postoperative subjective visual outcomes, objective visual performances using by iTrace analyzer and safety aspects, which were obtained after bilateral implantations of the AT LISA tri839MP and AcrySof IQ PanOptix TFNT00 during FLACS.
SUBJECTS AND METHODS
Ethical Approval
Ethics approval was granted by the ethics committee of Aier Eye Hospital, Jinan University (Guangzhou, China; No.GZAIERIRB2020003), which followed the Helsinki Declaration. Written informed consent forms were obtained from all participants.
Participants
Patients with cataract in Aier Eye Hospital, Jinan University (Guangzhou, China) between October 2020 and October 2022, who underwent bilateral implantations of the AT LISA tri839MP or AcrySof IQ PanOptix TFNT00 during FLACS were enrolled. The inclusive criteria included: 1) cataract patients older than 40y with good fundus conditions and no other eye diseases that affect vision; 2) patients with strong desire to get rid of spectacles for all distance; 3) the natural mesopic pupil diameter is 3.0 to 5.5 mm; 4) the Kappa and Alpha angle are <0.5 mm; 5) regular corneal astigmatism <0.75 D against-the -rule or 1.00 D with-the-rule. The exclusive criteria included: 1) patients with systemic disease (e.g., severe hypertension, coronary heart diseases, diabetes, connective tissue disorders, severe autoimmune diseases and so on); 2) patients with other eye diseases (e.g. uveitis, glaucoma, fundus ocular diseases, severe corneal opacity, severe dry eye, etc.); 3) patients with any ocular surgery history within 6mo; 4) patients with poor cooperation and compliance.
Ophthalmic Examinations
All ophthalmic examinations were performed by qualified technicians or doctors. Surgical, preoperative and postoperative data were collected retrospectively. Patients all underwent the second eye surgery within 2wk after the first one and received the routine ophthalmic examinations before surgery and were followed-up at 1d, 1wk, 1, and 3mo after the second eye surgery. Regular perioperative examinations included uncorrected distance visual acuity (UDVA, at 5 m), uncorrected near visual acuity (UNVA, at 30 cm and 40 cm), uncorrected intermediate visual acuity (UIVA, at 60 cm and 80 cm), spherical equivalent (SE), intraocular pressure (IOP), slit-lamp microscopy, and objective performances (total MTF10, MTF30 and the average height with or without correction, SRs with or without correction and HOAs) at every visit. Visual acuity was measured in decimal visual acuity under photopic conditions using international standard logarithmic eye chart (GB11533-2011) and then converted into the logarithm of minimum angle of resolution (logMAR) notation. The SEs were recorded by automatic optometry at very visit. The axial length (AL) was measured by IOL Master 700 (Carl Zeiss, Germany) before operation. The IOL power was calculated by Barrett Universal II formula, and target diopter was set between 0 and -1.0 D to account for the farsighted drift in the long axis eyes. Objective visual qualities were recorded by the iTrace analyzer (Tracey Technologies, USA), which is a powerful device using ray tracing to analyze visual function and sources of aberrations[17]–[18].
The total MTF10, MTF30 and the average height with or without correction, SRs with or without correction and HOAs (coma, spherical, secondary astigmatism, trefoil) were recorded at a 3-mm pupil size before surgery and 1wk, 1, and 3mo after surgery. The average data were taken after 3 measurements.
Study Lenses
The AT LISA tri839MP is a trifocal (+3.33 D for near; +1.66 D for intermediate), clear, aspheric (-0.18 µm), aberration-correcting IOL, which was made of 12% hydrophilic acrylate with hydrophobic surface. The IOL size is 11 mm, with a diffractive front surface and an optical diameter of 6 mm, in which a central 4.3 mm area with trifocal design, and the surrounding area with bifocal design, and its inner ring optical diameter is 1.04 mm. The range is from 0 to +32.0 D.
The AcrySof IQ PanOptix TFNT00 is another trifocal (+3.25 D for near; +2.17 D for intermediate), yellow, aspheric (-0.10 µm), aberration-correcting IOL, which was made of hydrophobic acrylate/methacrylate copolymer. The IOL size is 13 mm, with an optical diameter of 6 mm and a diffractive region of 4.5 mm, and its inner ring optical diameter is 1.164 mm. The range is from +6.0 to +34.0 D.
Surgical Procedures
All FLACSs were performed as following: patients under topical anesthesia (Benoxil; Santen Pharmaceutical Co. Ltd., China) lay on the operating bed, the femtosecond laser was used to perform capsulorhexis (5.0 mm) and nucleus fragmentation (energy parameter, 10 µJ) (LenSx; Alcon Laboratories, USA). A 2.2 mm main corneal incision and a 1.0 mm side incision were made manually. The anterior chamber was then filled with viscoelastic agent, and the isolated anterior capsule was removed and separated.
A phaco procedure was performed using the Centurion Vision System with Active Fluidics (Alcon Laboratories, USA). During I/A mode, residual cortices were sucked, anterior and posterior capsule were polished. Then an IOL (AT LISA tri839MP or AcrySof IQ PanOptix TFNT00) was implanted and adjusted to a normal position in the capsular bag. Following removal of the viscoelastic agent, the puncture site was hydrated to form anterior chamber. At the end of surgery, the Purkinje reflex was checked to ensure the optimal centralization of the IOLs in all patients. The operative eyes were bandaged after usage of dexamethasone and tobramycin eye ointment (Tobradex, Yangtze River Pharmaceutical Group Co. Ltd., China).
The same experienced surgeon (Wu ZM) performed all surgical procedures with the same equipment and instruments. All patients received topical levofloxacin (Shentian Pharmaceutical Co., Ltd., China) and diclofenac sodium eye drops (Shenyang Xingqi Pharmaceutical Co., Ltd., China) 4 times daily for 3d before surgery. The standard postoperative treatment was same to each eye and consisted of topical tobradex (0.3% tobramycin and 1% dexamethasone, Alcon, USA) 4 times per day for 2wk, and pranoprofen (Yangtze River Pharmaceutical Group Co., Ltd., China) 4 times per day for 2wk.
Sample Size
Before the research starts, we calculated the sample size according to our previous clinical observational results. UDVA outcome was observed 0.01±0.03 logMAR in the TFNT group and 0.04±0.03 logMAR in the tri839 group during 1mo follow-up. So, the mean and average values were set 0.01±0.03 and 0.04±0.03 respectively in experimental group and control group. The K value (experimental group sample size/control group sample size), alpha and power were set 1:1, 0.05 and 0.9 respectively. Based on these assumptions, 16 eyes were required in each group. Assuming the loss rate of the subjects is 20%, finally the sample size is at least 20.
Statistical Analysis
All statistical analyses were conducted using SPSS software (version 26, USA). To determine the normality of data distribution, Kolmogorov-Smirnov test was carried out. For normally distributed data, continuous and categorical variables were described as mean±standard deviation (SD) and number and percentage (%), respectively, while for non-normally distributed data, the median and interquartile range (IQR, P25-P75) were used. Paired t-test or χ2 test was chosen to assess between-group differences in normal distribution variables, and Mann-Whitney U test for non-normal distribution ones. Kruskal-Wallis and one-way ANOVA were used to evaluate the differences between the two IOL groups. A P value <0.05 was considered significant.
RESULTS
Preoperative Demographic Characteristics
At the beginning, 54 patients 108 eyes with cataract who underwent uneventful FLACS combined with bilateral implantation of the tri839 or TFNT were recorded. After 3mo' follow-up, only 28 patients' clinical records were available. Hence, data from 56 eyes of 28 patients were collected for analysis: 26 eyes of 13 patients in the tri839 group and 30 eyes of 15 patients in the TFNT group.
The patients' preoperative demographic characteristics are described in Table 1. The mean age was 59.3±11.8y and 61.5% (n=8) of patients were males in tri839 group, while the mean age was 56.2±8.3y and 73.3% (n=11) were males in TFNT00 group. The dysfunctional lens index (DLI), preoperative UDVA and expected SE did not differ statistically significantly between groups preoperatively. However, most patients were myopic with longer mean AL 27.32±3.05 mm in tri839 group than 25.85±2.54 mm in TFNT group (P=0.055). Accordingly, the IOL power implanted in both groups had a significant difference which was 10.40±7.40 D in tri839 group and 14.65±6.60 D in TFNT group respectively (P=0.027).
Table 1. Preoperative patient characteristics.
| Parameter | Group tri839 (n=26) | Group TFNT (n=30) | P |
| Age (y) | 59.3±11.8 | 56.2±8.3 | 0.424 |
| Gender, n (%) | 0.689 | ||
| Male | 8 (61.5) | 11 (73.3) | |
| Female | 5 (38.5) | 4 (26.7) | |
| Preoperative UDVA (logMAR) | 1.15±0.68 | 1.02±0.61 | 0.433 |
| Dysfunctional lens index | 3.77±2.30 | 4.23±1.85 | 0.419 |
| Axial length (mm) | 27.32±3.05 | 25.85±2.54 | 0.055 |
| IOL power implanted (D) | 10.40±7.40 | 14.65±6.60 | 0.027a |
| Expected spherical equivalent (D) | -0.32±0.24 | -0.23±0.19 | 0.116 |
SD: Standard deviation; UDVA: Uncorrected distant visual acuity; logMAR: Log of the minimum angle of resolution; IOL: Intraocular lens; D: Diopters. aP<0.05.
mean±SD
Visual Acuity
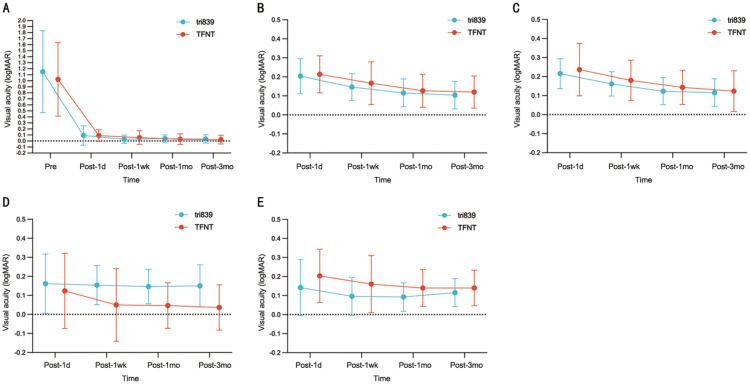
Postoperative visual acuities are described in Table 2, both groups had achieved satisfactory natural whole-course visual acuities. UDVA was not significantly different between groups except at 1wk postoperative visit, which was due to the cornea edema in 2 eyes in TFNT group (Figure 1A). Best-corrected distance visual acuity (BCVA) in both group was similarly good at 1mo follow-up (-0.02±0.09 vs -0.02±0.07 logMAR, P=0.839). TFNT group showed statistically significant better UNIA at 60 cm than tri839 group at the 1wk (0.05±0.19 vs 0.15±0.10 logMAR, P=0.013), 1mo (0.05±0.12 vs 0.15±0.09 logMAR, P=0.001) and 3mo (0.04±0.12 vs 0.15±0.11 logMAR, P=0.001) follow-up, while tri839 group showed statistically significant better UNIA at 80 cm than TFNT group at the 1d (0.14±0.15 vs 0.20±0.14 logMAR, P=0.041) and 1mo (0.09±0.07 vs 0.14±0.10 logMAR, P=0.042) follow-up (Figure 1D, 1E). Furthermore, UNVA outcomes both at 30 cm and 40 cm were seemly better in the TFNT group than in the tir839 group at very visit, but with non-significantly difference (all P>0.05; Figure 1B, 1C).
Table 2. The postoperative visual acuities of two groups.
| VA (logMAR) | Group tri839 (n=26) | Group TFNT (n=30) | P |
| Monocular VA at 1d postoperatively | |||
| UDVA at 5 m | 0.09±0.16 | 0.09±0.10 | 0.586 |
| UNVA at 30 cm | 0.20±0.09 | 0.21±0.10 | 0.705 |
| UNVA at 40 cm | 0.22±0.08 | 0.23±0.13 | 0.474 |
| UIVA at 60 cm | 0.16±0.16 | 0.12±0.20 | 0.430 |
| UIVA at 80 cm | 0.14±0.15 | 0.20±0.14 | 0.041a |
| Monocular VA at 1wk postoperatively | |||
| UDVA at 5 m | 0.03±0.07 | 0.09±0.10 | 0.011a |
| UNVA at 30 cm | 0.15±0.07 | 0.17±0.11 | 0.104 |
| UNVA at 40 cm | 0.16±0.06 | 0.18±0.11 | 0.443 |
| UIVA at 60 cm | 0.15±0.10 | 0.05±0.19 | 0.013a |
| UIVA at 80 cm | 0.10±0.10 | 0.16±0.15 | 0.141 |
| Monocular VA at 1mo postoperatively | |||
| UDVA at 5 m | 0.04±0.06 | 0.03±0.09 | 0.406 |
| BCVA at 5 m | -0.02±0.07 | -0.02±0.09 | 0.839 |
| UNVA at 30 cm | 0.12±0.07 | 0.13±0.09 | 0.864 |
| UNVA at 40 cm | 0.12±0.07 | 0.14±0.09 | 0.359 |
| UIVA at 60 cm | 0.15±0.09 | 0.05±0.12 | 0.001a |
| UIVA at 80 cm | 0.09±0.07 | 0.14±0.10 | 0.042a |
| Monocular VA at 3mo postoperatively | |||
| UNVA at 5 m | 0.03±0.07 | 0.02±0.07 | 0.556 |
| UNVA at 30 cm | 0.10±0.07 | 0.12±0.08 | 0.449 |
| UNVA at 40 cm | 0.11±0.07 | 0.12±0.11 | 0.745 |
| UIVA at 60 cm | 0.15±0.11 | 0.04±0.12 | 0.001a |
| UIVA at 80 cm | 0.12±0.07 | 0.14±0.09 | 0.282 |
VA: Visual acuity; logMAR: Log of the minimum angle of resolution; UDVA: Uncorrected distant visual acuity; BCVA: Best-corrected distant visual acuity; UNVA: Uncorrected near visual acuity; UIVA: Uncorrected intermediate visual acuity. aP<0.05.
Figure 1. The preoperative and postoperative visual acuity of two groups in 3mo.
A: Visual acuity at 5 m (logMAR); B: Postoperative visual acuity at 30 cm (logMAR); C: Postoperative visual acuity at 40 cm (logMAR); D: Postoperative visual acuity at 60 cm (logMAR); E: Postoperative visual acuity at 80 cm (logMAR).
Postoperative Refractive Stability
The SEs had a great significantly difference between the two groups at very follow-up (Table 3). However, postoperative refractive status in both groups were described and showed a stable status at every visit (Figures 2 and 3).
Table 3. The postoperative refractive stability of two groups.
| Time | Group tri839 (n=26) | Group TFNT (n=30) | P |
| SE at 1d postoperatively | -0.44±0.79 | -0.14±0.54 | 0.013a |
| SE at 1wk postoperatively | -0.50±0.37 | -0.11±0.32 | 0.000a |
| SE at 1mo postoperatively | -0.44±0.26 | -0.10±0.32 | 0.000a |
| SE at 3mo postoperatively | -0.46±0.26 | -0.05±0.24 | 0.000a |
SE: Spherical equivalent. aP<0.05.
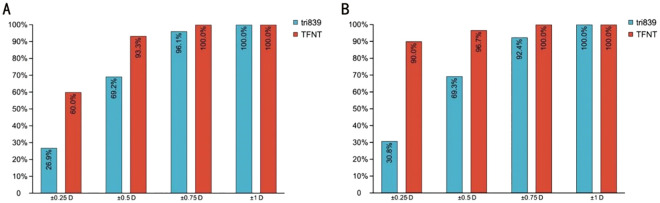
Figure 2. Distribution of postoperative spherical equivalence.
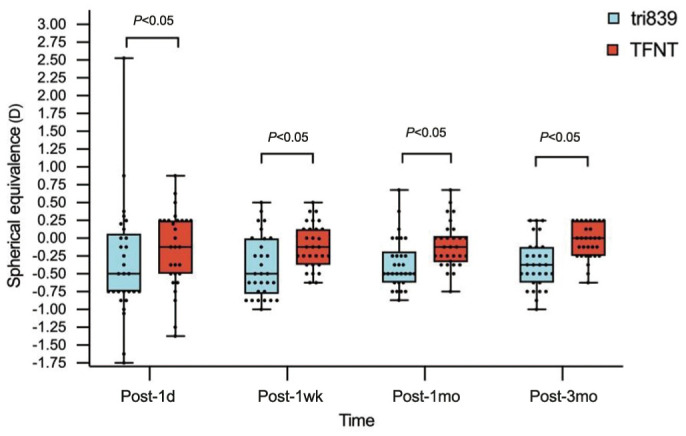
Figure 3. Postoperative refractive stability distribution of two groups.
A: Accumulated postoperative refractive error at 1mo; B: Accumulated postoperative refractive error at 3mo.
Objective Visual Quality
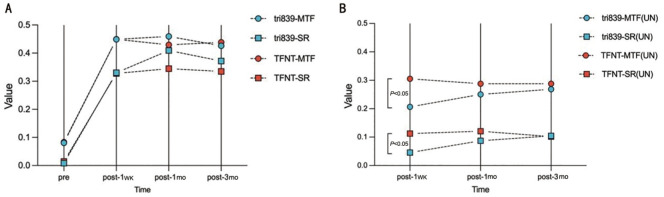
Compared with the preoperative total MTF and SR of the two groups, both postoperative values were significantly increased (P<0.05; Table 4, Figure 4A). However, during the 3mo follow-up, similar results and tendency were observed between groups, no matter with correction in sphere and cylinder or not (Figure 4B). Notably, when the sphere and cylinder were corrected, the total corrective average height of MTF and SR in tri839 group were better than those in TFNT group.
Table 4. The objective visual quality with iTrace analyzer of two groups.
| Objective visual quality | Group tri839 | Group TFNT | P |
| Preoperative | |||
| Total MTF10 | - | - | - |
| Total MTF30 | - | - | - |
| Total MTF avg | 0.081±0.059 | 0.083±0.072 | 0.874 |
| Total SR | 0.009±0.014 | 0.014±0.035 | 0.946 |
| Total MTF avg without correction | - | - | - |
| Total SR without correction | - | - | - |
| 1wk postoperatively | |||
| Total MTF10 | 0.533±0.208 | 0.533±0.160 | 0.517 |
| Total MTF30 | 0.204±0.123 | 0.183±0.755 | 0.490 |
| Total MTF avg | 0.450±0.175 | 0.449±0.127 | 0.599 |
| Total SR | 0.330±0.226 | 0.328±0.167 | 0.967 |
| Total MTF avg without correction | 0.207±0.072 | 0.344±0.178 | 0.000a |
| Total SR without correction | 0.046±0.042 | 0.113±0.070 | 0.000a |
| 1mo postoperatively | |||
| Total MTF10 | 0.540±0.149 | 0.534±0.164 | 0.813 |
| Total MTF30 | 0.193±0.083 | 0.191±0.081 | 0.971 |
| Total MTF avg | 0.459±0.139 | 0.429±0.160 | 0.637 |
| Total SR | 0.409±0.202 | 0.345±0.178 | 0.332 |
| Total MTF avg without correction | 0.250±0.082 | 0.287±0.099 | 0.256 |
| Total SR without correction | 0.087±0.074 | 0.121±0.092 | 0.161 |
| 3mo postoperatively | |||
| Total MTF10 | 0.550±0.147 | 0.524±0.131 | 0.688 |
| Total MTF30 | 0.203±0.100 | 0.196±0.112 | 0.891 |
| Total MTF avg | 0.429±0.160 | 0.446±0.113 | 0.696 |
| Total SR | 0.372±0.164 | 0.350±0.211 | 0.725 |
| Total MTF avg without correction | 0.269±0.139 | 0.286±0.045 | 0.364 |
| Total SR without correction | 0.105±0.121 | 0.010±0.031 | 0.265 |
MTF: Modulated transfer function; MTF avg: The average height of modulated transfer function; SR: Strehl ratio.
mean±SD
Figure 4. Preoperative and postoperative visual quality of two groups.
A: Corrective visual quality; B: Uncorrected visual quality. MTF: Modulated transfer function; SR: Strehl ratio.
High-Order Aberrations
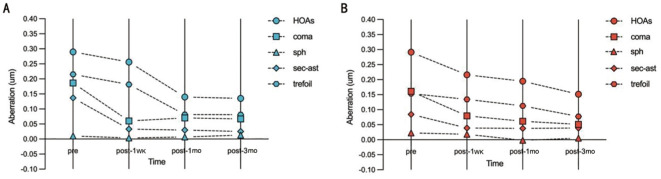
Compared with the preoperative total HOAs, coma, spherical, secondary astigmatism and trefoil of the two groups, both postoperative values were decreased, but without significant difference (P>0.05). And postoperative total HOAs, coma, spherical, secondary astigmatism and trefoil between two groups also showed no statistically significant difference at every visit time (P>0.05; Table 5, Figure 5).
Table 5. The HOAs with iTrace analyzer of two groups.
| Different HOAs | Group tri839 | Group TFNT | P |
| Preoperative | |||
| HOAs | 0.290±0.114 | 0.303±0.121 | 0.729 |
| Coma | 0.170±0.100 | 0.160±0.119 | 0.470 |
| Spherical | 0.103±0.086 | 0.330±0.109 | 0.473 |
| Secondary astigmatism | 0.120±0.100 | 0.084±0.065 | 0.232 |
| Trefoil | 0.188±0.108 | 0.171±0.110 | 0.478 |
| 1wk postoperatively | |||
| HOAs | 0.256±0.294 | 0.216±0.182 | 0.665 |
| Coma | 0.060±0.072 | 0.079±0.058 | 0.388 |
| Spherical | 0.003±0.037 | 0.018±0.054 | 0.419 |
| Secondary astigmatism | 0.033±0.028 | 0.039±0.034 | 0.607 |
| Trefoil | 0.181±0.234 | 0.135±0.123 | 0.452 |
| 1mo postoperatively | |||
| HOAs | 0.134±0.094 | 0.195±0.364 | 0.587 |
| Coma | 0.070±0.063 | 0.062±0.062 | 0.703 |
| Spherical | 0.007±0.033 | -0.002±0.028 | 0.407 |
| Secondary astigmatism | 0.030±0.016 | 0.038±0.034 | 0.428 |
| Trefoil | 0.082±0.058 | 0.113±0.224 | 0.618 |
| 3mo postoperatively | |||
| HOAs | 0.135±0.060 | 0.152±0.102 | 0.482 |
| Coma | 0.067±0.036 | 0.051±0.053 | 0.373 |
| Spherical | 0.013±0.014 | 0.005±0.030 | 0.402 |
| Secondary astigmatism | 0.025±0.011 | 0.039±0.025 | 0.334 |
| Trefoil | 0.081±0.068 | 0.077±0.068 | 0.703 |
HOA: Higher order aberration.
mean±SD
Figure 5. Preoperative and postoperative high order aberrations of two groups.
A: High order aberrations of group tri839; B: High order aberrations of group TFNT. HOA: Higher order aberration; Sph: Sperical; Sec-ast: Secondary astigmatism.
Postoperative Complications
The IOLs were successfully centered at every visit, and there were no serious postoperative complications during the 3mo period. However, there were 2 eyes in TFNT group with central cornea edema at 1d and 1wk visit due to the hard nucleus cataract and 1 eye in tri839 group with some signs of capsular opacification at 3mo visit which caused a little diminution of vision but did not yet need YAG laser posterior capsulotomy.
DISCUSSION
With the advances of IOL and development of surgical approaches, FLACS combined with trifocal IOLs implantation have been reported to make patients achieve clear, comfortable and lasting visual quality and optimal satisfaction[19]–[21]. Hence, the refractive cataract surgery has become a new trend, and the aim of this type of surgery is to restore and reconstruct binocular tertiary visual function with the guidance of binocular vision theory. However, there are few reports about the comparison of binocular visual performance (subjective and objective) after FLACS combined with bilateral implantation of trifocal IOLs. Therefore, our study was designed to compare the subjective and objective clinical visual outcomes of bilateral implantations with the tri839 or TFNT during FLACS.
Our study showed that postoperative visual acuities at all three distances were improved significantly compared with that preoperatively in both groups, which was similar to the previous studies with both identical trifocal IOLs[13],[16],[22]–[24], and confirmed that two trifocal IOLs could provide satisfactory results in whole-course visual acuities. Similarly, our data described both lenses (tri839 and TFNT00) provided excellent distance visual acuity (≤0.1 logMAR; 100% and 93.33%) and better intermediate visual performance at 60 cm in group TFNT (86.67%) and 80 cm in group tri839 (76.92%), respectively. Between-group comparison also found that TFNT group showed statistically significant better UNIA at 60 cm than tri839 group at the 1wk, 1, and 3mo follow-up while tri839 group showed statistically significant better UNIA at 80 cm than TFNT group at 1d and 1wk follow-up. This is due to the design of the TNFT00 IOLs with middle focus at 60 cm originally and the comfortable intermediate distance work for Chinese were at 60-70 cm[25]. Moreover, these results also indicate the importance of determining which intermediate distances are best for patients with different IOLs. UNVA outcomes both at 30 and 40 cm were seemly better in the tir839 group than TFNT group at very follow-up, but with non-significantly difference. Additionally, we noticed that the UNVA both at 30 and 40 cm in the two groups seemed not so good as the UIVA and UDVA. Several factors may contribute to these phenomena, such as the optical design of IOLs, limited patient sample, age, AL, examination protocol, environment and so on. In our study, since most patients were myopic with longer mean AL 27.32±3.05 mm in tri839 group than 25.85±2.54 mm in TFNT group (P=0.055), the target diopter was set between 0 and -1.0 D to account for the farsighted drift in the long axis eyes, and the SEs were -0.46±0.26 D in the tri839 group and -0.05±0.24 D in the TFNT group at 3mo follow-up, which also had a great significantly difference between the two groups at very follow-up. Liu et al[26] have found the similar results, in which they concluded that near vision would decrease in the extremely myopic eyes especially at lower luminance due to the IOLs distance-dominant design.
Refractive stability is an important indicative index for the visual performance and quality of life after cataract surgery[27]–[29]. Since the patients in our study were mostly high myopic especially in tri839 group, the target refraction was set between 0 and -1.0 D due to the tendency of hyperopic drift in long axis eyes, so our results showed the postoperative SE in group 839 were a little myopic, but SEs were in a very stable status at very visit in both groups (Figures 2 and 3). These results corroborate the findings of a great deal of the previous work. According to McNamara et al[27], visual and ocular parameters remained stable for two weeks after surgery. Khan et al[28] reported that refractive errors for most patients could be successfully measured and corrected one week after surgery, but need up to 4wk to stabilize due to the changes of central corneal thickness, effective lens position and so on; Charlesworth et al[29] found that the postoperative spherical and cylindrical degrees at 1 and 4wk had no statistical difference.
In our study at 3mo visit, 30.8% and 90.0% of the eyes had SE within ±0.25 D, 69.3% and 96.7% had SE within ±0.50 D, 92.4% and 100% had SE within ±0.75 D in tri839 group and TFNT group respectively, and both 100.00% had SE within ±1.00 D, and the results were similarly at 1mo visit (Figure 3), which implies the stability of the refractive status. Good refractive stability indicates that the cornea and other tissues were not damaged during the surgery, hence the corneal thickness, effective lens position were in a stable status. This result may be explained by the usage of femotosecond laser during the surgery procedure, which has an accuracy and advantages in small tissue damage[30].
Objective visual quality can be evaluated by the MTF curves and SRs[31]–[32]. The MTF curves reflect the different spatial frequencies in clear imaging degrees. In general, low spatial frequencies indicate the ability to see contours of objects, whereas high spatial frequencies indicate the ability to discern fine details. Shen et al[32] used the MTF values under a spatial frequency of 10 (MTF10) and 30 (MTF30) to evaluate far and near visual acuities in their study respectively. In this study, we not only used total MTF10 and MTF30 but also added the average height of total MTF (MTFavg) values to evaluate visual quality, and the average height less than 0.3 was implied not good visual acuity. An SR is obtained by taking the ratio of the peak intensity of an aberrated PSF to the diffraction-limited ideal PSF, and a value of 1.0 indicates the perfect optical quality[33]. SR reflects the level of image quality and has the highest correlations with the visual performance[34]–[35]. Besides, in our study, we also added the total MTF average height and SR without any correction in sphere and cylinder, which reflected the real and natural visual quality status.
Table 4 and Figure 4A showed that both postoperative total MTF and SR values at very visit time in two groups were significantly increased compared with the preoperative values, which indicated visual qualities in both group were greatly improved after surgery. These findings were similar to previously described data on MTF and SR[23],[32],[36]. However, similar results and tendency were observed between groups at very visit time, no matter with correction in sphere and cylinder or not, which implied that the stability of the visual quality. What is noteworthy is that when the sphere and cylinder were corrected, the total corrective MTFavg and SR in tri839 group were better than those in TFNT group, while when the sphere and cylinder were not corrected, the total uncorrected MTFavg and SR in tri839 group were worse than those in TFNT group. These changes were due to the longer AL and myopic shift in tri839 group, when the postoperative SEs were corrected, the visual quality could be improved. We also found the consistent existing research which showed that MTFcutoff and SR decreased with increasing AL, especially when AL≥28 mm because there was more intraocular light scattering when the AL increasing[26].
HOAs can cause blurred vision, glare, decreased night vision and so on even in patients with excellent postoperative vision[37]–[38]. Therefore, measuring the total HOAs postoperatively is useful to indicate the objective visual quality changes. Our findings showed the postoperative total HOAs were not high, which implied the patients were able to achieve satisfactory visual results. And the total HOAs, coma, spherical, secondary astigmatism and trefoil at very visit time were statistically lower than those before surgery, in accordance with the results of Zein El-Dein et al[36]. It is well known that total HOAs were comprised with cornea HOA and internal HOA. Therefore, one possible explanation for these results was the application of FLACS. Xu et al[39] reported that FLACS presented a significantly lower total HOAs and spherical aberration, because femtosecond laser was able to accurately control the size and shape of the capsulotomy and deliver a more centered IOL, resulting in lower internal HOAs and higher visual quality in comparison with conventional phacoemulsification. Similarly, Miháltz et al[40] also concluded that laser-assisted capsulotomy produced significantly fewer internal aberrations than conventional capsulotomy, which may be able to achieve a better optical quality.
The novelty of our study lies in two places: the first one is the comparison of both subjective and objective visual performance for bilateral implantation of two trifocal IOLs, which is focus on the binocular vision theory and aims to restore binocular tertiary visual function; the second one is the analysis of visual performance with visual acuity, refractive stability and MTF, SR, HOAs with iTrace analyzer after trifocal IOLs implantation using by femtosecond laser-assisted cataract procedures.
However, a number of limitations need to be noted in this study. First, it is a small sample size and short follow-up period study, which is inadequate to allow the generalizability of the results, hence a large-scale multicenter study and a longer follow-up period needs to be explored in future. Second, due to the lack of standardized reading charts, reading parameters were not tested. Finally, since the patient-related visual functioning questionnaires were mostly self-made and not standardized, visual disturbances and photic phenomena were difficult to determine.
In conclusion, FLACS with bilateral implantations of both tri839 and TFNT00 can achieve satisfactory natural whole-course vision, high postoperative refractive stability and good visual quality but without significantly difference. iTrace aberration instrument can accurately evaluate the visual quality under different status.
Acknowledgments
Foundations: Supported by Medical Science and Technology Research Foundation Project of Guangdong Province (No.C2021087); The Scientific Research Foundation Project of Guangzhou Aier Eye Hospital, Jinan University (No.GA2023004).
Conflicts of Interest: Ju RH, None; Qu HK, None; Wu ZM, None; Chen Y, None; Wu LN, None; Long Y, None; Wang Z, None.
REFERENCES
- 1.Feng JJ, Jan C, Peng YG, et al. Retrospective analysis of cataract surgery outcomes in China from 2009 to 2018: from a national registry system data. BMJ Open. 2023;13(4):e070989. doi: 10.1136/bmjopen-2022-070989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton MJ, Ramke J, Marques AP, et al. The lancet global health commission on global eye health: vision beyond 2020. Lancet Glob Health. 2021;9(4):e489–e551. doi: 10.1016/S2214-109X(20)30488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang HL, Chen XY, Xu JJ, Yao K. Comparison of femtosecond laser-assisted cataract surgery and conventional phacoemulsification on corneal impact: a meta-analysis and systematic review. PLoS One. 2023;18(4):e0284181. doi: 10.1371/journal.pone.0284181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ou YC, Wang YS, Wu T. Comparison of ultrasound energy consumption between low-energy femtosecond laser-assisted cataract surgery and conventional phacoemulsification cataract surgery in patients with different cataract densities. Eur J Ophthalmol. 2023;33(3):1373–1379. doi: 10.1177/11206721221147952. [DOI] [PubMed] [Google Scholar]
- 5.Kecik M, Schweitzer C. Femtosecond laser-assisted cataract surgery: update and perspectives. Front Med. 2023;10:1131314. doi: 10.3389/fmed.2023.1131314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoo SH, Zein M. Vision restoration: cataract surgery and surgical correction of myopia, hyperopia, and presbyopia. Med Clin North Am. 2021;105(3):445–454. doi: 10.1016/j.mcna.2021.01.002. [DOI] [PubMed] [Google Scholar]
- 7.Rich W, Reilly MA. A review of lens biomechanical contributions to presbyopia. Curr Eye Res. 2023;48(2):182–194. doi: 10.1080/02713683.2022.2088797. [DOI] [PubMed] [Google Scholar]
- 8.Davidson RS, Dhaliwal D, Hamilton DR, Jackson M, Patterson L, Stonecipher K, Yoo SH, Braga-Mele R, Donaldson K, ASCRS Refractive Cataract Surgery Subcommittee Surgical correction of presbyopia. J Cataract Refract Surg. 2016;42(6):920–930. doi: 10.1016/j.jcrs.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Łabuz G, Yan WJ, Baur ID, Khoramnia R, Auffarth GU. Comparison of five presbyopia-correcting intraocular lenses: optical-bench assessment with visual-quality simulation. J Clin Med. 2023;12(7):2523. doi: 10.3390/jcm12072523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moshirfar M, Martin DJ, Jensen JL, Payne CJ. Light adjustable intraocular lenses: an updated platform for cataract surgery. Curr Opin Ophthalmol. 2023;34(1):78–83. doi: 10.1097/ICU.0000000000000911. [DOI] [PubMed] [Google Scholar]
- 11.Villegas EA, Alcon E, Rubio E, Marín JM, Artal P. Refractive accuracy with light-adjustable intraocular lenses. J Cataract Refract Surg. 2014;40(7):1075–1084.e2. doi: 10.1016/j.jcrs.2013.10.046. [DOI] [PubMed] [Google Scholar]
- 12.Ozturkmen C, Kesim C, Sahin A. Evaluation of vision-related quality of life after unilateral implantation of a new trifocal intraocular lens. Beyoglu Eye J. 2022;7(3):167–172. doi: 10.14744/bej.2022.75768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu D, Ren SJ, Mills K, et al. Rate of complete spectacle independence with a trifocal intraocular lens: a systematic literature review and meta-analysis. Ophthalmol Ther. 2023;12(2):1157–1171. doi: 10.1007/s40123-023-00657-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doroodgar F, Niazi F, Sanginabadi A, et al. Visual performance of four types of diffractive multifocal intraocular lenses and a review of articles. Int J Ophthalmol. 2021;14(3):356–365. doi: 10.18240/ijo.2021.03.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosen E, Alió JL, Dick HB, Dell S, Slade S. Efficacy and safety of multifocal intraocular lenses following cataract and refractive lens exchange: Metaanalysis of peer-reviewed publications. J Cataract Refract Surg. 2016;42(2):310–328. doi: 10.1016/j.jcrs.2016.01.014. [DOI] [PubMed] [Google Scholar]
- 16.Sezgin AB. Visual and refractive outcomes, spectacle independence, and visual disturbances after cataract or refractive lens exchange surgery: comparison of 2 trifocal intraocular lenses. J Cataract Refract Surg. 2019;45(11):1539–1546. doi: 10.1016/j.jcrs.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 17.Pohlmann D, Pilger D, Bertelmann E, von Sonnleithner C. Corneal higher-order aberrations after cataract surgery: manual phacoemulsification versus femtosecond-laser assisted technique. Eur J Ophthalmol. 2021;31(6):2955–2961. doi: 10.1177/1120672121990611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Visser N, Berendschot TTJM, Verbakel F, et al. Evaluation of the comparability and repeatability of four wavefront aberrometers. Invest Ophthalmol Vis Sci. 2011;52(3):1302–1311. doi: 10.1167/iovs.10-5841. [DOI] [PubMed] [Google Scholar]
- 19.Wang W, Ni S, Li X, Chen X, Zhu YN, Xu W. Femtosecond laser-assisted cataract surgery with implantation of a diffractive trifocal intraocular lens after laser in situ keratomileusis: a case report. BMC Ophthalmol. 2018;18(1):160. doi: 10.1186/s12886-018-0834-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donmez O, Asena BS, Aydin Akova Y. Subjective and objective clinical outcomes of a new trifocal toric intraocular lens and effect of femtosecond laser cataract surgery. Eur J Ophthalmol. 2022;32(4):2225–2233. doi: 10.1177/11206721211046496. [DOI] [PubMed] [Google Scholar]
- 21.Teshigawara T, Meguro A, Yabuki K, Hata S, Mizuki N. Visual performance of the intraindividual implantation of a trifocal intraocular lens in the bag and a +4.0 D bifocal intraocular lens in the sulcus with optic capture created by femtosecond laser-assisted cataract surgery. Int Med Case Rep J. 2018;11:251–257. doi: 10.2147/IMCRJ.S176095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lapid-Gortzak R, Bhatt U, Sanchez JG, et al. Multicenter visual outcomes comparison of 2 trifocal presbyopia-correcting IOLs: 6-month postoperative results. J Cataract Refract Surg. 2020;46(11):1534–1542. doi: 10.1097/j.jcrs.0000000000000274. [DOI] [PubMed] [Google Scholar]
- 23.Shen JY, Cai L, Zhuo BX, Abulimiti A, Ni S, Zhang LM, Guo HK, Chen X, Yang J. Binocular visual outcomes comparison of two trifocal intraocular lenses in high-myopic cataract patients: a 1-year multicenter study. Am J Ophthalmol. 2023;254:1–10. doi: 10.1016/j.ajo.2023.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Velasco-Barona C, Corredor-Ortega C, Mendez-Leon A, Casillas-Chavarín NL, Valdepeña-López Velarde D, Cervantes-Coste G, Malacara-Hernández D, Gonzalez-Salinas R. Influence of angle κ and higher-order aberrations on visual quality employing two diffractive trifocal IOLs. J Ophthalmol. 2019;2019:7018937. doi: 10.1155/2019/7018937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kohnen T. First implantation of a diffractive quadrafocal (trifocal) intraocular lens. J Cataract Refract Surg. 2015;41(10):2330–2332. doi: 10.1016/j.jcrs.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 26.Liu H, Li FF, Xia HJ, Zhou J. Visual quality after implantation of trifocal intraocular lenses in highly myopic eyes with different axial lengths. Int J Ophthalmol. 2021;14(3):371–377. doi: 10.18240/ijo.2021.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNamara P, Hutchinson I, Thornell E, Batterham M, Iloski V, Agarwal S. Refractive stability following uncomplicated cataract surgery. Clin Exp Optom. 2019;102(2):154–159. doi: 10.1111/cxo.12837. [DOI] [PubMed] [Google Scholar]
- 28.Khan AM, Waldner DM, Luong M, Sanders E, Crichton ACS, Ford BA. Stabilization of refractive error and associated factors following small incision phacoemulsification cataract surgery. BMC Ophthalmol. 2022;22(1):13. doi: 10.1186/s12886-021-02221-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Charlesworth E, Alderson AJ, de Juan V, Elliott DB. When is refraction stable following routine cataract surgery? A systematic review and meta-analysis. Ophthalmic Physiol Opt. 2020;40(5):531–539. doi: 10.1111/opo.12719. [DOI] [PubMed] [Google Scholar]
- 30.Ma JL, Sun XQ, Liu Y, Liu YM. Observation of visual quality after femtosecond laser-assisted cataract surgery combined with trifocal intraocular lens implantation. Comput Math Methods Med. 2022;2022:1519416. doi: 10.1155/2022/1519416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Roda JA, Vilaseca M, Ondategui JC, Giner A, Burgos FJ, Cardona G, Pujol J. Optical quality and intraocular scattering in a healthy young population. Clin Exp Optom. 2011;94(2):223–229. doi: 10.1111/j.1444-0938.2010.00535.x. [DOI] [PubMed] [Google Scholar]
- 32.Shen JY, Zhang LM, Ni S, Cai L, Guo HK, Yang J. Comparison of visual outcomes and quality of life in patients with high myopic cataract after implantation of AT LISA tri 839MP and LS-313 MF30 intraocular lenses. J Ophthalmol. 2022;2022:5645752. doi: 10.1155/2022/5645752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bobroff N, Rosenbluth AE. Evaluation of highly corrected optics by measurement of the Strehl ratio. Appl Opt. 1992;31(10):1523–1536. doi: 10.1364/AO.31.001523. [DOI] [PubMed] [Google Scholar]
- 34.Alio JL, D'Oria F, Toto F, Balgos J, Palazon A, Versaci F, Alio Del Barrio JL. Retinal image quality with multifocal, EDoF, and accommodative intraocular lenses as studied by pyramidal aberrometry. Eye Vis. 2021;8(1):37. doi: 10.1186/s40662-021-00258-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valle P, Oti J, Canales V, Cagigal M. Visual axial PSF of diffractive trifocal lenses. Opt Express. 2005;13(7):2782–2792. doi: 10.1364/opex.13.002782. [DOI] [PubMed] [Google Scholar]
- 36.Zein El-Dein AA, Elmassry A, El-Hennawi HM, Mossallam EF. Objective and subjective evaluation of trifocal diffractive intraocular Lens after cataract extraction with phacoemulsification: a prospective clinical study. BMC Ophthalmol. 2021;21(1):179. doi: 10.1186/s12886-021-01937-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kretz FTA, Tandogan T, Khoramnia R, Auffarth GU. High order aberration and straylight evaluation after cataract surgery with implantation of an aspheric, aberration correcting monofocal intraocular lens. Int J Ophthalmol. 2015;8(4):736–741. doi: 10.3980/j.issn.2222-3959.2015.04.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vilarrodona L, Barrett GD, Johnson B. High-order aberrations in pseudophakia with different intraocular lenses. J Cataract Refract Surg. 2004;30(3):571–575. doi: 10.1016/j.jcrs.2003.12.041. [DOI] [PubMed] [Google Scholar]
- 39.Xu J, Li WB, Xu Z, Zhao BS, Zhong YY, Wang K, Liu X, Song XH, Yu YH, Zhu YN, Tang QM, Yao K. Comparative visual outcomes of EDOF intraocular lens with FLACS vs conventional phacoemulsification. J Cataract Refract Surg. 2023;49(1):55–61. doi: 10.1097/j.jcrs.0000000000001027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miháltz K, Knorz MC, Alió JL, Takács AI, Kránitz K, Kovács I, Nagy ZZ. Internal aberrations and optical quality after femtosecond laser anterior capsulotomy in cataract surgery. J Refract Surg. 2011;27(10):711–716. doi: 10.3928/1081597X-20110913-01. [DOI] [PubMed] [Google Scholar]